Abstract
Background
Despite more than 4 million cardiac noninvasive diagnostic tests (NIT) being performed annually for stable coronary artery disease in the United States, it is unclear whether they are associated with downstream improvements in outcomes when compared with no testing. We sought to determine whether NIT was associated with reduced downstream major adverse cardiovascular events when compared with not testing.
Methods and Results
We conducted a population‐based study of ≈1.5 million patients undergoing chest pain evaluation in Ontario, Canada. Patients were categorized into NIT and no‐testing groups. Cause‐specific proportional hazards models were used to compare the rate of major adverse cardiovascular events (composite outcome of unstable angina, acute myocardial infarction or cardiovascular mortality and each constituent) between the 2 groups after adjusting for clinically relevant covariates. The rate of the composite outcome was ≈25% lower for patients undergoing noninvasive testing (hazard ratio [HR], 0.77; 95% CI, 0.75–0.79). The benefits of testing were consistent for all 3 constituents of the composite; unstable angina (HR, 0.87; 95% CI, 0.82–0.93 for the NIT versus the no‐testing group), myocardial infarction (HR, 0.83; 95% CI, 0.79–0.86 for the NIT versus the no‐testing group) and cardiovascular mortality (HR, 0.68; 95% CI, 0.65–0.72 for the NIT versus the no‐testing group).
Conclusions
Our large population‐based study reports an ≈25% reduction in major adverse cardiovascular events that was independently associated with NIT in outpatients being evaluated for stable angina. This study demonstrates the prognostic importance of NIT versus no testing on the health of contemporary populations.
Keywords: cardiac noninvasive testing, chest pain, outpatient cardiology
Subject Categories: Diagnostic Testing, Exercise Testing, Quality and Outcomes
Nonstandard Abbreviations and Acronyms
- CCTA
coronary computed tomography angiography
- GXT
graded exercise stress test
- NIT
noninvasive testing
Clinical Perspective
What Is New?
Despite >4 million cardiac noninvasive diagnostic tests (NIT) performed annually for stable coronary artery disease in the United States, it is unclear whether they are associated with improvements in downstream outcomes.
Our real‐world, population‐based study of ≈1.5 million patients reported that the composite outcome (unstable angina, myocardial infarction, or cardiovascular mortality) was ≈25% lower for patients undergoing NIT compared with no testing (hazard ratio [HR], 0.77; 95% CI, 0.75–0.79).
The benefits of testing were consistent for all 3 composite constituents; unstable angina (HR, 0.87; 95% CI, 0.82–0.93), MI (HR, 0.83; 95% CI, 0.79–0.86) and cardiovascular mortality (HR, 0.68; 95% CI, 0.65–0.72).
What Are the Clinical Implications?
This study demonstrates the prognostic importance of NIT versus no testing on the health of contemporary populations.
Our results suggest that outpatients presenting with chest pain and being evaluated for stable angina may benefit significantly from NIT in the real world.
Our results therefore support current guidelines that advocate for NIT for outpatients being evaluated for stable coronary artery disease and argue against the notion that NIT in this population confers no discernable benefits when compared with no testing.
Stable coronary artery disease or stable angina is one of the most common presentations of patients with cardiovascular disease and leads to the performance of more than 4 million cardiac noninvasive diagnostic tests (NIT) annually in the United States.1, 2, 3 However, despite widespread use, questions remain regarding the association between NIT and downstream outcomes.3, 4, 5, 6, 7, 8 The PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) and SCOT‐HEART (Scottish Computed Tomography of the HEART) trials reported very low incidence of major adverse cardiovascular events (MACE) in patients undergoing different types of NIT.2, 9, 10 Given the very low number of events in contemporary cohorts being evaluated for chest pain, it is unclear if NIT can lead to downstream improvements in outcomes when compared with no testing in contemporary populations. Unfortunately, neither the PROMISE nor SCOT‐HEART trials included distinct “no testing” arms, although the latter may have included some untested patients in their “usual care” arm. As such, neither could address this important gap in knowledge regarding the comparison of outcomes between strategies of any NIT versus no testing in order to help shed light onto this question. Given the lack of proven effectiveness and the great costs conferred by NIT, researchers and policymakers have questioned the utility of NIT in the evaluation of patients for stable coronary artery disease (CAD).2
In order to explore the utility of NIT in patients evaluated for chest pain, we sought to evaluate whether any testing was associated with lower MACE when compared with no testing. In order to address this objective, we utilized a cohort comprising outpatients who were evaluated by physicians for chest pain. Based on the low number of events reported from the PROMISE and SCOT‐HEART clinical trials, we hypothesized that there would be no significant difference in MACE between those patients undergoing NIT when compared with those who were not tested. Our cohort was ≈150‐fold larger than that used in the PROMISE trial and we therefore believed that we would be extremely well positioned to detect differences between the NIT and no‐testing groups.
Methods
The data set from this study is held securely in coded form at the Institute for Clinical Evaluative Sciences (ICES). While data‐sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet prespecified criteria for confidential access, available at www.ices.on.ca/DAS. The full data set creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification. ICES is an independent, nonprofit research institute whose legal status under Ontario's health information privacy law allows it to collect and analyze healthcare and demographic data, without consent, for health system evaluation and improvement. Thus, patients enrolled in this study were exempt from providing informed consent.
Design
We conducted a retrospective cohort study of patients undergoing evaluation for chest pain (the index event) in Ontario, Canada between January 1, 2011 and September 30, 2015.
Derivation of the Cohort
Inclusion criteria included age ≥20 years and evaluation by a physician in Ontario for chest pain using 1 of 3 possible diagnostic codes (785: chest pain not yet diagnosed; 412: chest pain suggestive of CAD; and 413: angina). These are the only possible diagnostic codes that physicians in Ontario can use for the evaluation of chest pain. After inclusion in the cohort, patients were followed for 3 months to evaluate whether they received 1 of 4 noninvasive cardiac diagnostic tests for CAD available in Ontario (graded exercise stress test [GXT], stress echocardiography, myocardial perfusion imaging, or coronary computed tomography angiography [CCTA]). Those who underwent one of these tests were categorized in the NIT (testing) group and the others were categorized in the no‐testing group. As this was an inception cohort, we were interested in excluding those patients with a previous diagnosis of coronary artery disease. We therefore excluded those with a history of cardiovascular disease in the preceding 20 years using a previously validated algorithm that has been utilized in prior work.11, 12, 13, 14, 15 Furthermore, we implemented a 1‐year washout period whereby those who had a NIT in the year prior to evaluation for chest pain were excluded. Because our goal was to evaluate stable outpatients, and in the recognition that those evaluated in the emergency department or admitted to the hospital represent different populations, we excluded those patients who were evaluated for chest pain in the emergency department or while they were hospitalized.
Data Sources
Patient evaluation for chest pain as well as receipt of NIT was ascertained by the Ontario Health Insurance Plan Physician Billing Database as previously described.16, 17, 18, 19 The Registered Persons Database, a registry of Ontario residents who are registered for Ontario Health insurance coverage, was used to obtain demographic information. The presence of diabetes mellitus and hypertension were determined through the Ontario Diabetes and Ontario Hypertension databases, respectively. Total cholesterol values were determined via the Ontario Laboratories Information System. Smoking status was determined via the Canadian Community Health Survey. Hospitalizations were determined by the Canadian Institutes for Health Information Discharge Abstract database. Emergency Department visits were determined through the National Ambulatory Care Reporting System database. History of chronic obstructive pulmonary disease and cancer were determined via the Ontario COPD and Ontario Cancer Registry, respectively. The Immigration, Refugees and Citizenship Canada Permanent Resident Database and the Ontario Visible Minority database were utilized to determine ethnicity and immigration status. The Ontario Registrar General Death Register File was utilized to determine cause of death. The Ontario Drug Benefit Database was used to determine medication use among patients aged 65 years and older. We did not have data regarding medication use in those patients under the age of 65 years. The above‐listed databases were linked using unique encoded identifiers based on individual, de‐identified healthcare numbers and analyzed at ICES. ICES is an independent, nonprofit research institute funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care. As a prescribed entity under Ontario's privacy legislation, ICES is authorized to collect and use healthcare data for the purposes of health system analysis, evaluation, and decision support. Secure access to these data is governed by policies and procedures that are approved by the Information and Privacy Commissioner of Ontario. Given Canada's single‐payer government‐funded healthcare system, we were able to extract patient information with virtually 100% coverage of the population of Ontario.
Exposure
The exposure was receipt of NIT within 90 days of the evaluation for chest pain.
Outcomes
In order to mitigate issues caused by immortal time bias/survivorship bias, we performed landmark analyses for which time zero for follow‐up was at 90 days post initial chest pain evaluation. Patients were then followed up to December 31, 2016 for outcomes. This is the latest date that we can use in our database to ascertain cardiovascular mortality. The primary outcome was a composite of time to hospitalization for unstable angina, myocardial infarction, or cardiovascular mortality. Secondary outcomes were receipt of invasive angiography and coronary revascularization (percutaneous coronary intervention or coronary artery bypass grafting).
Covariates
Covariates were selected a priori based on clinical importance.12, 20, 21, 22, 23, 24, 25, 26 The following covariates were utilized in our statistical models: Cardiovascular risk factors: Age, sex, hypertension, dyslipidemia, diabetes mellitus, smoking status, income strata, and ethnicity. Measures of comorbidity: Chronic obstructive pulmonary disease, The Johns Hopkins Aggregated Diagnostic Groups, active cancer. Cardiac medications (in those aged 65 years and older): Use of aspirin, statins, beta‐blockers, nitrates, and angiotensin‐converting enzyme inhibitors.
Statistical Analysis
Descriptive Statistics
Characteristics of patients undergoing testing were compared with those not undergoing testing using the Pearson's χ2 test for categorical variables and the t test for continuous variables. Because of the large size of the cohort, we assessed for balance between the testing and nontesting arms using standardized differences. Standardized differences of <0.10 were considered to reflect adequate balance between the 2 groups. McNemar's test was used to compare medication use before and after NIT in those patients who underwent NIT and who were aged 65 years and older.
Unadjusted analyses
Grey's test was utilized to compare the cumulative incidence of the outcomes between the NIT and no‐testing groups, after accounting for the competing risk of noncardiovascular death.
Time to event‐adjusted analyses
We utilized a 90‐day landmark analysis with a cause‐specific proportional hazards model to compare the rate of the composite outcome between the NIT and no‐testing groups after adjusting for the clinically relevant covariates listed above. This cause‐specific model was used to account for the competing risk of noncardiovascular death.
Where neighborhood income, rurality, smoking status, or total cholesterol results were missing, we assumed that variables were missing at random and performed multiple imputation, creating 5 imputation data sets. Variables used in the imputation models were age on index date, sex, ethnic group, rurality, income quintile, baseline hypertension, baseline diabetes mellitus, baseline chronic obstructive lung disease, history of cancer, number of adjusted diagnostic groups, testing in 90 days, incidence of main composite outcome, and years of follow‐up. These variables were chosen for the imputation models for 1 of 3 reasons: (1) they have been shown to be either predictive of cholesterol levels or smoking status; (2) they were included in the final analytical model, or (3) they were an event in the final analytic model. Our imputation methods were similar to those previously utilized by our group in other projects and used a form of fully conditional specification (known as Multivariate Imputation using Chained Equations).13, 14
Sensitivity and Subanalyses
We performed sensitivity and subanalyses in order to evaluate the robustness of our results. First, we performed stratified analyses for both those < and ≥65 years of age. Second, we performed an analysis stratified by the different diagnostic codes that physicians used when evaluating patients with chest pain. Finally, we performed a propensity score matched analysis as a sensitivity analysis. The propensity score was estimated using a logistic regression model. Using this model, we regressed receipt of testing within 90 days of the index diagnosis of chest pain (versus no testing within 90 days) on the baseline covariates listed above in the covariates section. Propensity score matching was used to match NIT and nontested subjects. Subjects were matched on the logit of the propensity score using calipers with a width of 0.2 of the standard deviation of the logit of the propensity score.27 Exact matching was performed on sex and neighborhood income quintile. Standardized differences were used in the unmatched and the matched sample to assess for balance of baseline covariates between the testing and nontesting groups. A standardized difference of <0.10 was deemed to be acceptable. After matching was complete, outcomes were compared between the NIT and nontesting groups. Given the presence of competing risks (eg, noncardiovascular death), a cause‐specific hazards model was used to compare the rate of our composite outcome of time to myocardial infarction and/or cardiovascular death between the testing and no‐testing groups.28 A robust variance estimator was used to account for the matched nature of the sample.27 This model allows for a comparison of the cause‐specific hazard of cardiovascular death or myocardial infarction between the 2 groups. It is equivalent to the Cox proportional hazards model in the setting without competing risks. Propensity score matching analysis was performed separately in each imputed data set. The parameter estimates were pooled using Rubin's rules. All analyses were performed with SAS version 9.3. This study was approved by the research ethics board at Sunnybrook Health Sciences Centre.
Results
Derivation of the Study Cohort
During the study period, 2 494 173 subjects without coronary artery disease underwent evaluation for chest pain in Ontario, Canada. Of these, 189 032 subjects were excluded because of having a NIT in the preceding year. Also, 73 618 patients were excluded for having a hospitalization for cardiovascular disease before the index date. As part of the landmark analysis, 81 849 patients were excluded because of experiencing a primary or secondary outcome within 90 days of the index physician visit. An additional 658 032 patients were excluded because of being evaluated for chest pain while they were hospitalized or during an emergency department visit (Figure 1). Finally, 1 491 642 patients remained in our final cohort.
Figure 1. Derivation of the patient population.
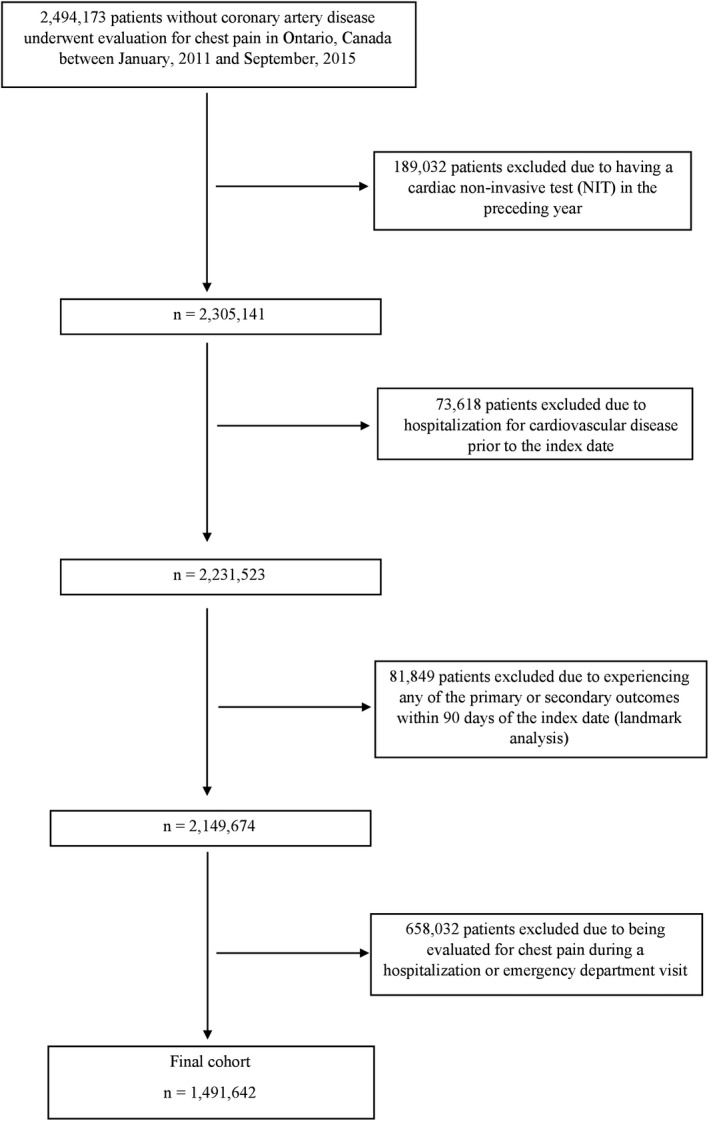
NIT indicates noninvasive testing.
Baseline Patient Characteristics
Of the 317 056 patients who underwent NIT, 186 223 (58.7%) underwent a GXT, 85 128 (26.9%) underwent a myocardial perfusion imaging scan, 44 926 (14.2%) underwent a stress echocardiography, and 779 (0.3%) underwent a CCTA.
Baseline patient characteristics are summarized in Table 1. Overall, the NIT and no‐testing groups were similar (standardized differences <0.10) in the majority of our measured variables in the categories of demographics, cardiovascular risk factors, comorbidities, cardiovascular medications, and ethnicity/immigration status. Subjects undergoing NIT were significantly less likely to be female (49.6% versus 57.7%, respectively, standardized difference=0.16) and to be prescribed beta‐blocking medications (among the elderly) (16.4%, versus 20.5%, respectively, standardized difference=0.11).
Table 1.
Baseline Patient Characteristics
| No Testing (N=1 174 586) | Noninvasive Testing (N=317 056) | Standardized Difference | |
|---|---|---|---|
| Demographics | |||
| Age, y, mean±SD | 55.74±16.54 | 56.11±13.18 | 0.02 |
| Female sex (%) | 677 905 (57.7%) | 157 381 (49.6%) | 0.16 |
| Patient evaluated in a rural location | 103 994 (8.9%) | 31 449 (9.9%) | 0.04 |
| Recent immigrants/ethnicities | |||
| Long‐term resident | 941 590 (80.2%) | 250 329 (79.0%) | 0.03 |
| Black | 25 336 (2.2%) | 5865 (1.8%) | 0.02 |
| East Asian | 38 766 (3.3%) | 8731 (2.8%) | 0.03 |
| Latin American | 15 929 (1.4%) | 4526 (1.4%) | 0.01 |
| South Asian | 64 694 (5.5%) | 21 210 (6.7%) | 0.05 |
| South East Asian | 21 276 (1.8%) | 5001 (1.6%) | 0.02 |
| West Asian | 24 815 (2.1%) | 7328 (2.3%) | 0.01 |
| White: Eastern European | 26 979 (2.3%) | 9543 (3.0%) | 0.04 |
| White: Western European | 12 992 (1.1%) | 3774 (1.2%) | 0.01 |
| Cardiovascular risk factors | |||
| Total cholesterol, mean±SD | 4.84±1.07 | 4.92±1.09 | 0.07 |
| Active smoker | 1842 (19.6%) | 462 (17.6%) | 0.05 |
| Hypertension | 488 754 (41.6%) | 137 981 (43.5%) | 0.04 |
| Diabetes mellitus | 197 429 (16.8%) | 60 207 (19.0%) | 0.06 |
| Income quintile | |||
| 1 | 225 011 (19.2%) | 56 670 (17.9%) | 0.03 |
| 2 | 240 503 (20.5%) | 63 576 (20.1%) | 0.01 |
| 3 | 240 086 (20.5%) | 64 560 (20.4%) | <0.01 |
| 4 | 240 681 (20.6%) | 66 913 (21.2%) | 0.02 |
| 5 | 224 777 (19.2%) | 64 440 (20.4%) | 0.03 |
| Comorbidities | |||
| COPD | 141 701 (12.1%) | 35 966 (11.3%) | 0.02 |
| Cancer | 94 337 (8.0%) | 20 779 (6.6%) | 0.06 |
| Adjusted diagnosis group, mean±SD | 10.64±4.0 | 10.24±3.9 | 0.09 |
| Medications (in subjects aged 65 y and older) | |||
| Angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker | 161 979 (45.4%) | 37 527 (45.1%) | 0.01 |
| Statin | 148 663 (41.6%) | 36 453 (43.8%) | 0.04 |
| Aspirin | 10 038 (2.8%) | 1961 (2.4%) | 0.03 |
| Beta‐blocker | 73 237 (20.5%) | 13 634 (16.4%) | 0.11 |
| Nitrate | 9920 (2.8%) | 2587 (3.1%) | 0.02 |
COPD indicates chronic obstructive pulmonary disease.
Outcomes
Patients were followed for a median of 4.1 years (interquartile range: 2.8–5.2 years) for ascertainment of outcomes.
Unadjusted Outcomes
Among those who did not undergo NIT testing, 26 756 (≈1.5%) underwent invasive coronary angiography within the 90‐day landmark period. Over 5 years of follow‐up after the landmark period, the incidence of downstream invasive angiography (3.8% versus 4.0%, P=0.01) and revascularization (1.5% versus 1.7%, P<0.01) via percutaneous coronary intervention or coronary artery bypass grafting was statistically lower in those undergoing NIT compared with those not undergoing testing. Given the small absolute difference between the NIT and no testing groups with respect to invasive angiography and revascularization; however, it is questionable whether or not these statistical differences are meaningful. Those undergoing NIT were significantly less likely to experience the primary composite outcome of unstable angina, acute myocardial infarction, or cardiovascular mortality (1.7% versus 2.8%, P<0.01). The same relationship was observed for each of the constituents of the primary outcome (Table 2, P<0.01).
Table 2.
Unadjusted Outcomes Comparing the NIT and No‐Testing Groups
| No Testing | Noninvasive Testing | |
|---|---|---|
| Invasive angiography | 46 666 (4.0%) | 11 950 (3.8%) |
| Coronary revascularization (%) | 19 569 (1.7%) | 4833 (1.5%) |
| Unstable angina, acute myocardial infarction, or cardiovascular mortality (%) | 32 853 (2.8%) | 5428 (1.7%) |
| Acute myocardial infarction (%) | 15 021 (1.3%) | 2979 (0.9%) |
| Unstable angina (%) | 5276 (0.5%) | 1123 (0.4%) |
| Cardiovascular mortality (%) | 15 941 (1.4%) | 1829 (0.6%) |
NIT indicates noninvasive diagnostic tests.
Adjusted Outcomes
After adjustment for clinically relevant covariates, the rate of the composite outcome of unstable angina, acute myocardial infarction, or cardiovascular mortality was ≈25% lower in patients undergoing NIT (hazard ratio [HR], 0.77; 95% CI, 0.75–0.79; Figure 2). Moreover, the benefits of testing were consistent for all 3 constituents of the composite outcome; unstable angina (HR, 0.87; 95% CI, 0.82–0.93 for the NIT versus the no‐testing group), myocardial infarction (HR, 0.83; 95% CI, 0.79–0.86 for the NIT versus the no‐testing group), and cardiovascular mortality (HR, 0.68; 95% CI, 0.65–0.72 for the NIT versus the no‐testing group).
Figure 2. Major adverse cardiovascular events compared between the NIT and no‐testing groups in the overall cohort, in patients <65 years of age, in patients aged 65 years and older, and stratified by different physician diagnostic codes.
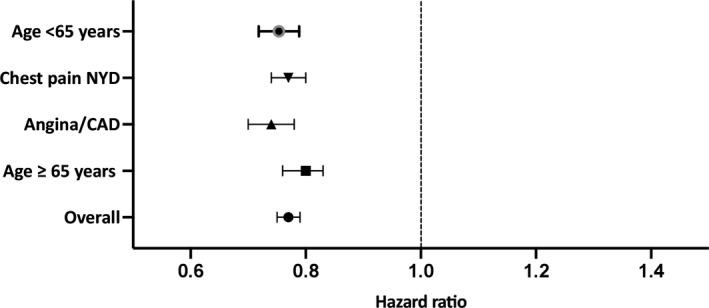
CAD indicates coronary artery disease; NIT, noninvasive diagnostic tests; and NYD, not yet diagnosed.
Medication Use After Testing
Patients undergoing NIT were significantly more likely to be on an angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker (50.8% versus 47.7%, P<0.01), a statin (51.7% versus 47.0%, P<0.01), and a beta‐blocker (22.3% versus 18.9%, P<0.01) after testing compared with before testing. However, they were slightly less likely to be on Aspirin (ASA) (2.4% versus 2.5%, P<0.01).
Sensitivity and Subanalyses
We performed multiple sensitivity and subanalyses in order to evaluate the robustness of our results. First, in both those patients <65 years of age (HR, 0.75; 95% CI, 0.72–0.79) and aged 65 years and older (HR, 0.80; 95% CI, 0.76–0.83) the results were similar to the overall group. Second, we repeated our analyses on those patients undergoing testing stratified by the different physician diagnostic codes. Our results were consistent across the different diagnostic codes (HR, 0.70; 95% CI, 0.74–0.78 for those undergoing testing after being evaluated by a physician for the diagnostic codes for angina/CAD and HR, 0.77; 95% CI, 0.74–0.80 for those undergoing testing after being evaluated by a physician for chest pain not yet diagnosed [Figure 2]).
Propensity Score Matched Analysis
Covariates were well matched between the testing and nontesting groups with standardized differences <0.10 for each covariate. The results of our matched analysis were consistent with our primary overall results as well as those of our other subanalyses, with patients undergoing NIT being significantly less likely to experience the composite main outcome (HR, 0.60; 95% CI, 0.58–0.63) when compared with those not undergoing NIT.
Discussion
In a large, population‐based study of outpatients with chest pain in Ontario, Canada, we observed a ≈25% reduction in the hazard of the composite outcome of unstable angina, acute myocardial infarction, or cardiovascular mortality associated with NIT. Our findings were consistent among all 3 constituents of the composite outcome and were robust across different physician diagnostic codes and in those patients aged 65 years and older.
Despite recent advances in care, CAD remains a major public health problem. The 2010 American Heart Association Heart Disease and Stroke Statistics Update reported that there are ≈18 million people in the United States who have been diagnosed with CAD.29 CAD is the second leading cause of death in Canada and is responsible for ≈30% of all deaths worldwide in people aged 35 and above and ≈17% of total deaths.30, 31, 32 A common presentation for CAD is stable angina, also known as stable CAD, which affects more than 8 million people in the United States each year.33, 34 Two large randomized controlled trials evaluated the efficacy of different NIT options among patients being assessed for stable CAD. The PROMISE trial randomly assigned 10 003 outpatients being evaluated for suspected stable CAD to either CCTA or functional stress testing. The primary end point of MACE (all‐cause mortality, myocardial infarction, hospitalization for unstable angina, or major cardiovascular procedural complication) did not significantly differ between the 2 groups.9 The SCOT‐HEART trial enrolled 4146 patients with stable chest pain to “usual care” (including a GXT) or “usual care” plus CCTA. While the initial results, based on median follow‐up of 1.7 years, reported that there was no significant difference between the 2 arms in terms of the clinical composite outcome of cardiovascular death or myocardial infarction, the subsequently published 5‐year results reported a reduction in the composite outcome associated with usual care plus CCTA.2, 10, 35 Interestingly, the event rates were lower than originally expected in both the anatomical and functional arms of the PROMISE and SCOT‐HEART trials. For example, the annual rate of all‐cause mortality and nonfatal myocardial infarction averaged between both studies was ≈1.4%.2, 9, 10 Given the low event rate, experts in the field have raised a further question of whether any testing leads to improved clinical outcomes when compared with no testing in real‐world populations.2 As far as we are aware, there are currently no data comparing outcomes with and without testing among outpatients being evaluated for stable CAD. The utility of cardiac NIT for chest pain is also questioned in other, related patient populations. For example, Natsui et al recently conducted a retrospective study of 24 459 patients who presented with chest pain to the emergency department of 13 sites across southern California and were subsequently discharged. They reported that there were very low rates of MACE, and there was no significant difference in MACE between those undergoing testing and those who did not. Specifically, there were no deaths and only ≈1.3% of all patients in their cohort developed either unstable angina or acute myocardial infarction within their follow‐up period.36
Similar to the Natsui and colleagues’ study, which focused on patients with chest pain discharged from the emergency department, we report low overall event rates in patients undergoing evaluation for chest pain in the outpatient setting. However, in contrast to the Natsui et al's findings, we report a significant reduction in MACE of ≈25% associated with testing when compared with those who were not tested. We speculate that the difference that we observed when compared with the Natsui article may be related to the different patient populations examined. While both our study and the Natsui article examined patients evaluated for chest pain and possible CAD, ours focused on outpatients evaluated for stable CAD while the latter focused on those patients presenting to the emergency department for chest pain who were discharged home, presumably in the absence of any high‐risk features (otherwise they likely would have been admitted to the hospital for further evaluation of the chest pain). As a result, the overall cohort utilized by Natsui et al was likely of significantly lower risk than ours. In this low‐risk cohort, it is conceivable that NIT did not confer any significant benefit.
Clinical Importance and Significance
Our results are important because they report a significant reduction in downstream MACE after NIT when compared with no testing in a large, contemporary, population‐based cohort. Interestingly, this reduction in MACE was observed despite the absence of increased rates of invasive angiography and/or revascularization associated with NIT. Thus, the lower MACE associated with NIT cannot be attributed to an increase in invasive/revascularization procedures. Our study was not designed to elucidate the possible mechanism for the observed lower MACE in the NIT group. We speculate that 1 possibility is that NIT may have triggered a series of events that ultimately led to better downstream medical management. Unfortunately, we were unable to evaluate medication use in the majority of our cohort (those aged 65 years and under), nor were we able to evaluate lifestyle interventions. Both of these factors may have contributed to our findings if they occurred preferentially in those patients undergoing NIT. Indeed, in those patients aged 65 years and older, use of some guideline‐recommended medications, shown to improve survival in patients with CAD, such as angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers and statins, was significantly higher after NIT when compared with before it. Similar to our study, the 5‐year follow‐up of the SCOT‐HEART study reported that patient receipt of NIT led to an escalation in downstream medication use. Specifically, the SCOT‐HEART study reported that CCTA led to an escalation of cardiovascular medications (“preventative therapies”) when compared with usual care, comprising mostly GXT. This was postulated as a potential mechanism leading to the reduction in the composite outcome associated with the usual care plus CCTA arm of the study. Unlike SCOT‐HEART where 50% of the study patients were randomized to receive CCTA, in our study, only a small percentage of patients (0.3%) received CCTA, with the majority receiving a GXT (58.7%). The small number of patients in our cohort receiving CCTA led to our decision not to compare outcomes between functional and anatomical groups because we felt that our ability to make valid comparisons between the 2 was extremely limited. The low percentage of patients receiving CCTA in our cohort is a reflection of the limited access and restricted capacity for CCTA in Ontario at present. Despite this fact, utilization of CCTA has been increasing in Ontario in recent years.16, 37 Based on the SCOT‐HEART 5‐year results, it is conceivable that NIT may have been associated with both a greater impact on downstream medication utilization, and a further reduction in MACE if CCTA played a more prominent role in testing. Given the trends of increasing utilization of CCTA in Ontario, this premise should be explored in a future study.
Our results suggest that outpatients presenting with chest pain and being evaluated for stable angina in the real world may significantly benefit from NIT. Furthermore, our results support current guidelines that advocate for NIT for outpatients being evaluated for stable CAD and argue against the notion that NIT in this population confers no discernable benefits when compared with no testing.38, 39, 40
Limitations
This study must be interpreted in the context of its limitations. First, because this is an observational study, there is the potential for selection bias in terms of who does and does not get tested. To address this limitation, we used a well‐defined outpatient population and utilized Cox regression analyses to account for observed differences between those tested and those who were not—allowing for adjustment for clinically important covariates. We also performed a propensity score matched analysis as a sensitivity analysis that supported our initial results and enhanced the robustness of our data. Second, our databases lacked granularity in a number of domains. For example, we lacked data on chest pain characteristics. We therefore could not account for the traditional pretest likelihood of obstructive CAD based on chest pain characteristics in our cohort. We also lacked data that would have allowed us to either measure testing for noncardiac causes of chest pain, or reliably determine whether empirical medical therapy was started, targeted at the presumed cause of the chest pain. Third, these results reflect patterns of care and outcomes in Ontario and may not necessarily be generalizable to other jurisdictions. With that said, Ontario is a large and diverse province consisting of ≈14 million people, similar in nature to many diverse populations around the world. The population‐level analyses that we performed (ie, having virtually 100% coverage of the population) further enhance the generalizability of our findings.
Conclusions
Our large, population‐based, real‐world study reported an ≈25% reduction in the rate of the composite outcome of cardiovascular mortality, acute myocardial infarction, or unstable angina that is independently associated with NIT in outpatients being evaluated for stable angina. As far as we are aware, ours is the first study to highlight the prognostic importance of NIT versus no testing on the health of populations.
Sources of Funding
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long‐Term Care (MOHLTC). Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (CIHI), Immigration, Refugees and Citizenship Canada (IRCC), and the Cancer Care Ontario (CCO). The opinions, results, and conclusions reported in this article are those of the authors and are independent from ICES, CIHI, IRCC, and CCO. No endorsement by ICES, the Ontario MOHLTC, CIHI, IRCC, or CCO is intended or should be inferred. We thank IMS Brogan Inc. for use of their Drug Information Database. This work was funded by an operating grant (Grant‐in‐Aid) from the Heart and Stroke Foundation of Canada (Dr Roifman, grant number G‐19‐0026297). This study was further supported by a Canadian Institutes for Health Research Foundation grant (Dr Jack Tu; FDN‐143313). Dr Roifman is also supported by National New Investigator and Ontario Clinician Scientist Phase 1 Awards from the Heart and Stroke Foundation of Canada. Drs Ko and Austin are supported by Mid‐Career Investigator Awards from the Heart and Stroke Foundation of Canada. Dr Wijeysundera is supported by the Ontario Clinician Scientist Phase 2 Award from the Heart and Stroke Foundation of Canada.
Disclosures
None.
Acknowledgments
The authors would like to acknowledge the generous support of Dr Jack Tu in this research. This project is dedicated to Dr Tu, who passed away prior to its completion. Parts of this report are based on Ontario Registrar General (ORG) information on deaths, the original source of which is ServiceOntario. The views expressed therein are those of the authors and do not necessarily reflect those of ORG or Ministry of Government Services.
(J Am Heart Assoc. 2020;9:e015724 DOI: 10.1161/JAHA.119.015724.)
For Sources of Funding and Disclosures, see page 9.
See Editorial by Butala
References
- 1. Douglas PS, Taylor A, Bild D, Bonow R, Greenland P, Lauer M, Peacock F, Udelson J. Outcomes research in cardiovascular imaging: report of a workshop sponsored by the National Heart, Lung, and Blood Institute. Circ Cardiovasc Imaging. 2009;2:339–348. [DOI] [PubMed] [Google Scholar]
- 2. Fordyce CB, Newby DE, Douglas PS. Diagnostic strategies for the evaluation of chest pain: clinical implications from SCOT‐HEART and PROMISE. J Am Coll Cardiol. 2016;67:843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levin DC, Rao VM. Turf wars in radiology: the overutilization of imaging resulting from self‐referral. J Am Coll Radiol. 2004;1:169–172. [DOI] [PubMed] [Google Scholar]
- 4. Levin DC, Rao VM, Parker L, Frangos AJ, Sunshine JH. Recent trends in utilization rates of noncardiac thoracic imaging: an example of how imaging growth might be controlled. J Am Coll Radiol. 2007;4:886–889. [DOI] [PubMed] [Google Scholar]
- 5. Levin DC, Rao VM, Parker L, Frangos AJ, Sunshine JH. Recent trends in utilization rates of abdominal imaging: the relative roles of radiologists and nonradiologist physicians. J Am Coll Radiol. 2008;5:744–747. [DOI] [PubMed] [Google Scholar]
- 6. Lucas FL, DeLorenzo MA, Siewers AE, Wennberg DE. Temporal trends in the utilization of diagnostic testing and treatments for cardiovascular disease in the United States, 1993–2001. Circulation. 2006;113:374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maitino AJ, Levin DC, Parker L, Rao VM, Sunshine JH. Practice patterns of radiologists and nonradiologists in utilization of noninvasive diagnostic imaging among the Medicare population 1993–1999. Radiology. 2003;228:795–801. [DOI] [PubMed] [Google Scholar]
- 8. Maitino AJ, Levin DC, Parker L, Rao VM, Sunshine JH. Nationwide trends in rates of utilization of noninvasive diagnostic imaging among the Medicare population between 1993 and 1999. Radiology. 2003;227:113–117. [DOI] [PubMed] [Google Scholar]
- 9. Douglas PS, Hoffmann U, Patel MR, Mark DB, Al‐Khalidi HR, Cavanaugh B, Cole J, Dolor RJ, Fordyce CB, Huang M, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015;372:1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. SCOT‐HEART Investigators . CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT‐HEART): an open‐label, parallel‐group, multicentre trial. Lancet. 2015;385:2383–2391. [DOI] [PubMed] [Google Scholar]
- 11. Tu K, Mitiku T, Lee DS, Guo H, Tu JV. Validation of physician billing and hospitalization data to identify patients with ischemic heart disease using data from the Electronic Medical Record Administrative data Linked Database (EMRALD). Can J Cardiol. 2010;26:e225–e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tu JV, Chu A, Rezai MR, Guo H, Maclagan LC, Austin PC, Booth GL, Manuel DG, Chiu M, Ko DT, et al. The incidence of major cardiovascular events in immigrants to Ontario, Canada: the CANHEART Immigrant Study. Circulation. 2015;132:1549–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tu JV, Chu A, Maclagan L, Austin PC, Johnston S, Ko DT, Cheung I, Atzema CL, Booth GL, Bhatia RS, et al. Regional variations in ambulatory care and incidence of cardiovascular events. CMAJ. 2017;189:E494–E501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tu JV, Chu A, Donovan LR, Ko DT, Booth GL, Tu K, Maclagan LC, Guo H, Austin PC, Hogg W, et al. The Cardiovascular Health in Ambulatory Care Research Team (CANHEART): using big data to measure and improve cardiovascular health and healthcare services. Circ Cardiovasc Qual Outcomes. 2015;8:204–212. [DOI] [PubMed] [Google Scholar]
- 15. Lee DS, Stitt A, Wang X, Yu JS, Gurevich Y, Kingsbury KJ, Austin PC, Tu JV. Administrative hospitalization database validation of cardiac procedure codes. Med Care. 2013;51:e22–e26. [DOI] [PubMed] [Google Scholar]
- 16. Roifman I, Rezai MR, Wijeysundera HC, Chow BJ, Wright GA, Tu JV. Utilization of cardiac computed tomography angiography and outpatient invasive coronary angiography in Ontario, Canada. J Cardiovasc Comput Tomogr. 2015;9:567–571. [DOI] [PubMed] [Google Scholar]
- 17. Roifman I, Wijeysundera HC, Austin PC, Maclagan LC, Rezai MR, Wright GA, Tu JV. Temporal trends in the utilization of noninvasive diagnostic tests for coronary artery disease in Ontario between 2008 and 2014: a population‐based study. Can J Cardiol. 2017;33:279–282. [DOI] [PubMed] [Google Scholar]
- 18. Roifman I, Wijeysundera HC, Austin PC, Rezai MR, Wright GA, Tu JV. Comparison of anatomic and clinical outcomes in patients undergoing alternative initial noninvasive testing strategies for the diagnosis of stable coronary artery disease. J Am Heart Assoc. 2017;6:e005462 DOI: 10.1161/JAHA.116.005462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roifman I, Austin PC, Qiu F, Wijeysundera HC. Impact of the publication of appropriate use criteria on utilization rates of myocardial perfusion imaging studies in Ontario, Canada: a population‐based study. J Am Heart Assoc. 2017;6:e005961 DOI: 10.1161/JAHA.117.005961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Anand SS, Islam S, Rosengren A, Franzosi MG, Steyn K, Yusufali AH, Keltai M, Diaz R, Rangarajan S, Yusuf S. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J. 2008;29:932–940. [DOI] [PubMed] [Google Scholar]
- 21. Gehani AA, Al‐Hinai AT, Zubaid M, Almahmeed W, Hasani MR, Yusufali AH, Hassan MO, Lewis BS, Islam S, Rangarajan S, et al. Association of risk factors with acute myocardial infarction in Middle Eastern countries: the INTERHEART Middle East study. Eur J Prev Cardiol. 2014;21:400–410. [DOI] [PubMed] [Google Scholar]
- 22. Guo J, Li W, Wang Y, Chen T, Teo K, Liu LS, Yusuf S. Influence of socioeconomic status on acute myocardial infarction in the Chinese population: the INTERHEART China study. Chin Med J (Engl). 2012;125:4214–4220. [PubMed] [Google Scholar]
- 23. Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, Blackett KN, Sitthi‐amorn C, Sato H, Yusuf S. Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:953–962. [DOI] [PubMed] [Google Scholar]
- 24. Teo KK, Ounpuu S, Hawken S, Pandey MR, Valentin V, Hunt D, Diaz R, Rashed W, Freeman R, Jiang L, et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case‐control study. Lancet. 2006;368:647–658. [DOI] [PubMed] [Google Scholar]
- 25. Kraus JF, Borhani NO, Franti CE. Socioeconomic status, ethnicity, and risk of coronary heart disease. Am J Epidemiol. 1980;111:407–414. [DOI] [PubMed] [Google Scholar]
- 26. Lee DS, Chiu M, Manuel DG, Tu K, Wang X, Austin PC, Mattern MY, Mitiku TF, Svenson LW, Putnam W, et al. Trends in risk factors for cardiovascular disease in Canada: temporal, socio‐demographic and geographic factors. CMAJ. 2009;181:E55–E66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Austin PC. The use of propensity score methods with survival or time‐to‐event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:1242–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lloyd‐Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, et al. Executive summary: heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:948–954. [DOI] [PubMed] [Google Scholar]
- 30. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. World Health Organization fact sheet on cardiovascular diseases. World Health Organization; 2017. Available at: http://wwwwhoint/mediacentre/factsheets/fs317/en/. Accessed November, 2019. [Google Scholar]
- 32. Mancini GB, Gosselin G, Chow B, Kostuk W, Stone J, Yvorchuk KJ, Abramson BL, Cartier R, Huckell V, Tardif JC, et al. Canadian Cardiovascular Society guidelines for the diagnosis and management of stable ischemic heart disease. Can J Cardiol. 2014;30:837–849. [DOI] [PubMed] [Google Scholar]
- 33. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, et al. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. [DOI] [PubMed] [Google Scholar]
- 34. Eisen A, Bhatt DL, Steg PG, Eagle KA, Goto S, Guo J, Smith SC, Ohman EM, Scirica BM. Angina and future cardiovascular events in stable patients with coronary artery disease: insights from the Reduction of Atherothrombosis for Continued Health (REACH) Registry. J Am Heart Assoc. 2016;5:e004080 DOI: 10.1161/JAHA.116.004080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Newby DE, Adamson PD, Berry C, Boon NA, Dweck MR, Flather M, Forbes J, Hunter A, Lewis S, MacLean S, et al. Coronary CT angiography and 5‐year risk of myocardial infarction. N Engl J Med. 2018;379:924–933. [DOI] [PubMed] [Google Scholar]
- 36. Natsui S, Sun BC, Shen E, Wu YL, Redberg RF, Lee MS, Ferencik M, Zheng C, Kawatkar AA, Gould MK, et al. Evaluation of outpatient cardiac stress testing after emergency department encounters for suspected acute coronary syndrome. Ann Emerg Med. 2019;74:216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vira T, Pechlivanoglou P, Connelly K, Wijeysundera HC, Roifman I. Cardiac computed tomography and magnetic resonance imaging vs. transoesophageal echocardiography for diagnosing left atrial appendage thrombi. Europace. 2019;21:e1–e10. [DOI] [PubMed] [Google Scholar]
- 38. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126:e354–e471. [DOI] [PubMed] [Google Scholar]
- 39. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126:3097–3137. [DOI] [PubMed] [Google Scholar]
- 40. Roifman I, Han L, Koh M, Wijeysundera HC, Austin PC, Douglas PS, Ko DT. Clinical effectiveness of cardiac noninvasive diagnostic testing in patients discharged from the emergency department for chest pain. J Am Heart Assoc. 2019;8:e013824 DOI: 10.1161/JAHA.119.013824. [DOI] [PMC free article] [PubMed] [Google Scholar]


