Abstract
Background
Aortic stenosis (AS) is highly prevalent in patients with atherosclerotic cardiovascular disease. Advanced glycation end products (AGEs) and the receptor for AGEs (RAGE) play a pivotal role for vascular calcification in atherosclerosis. We hypothesize that the AGEs–RAGE axis could also be involved in the pathophysiological mechanism of calcified AS.
Methods and Results
A total of 54 patients with calcified AS who underwent aortic valve replacement were prospectively enrolled from 2014 to 2016 (mean age 75.3±7.7 years). Aortic valve specimens were obtained from 47 patients and 16 deceased control subjects without aortic valve disease (mean age 63.2±14.5 years). The valvular expression of RAGE was evaluated by immunohistochemistry. Serum levels of AGEs and soluble RAGE were measured in 50 patients with calcified AS and 70 age‐matched and sex‐matched control subjects without heart disease. The valvular RAGE expression in patients with calcified AS was higher than controls (P=0.004) and was significantly associated with a decreased ankle‐brachial pressure index (P=0.007) and an increased intima‐media thickness (P=0.026). RAGE and α–smooth muscle actin were coexpressed and were partially costained with osteocalcin and alkaline phosphatase. The serum levels of AGEs and soluble RAGE were significantly higher in the patients with calcified AS than in the controls (P=0.013 and P<0.001, respectively). Soluble RAGE (inversely) and use of aspirin were independently correlated with changes in left ventricular systolic function after aortic valve replacement (P=0.012 and P=0.002, respectively).
Conclusions
Our present study suggests that RAGE may play a role in the pathogenesis of calcified AS, which is a prognostic marker in patients with AS after aortic valve replacement.
Keywords: advanced glycation end products, aortic valve stenosis, atherosclerosis, calcification, inflammation, receptor for advanced glycation end products
Subject Categories: Inflammation, Valvular Heart Disease, Atherosclerosis
Nonstandard Abbreviations and Acronyms
- ∆LVEF
change in left ventricular ejection fraction
- ABI
ankle‐brachial pressure index
- AGEs
advanced glycation end products
- ALP
alkaline phosphatase
- AS
aortic stenosis
- AVR
aortic valve replacement
- CVD
cardiovascular disease
- eGFR
estimate glomerular filtration rate
- IMT
intima‐media thickness
- RAGE
receptor for AGEs
- SMCs
smooth muscle cells
- SMemb
nonmuscle myosin heavy chain
- sRAGE
soluble RAGE
- αSMA
α–smooth muscle actin
Clinical Perspective
What Is New?
Serum levels of advanced glycation end products and the receptor for advanced glycation end products were significantly higher in calcified aortic stenosis patients than those in age‐matched and sex‐matched controls.
The soluble form of the receptor for advanced glycation end product was independently correlated with changes in left ventricular systolic function after aortic valve replacement.
The valvular receptor for advanced glycation end products expression in calcified aortic stenosis patients was significantly higher than that in controls and was associated with decreased ankle‐brachial index and increased carotid intima‐media thickness.
What Are the Clinical Implications?
The receptor for advanced glycation end products may play a role in the pathogenesis of calcified aortic stenosis, which is a prognostic marker in patients with calcified aortic stenosis after surgical valve replacement.
Aortic stenosis (AS) is one of the common cardiovascular diseases (CVD) following coronary artery disease and essential hypertension.1 Although rheumatic fever was a main cause of AS until the 1970 era, the prevalence and incidence of rheumatic fever were remarkably decreased.2 On the other hand, the number of patients with AS with a calcified aortic valve are rapidly increasing as a result of demographic aging.1 In developed countries, >25% of adults older than 65 years and almost 50% in those aged older than 85 years have some degree of AS.1, 3 For a long time, calcified AS has been regarded as a passive degenerative disorder of the aortic valve.4 However, several recent studies have shown that AS has active and multifaced processes during the progression of the calcification of valve leaflets.4 Histopathological changes and pathogenetic pathways in calcified AS resemble those in atherosclerosis.5 Indeed, oxidized lipid retention, inflammatory reaction, and osteoblastic transformation of valve interstitial cells are involved in the pathogenesis of calcified AS.5
Advanced glycation end products (AGEs) are senescent macromolecular derivatives formed during the process of nonenzymatic Maillard reaction.6 A cell surface receptor for AGEs (RAGE) is a signal‐transducing receptor for AGEs that belongs to the immunoglobulin superfamily.7 There is a growing body of evidence that the AGEs–RAGE axis is implicated in various aging‐related disorders, such as CVD, neurodegenerative disease, osteoporosis, and cancer growth and metastasis.8 Indeed, the engagement of RAGE with AGEs elicits oxidative stress generation and evokes inflammatory, thrombotic, and fibrotic reactions in a variety of cells and therefore involved in atherosclerotic CVD.9 Moreover, soluble RAGE (sRAGE) was considered one of the prognostic biomarkers for future cardiovascular events and death in humans.10 However, little is known about the pathological role of the AGEs–RAGE axis in calcified AS. In this study, we addressed the issue of whether the AGEs–RAGE axis could contribute to the pathogenesis of calcified AS and if sRAGE may be a prognostic marker that predicted the improvement of cardiac function after aortic valve replacement in these patients.
METHODS
The authors declare that all supporting results are available within the article.
Subjects
Patients with calcified AS who were hospitalized in Kurume University Hospital for aortic valve replacement (AVR) were prospectively enrolled from July 2014 to June 2016. Patients with Marfan's syndrome, other known connective tissue diseases, or acute or chronic aortic dissection and those undergoing a re‐replacement surgical procedure were excluded from the study. The severity of aortic valve disease was assessed by echocardiography according to the American Heart Association/American College of Cardiology Valvular Heart Disease Guideline with the aortic valve peak velocity ≥4.0 m/s or mean pressure gradient ≥40 mm Hg and aortic valve area ≤1.0 cm2 (or aortic valve area index ≤0.6 cm2/m2).11 The study protocol was approved by the Ethical Committee for the Clinical Research of Kurume University. Written informed consent was obtained from all patients.
Clinical Variables
Medical history, including drug intake and smoking habit, was confirmed by a questionnaire. Blood pressure was measured by an upright standard sphygmomanometer in the sitting position. Vigorous physical activity and smoking were avoided for at least 60 minutes before blood pressure and resting heart rate measurements. Intima‐media thickness (IMT) of the common carotid artery was determined according to a method described previously.12 Ankle‐brachial systolic pressure index (ABI) was measured simultaneously using a validated automatic device (VP‐1000; Colin Corporation, Hayashi, Komaki City, Japan).13 Patients underwent the ABI measurement after resting in the supine position for at least 5 minutes. A comprehensive 2‐dimensional Doppler echocardiographic examination was performed on all patients using commercially available ultrasound equipment (Vivid E90, GE Healthcare, USA) according to the American Society of Echocardiography guidelines. Aortic valve jet velocity was recorded in multiple transducer positions. The envelope of highest jet velocity signal was manually traced to obtain the peak velocity, mean pressure gradient, and velocity time integral. The aortic valve area was measured by the continuity equation. Left ventricular ejection fraction was obtained via the modified biplane Simpson method. Echocardiographic findings were blindly evaluated by 2 experienced sonographers. The European System for Cardiac Operative Risk Evaluation was used to calculate the surgical risk.14
Blood Biochemistry
Blood samples were drawn from the antecubital vein of 50 patients with calcified AS to determine lipid profiles (total cholesterol, high‐density lipoprotein cholesterol, and triglycerides), liver enzymes, uric acid, creatinine, C‐reactive protein, calcium, phosphorus, and NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide). Estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease study equation modified with a Japanese coefficient.15 Serum levels of AGEs were measured using a competitive ELISA as described previously.16 Serum levels of sRAGE and sclerostin were determined with commercially available ELISA kits (R&D Systems, Inc., Minneapolis, MN).17 Other blood chemistry was measured with standard methods at a commercially available laboratory (The Kyodo Igaku Laboratory, Fukuoka, Japan).18 The 70 age‐matched, sex‐matched, and eGFR‐matched subjects without heart disease who visited our hospital for medical health checks were also enrolled as controls. Because renal function affects the serum levels of AGEs and sRAGE,19, 20 we compared those values between the patients with AS and renal function (eGFR)‐matched control subjects.
Histological Evaluation of Aortic Valves
Aortic valve specimens were obtained from the patients with calcified AS and autopsy control subjects. Antemortem echocardiography confirmed that the control subjects had no aortic valve disease. The valve specimens were fixed in neutral buffered formalin and then embedded in paraffin sections. The sections cut to 4 μm were used for light microscopy and Elastica van Gieson and immunohistochemical staining. RAGE, α–smooth muscle actin (αSMA), myosin heavy chain's isozyme (SM2), nonmuscle myosin heavy chain (SMemb), osteocalcin, and alkaline phosphatase (ALP) in calcified aortic valves were evaluated with immunohistochemical or immunofluorescent staining. The primary antibodies used in the present experiments were as follows: anti‐RAGE (SC‐365154, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti‐αSMA (55135‐1‐AP, Proteintech, Rosemont, IL, USA), anti‐osteocalcin (23418‐1‐AP, Proteintech, Rosemont, IL, USA), anti‐ALP (95462, Abcam, Cambridge, UK), anti‐SMemb (7602, Yamasa Corporation, Chiba, Japan/204358, Abcam, Cambridge, UK), and anti‐SM2 (7601, Yamasa Corporation, Chiba, Japan). The nucleus was stained using Mayer's hematoxylin and a fluorescent dye, TO‐PRO3 (T3605, Thermo Fisher Scientific, Waltham, MA, USA). Digital images were obtained by a microscope attached to the imaging software (KEYENCE BZ‐9000, KEYENCE, Japan). The positive rate of RAGE was defined using the following formula: (RAGE positive area÷specimen area)×100.
Statistical Analysis
The values are presented as mean value±SD or median with the interquartile range. The Shapiro–Wilk test was performed to evaluate the assumption of normality. Statistical analysis was performed by means of appropriate parametric and nonparametric methods. Paired t test or chi‐square test was conducted for comparisons between the calcified AS group and the control group. Correlations between AGEs and sRAGE and clinical variables were determined by linear regression analysis. Serial echocardiographic variables are presented as mean (SE). Mixed effect models were employed to examine the effect of time in the echocardiographic variables at baseline and follow‐up. The overall time trend as well as pairwise comparisons were performed. The association of changes in the left ventricular ejection fraction from baseline to 7 days after AVR (ΔLVEF) with clinical parameters at baseline were also analyzed by linear regression analysis. To determine the independent correlates of the serum levels of AGEs, sRAGE, or ΔLVEF, multiple stepwise regression analyses were performed. All multivariate analyses were carried out by following process: Any risk factor with P<0.05 was entered into the multivariate model, and a stepwise procedure was employed to obtain the final model with the inclusion/exclusion criteria and a P value of 0.2. All statistical analyses were performed with the JMP Pro version 13.0 (SAS Institute Inc., Cary, NC, USA).
RESULTS
A total of 54 patients with calcified AS (21 men and 33 women; mean age 75.3±7.7 years) who underwent AVR at our hospital were recruited. The clinical characteristics of the 54 patients with calcified AS and 70 age‐matched, sex‐matched, and eGFR‐matched control subjects are summarized in Table 1. The European System for Cardiac Operative Risk Evaluation was 7.85 (4.97–11.78), and the serum levels of NT‐proBNP were 1100.5 (190.6–5253.9) pg/mL. The serum levels of AGEs and sRAGE were significantly higher in the patients with calcified AS than in the controls. The serum levels of calcium or phosphorus were comparable between the 2 groups, whereas the sclerostin levels in the patients with calcified AS were significantly lower than in the controls. The number of patients with calcified AS who received statin and antihypertensive agents was significantly higher than that of the control subjects. When the calcified AS group was divided into 2 groups, patients with statins and those without, the serum levels of C‐reactive protein were significantly lower in the patients with statins compared with nonusers (0.05 mg/dL versus 0.11 mg/dL; P=0.013). In patients with calcified AS, the ABI levels were significantly lower, whereas the IMT values were larger when compared with controls. Computed tomography revealed that almost all patients had arterial calcification in a coronary artery and another vessel; 75.9% of patients had a calcified lesion in the left coronary artery and 64.8% in the right coronary artery. Calcification was also observed in 92.6% of patients in the aortic arch, 72.2% in the cervical arteries, 77.8% in the abdominal aorta at the level of the renal artery, and 63.5% in the common femoral artery.
Table 1.
Clinical Characteristics of Patients With Calcified AS and Control Subjects
| Variable | Calcified AS (n=54) | Control (n=70) | P Value |
|---|---|---|---|
| Male, n (%) | 21 (38.9) | 39 (55.7) | 0.063 |
| Age, ±SD, y | 75.3±7.7 | 75.4±3.9 | 0.905 |
| Age range, y | 60–93 | 71–84 | ··· |
| Body mass index, ±SD | 22.6±3.9 | 23.0±2.8 | 0.517 |
| NYHA functional class I/II/III/IV, n | 6/36/12/0 | ··· | ··· |
| Heart rate, ±SD, beats/min | 69.5±13.4 | 64.6±12.3 | 0.039a |
| Systolic blood pressure, ±SD, mm Hg | 121.0±23.9 | 139.6±19.3 | <0.001a |
| Diastolic blood pressure, ±SD mm Hg | 66.2±12.1 | 79.4±10.0 | <0.001a |
| Estimate glomerular filtration rate, ±SD, mL/min/1.73 m2 | 56.5±32.5 | 63.8±10.7 | 0.082 |
| EuroSCORE, median (IQR) | 7.85 (4.97–11.78) | ··· | ··· |
| C‐reactive protein, median (IQR), mg/dL | 0.09 (0.04–0.18) | ··· | ··· |
| Calcium, median (IQR), mg/dL | 9.0 (8.8–9.4) | 9.1 (9.0–9.3) | 0.335 |
| Phosphorus, median (IQR), mg/dL | 3.7 (3.2–4.2) | 3.4 (3.0–3.8) | 0.062 |
| Sclerostin, median (IQR), pg/mL | 199.0 (145.8–323.3) | 437.3 (272.1–766.5) | <0.001a |
| Total cholesterol, median (IQR), mg/dL | 178.0 (154.0–198.5) | 196.5 (177.0–221.3) | <0.001a |
| High‐density lipoprotein cholesterol, ±SD, mg/dL | 56.8±13.3 | 56.0±12.9 | 0.766 |
| Triglycerids, median (IQR), mg/dL | 91.0 (76.0–133.0) | 92.5 (71.8–123.5) | 0.740 |
| Glycated hemoglobin, median (IQR) | 5.7 (5.5–6.1) | 5.4 (5.2–5.7) | 0.025a |
| NT‐proBNP, median (IQR), pg/mL | 1100.5 (190.6–5253.9) | 92.9 (45.6–218.9) | <0.001a |
| Advanced glycation end products, median (IQR), μg/mL | 9.93 (8.31–12.19) | 8.32 (7.10–10.06) | 0.013a |
| sRAGE, median (IQR), pg/mL | 1054.0 (640.3–1426.8) | 679.8 (488.3–1021.7) | <0.001a |
| Minimum ankle‐brachial pressure index, ±SD | 1.03±0.18 | 1.11±0.08 | 0.001a |
| Maximum IMT of carotid artery, median (IQR), mm | 1.10 (0.98–1.43) | 0.87 (0.81–0.98) | <0.001a |
| Current smoking, n (%) | 11 (20.4) | 6 (8.6) | 0.058 |
| Hemodialysis, n (%) | 9 (16.7) | 0 | <0.001a |
| Coronary artery disease, n (%) | 20 (37.0) | 0 | <0.001a |
| Medications, n (%) | |||
| Statin use | 26 (48.2) | 20 (28.6) | 0.025a |
| Aspirin use | 15 (27.8) | 13 (18.6) | 0.224 |
| Antihypertensive agents | 42 (77.8) | 36 (51.4) | 0.003a |
| Oral hypoglycemic agents | 9 (16.7) | 7 (10.0) | 0.272 |
| Echocardiographic variables | |||
| Left ventricular ejection fraction, ±SD, % | 62.0±11.1 | 70.4±5.2 | <0.001a |
| Left ventricular diastolic diameter, median (IQR), mm | 44.0 (41.7–49.0) | 46.0 (42.9‐49.0) | 0.817 |
| Left ventricular systolic diameter, median (IQR), mm | 27.9 (25.8–31.0) | 28.0 (24.9‐30.0) | 0.111 |
| Interventricular septal wall thickness, mm | 11.9±2.2 | 9.7±1.3 | <0.001a |
| Posterior wall thickness, mm | 11.7±1.9 | 9.9±1.1 | <0.001a |
| Bicuspid valve, n (%) | 12 (22.2) | ··· | ··· |
| Aortic valve peak velocity, ±SD, m/s | 4.5±1.0 | ··· | ··· |
| Aortic valve peak pressure gradient, median (IQR), mm Hg | 74.0 (60.1–108.2) | ··· | ··· |
| Aortic valve mean pressure gradient, median (IQR), mm Hg | 44.1 (31.8–60.7) | ··· | ··· |
| Aortic valve area, median (IQR), cm2 | 0.73 (0.47–0.88) | ··· | ··· |
AS indicates aortic stenosis; EuroSCORE, European System for Cardiac Operative Risk Evaluation; IMT, intima‐media thickness; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; and sRAGE, soluble receptor for advanced glycation end products.
Statistically significant values.
Correlation of Clinical Variables With Serum AGEs or sRAGE
We first investigated which clinical variables were independently correlated with serum levels of AGEs or sRAGE. In the univariate analysis, body mass index (P=0.015) and glycated hemoglobin (P=0.038) were significantly correlated with serum levels of AGEs (Table 2). Because these significant parameters could be closely correlated with each other, multiple stepwise regression analysis was performed to determine the independent correlates of the serum levels of AGEs (Table 2). As a result, body mass index had an independent association with the serum levels of AGEs (P=0.034, R 2=0.175). As shown in Table 3, univariate analysis revealed that body mass index (inversely), eGFR (inversely), sclerostin, total cholesterol (inversely), glycated hemoglobin (inversely), and NT‐proBNP were associated with the serum levels of sRAGE. Multiple stepwise regression analysis revealed that eGFR was a sole independent correlate of serum sRAGE levels (Table 3; P=0.013, R 2=0.586).
Table 2.
Correlation of Clinical Variables With AGEs
| Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| r | P Value | Estimate | SE | P Value | |
| Sexa | 0.149 | 0.412 | ··· | ··· | ··· |
| Age | 0.002 | 0.989 | ··· | ··· | ··· |
| Body mass index | 0.344b | 0.015b | 0.028b | 0.012b | 0.034b |
| Heart rate | 0.131 | 0.370 | ··· | ··· | ··· |
| Systolic blood pressure | −0.078 | 0.596 | ··· | ··· | ··· |
| Diastolic blood pressure | −0.052 | 0.722 | ··· | ··· | ··· |
| Estimate glomerular filtration rate | 0.132 | 0.361 | ··· | ··· | ··· |
| C‐reactive proteinc | −0.035 | 0.810 | ··· | ··· | ··· |
| Calciumc | −0.039 | 0.787 | ··· | ··· | ··· |
| Phosphorusc | −0.017 | 0.907 | ··· | ··· | ··· |
| Sclerostinc | −0.033 | 0.822 | ··· | ··· | ··· |
| Total cholesterol | 0.040 | 0.782 | ··· | ··· | ··· |
| High‐density lipoprotein cholesterol | −0.164 | 0.260 | ··· | ··· | ··· |
| Triglyceridsc | 0.162 | 0.266 | ··· | ··· | ··· |
| Glycated hemoglobinc | 0.298b | 0.038b | 0.907 | 0.540 | 0.099 |
| NT‐proBNPc | −0.186 | 0.200 | ··· | ··· | ··· |
| Receptor for AGEsc | −0.061 | 0.676 | ··· | ··· | ··· |
| Minimum ankle‐brachial pressure index | 0.014 | 0.925 | ··· | ··· | ··· |
| Maximum IMT of carotid arteryc | −0.036 | 0.819 | ··· | ··· | ··· |
| Current smokinga | −0.283 | 0.165 | ··· | ··· | ··· |
| Coronary artery diseasea | 0.316 | 0.624 | ··· | ··· | ··· |
| Medications | ··· | ··· | ··· | ||
| Statin usea | 0.033 | 0.857 | ··· | ··· | ··· |
| Aspirin usea | −0.147 | 0.451 | ··· | ··· | ··· |
| Antihypertensive agentsa | −0.016 | 0.943 | ··· | ··· | ··· |
| Oral hypoglycemic agentsa | 0.015 | 0.950 | ··· | ··· | ··· |
| Echocardiographic variables | ··· | ··· | ··· | ||
| Left ventricular ejection fraction | 0.076 | 0.599 | ··· | ··· | ··· |
| Left ventricular diastolic diameter | −0.084 | 0.560 | ··· | ··· | ··· |
| Left ventricular systolic diameter | −0.105 | 0.469 | ··· | ··· | ··· |
| Interventricular septal wall thickness | 0.137 | 0.344 | ··· | ··· | ··· |
| Posterior wall thickness | 0.137 | 0.344 | ··· | ··· | ··· |
| Number of aortic valve | 0.216 | 0.329 | ··· | ··· | ··· |
| Aortic valve peak velocity | 0.091 | 0.528 | ··· | ··· | ··· |
| Aortic valve peak pressure gradientc | 0.089 | 0.538 | ··· | ··· | ··· |
| Aortic valve mean pressure gradientc | −0.004 | 0.978 | ··· | ··· | ··· |
| Aortic valve areac | −0.048 | 0.742 | ··· | ··· | ··· |
R 2=0.175. AGEs indicates advanced glycation end products; IMT, intima‐media thickness; and NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Male=0, female=1 or no=0, yes=1.
Statistically significant values.
Log‐transformed value was used.
Table 3.
Correlation of Clinical Variables With sRAGE
| Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| r | P Value | Estimate | SE | P Value | |
| Sexa | −0.071 | 0.691 | ··· | ··· | ··· |
| Age | −0.064 | 0.659 | ··· | ··· | ··· |
| Body mass index | −0.412b | 0.003b | −0.035 | 0.017 | 0.052 |
| Heart rate | 0.131 | 0.370 | ··· | ··· | ··· |
| Systolic blood pressure | −0.132 | 0.365 | ··· | ··· | ··· |
| Diastolic blood pressure | −0.165 | 0.258 | ··· | ··· | ··· |
| Estimate glomerular filtration rate | −0.699b | <0.001b | −0.008b | 0.003b | 0.013b |
| C‐reactive proteinc | 0.188 | 0.192 | ··· | ··· | ··· |
| Calciumc | 0.100 | 0.489 | ··· | ··· | ··· |
| Phosphorusc | −0.088 | 0.543 | ··· | ··· | ··· |
| Sclerostinc | 0.434b | 0.002b | 0.123 | 0.132 | 0.358 |
| Total cholesterol | −0.368b | 0.009b | −0.002 | 0.002 | 0.390 |
| High‐density lipoprotein cholesterol | 0.045 | 0.761 | ··· | ··· | ··· |
| Triglyceridsc | −0.252 | 0.080 | ··· | ··· | ··· |
| Glycated hemoglobinc | −0.386b | 0.006b | −0.666 | 0.780 | 0.398 |
| NT‐proBNPc | 0.543b | <0.001b | 0.047 | 0.037 | 0.211 |
| Advanced glycation end productsc | −0.061 | 0.676 | ··· | ··· | ··· |
| Minimum ankle‐brachial pressure index | 0.039 | 0.793 | ··· | ··· | ··· |
| Maximum IMT of carotid arteryc | −0.061 | 0.692 | ··· | ··· | ··· |
| Current smokinga | 0.040 | 0.836 | ··· | ··· | ··· |
| Coronary artery diseasea | 0.093 | 0.523 | ··· | ··· | ··· |
| Medications | ··· | ··· | ··· | ||
| Statin usea | 0.097 | 0.581 | ··· | ··· | ··· |
| Aspirin usea | −0.051 | 0.804 | ··· | ··· | ··· |
| Antihypertensive agentsa | −0.053 | 0.795 | ··· | ··· | ··· |
| Oral hypoglycemic agentsa | −0.061 | 0.782 | ··· | ··· | ··· |
| Echocardiographic variables | ··· | ··· | ··· | ||
| Left ventricular ejection fraction | 0.130 | 0.368 | ··· | ··· | ··· |
| Left ventricular diastolic diamete | 0.019 | 0.894 | ··· | ··· | ··· |
| Left ventricular systolic diameter | −0.108 | 0.457 | ··· | ··· | ··· |
| Interventricular septal wall thickness | −0.017 | 0.906 | ··· | ··· | ··· |
| Posterior wall thickness | 0.013 | 0.928 | ··· | ··· | ··· |
| Number of aortic valve | 0.341 | 0.093 | ··· | ··· | ··· |
| Aortic valve peak velocity | 0.126 | 0.385 | ··· | ··· | ··· |
| Aortic valve peak pressure gradientc | 0.161 | 0.265 | ··· | ··· | ··· |
| Aortic valve mean pressure gradientc | 0.203 | 0.166 | ··· | ··· | ··· |
| Aortic valve areac | 0.005 | 0.975 | ··· | ··· | ··· |
R 2=0.586. IMT indicates intima‐media thickness; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and sRAGE, soluble receptor for advanced glycation end products.
Male=0, female=1 or no=0, yes=1.
Statistically significant values.
Log‐transformed value was used.
Effects of AVR on Cardiac Function
Patients with calcified AS underwent isolated AVR and concomitant other cardiac surgeries. After surgical interventions, no serious perioperative adverse events were observed in our patients. Echocardiographic variables at baseline and follow‐up are shown in Table 4. Seven days after AVR, left ventricular dimensions at the end‐systolic and end‐diastolic phases were significantly decreased from 45.6 (0.9) to 41.2 (0.9) mm (P<0.001) and from 29.4 (0.8) to 26.3 (0.8) mm (P<0.001), respectively. Left ventricular ejection fraction was significantly increased from 62.0% (1.4) at baseline to 66.1% (1.4) at 7 days after AVR (P=0.005) and to 70.1% (1.4) at 3 months after AVR (P<0.001). Similar to the left ventricular dimensions, the left atrial diameter also gradually decreased after AVR (Table 4).
Table 4.
Echocardiographic Variables at Baseline and Follow‐Up
| Variable | Baseline, Mean (SE) | 7 Days After AVR, Mean (SE) | Versus Baseline | 3 Months After AVR, Mean (SE) | Versus Baseline | Overall Time Trend |
|---|---|---|---|---|---|---|
| Left atrial diameter, mm | 42.5 (1.1) | 40.2 (1.1) | 0.004a | 39.5 (1.1) | <0.001a | P<0.001a |
| Interventricular septal wall thickness, mm | 11.9 (0.3) | 11.9 (0.3) | 0.801 | 11.3 (0.3) | 0.042a | P=0.050 |
| Posterior wall thickness, mm | 11.7 (0.3) | 11.9 (0.3) | 0.369 | 11.3 (0.3) | 0.195 | P=0.101 |
| Left ventricular diastolic diameter, mm | 45.6 (0.9) | 41.2 (0.9) | <0.001a | 39.9 (0.9) | <0.001a | P<0.001a |
| Left ventricular systolic diameter, mm | 29.4 (0.8) | 26.3 (0.8) | <0.001a | 24.3 (0.8) | <0.001a | P<0.001a |
| Left ventricular ejection fraction, % | 62.0 (1.4) | 66.1 (1.4) | 0.005a | 70.1 (1.4) | <0.001a | P<0.001a |
AVR indicates aortic valve replacement.
Statistically significant values.
Correlation of Baseline Clinical Variables With ∆LVEF
Next we examined the association of baseline clinical variables with ΔLVEF. As shown in Table 5, univariate analysis revealed that eGFR, phosphorus, total cholesterol, sRAGE (inversely), and use of aspirin were significantly correlated with ∆LVEF values. Phosphorus, sRAGE (inversely), and use of aspirin at baseline still remained significant and were independently correlated with ∆LVEF values (R 2=0.478). There was no significant correlation between the serum levels of AGEs and ΔLVEF.
Table 5.
Correlation of Baseline Clinical Variables With the Change in Left Ventricular Ejection Fraction
| Baseline Variable | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| r | P Value | Estimate | SE | P Value | |
| Sexa | −0.189 | 0.290 | ··· | ··· | ··· |
| Age | −0.170 | 0.228 | ··· | ··· | ··· |
| Body mass index | 0.095 | 0.502 | |||
| Heart rate | −0.028 | 0.846 | ··· | ··· | ··· |
| Systolic blood pressure | 0.012 | 0.936 | ··· | ··· | ··· |
| Diastolic blood pressure | 0.175 | 0.229 | ··· | ··· | ··· |
| Estimate glomerular filtration rate | 0.323b | 0.025b | ··· | ··· | ··· |
| C‐reactive proteinc | 0.054 | 0.715 | ··· | ··· | ··· |
| Calciumc | 0.033 | 0.823 | ··· | ··· | ··· |
| Phosphorusc | 0.380b | 0.008b | 17.717b | 5.964b | 0.005b |
| Sclerostinc | −0.168 | 0.253 | ··· | ··· | ··· |
| Total cholesterol | 0.395b | 0.006b | 0.057 | 0.040 | 0.156 |
| High‐density lipoprotein cholesterol | 0.093 | 0.535 | ··· | ··· | ··· |
| Triglyceridsc | 0.054 | 0.720 | ··· | ··· | ··· |
| Glycated hemoglobinc | 0.043 | 0.775 | ··· | ··· | ··· |
| NT‐proBNPc | −0.170 | 0.254 | ··· | ··· | ··· |
| Advanced glycation end productsc | −0.039 | 0.790 | ··· | ··· | ··· |
| sRAGEc | −0.431b | 0.002b | −5.549b | 2.123b | 0.012b |
| Minimum ankle‐brachial pressure index | 0.017 | 0.905 | ··· | ··· | ··· |
| Maximum IMT of carotid arteryc | 0.231 | 0.132 | ··· | ··· | ··· |
| Current smokinga | 0.031 | 0.873 | ··· | ··· | ··· |
| Coronary artery diseasea | 0.139 | 0.324 | ··· | ··· | ··· |
| Medication, na | |||||
| Statin usea | −0.232 | 0.175 | ··· | ··· | ··· |
| Aspirin usea | 0.415b | 0.025b | −4.646b | 1.374b | 0.002b |
| Antihypertensive agentsa | −0.053 | 0.783 | ··· | ··· | ··· |
| Oral hypoglycemic agentsa | 0.324 | 0.142 | ··· | ··· | ··· |
| Echocardiographic variables | |||||
| Number of aortic valve | −0.289 | 0.134 | ··· | ··· | ··· |
| Aortic valve peak velocity | 0.021 | 0.883 | ··· | ··· | ··· |
| Aortic valve peak pressure gradientc | 0.027 | 0.850 | ··· | ··· | ··· |
| Aortic valve mean pressure gradientc | 0.046 | 0.750 | ··· | ··· | ··· |
| Aortic valve areac | −0.177 | 0.215 | ··· | ··· | ··· |
R 2=0.478. IMT indicates intima‐media thickness; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; and sRAGE, soluble receptor for advanced glycation end products.
Male=0, female=1 or no=0, yes=1.
Statistically significant values.
Log transformed value was used.
RAGE Expression in the Calcified Aortic Valve
Aortic valve specimens from 47 of the 54 patients with calcified AS (18 men and 29 women; mean age 74.9±7.2 years) and 16 autopsy control subjects (11 men and 5 women; mean age 63.2±14.5 years) were evaluated. Elastica van Gieson staining was performed to confirm the structure of the calcified aortic valve leaflet. The aortic valve has the following 3 layers: fibrosa, spongiosa, and ventricularis.4 A large amount of calcium deposition was observed in the aortic side. RAGE was expressed in the calcified aortic valves, which was expressed in all 3 layers, especially in the spongiosa and the ventricularis (Figure 1A). The least square mean of the valvular RAGE‐positive area adjusted for age and sex by using an analysis of covariance was significantly higher in the patients with calcified AS than that in the controls (8.40% versus 2.31%; P=0.004) (Figure 1B) and was correlated with ABI (inversely, r=−0.395, P=0.007) and IMT (r=0.356, P=0.026). In addition, the RAGE‐positive area was significantly lower in patients who received statins than in those without (r=0.298, P=0.042). The calcified aortic valves contained smooth muscle cells (SMCs), which were mainly composed of SMemb‐positive and SM2‐positive cells, markers of synthetic and contractile SMCs, respectively21 (Figure 2A and 2B). An immunofluorescent study revealed that αSMA which is a marker of SMCs22 and RAGE were colocalized (Figure 3A and 3B). In addition, RAGE were costained with ALP, osteocalcin, and SMemb (Figure 3C) in calcified AS.
Figure 1. Expression of RAGE in calcified AS valves.
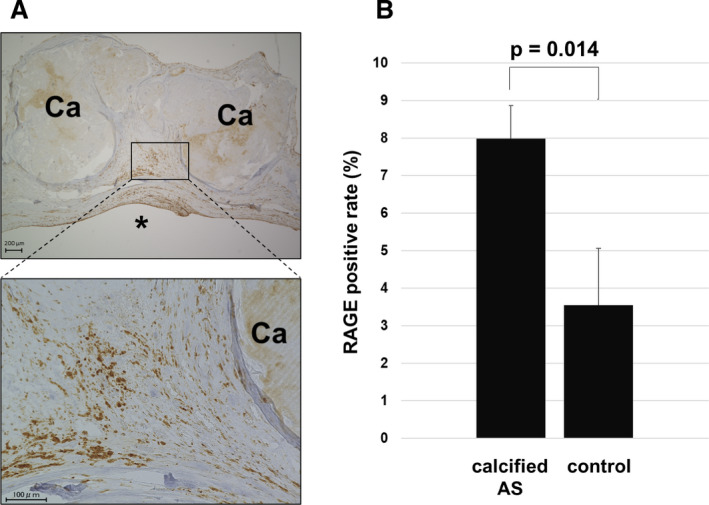
A, Expression of RAGE in calcified AS valves. *Left ventricle side. B, RAGE positive area (percentage) in calcified AS and controls (P=0.014). AS indicates aortic stenosis; Ca, calcification; and RAGE, receptor for advanced glycation end products.
Figure 2. Expression of SMCs in calcified AS valves.
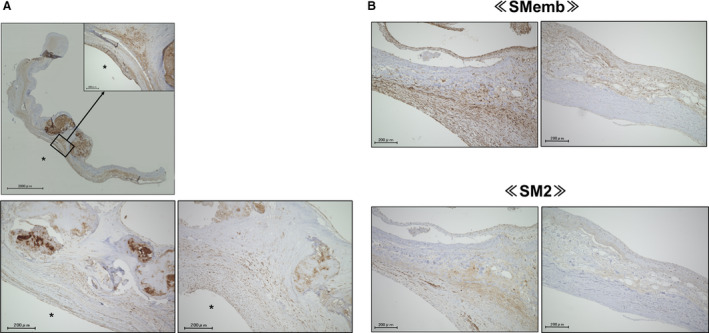
A and B, Calcified aortic valve contained SMCs, which were mainly composed of SMemb‐positive or SM2‐positive cells (n=6). *Left ventricle side. AS indicates aortic stenosis; SM2, myosin heavy chain; SMCs, smooth muscle cells; and SMemb, nonmuscle myosin heavy chain.
Figure 3. αSMA and RAGE were colocalized, part of which were costained with ALP, osteocalcin, and SMemb in calcified aortic stenosis valves.
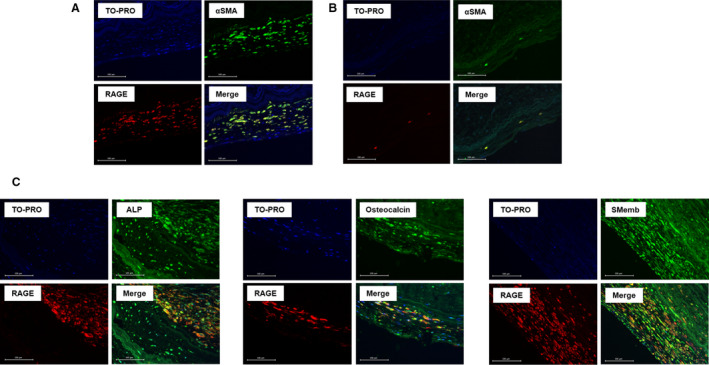
A and B, Immunofluorescence imaging of αSMA and RAGE in aortic stenosis patients (n=6) and controls (n=2). C, Immunofluorescent staining of ALP (n=5), osteocalcin (n=5), and SMemb (n=2) in aortic stenosis valves. TOPRO‐3 was used for nuclear staining. αSMA indicates α–smooth muscle actin; ALP, alkaline phosphatase; RAGE, receptor for advanced glycation end products; and SMemb, nonmuscle myosin heavy chain.
DISCUSSION
The major findings of our study were that (1) serum levels of AGE or sRAGE were significantly higher in patients with calcified AS than in control subjects, (2) low sRAGE values and the use of aspirin at baseline were independently correlated with ∆LVEF, (3) RAGE expression in calcified AS valves was significantly higher than in controls, and (4) RAGE and αSMA were coexpressed in AS tissues, parts of which were positively stained with markers of osteoblasts, such as ALP and osteocalcin.
Serum Levels of AGEs and sRAGE in Patients With Calcified AS
Several cross‐sectional and prospective studies have shown that the serum levels of AGEs were associated with CVD and become a predictor of future cardiovascular events and death.8, 23, 24, 25 In addition, the serum levels of AGEs were associated with endothelial dysfunction, vascular inflammation, and sRAGE in patients high risk for CVD.8, 10, 16, 17, 26, 27 However, there is still some controversy about the clinical significance of sRAGE in humans.10 Some researchers stated that sRAGE may protect against the AGEs‐elicited vascular damage by acting as a decoy because exogenously administered sRAGE may capture and eliminate circulating AGEs.28 On the contrary, other researchers have the opposite opinion. They demonstrated that sRAGE levels were positively associated with serum levels of AGEs, inflammatory biomarkers, and CVD in both diabetic and nondiabetic patients; therefore, it could reflect tissue RAGE expression.8, 10, 29, 30, 31, 32 In support of the latter speculation, several prospective studies have recently shown that higher serum levels of sRAGE could predict future cardiovascular events and death.33, 34 In this study, serum levels of AGEs and sRAGE and valvular RAGE expression were significantly higher in patients with calcified AS compared with controls. Furthermore, we found that low sRAGE at baseline was independently associated with the improvement of the left ventricular ejection fraction after aortic valve replacement. Because early postoperative improvement of the left ventricular ejection fraction was associated with a significant relief of heart failure symptoms and favorable prognosis,35 our present findings suggest that sRAGE may be a marker for AGEs–RAGE axis activation and identify patients with AS who may benefit from valve replacement surgery.
Phenotypic Change of SMCs Within Calcified Aortic Valves
Transdifferentiation of valvular interstitial cells into osteoblastic and myofibroblastic cells is supposed to play a role in the development and progression of valvular calcification in AS.4, 5 Indeed, the expression levels of markers of myofibroblast cells and SMCs in calcified aortic valves are correlated to the severity of calcification in AS.22 In the initial and early phase of atherosclerosis, vascular SMCs undergo a phenotypic change from contractile type to synthetic type in response to a variety of atherogenic stimuli, such as oxidative stress shear stress and inflammatory, cytokine, and growth factors.36 Our present study revealed that SMemb‐positive SMCs, a marker of the synthetic SMCs type, were rich in the calcified aortic valves. Therefore, the phenotypic change of SMCs may also be involved in calcified AS.
RAGE Expression in Calcified Aortic Valves and Atherosclerosis
Carotid artery IMT and ABI are representative surrogate markers that could predict future atherosclerotic cardiovascular events in humans.37, 38 In the present study, valvular RAGE expression in patients with calcified AS was correlated with low ABI and high carotid artery IMT values. In addition, the valvular RAGE expression was significantly lower in patients taking statins than those without. Besides lowering low‐density lipoprotein cholesterol levels, statins have been shown to exhibit anti‐inflammatory properties on vascular wall cells.39 Indeed, the serum levels of C‐reactive protein in patients with statins were significantly lower than those without statins (P=0.013). Because statins delayed the progression of AS,40 statins may have protective effects against AS partly via the suppression of the AGEs–RAGE axis.
Osteogenic Differentiation of SMCs in Calcified Aortic Valves Via RAGE
Engagement of RAGE with AGEs evokes inflammatory and oxidative stress reactions and could promote the osteoblastic differentiation of SMCs, as evidence by ALP, osteopontin, and osteocalcin overexpression.41, 42 Furthermore, RAGE activation in aortic valves has been shown to stimulate the production of proinflammatory cytokines and promote the progression of aortic valve calcification.43, 44 RAGE‐deficient mice were resistant to aortic valve stenosis while on a high‐fat diet.45 In our study, RAGE and αSMA were coexpressed in calcified aortic valves, part of which were costained with ALP and osteocalcin, markers of osteoblasts as well as SMemb. Taken together, although our present study showed a correlation, not causation, activation of the AGEs–RAGE axis may contribute to the development and progression of calcified AS.
Limitations
There are some limitations in this study. First, this was a relatively small study with short observational periods. We could not quantify the immunofluorescence images because of a lack of samples. Second, although age‐matched, sex‐matched, and eGFR‐matched subjects without organic heart disease were used as controls in the present study, many unadjusted factors could confound the present findings. Third, because we could not measure the calcium score, we did not clarify whether the levels of calcification in the valves were consistent among patients. Fourth, the postoperative levels of sRAGE were not measured in this study. Further longitudinal studies are needed to clarify whether the suppression of the AGEs–RAGE axis by statins could actually slow down the process of AS and improve survival in these patients.
CONCLUSIONS
Our present study suggests that RAGE may play a role in the pathogenesis of calcified AS, which is a prognostic marker in patients with calcified AS after surgical valve replacement.
Sources of Funding
This work was supported by research grants from a Grant‐in‐Aid for Scientific Research (Grant 17K16601 to K.S.) from the Japan Society for the Promotion of Science, Tokyo, Japan.
Disclosures
None.
Acknowledgments
We thank Yoko Motomura, Department of Surgery, Kurume University School of Medicine; and Mami Nakayama, Miho Nakao‐Kogure, Katsue Shiramizu, Miyuki Nishikata, and Makiko Kiyohiro, Division of Cardiovascular Medicine, Department of Medicine, Kurume University School of Medicine.
(J Am Heart Assoc. 2020;9:e015261 DOI: 10.1161/JAHA.119.015261.)
For Sources of Funding and Disclosures, see page 11.
References
- 1. Lindman BR, Clavel MA, Mathieu P, Iung B, Lancellotti P, Otto CM, Pibarot P. Calcific aortic stenosis. Nat Rev Dis Primers. 2016;2:16006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carabello BA. Introduction to aortic stenosis. Circ Res. 2013;113:179–185. [DOI] [PubMed] [Google Scholar]
- 3. Supino PG, Borer JS, Preibisz J, Bornstein A. The epidemiology of valvular heart disease: a growing public health problem. Heart Fail Clin. 2006;2:379–393. [DOI] [PubMed] [Google Scholar]
- 4. Rajamannan NM, Evans FJ, Aikawa E, Grande‐Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O'Brien KD, et al. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group, executive summary: calcific aortic valve disease—2011 update. Circulation. 2011;124:1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Deck MR, Boon NA, Newby DE. Calcific aortic stenosis. J Am Coll Cardiol. 2012;60:1854–1863. [DOI] [PubMed] [Google Scholar]
- 6. Yamagishi S. Role of advanced glycation end products (AGEs) and receptor for AGEs (RAGE) in vascular damage in diabetes. Exp Gerontol. 2011;46:217–224. [DOI] [PubMed] [Google Scholar]
- 7. Fukami K, Yamagishi S, Okuda S. Role of AGEs‐RAGE system in cardiovascular disease. Curr Pharm Des. 2014;20:2395–2402. [DOI] [PubMed] [Google Scholar]
- 8. Yamagishi S, Nakamura N, Suematsu M, Kaseda K, Matsui T. Advanced glycation end products: a molecular target for vascular complications in diabetes. Mol Med. 2015;21:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yamagishi S, Imaizumi T. Diabetic vascular complications: pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des. 2005;11:2279–2299. [DOI] [PubMed] [Google Scholar]
- 10. Yamagishi S, Matsui T. Soluble form of a receptor for advanced end products (sRAGE) as a biomarker. Front Biosci. 2010;2:1184–1195. [DOI] [PubMed] [Google Scholar]
- 11. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:57–185. [DOI] [PubMed] [Google Scholar]
- 12. Tahara N, Kai H, Nakaura H, Mizoguchi M, Ishibashi M, Kaida H, Baba K, Hayabuchi N, Imaizumi T. The prevalence of inflammation in carotid atherosclerosis: analysis with fluorodeoxyglucose‐positron emission tomography. Eur Heart J. 2007;28:2243–2248. [DOI] [PubMed] [Google Scholar]
- 13. Al‐Qaisi M, Nott DM, King DH, Kaddoura S. Ankle brachial pressure index (ABPI): an update for practitioners. Vasc Health Risk Manag. 2009;5:833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nashef SA, Roques F, Hammill BG, Peterson ED, Michel P, Grover FL, Wyse RK, Ferguson TB; EurpSCORE Project Group . Validation of European system for cardiac operative risk evaluation (EuroSCORE) in North American cardiac surgery. Eur J Cardiothorac Surg. 2002;22:101–105. [DOI] [PubMed] [Google Scholar]
- 15. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A; Collaborators Developing the Japanese Equation for Estimated GFR . Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. [DOI] [PubMed] [Google Scholar]
- 16. Tahara N, Kojima R, Yoshida R, Bekki M, Sugiyama Y, Tahara A, Maeda S, Honda A, Igata S, Nakamura T, et al. Serum levels of protein‐bound methylglyoxal‐derived hydroimidazolone‐1 are independently correlated with asymmetric dimethylarginine. Rejuvenation Res. 2019;22:431–438. [DOI] [PubMed] [Google Scholar]
- 17. Yamagishi S, Adachi H, Nakamura K, Matsui T, Jinnouchi Y, Takenaka K, Takeuchi M, Enomoto M, Furuki K, Hino A, et al. Positive association between serum levels of advanced glycation end products and the soluble form of receptor for advanced glycation end products in nondiabetic subjects. Metabolism. 2006;55:1227–1231. [DOI] [PubMed] [Google Scholar]
- 18. Yamagishi S, Adachi H, Abe A, Yashiro T, Enomoto M, Furuki K, Hino A, Jinnouchi Y, Takenaka K, Matsui T, et al. Elevated serum levels of pigment epithelium‐derived factor in the metabolic syndrome. J Clin Endocrinol Metab. 2006;91:2447–2450. [DOI] [PubMed] [Google Scholar]
- 19. Makita Z, Radoff S, Rayfield EJ, Yang Z, Skolnik E, Delaney V, Friedman EA, Cerami A, Vlassara H. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med. 1991;325:836–842. [DOI] [PubMed] [Google Scholar]
- 20. Kalousová M, Hodková M, Kazderová M, Fialová J, Tesar V, Dusilová‐Sulková S, Zima T. Soluble receptor for advanced glycation end products in patients with decreased renal function. Am J Kidney Dis. 2006;47:406–411. [DOI] [PubMed] [Google Scholar]
- 21. Aikawa M, Sivam PN, Kuro‐o M, Kimura K, Nakahara K, Takewaki S, Ueda M, Yamaguchi H, Yazaki Y, Periasamy M, et al. Human smooth muscle myosin heavy chain isoform as molecular markers for vascular development and atherosclerosis. Circ Res. 1993;73:1000–1012. [DOI] [PubMed] [Google Scholar]
- 22. Latif N, Sarathchandra P, Chester AH, Yacoub MH. Expression of smooth muscle cell markers and co‐activators in calcified aortic valves. Eur Heart J. 2015;36:1335–1345. [DOI] [PubMed] [Google Scholar]
- 23. Kilhovd BK, Berg TJ, Birkeland KI, Thorsby P, Hanssen KF. Serum levels of advanced glycation end products are increased in patients with type 2 diabetes and coronary heart disease. Diabetes Care. 1999;22:1543–1548. [DOI] [PubMed] [Google Scholar]
- 24. Kilhovd BK, Juutilainen A, Lehto S, Rönnemaa T, Torjesen PA, Birkeland KI, Berg TJ, Hanssen KF, Laakso M. High serum levels of advanced glycation end products predict increased coronary heart disease mortality in nondiabetic women but not in nondiabetic men: a population‐based 18‐year follow‐up study. Arterioscler Thromb Vasc Biol. 2005;25:815–820. [DOI] [PubMed] [Google Scholar]
- 25. Kilhovd BK, Juutilainen A, Lehto S, Rönnemaa T, Torjesen PA, Hanssen KF, Laakso M. Increased serum levels of methylglyoxal‐derived hydroimidazolone‐AGE are associated with increased cardiovascular disease mortality in nondiabetic women. Atherosclerosis. 2009;205:590–594. [DOI] [PubMed] [Google Scholar]
- 26. Tahara N, Yamagishi S, Takeuchi M, Honda A, Tahara A, Nitta Y, Kodama N, Mizoguchi M, Kaida H, Ishibashi M, et al. Positive association between serum level of glyceraldehyde‐derived advanced glycation end products and vascular inflammation evaluated by [(18)F] fluorodeoxyglucose positron emission tomography. Diabetes Care. 2012;35:2618–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kajikawa M, Nakashima A, Fujimura N, Maruhashi T, Iwamoto Y, Iwamoto A, Matsumoto T, Oda N, Hidaka T, Kihara Y, et al. Ratio of serum levels of AGEs to soluble form of RAGE is a predictor of endothelial function. Diabetes Care. 2015;38:119–125. [DOI] [PubMed] [Google Scholar]
- 28. Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ Jr, Chow WS, Stern D, Schmidt AM. Suppession of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025–1031. [DOI] [PubMed] [Google Scholar]
- 29. Nakamura K, Yamagishi S, Adachi H, Kurita‐Nakamura Y, Matsui T, Yoshida T, Imaizumi T. Serum levels of sRAGE, the soluble form of receptor for advanced glycation end products, are associated with inflammatory markers in patients with type 2 diabetes. Mol Med. 2007;13:185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nakamura K, Yamagishi S, Adachi H, Matsui T, Kurita‐Nakamura Y, Takeuchi M, Inoue H, Imaizumi T. Circulating advanced glycation end products (AGEs) and soluble form of receptor for AGEs (sRAGE) are independent determinants of serum monocyte chemoattractant protein‐1 (MCP‐1) levels in patients with type 2 diabetes. Diabetes Metab Res Rev. 2008;24:109–114. [DOI] [PubMed] [Google Scholar]
- 31. Yamagishi S, Matsui T, Nakamura K. Kinetics, role and therapeutic implications of endogenous soluble form of receptor for advanced glycation end products (sRAGE) in diabetes. Curr Drug Targets. 2007;8:1138–1143. [DOI] [PubMed] [Google Scholar]
- 32. Nakamura K, Yamagishi S, Adachi H, Kurita‐Nakamura Y, Matsui T, Yoshida T, Sato A, Imaizumi T. Elevation of soluble form of receptor for advanced glycation end products (sRAGE) in diabetic subjects with coronary artery disease. Diabetes Metab Res Rev. 2007;23:368–371. [DOI] [PubMed] [Google Scholar]
- 33. Fujisawa K, Katakami N, Kaneto H, Naka T, Takahara M, Sakamoto F, Irie Y, Miyashita K, Kubo F, Yasuda T, et al. Circulating soluble RAGE as a predictive biomarker of cardiovascular event risk in patients with type 2 diabetes. Atherosclerosis. 2013;227:425–428. [DOI] [PubMed] [Google Scholar]
- 34. Colhoun HM, Betteridge DJ, Durrington P, Hitman G, Neil A, Livingstone S, Charlton‐Menys V, Bao W, Demicco DA, Preston GM, et al. Total soluble and endogenous secretory receptor for advanced glycation end products as predictive biomarkers of coronary heart disease risk in patients with type 2 diabetes: an analysis from the CARDS trial. Diabetes. 2011;60:2379–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vaquette B, Corbineau H, Laurent M, Lelong B, Langanay T, de Place C, Froger‐Bompas C, Leclercq C, Daubert C, Leguerrier A. Valve replacement in patients with critical aortic stenosis and depressed left ventricular function: predictors of operative risk, left ventricular function recovery, and long term outcome. Heart. 2005;91:1324–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miura T, Minamisawa M, Ueki Y, Abe N, Nishimura H, Hashizume N, Mochidome T, Harada M, Oguchi Y, Yoshie K, et al. Impressive predictive value of ankle‐brachial index for very long‐term outcomes in patients with cardiovascular disease: IMPACT‐ABI study. PLoS One. 2017;12:e0177609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dijk JM, van der Graaf Y, Bots ML, Grobbee DE, Algra A. Carotid intima‐media thickness and the risk of new vascular events in patients with manifest atherosclerotic disease: the SMART study. Eur Heart J. 2006;27:1971–1978. [DOI] [PubMed] [Google Scholar]
- 39. Libby P, Ridker PM, Hansson GK; Leducq Transatlantic Network on Atherothrombosis . Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosenhek R, Rader F, Loho N, Gabriel H, Heger M, Klaar U, Schemper M, Binder T, Maurer G, Baumgartner H. Statins but not angiotensin‐converting enzyme inhibitors delay progression of aortic stenosis. Circulation. 2004;110:1291–1295. [DOI] [PubMed] [Google Scholar]
- 41. Yamagishi S, Nakamura K, Matsui T, Noda Y, Imaizumi T. Receptor for advanced glycation end products (RAGE): a novel therapeutic target for diabetic vascular complication. Curr Pharm Des. 2005;14:487–495. [DOI] [PubMed] [Google Scholar]
- 42. Suga T, Iso T, Shimizu T, Tanaka T, Yamagishi S, Takeuchi M, Imaizumi T, Kurabayashi M. Activation of receptor for advanced glycation end products induces osteogenic differentiation of vascular smooth muscle cells. J Atheroscler Thromb. 2011;18:670–683. [DOI] [PubMed] [Google Scholar]
- 43. Li F, Cai Z, Chen F, Shi X, Zhang Q, Chen S, Shi J, Wang DW, Dong N. Pioglitazone attenuates progression of aortic valve calcification via down‐regulating receptor for advanced glycation end products. Basic Res Cardiol. 2012;107:306. [DOI] [PubMed] [Google Scholar]
- 44. Li F, Zhao Z, Cai Z, Dong N, Liu Y. Oxidized low‐density lipoprotein promotes osteoblastic differentiation of valvular interstitial cells through RAGE/MAPK. Cardiology. 2015;130:55–61. [DOI] [PubMed] [Google Scholar]
- 45. Hofmann B, Yakobus Y, Indrasari M, Nass N, Santos AN, Kraus FB, Silber RE, Simm A. RAGE influences the development of aortic valve stenosis in mice on a high fat diet. Exp Gerontol. 2014;59:13–20. [DOI] [PubMed] [Google Scholar]


