Abstract
Background
The efficacy of low‐voltage‐area (LVA) ablation has not been well determined. This study aimed to investigate the efficacy of LVA ablation in addition to pulmonary vein isolation on rhythm outcomes in patients with paroxysmal atrial fibrillation (AF).
Methods and Results
VOLCANO (Catheter Ablation Targeting Low‐Voltage Areas After Pulmonary Vein Isolation in Paroxysmal Atrial Fibrillation Patients) trial included paroxysmal AF patients undergoing initial AF ablation. Of 398 patients in whom a left atrial voltage map was obtained after pulmonary vein isolation, 336 (85%) had no LVA (group A). The remaining 62 (15%) patients with LVAs were randomly allocated to undergo LVA ablation (group B, n=30) or not (group C, n=32) in a 1:1 fashion. Primary end point was 1‐year AF‐recurrence‐free survival rate. No adverse events related to LVA ablation occurred. Procedural (124±40 versus 95±33 minutes, P=0.003) and fluoroscopic times (29±11 versus 24±8 minutes, P=0.034) were longer in group B than group C. Patients with LVAs demonstrated lower AF‐recurrence‐free survival rates (88%) than those without LVA (B, 57%, P<0.0001; C, 53%, P<0.0001). However, LVA ablation in addition to pulmonary vein isolation did not impact AF‐recurrence‐free survival rate (group B versus C, P=0.67).
Conclusions
The presence of LVA was a strong predictor of AF recurrence after pulmonary vein isolation in patients with paroxysmal AF. However, LVA ablation had no beneficial impact on 1‐year rhythm outcomes.
Registration
URL: https://www.umin.ac.jp/ctr; Unique identifier: UMIN000023403.
Keywords: ablation, atrial fibrillation, low‐voltage area, paroxysmal, recurrence
Subject Categories: Arrhythmias, Atrial Fibrillation, Catheter Ablation and Implantable Cardioverter-Defibrillator
Nonstandard Abbreviations and Acronyms
- AF
atrial fibrillation
- LVA
low‐voltage area
- PVI
pulmonary vein isolation
Clinical Perspective
What Is New?
Low‐voltage‐area ablation in addition to pulmonary vein isolation had no beneficial impact on rhythm outcomes in patients with paroxysmal atrial fibrillation, although low‐voltage‐area existence strongly predicted atrial fibrillation recurrence.
What Are the Clinical Implications?
This result indicates that a different ablation strategy should be explored for paroxysmal atrial fibrillation with low‐voltage areas.
In addition, preprocedural estimation of low‐voltage area presence may be useful in distinguishing patients with excellent outcomes after ablation from those with poor outcomes.
Catheter ablation has become a mainstream treatment option for paroxysmal atrial fibrillation (AF).1 Electrical pulmonary vein isolation (PVI) is well established as the cornerstone of AF ablation. However, frequent AF recurrence after ablation remains an unsolved problem, with reported 1‐year AF recurrence rates of 11% to 41%.1
Recent studies have shown that the presence of left atrial low‐voltage area (LVA) after PVI is a powerful predictor of AF recurrence, not only in persistent AF2, 3, 4, 5, 6 but also in paroxysmal AF.7, 8 Two randomized controlled trials demonstrated comparable or better efficacies with LVA ablation in addition to PVI than with conventional complex‐electrogram guided ablation or linear ablation strategies, mainly in persistent AF patients.9, 10 To date, however, no randomized controlled investigation of the efficacy and safety of LVA ablation in patients with paroxysmal AF has been reported.
Here, we compared the efficacy and safety of PVI plus LVA ablation with PVI alone in patients with paroxysmal AF.
Methods
Data Disclosure
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design
Patients with symptomatic paroxysmal AF who were scheduled to undergo catheter ablation were eligible for participation in the prospective open‐label randomized controlled VOLCANO (Catheter Ablation Targeting Low‐Voltage Areas After Pulmonary Vein Isolation in Paroxysmal Atrial Fibrillation Patients) trial (UMIN‐CTR, UMIN000023403). Paroxysmal AF was defined as recurrent AF that was self‐terminating within 7 days. Exclusion criteria were aged <20 years, prior cardiac surgery, prior catheter ablation, or severe mitral valve disease. Study patients underwent PVI followed by left atrial voltage mapping. Patients without LVAs were classified into group A. Patients with LVAs were randomly allocated to group B (adjunct LVA ablation) or group C (no LVA ablation), with randomization performed in a 1:1 fashion during the ablation procedure. If voltage mapping failed to complete because of an unstable heart rhythm, the patient was categorized into group D.
The primary end point was AF‐recurrence‐free survival rate without any antiarrhythmic drugs during a 12‐month follow‐up period after a single ablation procedure. The secondary end points encompassed procedural characteristics including procedural time, fluoroscopic time, and severe complications.
We calculated the sample size on the basis of power analysis, assuming a 15% incidence of LVAs among the paroxysmal AF population7 and an AF‐recurrence‐free survival rate of 90% in group B and 60% in group C.2 Considering that the inclusion of 30 patients each in groups B and C allowed for a statistical power of 80% with a type I error of 0.05, the estimated total sample size was 400 patients.
This study complied with the Declaration of Helsinki. Written informed consent for ablation and participation in the study was obtained from all patients, and the protocol was approved by our institutional review board.
Electrophysiological Study and Ablation Procedure
An oral anticoagulant was prescribed at least 1 month before and 2 months after the ablation as recommended by an international expert consensus statement.1 Antiarrhythmic drugs were discontinued ≥48 hours before ablation. Preprocedural transesophageal echocardiography was performed to exclude left atrial thrombi. The ablation procedure was performed under intravenous sedation with dexmedetomidine, and repetitive intravenous heparin boli were administered to maintain an activated clotting time of 300 to 350 seconds. Esophageal temperature was monitored in real time using an esophageal temperature probe (Sensitherm; Abbott, St. Paul, Minnesota, USA).
Trans‐septal left atrial access was obtained using a standard technique, with CARTO 3 (Biosense Webster, Diamond Bar, CA, USA) or Ensite Precision system (Abbott, St. Paul, MN, USA) used for catheter navigation and mapping. PVI was performed as the initial step using a second‐generation cryoballoon catheter (Arctic Front Advance®; Medtronic, Minneapolis, MN) or 3.5‐mm open‐irrigated ablation catheter with a contact‐force sensor (Thermocool ST/SF, Biosense Webster, or Flexibility; Abbott). For the cryoballoon catheter, complete pulmonary vein occlusion by cryoballoon was confirmed by injecting contrast medium from the balloon injection lumen, followed by a single application of 180‐second freezing. Cryo applications were repeated until the pulmonary vein electrogram disappeared on a spiral mapping catheter (Achieve; Medtronic). Radiofrequency application was set at 30 W using a dragging technique with a maximum temperature of 42°C and irrigation rate of 8 mL/min. Operators attempted to maintain an appropriate contact force between the catheter and endocardium of between 5 and 20 g. Bidirectional conduction block was confirmed using a 20‐pole circular mapping catheter (Lasso Nav, Biosense webstar, or Inquiry Optima; Abbott) and served as the procedural end point. Completion of PVI was re‐examined following a waiting time of ≥20 minutes after the initial completion of PVI.
Voltage Mapping and LVA Ablation
Following PVI, left atrial voltage mapping during sinus rhythm was performed using a 20‐pole circular mapping catheter (Lasso Nav or Inquiry Optima) via a steerable sheath (Agilis; Abbott). Electrical cardioversion was performed when AF persisted during voltage mapping. Mapping points were automatically acquired using the criteria of cycle length stability, catheter position stability, and point density. Mapping points were acquired to fill all color gaps on the entire left atrial surface under the interpolation color threshold of 7 mm. The band pass filter was set at 30 to 500 Hz. LVAs were defined as sites with a bipolar peak‐to‐peak voltage of <0.50 mV and covering >5.0 cm2. LVA size was manually measured on each voltage map.
Patients in group B underwent ablation targeting LVAs. LVAs were homogeneously ablated using the open‐irrigated radiofrequency catheter with the power set at 30 W. The ablation catheter was moved in a point‐by‐point fashion. The end point of each radiofrequency application was an electrogram voltage reduction of >50%. Isolation of the posterior LVA by PVI, roof, and bottom lines (box isolation) to avoid esophageal injury was permitted. In such cases, both entrance and exit blocks between the posterior wall and other left atrium were confirmed. In addition, LVA ablation in areas where ablation could potentially result in or cause a conduction disturbance was avoided. Examples of this include anterior wall broad ablation that may eliminate or delay appendage contraction and septal wall broad ablation that may impair atrial‐ventricular conduction.
Atrial Tachyarrhythmia Induction Test and Additional Ablation
After the procedure above, constant burst pacing was performed for 5 seconds at each cycle length, starting with 300 ms and a subsequent decrement of 20 to 200 ms or the shortest cycle length that resulted in 1:1 atrial capture. This was followed by a high‐dose isoproterenol provocation test (infusion of 5, 10, and 20 μg/min isoproterenol for 2 minutes each) to induce AF or atrial tachycardia. Ablation of induced and spontaneously developing AF‐triggering ectopies and atrial tachycardia was attempted at the earliest activation site for AF trigger or centrifugal atrial tachycardia, and across the reentrant circuit for macro‐reentrant atrial tachycardia.
Follow‐Up
Patients were followed up at 1, 3, 6, 9, and 12 months after the ablation procedure. Routine ECGs were conducted at each outpatient visit, and 24‐hour ambulatory Holter monitoring was performed 6‐ and 12‐months post‐ablation. When patients experienced symptoms suggestive of an arrhythmia, surface ECG, ambulatory ECG, and/or cardiac event recording were also conducted. AF recurrence was defined as the occurrence of 1 of the following events from 3 months after the initial ablation (blanking period): (1) AF and/or atrial tachycardia indicated on a routine or symptom‐triggered ECG during an outpatient visit; or (2) AF and/or atrial tachycardia of at least 30‐second duration on ambulatory ECG monitoring. No antiarrhythmic drugs were prescribed after the ablation procedure unless AF recurrence was observed.
Statistical Analysis
Continuous data are expressed as the mean±SD or median (interquartile range). Categorical data are presented as absolute values and percentages. Tests for significance were conducted using the unpaired t test, repeated measures ANOVA, or a non‐parametric test (Mann–Whitney U test) for continuous variables and the Chi‐squared test or Fisher exact test for categorical variables. AF‐recurrence‐free survival rates were calculated using the Kaplan–Meier method. Comparison of survival curves between the groups was performed using a 2‐sided Mantel–Haenszel (log‐rank) test. A P<0.05 was considered statistically significant. Clinical factors associated with AF recurrence were determined by univariate and multivariate Cox proportional hazards models. Variables with a P≤0.10 in the univariate models were included in the multivariate analysis. The analyses were exploratory and no adjustments were done. All analyses were performed using commercial software (SPSS version 25.0; SPSS, Inc., Chicago, IL, USA).
Results
Patients
From August 2016 to October 2018, 426 patients fulfilled the inclusion criteria (Figure 1). After excluding 23 patients who met ≥1 exclusion criteria, 403 patients underwent catheter ablation. One patient developed cardiac tamponade during the PVI procedure, and a voltage map was not obtained. Finally, 402 patients were enrolled in the study. Voltage mapping after PVI was not completed in 4 patients (group D) because AF repeatedly developed just after electric cardioversion. Among 398 patients in whom voltage mapping was completed, 336 patients had no LVA (group A). The remaining 62 patients had LVAs after PVI, and were randomly allocated to undergo LVA ablation (group B, 30 patients) or not (group C, 32 patients).
Figure 1. Patient flowchart.
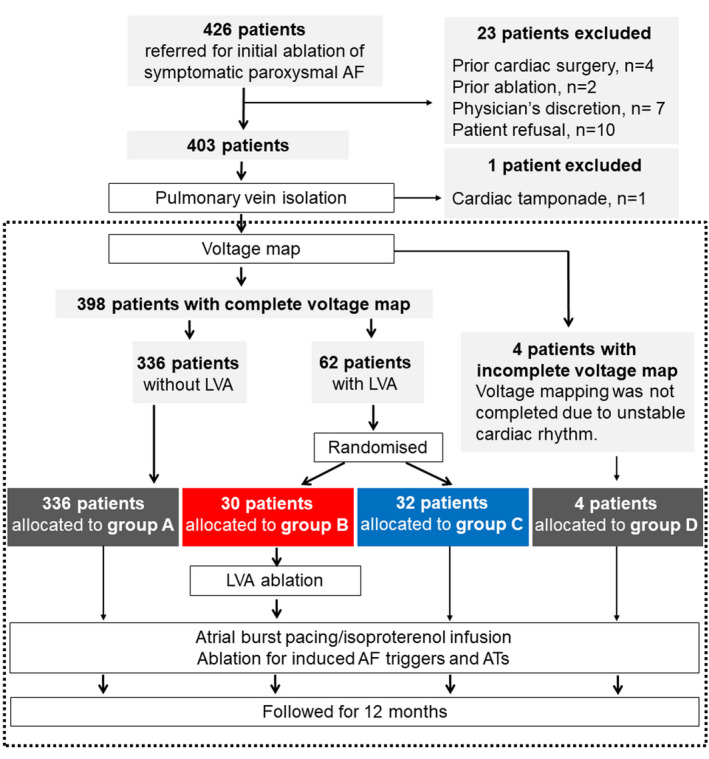
Final patient enrollment was 402 patients, as framed by a dashed rectangle. Among 398 patients in whom voltage mapping was completed, 332 patients had no low‐voltage area (group A). The remaining 62 patients had low‐voltage areas after pulmonary vein isolation and were randomly allocated to undergo low‐voltage area ablation (group B) or not (group C). Voltage mapping was not completed in 4 patients (group D) because of unstable heart rhythm. AF indicates atrial fibrillation; AT, atrial tachycardia; LVA, low‐voltage area.
Baseline patient characteristics are shown in Table 1. Patients with LVAs (groups B and C) were more likely to be elderly, and have diabetes mellitus, heart failure, enlarged left atrium, and elevated estimated left ventricular filling pressure. No differences were seen between groups B and C.
Table 1.
Patient Characteristics
| Group A | Group B | Group C | Group D | P Value | ||
|---|---|---|---|---|---|---|
| n=336 | n=30 | n=32 | n=4 | Group A vs B and C | Group B vs C | |
| Age, y | 67.8±11.6 | 75.3±7.2 | 74.7±8.0 | 74.0±6.0 | <0.001 | 0.74 |
| Women, n (%) | 131 (39) | 21 (70) | 23 (72) | 1 (20) | <0.001 | 0.87 |
| Body mass index, kg/m2 | 23.7±3.7 | 22.3±3.5 | 22.1±4.8 | 26.0±6.4 | 0.11 | 0.88 |
| AF duration, mo | 6 (2, 35) | 4 (2, 14) | 5 (2, 23) | 10 (7, 32) | 0.08 | 0.29 |
| Hypertension, n (%) | 195 (58) | 20 (67) | 16 (50) | 4 (100) | 0.997 | 0.18 |
| Diabetes mellitus, n (%) | 51 (15) | 10 (33) | 6 (19) | 1 (25) | 0.040 | 0.19 |
| Heart failure, n (%) | 30 (9) | 5 (17) | 6 (19) | 0 (0) | 0.036 | 0.83 |
| CHA2DS2‐VASc score | 2.4±1.4 | 3.6±1.2 | 3.3±1.3 | 3.3±1.0 | <0.001 | 0.32 |
| NT‐pro BNP, pg/mL | 125 (59, 409) | 457 (242, 908) | 305 (186, 1246) | 789 (150, 1132) | 0.09 | 0.84 |
| eGFR, mL/min | 60±19 | 54±20 | 52±16 | 58±39 | 0.008 | 0.050 |
| Echocardiography | ||||||
| Left atrial diameter, mm | 37±6 | 40±6 | 38±5 | 43±6 | 0.040 | 0.31 |
| Ejection fraction, % | 66±9 | 64±14 | 65±10 | 57±17 | 0.41 | 0.77 |
| Left ventricular mass, g | 174±49 | 179±71 | 183±67 | 181±25 | 0.30 | 0.80 |
| E/A | 1.0±0.5 | 1.4±0.7 | 1.2±0.7 | 1.2±0.3 | 0.002 | 0.34 |
| E/e′ | 10.3±3.8 | 13.7±5.8 | 13.9±7.5 | 15.4±2.9 | <0.001 | 0.91 |
| Medications | ||||||
| Vitamin K antagonist, n (%) | 36 (11) | 3 (10) | 4 (13) | 1 (25) | 0.91 | 0.54 |
| Antiarrhythmic drugs, n (%) | 175 (52) | 20 (67) | 19 (59) | 4 (100) | 0.12 | 0.55 |
A indicates diastolic late transmitral flow velocity; E, diastolic early transmitral flow velocity; e′, diastolic early mitral annular velocity; eGFR, estimated glomerular filtration rate; and NT‐pro BNP, N‐terminal pro‐B‐type natriuretic peptide.
Voltage Mapping and LVA Distribution
Voltage mapping was performed after PVI using CARTO or Ensite (CARTO; 56% in group A, 60% in group B, 63% in group C, and 100% in group D, P=0.31). LVA sizes did not differ between groups B and C (Table 2). LVAs were predominantly observed in the anterior‐septal wall in 57 of 62 (92%) patients, followed by the roof in 26 (42%), posterior wall in 18 (29%), inferior wall in 7 (11%), and lateral wall in 5 (8%).
Table 2.
Procedural Characteristics
| Group A | Group B | Group C | Group D | P Value | ||
|---|---|---|---|---|---|---|
| n=336 | n=30 | n=32 | n=4 | Group A vs B and C | Group B vs C | |
| Pulmonary vein isolation, n (%) | 336 (100) | 30 (100) | 32 (100) | 4 (100) | >0.999 | >0.999 |
| Left atrial linear ablation, n (%) | 6 (2) | 6 (20) | 1 (3) | 0 (0) | <0.001 | 0.043 |
| Roof, n (%) | 6 (2) | 5 (17) | 0 (0) | 0 (0) | 0.006 | 0.022 |
| Bottom, n (%) | 2 (1) | 2 (7) | 0 (0) | 0 (0) | 0.12 | 0.23 |
| Mitral isthmus, n (%) | 0 (0) | 2 (7) | 1 (3) | 0 (0) | 0.004 | 0.48 |
| Cavo‐tricuspid isthmus, n (%) | 45 (13) | 4 (13) | 5 (16) | 1 (20) | 0.81 | 0.54 |
| Non‐pulmonary‐vein trigger ablation, n (%) | 12 (4) | 3 (10) | 4 (13) | 4 (100) | 0.009 | 0.54 |
| Superior vena cava isolation, n (%) | 5 (2) | 0 (0) | 1 (3) | 0 (0) | >0.999 | 0.52 |
| No. of mapping points, n | 1156 (900, 1419) | 1461 (1029, 1858) | 1279 (881, 1625) | N.A. | 0.50 | 0.55 |
| LVA size, cm2 | N.A. | 15.8±15.4 | 16.9±10.0 | N.A. | N.A. | 0.75 |
| Inducibility of atrial fibrillation or regular tachycardias, n (%) | 102 (30) | 14 (47) | 17 (53) | 4 (100) | 0.003 | 0.61 |
| Procedural time, min | 83±42 | 124±40 | 95±33 | 85±26 | <0.001 | 0.003 |
| Fluoroscopic time, min | 22±9 | 29±11 | 24±8 | 27±13 | <0.001 | 0.050 |
LVA indicates low‐voltage area.
Ablation Procedures
PVI was completed in all patients using a cryoballoon or radiofrequency ablation catheter (cryoballoon; 83% in group A, 77% in group B, 84% in group C, and 50% in group D, P=0.29). LVA ablation was performed in group B with a mean delivered radiofrequency energy of 17 780±13 362 J. An example case is shown in Figure 2. Some portions of the LVA were not ablated in 3 of 30 (10%) patients, in consideration of the risk of esophageal injury in 1 patient and conduction disturbance of the left atrial appendage in 2. Ablation targeting non‐pulmonary‐vein AF triggers was more frequently performed in patients with LVA (groups B and C) than in those without (group A, Table 2).
Figure 2. Example of low‐voltage area ablation in addition to pulmonary vein isolation.
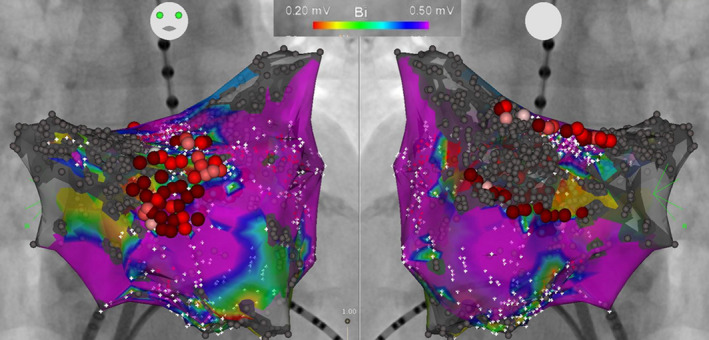
Left atrial voltage map after pulmonary vein isolation in a 76‐year‐old female patient. Low‐voltage areas were observed in the anterior‐septal wall and posterior wall. Low‐voltage area ablation consisted of voltage homogenization covering a low‐voltage area in the anterior‐septal wall, and roof and bottom linear ablation isolating a low‐voltage area in the posterior wall.
Left atrial linear ablation in addition to PVI was performed to isolate the posterior wall with LVAs (n=2), isolate the posterior wall with non‐pulmonary vein foci (n=2), or block macro‐reentrant atrial tachycardias that were induced after PVI (n=9).
Inducibility of AF or regular atrial tachycardias by atrial programmed stimuli at the end of the ablation procedure was higher in patients with LVA (groups B and C) than in those without (group A), and was comparable between groups B and C (Table 2).
Procedure and fluoroscopic times were longer in group B than in group C. Severe complication developed in 2 patients in group A. Gastric hypomobility requiring a 7‐day fasting cure developed in 1 patient who underwent cryoballoon PVI. The other complication was femoral anterior‐venous anastomosis requiring surgical repair. No severe complication related to LVA ablation developed.
AF Recurrence
All patients were followed for 1 year, except for 2 patients in group A who were lost to follow‐up at 3 and 6 months after ablation, respectively. One patient died of heart failure 6 months after ablation in group D.
There was no difference in AF‐recurrence‐free survival rate between group B (57%) and C (53%, P=0.67, Figure 3). Patients in group A demonstrated higher AF‐recurrence‐free survival rate (88%) than those in group B (P<0.0001) or C (P<0.0001). All patients in group D experienced AF recurrence soon after ablation. Multivariate analysis among patients with a completed voltage map (group A, B, and C) revealed that female sex, large left atrium, and presence of LVA were independently associated with AF recurrence (Table 3).
Figure 3. Atrial fibrillation recurrence‐free survival rates.
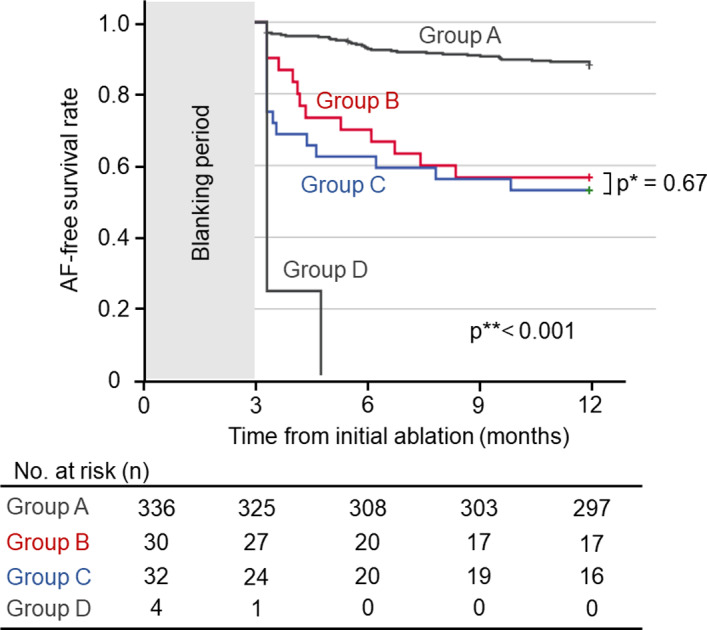
Kaplan–Meier curves for atrial fibrillation‐recurrence‐free survival are shown. Blue line, patients without low‐voltage area (LVA) (group A); red line, patients allocated to pulmonary vein isolation plus LVA ablation (group B); green line, patients allocated to pulmonary vein isolation alone; orange line, patients in whom voltage mapping was not completed because of unstable heart rhythm. Patients without LVA (group A) demonstrated excellent rhythm outcomes. In contrast, those with LVAs had a significantly lower atrial fibrillation‐recurrence‐free survival rate. Allocation to additional LVA ablation or not did not influence atrial fibrillation‐recurrence‐free survival rates. AF indicates atrial fibrillation.
Table 3.
Factors Associated With AF Recurrencea Among Patients With a Complete Voltage Map (Groups A, B, and C)
| Recurrence | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| With (n=68) | Without (n=330) | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Age, y | 70.1±10.6 | 68.7±11.5 | 1.01 | 0.99–1.03 | 0.32 | 0.99 | 0.96–1.02 | 0.56 |
| Women, n (%) | 42 (62) | 133 (40) | 2.22 | 1.39–3.61 | 0.001 | 1.88 | 1.06–3.32 | 0.031 |
| Body mass index | 23.1±4.1 | 23.5±3.7 | 0.97 | 0.91–1.04 | 0.40 | |||
| AF period, mo | 5 (2, 16) | 7 (3, 21) | 0.99 | 0.98–1.003 | 0.18 | |||
| Heart failure, n (%) | 10 (15) | 31 (9) | 1.52 | 0.77–2.96 | ||||
| CHA2DS2‐VASc score | 2.9±1.5 | 2.5±1.4 | 1.17 | 0.99–1.38 | 0.07 | 0.96 | 0.74–1.24 | 0.96 |
| Estimated GFR, pg/mL | 60±17 | 59±20 | 1.004 | 0.99–1.02 | 0.53 | |||
| Left atrial diameter, mm | 39.5±5.6 | 37.4±6.1 | 1.05 | 1.01–1.09 | 0.008 | 1.05 | 1.01–1.10 | 0.012 |
| Cryoballoon, n (%) | 54 (79) | 274 (83) | 0.80 | 0.44–1.43 | 0.45 | |||
| LVA presence, n (%) | 28 (41) | 34 (10) | 4.83 | 2.98–7.85 | <0.001 | 4.17 | 2.47–7.04 | 0.001 |
Factors with P<0.10 in the univariate analysis were incorporated in the multivariate analysis. AF indicates atrial fibrillation; GFR, glomerular filtration rate; HR, hazard ratio; and LVA, low‐voltage area.
AF recurrence indicates recurrence of both atrial fibrillation and atrial tachycardia.
Among patients with LVA (group B and C), a large left atrium and broad LVA were associated with AF recurrence (Table 4). Subgroup analyses demonstrated that LVA ablation did not reduce AF recurrence in any patient category (Figure 4).
Table 4.
Factors Associated With AF Recurrencea Among Patients With LVA (Groups B and C)
| Recurrence | Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|---|
| With (n=28) | Without (n=34) | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Age, y | 74.5±7.7 | 75.4±7.5 | 0.99 | 0.94–1.04 | 0.72 | 1.01 | 0.96–1.06 | 0.82 |
| Women, n (%) | 22 (79) | 22 (65) | 1.74 | 0.70–4.29 | 0.23 | 2.34 | 0.87–6.32 | 0.93 |
| Body mass index | 22.6±5.0 | 21.9±3.4 | 1.04 | 0.95–1.14 | 0.40 | |||
| AF period, mo | 5 (3, 13) | 9 (3, 17) | 0.99 | 0.96–1.02 | 0.38 | |||
| Heart failure | 7 (25) | 4 (12) | 1.66 | 0.70–3.90 | 0.25 | |||
| CHA2DS2‐VASc score | 3.6±1.3 | 3.3±1.2 | 1.20 | 0.90–1.60 | 0.21 | |||
| Estimated GFR, pg/mL | 58±17 | 50±19 | 1.02 | 0.996–1.04 | 0.11 | |||
| Left atrial diameter, mm | 41.1±5.1 | 37.5±5.4 | 1.08 | 1.01–1.16 | 0.023 | 1.10 | 1.02–1.18 | 0.017 |
| Cryoballoon, n (%) | 21 (75) | 29 (85) | 0.69 | 0.29–1.62 | 0.40 | |||
| LVA size, cm2 | 20.0±15.9 | 13.3±8.7 | 1.03 | 1.01–1.06 | 0.009 | 1.04 | 1.01–1.06 | 0.010 |
| LVA ablation (group B) | 13 (46) | 17 (50) | 0.86 | 0.41–1.80 | 0.68 | 0.81 | 0.38–1.73 | 0.58 |
Factors with P<0.10 in the univariate analysis were incorporated in the multivariate analysis. AF indicates atrial fibrillation; GFR, glomerular filtration rate; HR, hazard ratio; and LVA, low‐voltage area.
AF recurrence indicates recurrence of both atrial fibrillation and atrial tachycardia.
Figure 4. Forrester plots displaying the impact of low‐voltage area ablation on atrial fibrillation recurrence stratified according by subgroup.
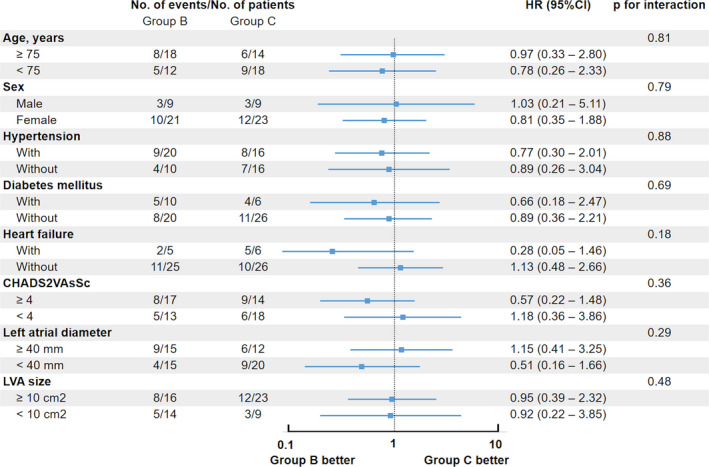
Hazard ratios and P value for interactions stratified according to subgroup. No beneficial impact of low‐voltage area ablation was observed in any subgroup analysis. HR indicates hazard ratio; and LVA, low‐voltage area.
Discussion
This randomized controlled trial demonstrated that LVA ablation in addition to PVI had no beneficial impact on rhythm outcomes in patients with paroxysmal AF undergoing AF ablation. In contrast, LVA presence strongly predicted AF recurrence. To our knowledge, this is the first randomized controlled trial to compare the efficacy of PVI plus LVA ablation with PVI alone in patients with paroxysmal AF.
Impact of LVA Presence in Patients With Paroxysmal AF
LVAs were observed in patients with paroxysmal AF, with a prevalence of 15% in this study, which was comparable with those in previous studies.2, 7 The poor rhythm outcomes in patients with LVAs clearly contrasted to the excellent outcomes in those without LVAs.
The characteristic pathophysiologic mechanism of LVA generation in paroxysmal AF likely explains the strong association between LVA presence and AF recurrence after PVI. The generation of LVAs in paroxysmal AF is more likely dependent on upstream factors causing atrial remodeling such as aging, female sex, and elevated atrial pressure than in persistent AF.11, 12, 13 These upstream factors would likely continue to remodel the atrium even after ablation and might contribute to the poor rhythm outcome in patients with LVA. On the other hand, as in cases with persistent AF in which atrial remodeling is partially caused by the AF persistence itself, elimination of AF by catheter ablation may suppress the progression of AF substrate.
LVA Ablation in Paroxysmal AF Patients
LVA ablation failed to reduce AF recurrence in the present study. Two previous observational studies reported the conflicting results that LVA ablation is effective14 and not effective15 in paroxysmal AF patients. These results suggest that LVA ablation does not always suppress arrhythmogenic substrate sufficiently to reduce paroxysmal AF recurrence.
The lack of efficacy of LVA ablation in paroxysmal AF patients may be explained as follows. First, the presence of LVA likely indicates the existence of upstream factors which cause atrial fibrosis. As mentioned above, the influence of upstream factors and atrial fibrosis is likely greater in paroxysmal AF than persistent AF. Therefore, the continuous progression of arrhythmogenic substrate even after LVA ablation might reduce the effect of LVA ablation. Second, the pathophysiological mechanism of LVA in AF has not been well determined. Although conduction slowing within an LVA is believed to play a role as AF substrate, data on whether LVAs obtained during sinus rhythm truly contribute to AF substrate such as rotors and focal sources is scarce. Third, AF recurrence in paroxysmal AF would highly depend on the presence of AF‐triggering ectopies rather than substrate maintaining AF, possibly attenuating the importance of LVA ablation.
In contrast to our study, 2 previous randomized controlled trials that mainly included persistent AF patients demonstrated better or equal rhythm outcomes by LVA ablation than other additional ablations such as ablation guided by complex fractionated electrogram or linear ablation, consistently showing the efficacy of LVA ablation in addition to PVI.9, 10 These results support the hypothesis that the development of AF in patients with persistent AF depends more on the atrial substrate caused by AF burden and less on non‐pulmonary‐vein AF triggers than in those with paroxysmal AF. The efficacy of LVA ablation is accordingly greater in persistent AF.
Clinical Implications and Future Perspective
In patients with paroxysmal AF and LVAs, rhythm outcomes after catheter ablation were markedly poor even after PVI plus LVA ablation. This result indicates that a different ablation strategy should be explored for paroxysmal AF with LVAs. Possibly, ablation for non‐pulmonary‐vein triggers induced by intense provocation test using high‐dose isoproterenol and/or adenosine may improve rhythm outcomes. In addition, preprocedural estimation of LVA presence may be useful in distinguishing patients with excellent outcomes after ablation from those with poor outcomes.
Although several randomized controlled trials have investigated LVA ablation, the relatively small number of patients with LVA in each trial means that efficacy remains uncertain: 35 patients in the study by Kircher et al,9 47 in the STABLE‐SR study,10 and 62 in the present study. The role of LVA ablation in AF patients is expected to be clarified by several randomized controlled trials now underway, such as SUPPRESS AF (UMIN‐CTR, UMIN000035940), which plans to include 340 patients with persistent AF and left atrial LVA.
Limitations
Several limitations of this study warrant mention. First, PVI durability and isolated areas of pulmonary vein antrum may differ between PVI done using a cryoballoon or radiofrequency ablation catheter. Second, voltage maps were created using 2 different mapping systems. LVAs detected by one mapping system may not be identically detected using the other system. Third, a voltage map was obtained using a circular mapping catheter that may not have been optimal for left atrial mapping. Fourth, the definition of low‐voltage areas (areas with a voltage <0.5 mV covering 5.0 cm2 of the left atrial surface) was somewhat arbitrary. Fifth, AF recurrence after discharge was quantified on the basis of patient symptoms, giving rise to the possibility that asymptomatic episodes of AF might have been missed. Finally, the small sample size could bias patient characteristics and limits the statistical accuracy of our results. Multicenter randomized controlled trials in sufficient numbers of patients are warranted.
Conclusions
The presence of LVA was a strong predictor of AF recurrence after PVI in patients with paroxysmal AF. However, LVA ablation had no beneficial impact on 1‐year rhythm outcomes.
Sources of Funding
None.
Disclosures
Masuda has received personal fees from Johnson & Johnson outside the submitted work. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2020;9:e015927 DOI: 10.1161/JAHA.120.015927.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;e1–e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rolf S, Kircher S, Arya A, Eitel C, Sommer P, Richter S, Gaspar T, Bollmann A, Altmann D, Piedra C, et al. Tailored atrial substrate modification based on low‐voltage areas in catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2014;825–833. [DOI] [PubMed] [Google Scholar]
- 3. Yang G, Yang B, Wei Y, Zhang F, Ju W, Chen H, Li M, Gu K, Lin Y, Wang B, et al. Catheter ablation of nonparoxysmal atrial fibrillation using electrophysiologically guided substrate modification during sinus rhythm after pulmonary vein isolation. Circ Arrhythm Electrophysiol. 2016;e003382 DOI: 10.1161/CIRCEP.115.003382. [DOI] [PubMed] [Google Scholar]
- 4. Kottkamp H, Berg J, Bender R, Rieger A, Schreiber D. Box isolation of fibrotic areas (BIFA): a patient‐tailored substrate modification approach for ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2016;22–30. [DOI] [PubMed] [Google Scholar]
- 5. Jadidi AS, Lehrmann H, Keyl C, Sorrel J, Markstein V, Minners J, Park CI, Denis A, Jaïs P, Hocini M, et al. Ablation of persistent atrial fibrillation targeting low‐voltage areas with selective activation characteristics. Circ Arrhythm Electrophysiol. 2016;e002962 DOI: 10.1161/CIRCEP.115.002962. [DOI] [PubMed] [Google Scholar]
- 6. Yamaguchi T, Tsuchiya T, Nakahara S, Fukui A, Nagamoto Y, Murotani K, Eshima K, Takahashi N. Efficacy of left atrial voltage‐based catheter ablation of persistent atrial fibrillation. J Cardiovasc Electrophysiol. 2016;1055–1063. [DOI] [PubMed] [Google Scholar]
- 7. Masuda M, Fujita M, Iida O, Okamoto S, Ishihara T, Nanto K, Kanda T, Tsujimura T, Matsuda Y, Okuno S, et al. Left atrial low‐voltage areas predict atrial fibrillation recurrence after catheter ablation in patients with paroxysmal atrial fibrillation. Int J Cardiol. 2018;97–101. [DOI] [PubMed] [Google Scholar]
- 8. Vlachos K, Efremidis M, Letsas KP, Bazoukis G, Martin R, Kalafateli M, Lioni L, Georgopoulos S, Saplaouras A, Efremidis T, et al. Low‐voltage areas detected by high‐density electroanatomical mapping predict recurrence after ablation for paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2017;1393–1402. [DOI] [PubMed] [Google Scholar]
- 9. Kircher S, Arya A, Altmann D, Rolf S, Bollmann A, Sommer P, Dagres N, Richter S, Breithardt OA, Dinov B, et al. Individually tailored vs. standardized substrate modification during radiofrequency catheter ablation for atrial fibrillation: a randomized study. Europace. 2018;1766–1775. [DOI] [PubMed] [Google Scholar]
- 10. Yang B, Jiang C, Lin Y, Yang G, Chu H, Cai H, Lu F, Zhan X, Xu J, Wang X, et al. STABLE‐SR (Electrophysiological Substrate Ablation in the Left Atrium During Sinus Rhythm) for the treatment of nonparoxysmal atrial fibrillation: a prospective, multicenter randomized clinical trial. Circ Arrhythm Electrophysiol. 2017;e005405 DOI: 10.1161/CIRCEP.117.005405. [DOI] [PubMed] [Google Scholar]
- 11. Masuda M, Fujita M, Iida O, Okamoto S, Ishihara T, Nanto K, Kanda T, Shiraki T, Sunaga A, Matsuda Y, et al. Influence of underlying substrate on atrial tachyarrhythmias after pulmonary vein isolation. Heart Rhythm. 2016;870–878. [DOI] [PubMed] [Google Scholar]
- 12. Kosiuk J, Dinov B, Kornej J, Acou WJ, Schönbauer R, Fiedler L, Buchta P, Myrda K, Gąsior M, Poloński L, et al. Prospective, multicenter validation of a clinical risk score for left atrial arrhythmogenic substrate based on voltage analysis: DR‐FLASH score. Heart Rhythm. 2015;2207–2212. [DOI] [PubMed] [Google Scholar]
- 13. Park J, Joung B, Uhm JS, Young Shim C, Hwang C, Hyoung Lee M, Pak HN. High left atrial pressures are associated with advanced electroanatomical remodeling of left atrium and independent predictors for clinical recurrence of atrial fibrillation after catheter ablation. Heart Rhythm. 2014;953–960. [DOI] [PubMed] [Google Scholar]
- 14. Zhou W, Wang L, Zhou B, Wu L. Catheter ablation of paroxysmal atrial fibrillation using high‐density mapping‐guided substrate modification. Pacing Clin Electrophysiol. 2018;1630–1634. [DOI] [PubMed] [Google Scholar]
- 15. Mohanty S, Mohanty P, Di Biase L, Trivedi C, Morris EH, Gianni C, Santangeli P, Bai R, Sanchez JE, Hranitzky P, et al. Long‐term follow‐up of patients with paroxysmal atrial fibrillation and severe left atrial scarring: comparison between pulmonary vein antrum isolation only or pulmonary vein isolation combined with either scar homogenization or trigger ablation. Europace. 2017;1790–1797. [DOI] [PubMed] [Google Scholar]


