Abstract
Background
The rate of sudden cardiac death in the hemodialysis population exceeds that of the general population by >20‐fold. Hemodialysis patients may be particularly susceptible to sudden cardiac death provoked by drug‐induced QT prolongation because of their substantial cardiovascular disease burden, exposure to electrolyte shifts during dialysis, and extensive polypharmacy. However, population‐specific data regarding the frequency and patterns of QT prolonging medication use are limited.
Methods and Results
We conducted a descriptive drug utilization study using 3 administrative databases, the United States Renal Data System, MarketScan, and Medicare claims. We characterized the extent and patterns of QT prolonging medication use by adult hemodialysis patients and individuals without end‐stage kidney disease annually from 2012 to 2016. We also identified instances of high‐risk QT prolonging medication use among hemodialysis patients. In total, 338 515 hemodialysis patients and 40.7 million individuals without end‐stage kidney disease were studied. Annual utilization rates of QT prolonging medications with known torsades de pointes risk in hemodialysis patients were ~1.4 to ~2.5 times higher than utilization rates in individuals without end‐stage kidney disease. Hemodialysis patients with demographic and clinical risk factors for drug‐induced QT prolongation were exposed to medications with known torsades de pointes risk more often than patients without risk factors.
Conclusions
Hemodialysis patients use QT prolonging medications with known torsades de pointes risk more extensively than individuals without end‐stage kidney disease. Given the widespread use and instances of high‐risk prescribing, future studies evaluating the cardiac safety of these drugs in the hemodialysis population are needed.
Keywords: Hemodialysis, patterns of use, QT prolonging medications
Subject Categories: Epidemiology, Arrhythmias
Nonstandard Abbreviations and Acronyms
- ESKD
end‐stage kidney disease
- SCD
sudden cardiac death
- TdP
torsades de pointes
Clinical Perspective
What is New?
This descriptive drug utilization study shows that hemodialysis patients, a population with an extraordinarily high rate of cardiac arrhythmias and sudden cardiac death, use QT prolonging medications to a much greater extent than similarly aged individuals without end‐stage kidney disease.
Use of QT prolonging medications with known torsades de pointes risk was particularly high among hemodialysis patients at risk for drug‐induced QT prolongation (eg. the elderly, women, and individuals with underlying heart disease), and frequent exposures to potential drug interactions occurred.
What are the Clinical Implications?
Given the widespread use and frequent instances of high‐risk prescribing, future studies evaluating the cardiac safety of QT prolonging drugs in the hemodialysis population are needed.
Sudden cardiac death (SCD) is the leading cause of mortality among individuals receiving maintenance hemodialysis, accounting for ~30% of all deaths.1 SCD typically occurs when a vulnerable myocardium is exposed to a pro‐arrhythmic trigger.2 Structural heart disease is highly prevalent in end‐stage kidney disease (ESKD) and can alter cardiac conduction pathways,3, 4, 5 making the heart more likely to produce fatal arrhythmias when it's exposed to pro‐arrhythmic triggers (eg, medications that prolong the QT interval, electrolyte abnormalities). Unfortunately, traditional preventative therapies, such as prophylactic implantable cardioverter defibrillators, have limited efficacy in hemodialysis patients.6 Therefore, it is of utmost importance to identify modifiable population‐specific SCD risk factors.
Drug‐induced SCD may be preventable. Many medications can induce QT interval prolongation, an electrocardiographic manifestation of delayed ventricular repolarization that increases the risk of rapidly fatal arrhythmias like torsades de pointes (TdP). More than 175 drugs on the United States market have documented QT prolonging effects, including antiarrhythmic and non‐antiarrhythmic agents.7 Data from the general population indicate that QT prolonging medication use associates with a higher risk of SCD,8 especially among individuals with multiple pro‐arrhythmic risk factors and/or existing QT prolongation.9, 10 Hemodialysis patients may be particularly susceptible to drug‐induced arrhythmias and SCD because of their substantial cardiovascular disease burden, recurrent exposure to electrolyte shifts during thrice‐weekly hemodialysis treatments, and extensive polypharmacy, among other factors.11, 12 However, despite studies indicating that 65% to 75% of hemodialysis patients have prolonged QT intervals,13, 14 we lack data on the frequency and patterns of QT prolonging medication use in this vulnerable population.
To address this evidence gap, we aimed to: characterize the (1) extent and (2) patterns of QT prolonging medication use in the hemodialysis population relative to individuals without ESKD; and (3) identify instances of high‐risk QT prolonging medication use among hemodialysis patients.
Methods
Because of contractual data use and licensing agreements, the authors cannot make the data and materials used in this study available to other investigators for the purposes of reproducing results. Interested parties can contact: the United States Renal Data System Coordinating Center to obtain United States Renal Data System data; the Centers for Medicare & Medicaid Services to obtain Medicare data; and Truven Health Analytics to obtain the MarketScan Commercial Claims and Encounters Database.
Study Design and Populations
The University of North Carolina at Chapel Hill Institutional Review Board approved this study (#18‐3175). A waiver of consent was granted because of the study's large size, data anonymity, and retrospective nature.
When information on medication use in a given population is unknown, initial descriptive drug utilization studies help to identify areas requiring more in‐depth evaluation in future investigations (eg, comparative safety and effectiveness studies).15 Thus, we conducted a drug utilization study (Figure S1) to describe the magnitude of prescription QT prolonging medication use by the hemodialysis population on an annual basis from 2012 to 2016. Since medication use is dynamic, we tracked QT prolonging medication use starting from January 1 (the index date) until December 31 in each study year. We defined the baseline period as the 180 days before January 1. To contextualize the observed level of QT prolonging medication use in the hemodialysis population, we also described QT prolonging medication use in individuals without ESKD (ie, the non‐ESKD population) during the same time period using the same study design and approach.
Annual Hemodialysis Cohorts
We used data from the United States Renal Data System, a national ESKD surveillance system, linked with Medicare claims to generate annual cohorts of adult hemodialysis patients from 2012 to 2016. In each study year, we identified individuals aged ≥18 years who received in‐center hemodialysis on January 1 and during the 180‐day baseline period. We excluded patients if they had a dialysis vintage (ie, total time on maintenance dialysis therapy) ≤90 days at the start of baseline and those who lacked continuous insurance enrollment (Medicare Parts A, B, and D) or received hospice care during baseline. We also created annual sub‐cohorts of younger (aged 18–64 years) and older (aged ≥66 years) hemodialysis patients for comparison with similarly aged individuals without ESKD, as further described below.
Annual Non‐ESKD Comparator Cohorts
To facilitate consideration of non‐ESKD comparator cohorts that spanned the adult age range, we used 2 distinct US administrative claims data sources, the MarketScan Commercial Claims and Encounters Database and a 20% random sample of Medicare fee‐for‐service beneficiaries. We generated annual cohorts (2012–2016) of younger and older adults without ESKD (i.e. individuals without a relevant ESKD diagnosis or procedure code during the 180‐day baseline period, Table S1) using Marketscan and Medicare data, respectively. We identified adults without ESKD who met age specifications (18–64 years for Marketscan and ≥66 years for Medicare) on January 1 of each year. In both cohorts, we excluded individuals if they lacked continuous insurance enrollment (commercial medical and prescription coverage for MarketScan, and Medicare Parts A, B, and D for Medicare) or received hospice care during baseline.
Cohort Characterization
Covariates of interest were ascertained during the baseline period and included patient demographics, comorbid conditions, medication use, and metrics of healthcare utilization. Comorbid conditions were considered present if an applicable discharge diagnosis code (located in any billing position) was associated with ≥1 inpatient claim or ≥2 outpatient claims during the 180‐day baseline period (Table S2). Medication use was determined on the last day of the baseline period and polypharmacy was defined as taking ≥5 medications.16 We present the baseline characteristics of the most contemporary (2016) hemodialysis and non‐ESKD cohorts.
QT Prolonging Medication Use
We compiled a comprehensive list of QT prolonging medications using the CredibleMeds website, a reliable online clinical resource with up‐to‐date information about medications that can cause QT prolongation and/or TdP.7 Based upon published literature, medication package inserts, data from the US Food and Drug Administration's Adverse Event Reporting System, and other sources, CredibleMeds classifies QT prolonging medications as having a known, possible, or conditional TdP risk (Table 1 and Table S3).7 In each study year, we used prescription claims data to longitudinally track the daily use of outpatient medications with a known, possible, or conditional TdP risk for each individual in the hemodialysis and non‐ESKD populations.
Table 1.
CredibleMeds Definitions for Medications With Known, Possible, and Conditional TdP Risk
| CredibleMeds Classification7 | Definition |
|---|---|
| Known TdP risk | Drugs that prolong the QT interval and are clearly associated with a known risk of TdP, even when taken as recommended. |
| Possible TdP risk | Drugs that can cause QT prolongation but currently lack evidence for a risk of TdP when taken as recommended. |
| Conditional TdP risk | Drugs that are associated with TdP only under certain conditions (eg, excessive dose, in patients with conditions such as hypokalemia, or when taken with interacting drugs) or drugs that create conditions that facilitate or induce TdP (eg, cause an electrolyte disturbance that induces TdP). |
CredibleMeds classifies medications that can prolong the QT interval as having a known, possible, or conditional TdP risk. Lists of medications falling into each CredibleMeds category are provided in Table S3. TdP indicates torsades de pointes.
To quantify the extent of prescription QT prolonging medication use in each population from 2012 to 2016, we determined the annual rate of exposure to ≥1 QT prolonging medication, overall and by CredibleMeds class (known, possible, or conditional TdP risk). We also conducted a supplemental extent of use analysis excluding QT prolonging thiazide/thiazide‐like diuretics (eg, hydrochlorothiazide, indapamide, metolazone; Table S3) since these agents have limited efficacy in ESKD,17 but are frequently used by individuals without ESKD.18
In addition, we characterized the patterns of QT prolonging medication use in the hemodialysis and non‐ESKD populations by identifying the top 5 medications prescribed in each CredibleMeds class and determining the rate of concurrent (ie, simultaneous) use of medications with known TdP risk with other QT prolonging drugs. Given that QT prolonging medication use was stable across time, these analyses focused on the most contemporary study year, 2016.
Finally, we identified instances of high‐risk QT prolonging medication use in the hemodialysis population, including the use of QT prolonging medications by patients with risk‐factors for drug‐induced QT prolongation and exposure to potential drug interactions. In these analyses, we focused on medications with known TdP risk since these drugs are associated with QT prolongation and TdP when taken as recommended (ie, at typical therapeutic doses).7 Using the 2016 hemodialysis cohort, we determined the rate of exposure to ≥1 medication with known TdP risk among hemodialysis patients with and without demographic and clinical risk factors for drug‐induced QT prolongation (advanced age, female sex, conduction disorder, ischemic heart disease, heart failure, and liver disease).9, 10 Additionally, since concurrent use of multiple QT prolonging medications can lead to more extensive QT prolongation (ie, a potential pharmacodynamic drug interaction),9, 10 we identified medications with known TdP risk that are frequently used together by computing the rate of concurrent use. Finally, concurrent use of a QT prolonging medication with a drug that inhibits its metabolism (ie, a potential pharmacokinetic drug interaction) can raise serum concentrations of the QT prolonging drug, enhancing its arrhythmogenicity.9, 10 Thus, we identified the most commonly prescribed medications with known TdP risk that are major substrates of cytochrome P450 isoenzymes and calculated the rate (95% CI) of concurrent use of these drugs and pertinent cytochrome inhibitors (Table S4).
Statistical Analysis
We described the baseline characteristics of the hemodialysis and non‐ESKD cohorts as mean ± SD or median [quartile 1, quartile 3] for continuous variables and as count (%) for categorical variables. In each annual cohort, individuals were followed forward in historical time from January 1 until December 31 or the occurrence of a censoring event. Censoring events common to both populations included loss of insurance, hospice entry, and death. A change of dialysis modality to home hemodialysis or peritoneal dialysis, kidney transplantation, and recovery of kidney function were additional censoring events for the hemodialysis population, whereas the development of ESKD was an additional censoring event for the non‐ESKD population.
Across all analyses, we calculated medication utilization rates in each annual hemodialysis and non‐ESKD cohort as the: [total # of days exposed / total follow‐up time] and estimated Wald 95% CIs. We expressed the resultant QT prolonging medication utilization rates as the number of days exposed per person year (a descriptor of medication use across time). To facilitate comparisons between the hemodialysis and non‐ESKD populations, we age‐ and sex‐standardized medication utilization rate estimates using standardized mortality ratio weighting.19 In analyses of younger individuals, we standardized estimates to the age and sex distribution of the 2016 younger hemodialysis cohort (aged 18–64 years). In analyses of older individuals, we standardized estimates to the age and sex distribution of the 2016 older hemodialysis cohort (aged ≥66 years). All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Overall, 338 515 hemodialysis patients and 40 663 741 individuals without ESKD were studied. Annual cohort sizes ranged from 96 447 to 102 786 for the younger hemodialysis population; 64 636 to 79 037 for the older hemodialysis population; 13 992 738 to 20 358 190 for the younger non‐ESKD population; and 2 341 761 to 3 134 841 for the older non‐ESKD population (Tables S5, S6 and Figures S2 through S4).
Table 2 displays baseline characteristics of the 2016 hemodialysis and non‐ESKD cohorts. The adult hemodialysis population had a high prevalence of cardiovascular comorbidities, and 80.9% of patients had ≥1 demographic or clinical risk factor for drug‐induced QT prolongation. Cardiac comorbidities, such as arrhythmias, conduction disorders, and heart failure, were more common in hemodialysis patients compared with similarly aged individuals without ESKD. In addition, >50% of the hemodialysis cohort was exposed to polypharmacy, and a higher proportion of these patients were using QT prolonging medications at baseline compared with the non‐ESKD population. For example, 10.4% of younger hemodialysis patients were using ≥1 medication with known TdP risk versus 3.8% of similarly aged individuals without ESKD. Analogous patterns were observed among older individuals.
Table 2.
Baseline Characteristics of the 2016 Hemodialysis and General Population Cohorts
| Characteristic | Younger Adults | Older Adults | All Adults | ||
|---|---|---|---|---|---|
| Hemodialysisn=100 440 | Non‐ESKDn=13 992 738 | Hemodialysisn=79 037 | Non‐ESKDn=3 134 841 | Hemodialysisn=184 573 | |
| Age, y | 51.5±9.8 | 42.3±13.7 | 75.0±6.8 | 75.6±7.5 | 61.9±14.3 |
| Female | 40 824 (40.6%) | 7 362 983 (52.6%) | 40 764 (51.6%) | 1 900 124 (60.6%) | 83 931 (45.5%) |
| Cause of ESKD | |||||
| Diabetes mellitus | 42 905 (42.7%) | … | 38 386 (48.6%) | … | 84 086 (45.6%) |
| Hypertension | 29 093 (29.0%) | … | 25 655 (32.5%) | … | 56 106 (30.4%) |
| Glomerular disease | 14 091 (14.0%) | … | 4974 (6.3%) | … | 19 434 (10.5%) |
| Other | 14 351 (14.3%) | … | 10 022 (12.7%) | … | 24 947 (13.5%) |
| Time on maintenance hemodialysis (years) | 4.45 [2.23, 8.07] | 3.52 [1.67, 6.35] | 4.05 [1.95, 7.25] | ||
| Arrhythmia | 13 003 (12.9%) | 89 762 (0.6%) | 21 269 (26.9%) | 621 170 (19.8%) | 35 282 (19.1%) |
| Conduction disorder | 3153 (3.1%) | 12 058 (0.1%) | 4112 (5.2%) | 144 389 (4.6%) | 7470 (4.0%) |
| Heart failure | 24 119 (24.0%) | 25 771 (0.2%) | 26 548 (33.6%) | 364 289 (11.6%) | 52 223 (28.3%) |
| Ischemic heart disease | 24 438 (24.3%) | 103 103 (0.7%) | 30 323 (38.4%) | 756 559 (24.1%) | 56 525 (30.6%) |
| Chronic liver disease | 6522 (6.5%) | 38 440 (0.3%) | 4594 (5.8%) | 86 025 (2.7%) | 11 454 (6.2%) |
| Has a cardiac pacemaker | 1741 (1.7%) | 4507 (0.0%) | 4671 (5.9%) | 127 477 (4.1%) | 6575 (3.6%) |
| Has an implantable cardiac defibrillator | 1968 (2.0%) | 5412 (0.0%) | 2154 (2.7%) | 42 078 (1.3%) | 4257 (2.3%) |
| # of baseline hospitalizations | |||||
| 0 | 64 382 (64.1%) | 13 660 222 (97.6%) | 50 134 (63.4%) | 2 847 101 (90.8%) | 117 781 (63.8%) |
| 1 | 18 448 (18.4%) | 293 633 (2.1%) | 15 850 (20.1%) | 214 620 (6.8%) | 35 259 (19.1%) |
| ≥2 | 17 610 (17.5%) | 38 883 (0.3%) | 13 053 (16.5%) | 73 121 (2.3%) | 31 533 (17.1%) |
| Polypharmacya | 54 594 (54.4%) | 976 494 (7.0%) | 47 208 (59.7%) | 1 200 903 (38.3%) | 104 856 (56.8%) |
| # of medications used with any level of TdP riskb | |||||
| 0 | 53 430 (53.2%) | 11 393 516 (81.4%) | 35 609 (45.1%) | 1 489 263 (47.5%) | 91 454 (49.5%) |
| 1 | 27 919 (27.8%) | 1 972 503 (14.1%) | 24 645 (31.2%) | 982 888 (31.4%) | 54 146 (29.3%) |
| ≥2 | 19 091 (19.0%) | 626 719 (4.5%) | 18 783 (23.8%) | 662 691 (21.1%) | 38 973 (21.1%) |
| Use of ≥1 medication with known TdP riskb | 10 493 (10.4%) | 538 546 (3.8%) | 12 655 (16.0%) | 367 736 (11.7%) | 23 818 (12.9%) |
| Use of ≥1 medication with possible TdP riskb | 8980 (8.9%) | 427 635 (3.1%) | 7928 (10.0%) | 294 176 (9.4%) | 17 385 (9.4%) |
| Use of ≥1 medication with conditional TdP riskb | 39 759 (39.6%) | 1 992 865 (14.2%) | 36 160 (45.8%) | 1 410 190 (45.0%) | 78 186 (42.4%) |
Values given are mean±SD or median [quartile 1, quartile 3] for continuous variables and as count (%) for categorical variables. ESKD indicates end‐stage kidney disease; and TdP, torsades de pointes.
Polypharmacy was defined as taking 5 or more medications.16
CredibleMeds classifies medications that can prolong the QT interval as having a known, possible, or conditional TdP risk. Corresponding definitions are provided in Table 1 and lists of medications falling into each category are provided in Table S3. Medications classified as having any level of TdP risk are those falling into any of the 3 CredibleMeds categories.
The extent and patterns of QT prolonging medication use differed between the hemodialysis and non‐ESKD populations. In each study year (2012–2016), annual standardized rates of exposure to ≥1 QT prolonging medication in any CredibleMeds class as well as those with known and possible TdP risk (separately) were higher in younger and older hemodialysis patients compared with similarly aged individuals without ESKD Figure 1 and Tables S7 through S9). However, the magnitude of these population‐specific differences in utilization rates varied across age groups. For example, in 2016, the standardized rate (95% CI) of exposure to ≥1 QT prolonging medication with known TdP risk in the younger hemodialysis population was 2.5 times higher than that of the younger non‐ESKD population (38.6 [37.8–39.4] versus 15.4 [15.0–15.9] days exposed per person‐year), and the rate in the older hemodialysis population was 1.4 times higher than that of the older non‐ESKD population (58.3 [57.2–59.4] versus 42.0 [41.1–42.9] days exposed per person‐year). The use of QT prolonging medications with conditional TdP risk in the hemodialysis population relative to the non‐ESKD population varied by age group (Figure 1 and Tables S7 through S9). Annual standardized rates of exposure to ≥1 medication with conditional TdP risk was higher in younger hemodialysis patients compared with similarly aged individuals without ESKD. In contrast, rates of exposure to ≥1 medication with conditional TdP risk were similar in the older hemodialysis and non‐ESKD populations. However, when thiazide/thiazide‐like diuretics were excluded, annual rates of exposure to ≥1 medication with conditional TdP risk were 1.4 to 1.5 times higher in older hemodialysis patients compared with similarly aged individuals without ESKD (Figure S5 and Tables S10 through S12).
Figure 1. Use of ≥1 prescription QT prolonging medication by the hemodialysis and non‐ESKD populations, 2012 to 2016.

A and B, Depict annual standardized rates of exposure to ≥1 QT prolonging medication in the younger hemodialysis and non‐ESKD populations, respectively. C and D, Depict analogous annual rates of QT prolonging medication exposure in the older hemodialysis and non‐ESKD populations. CredibleMeds classifies medications that can prolong the QT interval as having a known, possible, or conditional TdP risk. Corresponding definitions are provided in Table 1 and lists of medications falling into each category are provided in Table S3. Medications classified as having any TdP risk are those falling into any of the 3 CredibleMeds categories. ESKD indicates end‐stage kidney disease; and TdP, torsades de pointes.
Table 3 and Table S13 display the top 5 medications with known, possible, and conditional TdP risk used by the hemodialysis and non‐ESKD populations in 2016. Overall, non‐antiarrhythmic QT prolonging medications, including psychotropics, antiemetics, antibiotics, diuretics, and acid suppressants, were frequently used by the hemodialysis and non‐ESKD populations. However, the individual drugs comprising the top 5 medications with known, possible, and conditional TdP risk used and their respective rankings differed. For example, omeprazole was the top medication with conditional TdP risk used by younger and older hemodialysis patients. In contrast, hydrochlorothiazide was the top medication used by similarly aged individuals without ESKD.
Table 3.
Top 5 Medications in Each CredibleMeds Class Used by the Hemodialysis and General Populations in 2016
| Younger Adults | Older Adults | All Adults | |||
|---|---|---|---|---|---|
| Hemodialysis | Non‐ESKD | Hemodialysis | Non‐ESKD | Hemodialysis | |
| Known TdP risk | |||||
| 1 | Citalopram | Escitalopram | Amiodarone | Citalopram | Amiodarone |
| 2 | Escitalopram | Citalopram | Citalopram | Donepezil | Citalopram |
| 3 | Amiodarone | Azithromycin | Donepezil | Escitalopram | Escitalopram |
| 4 | Ondansetron | Ondansetron | Escitalopram | Amiodarone | Ondansetron |
| 5 | Levofloxacin | Ciprofloxacin | Ondansetron | Sotalol | Donepezil |
| Possible TdP risk | |||||
| 1 | Tramadol | Venlafaxine | Tramadol | Tramadol | Tramadol |
| 2 | Mirtazapine | Tramadol | Mirtazapine | Memantine | Mirtazapine |
| 3 | Promethazine | Tizanidine | Memantine | Mirtazapine | Venlafaxine |
| 4 | Venlafaxine | Aripiprazole | Venlafaxine | Venlafaxine | Promethazine |
| 5 | Tizanidine | Nortriptyline | Risperidone | Risperidone | Risperidone |
| Conditional TdP risk | |||||
| 1 | Omeprazole | Hydrochlorothiazide | Omeprazole | Hydrochlorothiazide | Omeprazole |
| 2 | Pantoprazole | Omeprazole | Pantoprazole | Omeprazole | Pantoprazole |
| 3 | Furosemide | Sertraline | Furosemide | Furosemide | Furosemide |
| 4 | Sertraline | Pantoprazole | Sertraline | Pantoprazole | Sertraline |
| 5 | Esomeprazole | Fluoxetine | Famotidine | Sertraline | Esomeprazole |
CredibleMeds classifies medications that can prolong the QT interval as having a known, possible. or conditional TdP risk. Corresponding definitions are provided in Table 1 and lists of medications falling into each category are provided in Table S3. Corresponding medication utilization rates (days exposed per person‐year) for each QT prolonging drug are presented in Table S13. ESKD indicates end‐stage kidney disease; and TdP, torsades de pointes.
Table 4 shows standardized rates (95% CIs) of concurrent exposure to ≥2 QT prolonging medications in the 2016 hemodialysis and non‐ESKD cohorts. Hemodialysis patients used multiple QT prolonging medications more often. For example, the standardized rate (95% CI) of concurrent exposure to ≥2 distinct medications with known TdP risk among younger hemodialysis patients was 7 times higher that of similarly aged individuals without ESKD (2.8 [2.6–3.0] versus 0.4 [0.3–0.5] days exposed per person‐year). Comparable utilization patterns were seen among older individuals, albeit of lower magnitude.
Table 4.
Concurrent Use of Prescription Medications With Known TdP Risk and Other Drugs That Can Prolong the QT Interval by the Hemodialysis and General Populations in 2016
| Medication Combinations | Younger Adults | Older Adults | All Adults | ||
|---|---|---|---|---|---|
| Hemodialysis n=100 440 | Non‐ESKD n=13 992 738 | Hemodialysis n=79 037 | Non‐ESKD n=3 134 841 | Hemodialysis n=184 573 | |
| Known TdP risk+Any TdP risk | 25.6 (25.0–26.2) | 6.5 (6.2–6.9) | 39.6 (38.7–40.5) | 28.5 (27.8–29.2) | 31.6 (31.1–32.2) |
| Known TdP risk+Known TdP risk | 2.8 (2.6–3.0) | 0.4 (0.3–0.5) | 5.4 (5.1–5.7) | 3.3 (3.1–3.6) | 3.9 (3.7–4.1) |
| Known TdP risk+Possible TdP risk | 6.7 (6.4–7.0) | 1.4 (1.2–1.5) | 10.3 (9.9–10.8) | 9.0 (8.6–9.4) | 8.2 (8.0–8.5) |
| Known risk TdP+Conditional TdP risk | 22.8 (22.2–23.4) | 5.7 (5.4–6.0) | 34.9 (34.1–35.8) | 24.6 (23.9–25.2) | 28.0 (27.6–28.5) |
Values presented are standardized rates (95% CIs) of exposure to specific medication combinations (ie, rates of exposure to ≥2 QT prolonging medications) in 2016 and are expressed as the number of days exposed per person‐year. CredibleMeds classifies medications that can prolong the QT interval as having a known, possible, or conditional TdP risk. Corresponding definitions are provided in Table 1 and lists of medications in each category are provided in Table S3. Medications classified as having any TdP risk are those in any of the 3 CredibleMeds categories. ESKD indicates end‐stage kidney disease; and TdP, torsades de pointes.
Among adults with hemodialysis dependent ESKD, several high‐risk patterns of QT prolonging medication use were identified. In 2016, hemodialysis patients with risk factors for drug‐induced QT prolongation (eg, advanced age, female sex, heart failure) were exposed to medications with known TdP risk more often than those without such risk factors (Figure 2 and Table S14). The observed subgroup utilization patterns were consistent when we excluded antiarrhythmic medications. In addition, the hemodialysis population was exposed to potential drug interactions involving QT prolonging medications. Concurrent use of multiple medications with known TdP risk (Figure 3 and Table S15), as well as concurrent use of medications known TdP risk and metabolic inhibitors (Figure 4 and Table S16) occurred. Citalopram and escitalopram were the QT prolonging medications with known TdP risk most frequently involved in potential pharmacodynamic and pharmacokinetic drug interactions. With regard to potential pharmacokinetic interactions, proton pump inhibitors, including omeprazole, pantoprazole, and esomeprazole, were the most common cytochrome 2C19 inhibitors used with citalopram and escitalopram.
Figure 2. Use of ≥1 prescription medication with known TdP risk by hemodialysis patients with and without risk factors for drug‐induced QT prolongation in 2016.

A, Depicts standardized rates of exposure to ≥1 medication with known TdP risk by hemodialysis patients with and without risk factors for drug‐induced QT prolongation. B, Depicts analogous rates of exposure to ≥1 non‐antiarrhythmic medication with known TdP risk in each subgroup. Medications with known TdP risk are listed in Table S3. Advanced age was defined as ≥65 years of age.9 TdP indicates torsades de pointes.
Figure 3. Concurrent use of medications with known TdP risk by the 2016 hemodialysis population.

Values presented are crude rates of exposure to a given medication combination expressed as the number of days exposed per person‐year. TdP indicates torsades de pointes.
Figure 4. Concurrent use of CYP metabolized medications with known risk TdP risk and relevant metabolic inhibitors by the 2016 hemodialysis population.
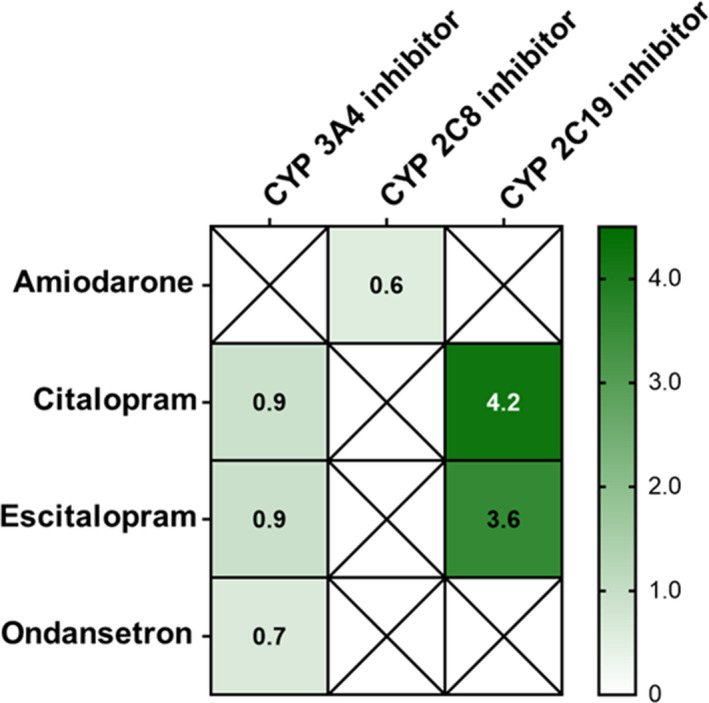
Values presented are crude rates of exposure to a given medication combination expressed as the number of days exposed per person‐year. Of the top 5 medications with a known TdP risk used by the adult hemodialysis population, amiodarone, citalopram, escitalopram, and ondansetron are major substrates of cytochrome isoenzymes. An “X” on the figure indicates that the QT prolonging medication is not a major substrate of specified CYP isoenzyme. Relevant CYP inhibitors are listed in Table S4. CYP indicates cytochrome P450; and TdP, torsades de pointes.
Discussion
Our study demonstrated that hemodialysis patients, a population with an extraordinarily high rate of cardiac arrhythmias20 and SCD,1 utilize medications with known and possible TdP risk to a much greater extent than similarly aged individuals in the non‐ESKD population. The use of QT prolonging medications with known TdP risk was particularly high in hemodialysis patient subgroups at risk for drug‐induced QT prolongation such as the elderly, women, and individuals with underlying heart failure and ischemic heart disease. Moreover, there were frequent exposures to potential drug interactions. These findings raise concerns about high‐risk and potentially unsafe prescribing patterns in the hemodialysis population. In many instances, QT prolonging medications have comparable therapeutic alternatives without QT prolonging effects, rendering alternative, and potentially safer prescribing decisions possible. Our findings underscore the need for additional research assessing the comparative safety of QT prolonging medications in the hemodialysis population.
Between the late 1980s and the early 2000s, the Food and Drug Administration removed several non‐antiarrhythmic drugs (eg, terfenadine, astemizole, cisapride) from the US market because of pro‐arrhythmic concerns, specifically an increased risk of TdP and SCD.10, 21 Since then, the Food and Drug Administration has required sponsors to conduct in vitro and in vivo experiments22 as well as clinical assessments in humans23 to evaluate and define a medication's pro‐arrhythmic potential before regulatory approval. For all new drugs with systemic bioavailability, the Food and Drug Administration requires sponsors to conduct a thorough QT/QTc study, a randomized, placebo‐ and positive‐controlled trial to determine if a drug can induce QT prolongation at therapeutic and/or supratherapeutic doses.23 However, while informative, these studies are typically conducted in healthy volunteers, and their findings may not generalize to individuals with baseline cardiovascular vulnerability. Data from the general population support this notion. The presence of pro‐arrhythmic risk factors, such as advanced age, female sex, left ventricular hypertrophy, and prior QT interval prolongation, augments the QT prolonging effects of medications with known TdP risk.24 In fact, at least 1 such risk factor was present in >90% of drug‐induced TdP cases reported in the literature.25 Therefore, it is likely that the extent of drug‐induced QT prolongation is more pronounced in populations with multiple pro‐arrhythmic risk factors, such as hemodialysis patients.
Existing cardiac safety warnings on product labels26, 27 as well as a scientific statement from the American Heart Association/American College of Cardiology28 call attention to patient populations at heightened risk for drug‐induced QT prolongation, TdP, and SCD. However, these warnings do not specifically identify hemodialysis patients. The hemodialysis population carries a substantial cardiac burden. We found that >80% of hemodialysis patients had at least 1 demographic (advanced age, female sex) or clinical (arrhythmia, conduction disorder, heart failure, ischemic heart disease, liver disease) risk factor for drug‐induced QT prolongation. In addition, we found that hemodialysis patients with such risk factors were exposed to drugs with known TdP risk more often than those without. While it is certainly possible that prescribing clinicians determined that the therapeutic benefits of QT prolonging medications outweighed potential pro‐arrhythmic risks, prior studies indicate that medical providers often have limited knowledge about drugs with QT liability and associated risk factors.29, 30, 31 Thus, future investigations are needed to determine the frequency of inappropriate QT prolonging medication prescribing among high‐risk hemodialysis patients and to identify effective interventions to promote safer prescribing practices.
Hemodialysis patients are clinically complex, and on average, require 10 to 12 medications per day to manage multiple comorbid conditions.32 Such extensive polypharmacy increases the likelihood that drug interactions and adverse drug events will occur. Pharmacodynamic and pharmacokinetic drug interactions involving QT prolonging medications can result in more profound QT interval lengthening, increasing the risk of TdP and SCD.9, 10 We found that the hemodialysis population had a higher prevalence of polypharmacy and used multiple QT prolonging medications more often than the general population. Notably, among hemodialysis patients, the antidepressants citalopram and escitalopram were the medications with known TdP risk most frequently involved in potential pharmacodynamic and pharmacokinetic drug interactions. Exposure to such drug interactions may have devastating consequences. Recent pharmacoepidemiologic studies indicate that the risk of SCD associated with citalopram and escitalopram therapy is more pronounced in the setting of concurrent QT prolonging medication and metabolic inhibitor use.12, 33
The hemodialysis population experiences an overwhelmingly high rate of SCD, which exceeds that of the general population by 20‐ to 30‐fold.34 To date, efforts to identify modifiable SCD risk factors have mainly focused on the dialysis procedure.35 Despite sound biologic plausibility, the potential role of drug‐induced QT prolongation in SCD among hemodialysis patients has been underappreciated.36 Given the widespread use of QT prolonging medications in the hemodialysis population as well as their broad range of clinical indications (eg, depression, infections, nausea/vomiting), future large‐scale cardiac safety studies are needed to assess the association between specific QT prolonging drugs, therapeutic alternatives, and clinical outcomes, such as sudden cardiac death.
Our findings should be considered within the context of study limitations. First, our data sources do not capture prescription medications purchased without insurance or over‐the‐counter medications, and thus may underestimate the frequency of QT prolonging medication use. Second, laboratory parameters, including serum electrolytes, were not available, precluding evaluation of QT prolonging medication use among hemodialysis patients with various electrolyte abnormalities associated with QT prolongation and TdP. Third, information on QT interval measurements from ECGs was not available, and thus, we were unable to determine if drug‐induced QT prolongation occurred. Finally, this was a drug utilization study. Our analyses focused on patterns of medication use and did not investigate potential associations between QT prolonging drug use and clinical outcomes such as SCD.
Conclusions
Our study establishes that hemodialysis patients use QT prolonging medications with known and possible TdP risk to a greater extent than individuals without ESKD. Non‐antiarrhythmic drugs (eg, psychotropics, antiemetics, acid suppressants) were the most commonly prescribed agents. Our findings highlight high‐risk and potentially unsafe prescribing patterns, underscoring the need for future studies evaluating the cardiac safety of QT prolonging medications, especially non‐antiarrhythmic agents, in the clinically complex hemodialysis population.
Sources of Funding
The project described was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Award Number UL1 TR002489. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. M.M.A. and J.E.F. are supported grant R03 HS026801 awarded by the Agency for Healthcare Research and Quality. M.M.A., L.W., P.H.P., W.C.W., and J.E.F. are supported by grant R01 HL152034 awarded by the National Heart, Lung, and Blood Institute of the National Institutes of Health. J.E.F. is supported by grant K23 DK109401 awarded by the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH. The database infrastructure (MarketScan and Medicare data) used for this project was funded by the Department of Epidemiology, University of North Carolina Gillings School of Global Public Health; the Cecil G. Sheps Center for Health Services Research; the Comparative Effectiveness Research Strategic Initiative of University of North Carolina's Clinical and Translational Science Award (UL1 TR002489); and the University of North Carolina School of Medicine.
Disclosures
M.M.A. has received investigator‐initiated research funding from the Renal Research Institute, a subsidiary of Fresenius Medical Care, North America and honoraria from the International Society of Nephrology. P.H.P. has received consulting fees from AstraZeneca, Janssen, and Relypsa. W.C.W. has served as an advisor to and received consulting fees from Akebia, Amgen, AstraZeneca, Bayer, Daichii‐Sankyo, Janssen, Relypsa, Vifor FMC Renal Pharma, and ZS Pharma. J.E.F. has received investigator‐initiated research funding from the Renal Research Institute, a subsidiary of Fresenius Medical Care, North America. In the past 2 years, J.E.F. has received speaking honoraria from American Renal Associates, the American Society of Nephrology, Dialysis Clinic, Inc, the National Kidney Foundation, and multiple universities. J.E.F. is on the medical advisory board of NxStage Medical, Inc and has received consulting fees from Fresenius Medical Care, North America, and AstraZeneca. The remaining authors have no disclosures to report..
Supporting information
Tables S1–S16 Figures S1–S5
Acknowledgments
Some of the data reported here have been provided by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government.
(J Am Heart Assoc. 2020;9:e015969 DOI: 10.1161/JAHA.120.015969.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.015969
For Sources of Funding and Disclosures, see page 11.
References
- 1. Saran R, Robinson B, Abbott KC, Agodoa LYC, Bragg‐Gresham J, Balkrishnan R, Bhave N, Dietrich X, Ding Z, Eggers PW, et al. US Renal data system 2018 Annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2019;A7–A8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;1473–1482. [DOI] [PubMed] [Google Scholar]
- 3. Paoletti E, Specchia C, Di Maio G, Bellino D, Damasio B, Cassottana P, Cannella G. The worsening of left ventricular hypertrophy is the strongest predictor of sudden cardiac death in haemodialysis patients: a 10 year survey. Nephrol Dial Transplant. 2004;1829–1834. [DOI] [PubMed] [Google Scholar]
- 4. Mark PB, Johnston N, Groenning BA, Foster JE, Blyth KG, Martin TN, Steedman T, Dargie HJ, Jardine AG. Redefinition of uremic cardiomyopathy by contrast‐enhanced cardiac magnetic resonance imaging. Kidney Int. 2006;1839–1845. [DOI] [PubMed] [Google Scholar]
- 5. Schietinger BJ, Brammer GM, Wang H, Christopher JM, Kwon KW, Mangrum AJ, Mangrum JM, Kramer CM. Patterns of late gadolinium enhancement in chronic hemodialysis patients. JACC Cardiovasc Imaging. 2008;450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jukema JW, Timal RJ, Rotmans JI, Hensen LCR, Buiten MS, de Bie MK, Putter H, Zwinderman AH, van Erven L, Krol‐van Straaten MJ, et al. Prophylactic use of implantable cardioverter‐defibrillators in the prevention of sudden cardiac death in dialysis patients. Circulation. 2019;2628–2638. [DOI] [PubMed] [Google Scholar]
- 7. Woosley RK, Heise CW, Romero KA. QT Drugs List. AZCERT, Inc. 1822 Innovation Park Dr., Oro Valley, AZ 85755. 2019. Available at: www.Crediblemeds.org. Accessed July 10, 2019.
- 8. Straus SM, Sturkenboom MC, Bleumink GS, Dieleman JP, van der Lei J, de Graeff PA, Kingma JH, Stricker BH. Non‐cardiac QTc‐prolonging drugs and the risk of sudden cardiac death. Eur Heart J. 2005;2007–2012. [DOI] [PubMed] [Google Scholar]
- 9. Trinkley KE, Page RL, Lien H, Yamanouye K, Tisdale JE. Qt interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin. 2013;1719–1726. [DOI] [PubMed] [Google Scholar]
- 10. Turner JR, Rodriguez I, Mantovani E, Gintant G, Kowey PR, Klotzbaugh RJ, Prasad K, Sager PT, Stockbridge N, Strnadova C, et al. Drug‐induced proarrhythmia and torsade de pointes: a primer for students and practitioners of medicine and pharmacy. J Clin Pharmacol. 2018;997–1012. [DOI] [PubMed] [Google Scholar]
- 11. Gussak I, Gussak HM. Sudden cardiac death in nephrology: focus on acquired long qt syndrome. Nephrol Dial Transplant. 2007;12–14. [DOI] [PubMed] [Google Scholar]
- 12. Assimon MM, Brookhart MA, Flythe JE. Comparative cardiac safety of selective serotonin reuptake inhibitors among individuals receiving maintenance hemodialysis. J Am Soc Nephrol. 2019;611–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nie Y, Zou J, Liang Y, Shen B, Liu Z, Cao X, Chen X, Ding X. Electrocardiographic abnormalities and QTc interval in patients undergoing hemodialysis. PLoS ONE. 2016;e0155445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sherif KA, Abo‐Salem E, Panikkath R, Nusrat M, Tuncel M. Cardiac repolarization abnormalities among patients with various stages of chronic kidney disease. Clin Cardiol. 2014;417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elseviers M. Drug utilization research: methods and applications. Chichester, West Sussex Hoboken, NJ: John Wiley & Sons Inc; 2016. [Google Scholar]
- 16. Masnoon N, Shakib S, Kalisch‐Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brater DC. Pharmacology of diuretics. Am J Med Sci. 2000;38–50. [DOI] [PubMed] [Google Scholar]
- 18. Shah SJ, Stafford RS. Current trends of hypertension treatment in the United States. Am J Hypertens. 2017;1008–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tripepi G, Jager KJ, Dekker FW, Zoccali C. Stratification for confounding–part 2: direct and indirect standardization. Nephron Clin Pract. 2010;c322–c325. [DOI] [PubMed] [Google Scholar]
- 20. Roy‐Chaudhury P, Tumlin JA, Koplan BA, Costea AI, Kher V, Williamson D, Pokhariyal S, Charytan DM; on behalf of the MiD investigators and committees . Primary outcomes of the monitoring in dialysis study indicate that clinically significant arrhythmias are common in hemodialysis patients and related to dialytic cycle. Kidney Int. 2018;941–951. [DOI] [PubMed] [Google Scholar]
- 21. Turner JR, Karnad DR, Cabell CH, Kothari S. Recent developments in the science of proarrhythmic cardiac safety of new drugs. Eur Heart J Cardiovasc Pharmacother. 2017;118–124. [DOI] [PubMed] [Google Scholar]
- 22. U. S. Food and Drug administration . Guidance for industry – S7B Nonclinical Evaluation of the Potential for Delayed Ventricular Repolarization (QT Interval Prolongation) by Human Pharmaceuticals. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/s7b-nonclinical-evaluation-potential-delayed-ventricular-repolarization-qt-interval-prolongation. Accessed December 10, 2019. [PubMed]
- 23. U. S. Food and drug administration . E14 Clinical Evaluation of QT/QTc Interval Prolongation and Proarrhythmic Potential for Non‐Antiarrhythmic Drugs. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/e14-clinical-evaluation-qtqtc-interval-prolongation-and-proarrhythmic-potential-non-antiarrhythmic-0. Accessed December 10, 2019.
- 24. Alburikan KA, Aldemerdash A, Savitz ST, Tisdale JE, Whitsel EA, Soliman EZ, Thudium EM, Sueta CA, Kucharska‐Newton AM, Stearns SC, et al. Contribution of medications and risk factors to QTc interval lengthening in the Atherosclerosis Risk in Communities (ARIC) study. J Eval Clin Pract. 2017;1274–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zeltser D, Justo D, Halkin A, Prokhorov V, Heller K, Viskin S. Torsade de pointes due to noncardiac drugs: Most patients have easily identifiable risk factors. Medicine (Baltimore). 2003;282–290. [DOI] [PubMed] [Google Scholar]
- 26. Celexa® (citalopram hydrobromide) [package insert] . Irvine, CA: Allergan USA, Inc; 2017. [Google Scholar]
- 27. Zofran® (ondansetron hydrochloride) [package insert] . East Hanover, NJ: Novartis pharmaceuticals corporation; 2017. [Google Scholar]
- 28. Drew BJ, Ackerman MJ, Funk M, Gibler WB, Kligfield P, Menon V, Philippides GJ, Roden DM, Zareba W; on behalf of the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology, the Council on Cardiovascular Nursing, and the American College of Cardiology Foundation . Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2010;1047–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. LaPointe NM, Al‐Khatib SM, Kramer JM, Califf RM. Knowledge deficits related to the QT interval could affect patient safety. Ann Noninvasive Electrocardiol. 2003;157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Al‐Khatib SM, Allen LaPointe NM, Kramer JM, Chen AY, Hammill BG, Delong L, Califf RM. A survey of health care practitioners’ knowledge of the QT interval. J Gen Intern Med. 2005;392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Buss VH, Lee K, Naunton M, Peterson GM, Kosari S. Identification of patients at‐risk of QT interval prolongation during medication reviews: a missed opportunity? J Clin Med. 2018;533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Manley HJ, Garvin CG, Drayer DK, Reid GM, Bender WL, Neufeld TK, Hebbar S, Muther RS. Medication prescribing patterns in ambulatory haemodialysis patients: comparisons of USRDS to a large not‐for‐profit dialysis provider. Nephrol Dial Transplant. 2004;1842–1848. [DOI] [PubMed] [Google Scholar]
- 33. Wu WT, Tsai CT, Chou YC, Ku PM, Chen YC, You SL, Hung CF, Sun CA. Cardiovascular outcomes associated with clinical use of citalopram and omeprazole: a nationwide population‐based cohort study. J Am Heart Assoc. 2019;e011607. doi: 10.1161/jaha.118.011607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Makar MS, Pun PH. Sudden cardiac death among hemodialysis patients. Am J Kidney Dis. 2017;684–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Turakhia MP, Blankestijn PJ, Carrero JJ, Clase CM, Deo R, Herzog CA, Kasner SE, Passman RS, Pecoits‐Filho R, Reinecke H, et al. Chronic kidney disease and arrhythmias: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J. 2018;2314–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ritz E, Wanner C. The challenge of sudden death in dialysis patients. Clin J Am Soc Nephrol. 2008;920–929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S16 Figures S1–S5


