INTRODUCTION
Gastroesophageal adenocarcinomas (GEAs) represent a significant global health concern, often presenting in late, metastatic stages resulting in high levels of morbidity and mortality.1-3 Diffuse-type gastric cancers characterized by poorly cohesive cells and often signet ring morphology have a worse prognosis as the result of earlier age of onset, rapid disease progression, high rates of metastases, and decreased response rates to standard therapies.4-6 The genomic heterogeneity of GEAs between and within patients has been well described, and the identification of actionable mutations is a field of study with novel clinical trial designs.7-12 Comprehensive genomic profiling using tissue-based next-generation sequencing (NGS) of GEAs has demonstrated recurrent genomic alterations, including frequently observed gene amplifications of receptor tyrosine kinases (RTKs) such as HER2, MET, EGFR, FGFR2, and also downstream KRAS, each of which may benefit from targeted treatment.13,14 NGS of cell-free circulating tumor DNA (ctDNA-NGS) can provide further disease characterization and important prognostic information when correlated with serial ctDNA response.15,16
EGFR is a well-recognized genomically activated oncogenic driver. EGFR monoclonal antibodies and tyrosine kinase inhibitors have been approved for numerous malignancies. Early-phase clinical trials suggested potential benefit in GEAs17-19; however, larger phase III trials incorporating EGFR inhibition in first- and later-line settings were subsequently negative, although notably performed in biomarker-unselected patient populations.20-22 The clinical activity of EGFR inhibitors in GEAs with genomically activated EGFR amplification has been previously described, including in cases where conventional therapies have been exhausted.23,24 Although response rates are notably high in EGFR-amplified tumors, widespread potential for anti-EGFR treatment resistance, including genomic activation of downstream MAPK and PIKCA pathways, is common.23
An immune checkpoint inhibitor (ICI), pembrolizumab, is approved for third-line treatment of microsatellite stable GEA tumors with PD-L1 expression by combined positivity score (CPS) ≥ 1, although response rate is only 13.3%.25,26 Recently, the strategy of ICIs in combination with anti-HER2 antibodies for HER2-amplified tumors has demonstrated efficacy.27-29 For GEA tumors harboring other RTK amplifications, such as EGFR, this strategy of dual-target inhibition toward the RTK in combination with ICIs to harness both the innate and adaptive immune systems to potentially overcome resistance mechanisms toward either agent alone may represent an important novel therapeutic strategy. Herein, we report the first case, to our knowledge, of an exceptional response to combination anti-EGFR and anti–PD-1 dual antibody therapy in a patient with chemorefractory GEA harboring EGFR amplification and low-level PD-L1 expression.
CASE REPORT
A 43-year-old, previously healthy woman presented with abdominal pain in fall 2018. On September 27, 2018, she underwent endoscopy and was diagnosed with a gastric fundus ulcer demonstrating a poorly differentiated adenocarcinoma, Epstein-Barr virus negative, with HER2 immunohistochemistry (IHC) 3+ expression. She received neoadjuvant chemotherapy with FLOT (fluorouracil, leucovorin, oxaliplatin, docetaxel) for four cycles, which she tolerated poorly because of nausea/vomiting, fatigue, alopecia, and neuropathy. On January 10, 2019, she underwent a total gastrectomy, esophagojejunostomy, and cholecystectomy. Final pathology demonstrated ypT4aN3aM0R0, grade 2 pathologic response, poorly differentiated adenocarcinoma with signet ring cell features (mixed type), extending to the serosal surface with 12 of 16 lymph node involvement, and HER2 IHC 3+ expression. Given the poor pathologic response to neoadjuvant therapy, per the outside treating physician she then received 5 weeks of adjuvant chemoradiation with capecitabine, which was completed in May 2019.
On August 28, 2019, a computed tomography (CT) scan of the abdomen and pelvis demonstrated postsurgical changes and high-density adnexal lesions. By November 2019, an abdominal magnetic resonance image demonstrated clear masses consistent with recurrent metastatic peritoneal disease. Given her symptoms of abdominal pain, on December 16, 2019, palliative bilateral ovarian tumor debulking and salpingectomy were performed. The final pathology showed extensive metastatic involvement of focally necrotic grade 3, poorly differentiated adenocarcinoma with more than 50% signet rings. Lymphovascular invasion was present in all specimens, including the ovaries, cul-de-sac, infra-colic omental remnant, and small bowel mesentery biopsies.
She presented to University of Chicago Medical Oncology for an initial consult on January 2, 2020. She endorsed fatigue and weight loss of more than 50 pounds since the initial primary tumor resection in January 2019. A restaging CT scan on January 24, 2020, demonstrated rapid progression from scans obtained in December 2019, with encasement of the left ureter and peritoneal, pleural, and intrahepatic metastases. Treatment options for recurrent metastatic disease were discussed. However, she declined further chemotherapy or port-a-cath replacement given prior chemotherapy intolerance, infection of her first central venous access, and declining performance status.
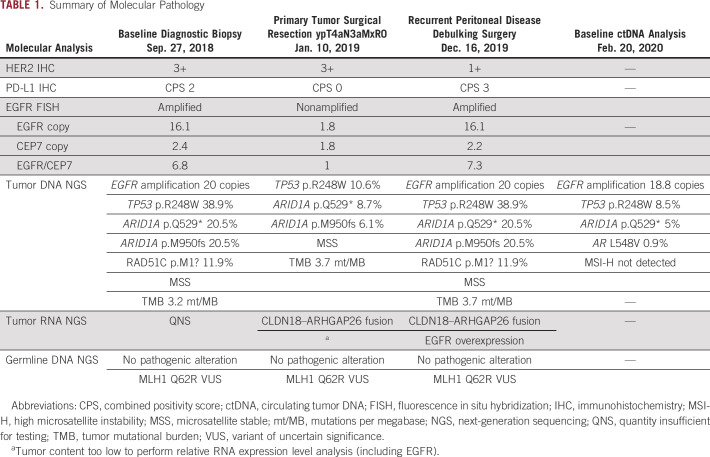
Molecular testing was performed with ctDNA with Guardant 360 on February 20, 2020, and Tempus xT on the DNA/RNA from the December 16, 2019, palliative metastasectomy. Tumor-NGS results demonstrated EGFR amplification (20 copies) by DNA-NGS and CLDN18-ARHGAP26 chromosomal rearrangement and EGFR overexpression by RNA-NGS. PD-L1 IHC was CPS 3 by Dako PD-L1 22C3 clone 3. Analysis of ctDNA-NGS demonstrated EGFR amplification (18.8 copies; Table 1, Figs 1 and 2).
TABLE 1.
Summary of Molecular Pathology
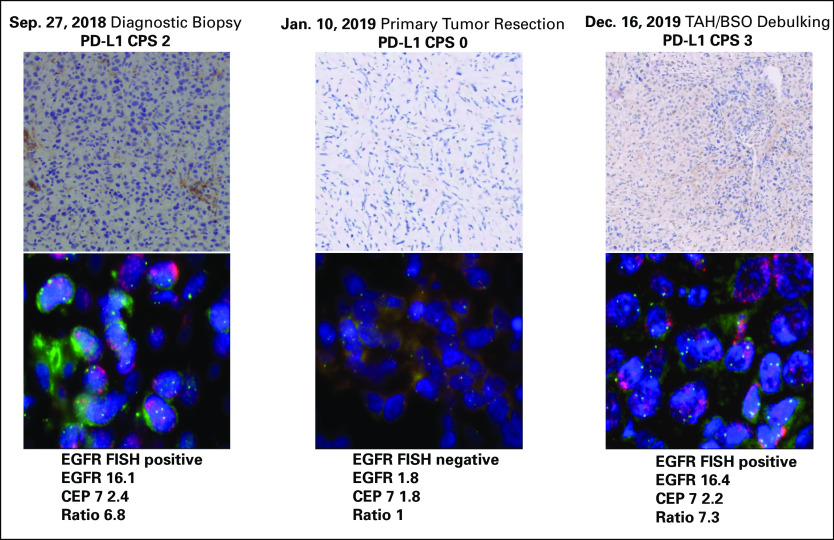
FIG 1.
Immunohistochemistry (IHC) analysis of PD-L1 and fluorescence in situ hybridization (FISH) analysis of EGFR on three time points obtained before initiation of anti-EGFR and anti–PD-1 combination therapy. CPS, combined positivity score; TAH/BSO, total abdominal hysterectomy–bilateral salpingo-oophorectomy.
FIG 2.
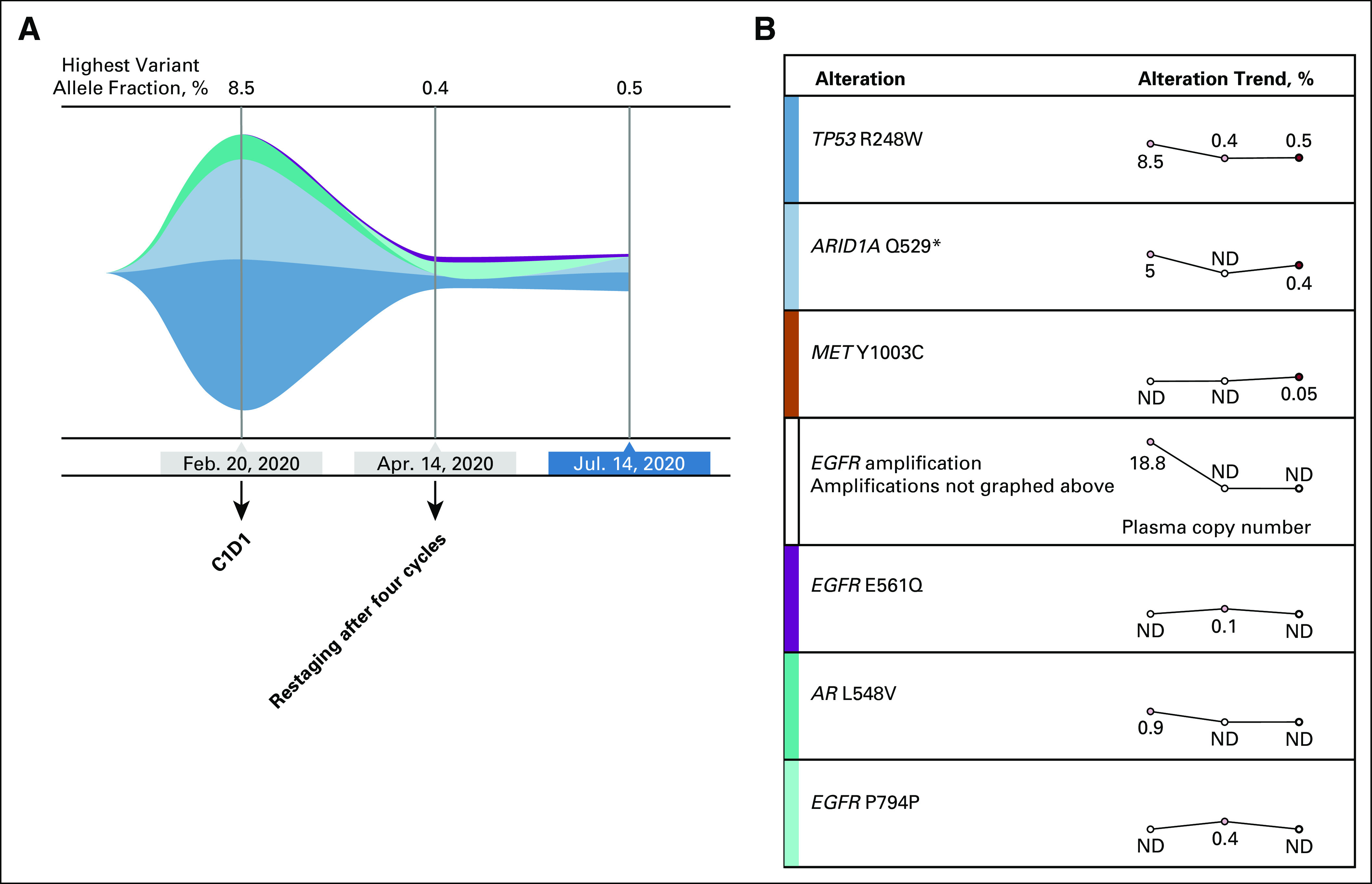
Circulating tumor DNA analysis before and after cetuximab and nivolumab therapy. (A) Tumor response map with trends in mutation allele frequency. (B) Trends over the same time points of the specific genomic events. C1D1, cycle one day 1 of therapy; ND, not detected.
The patient had previously experienced rapid progression after curative-intent chemotherapy and chemoradiotherapy and now declined further chemotherapy. Given the findings of high EGFR amplification in her tumor and blood; PD-L1 positivity in the tumor; and after discussing the rationale and potential risks and benefits, the decision was made to proceed with cetuximab 500 mg/kg and nivolumab 240 mg every 2 weeks for four cycles. While awaiting insurance approval of this planned treatment, she experienced increased bloating and underwent palliative paracentesis on February 11, 2020, with 2.6 L drained but with rapid ascites re-accumulation. She presented to an emergency department on February 15, 2020, with nausea, vomiting, anorexia, and lower-extremity edema. She weighed 80 pounds, had a serum albumin level of 3.0 g/dL, and elevated carcinoembryonic antigen level to 8.2 ng/mL. On February 20, 2020, a restaging CT scan demonstrated worsening metastatic disease with multiple new hepatic lesions, worsening bilateral pleural effusions and pleural nodularity, peritoneal lining thickening with soft tissue nodules/serosal implants, and left ureteral encasement with associated hydronephrosis (Fig 3). Her symptoms remained severe up to and including the day of the first dose of nivolumab/cetuximab on February 20, 2020. On February 25, 2020, she received an additional paracentesis, with 4.2 L drained.
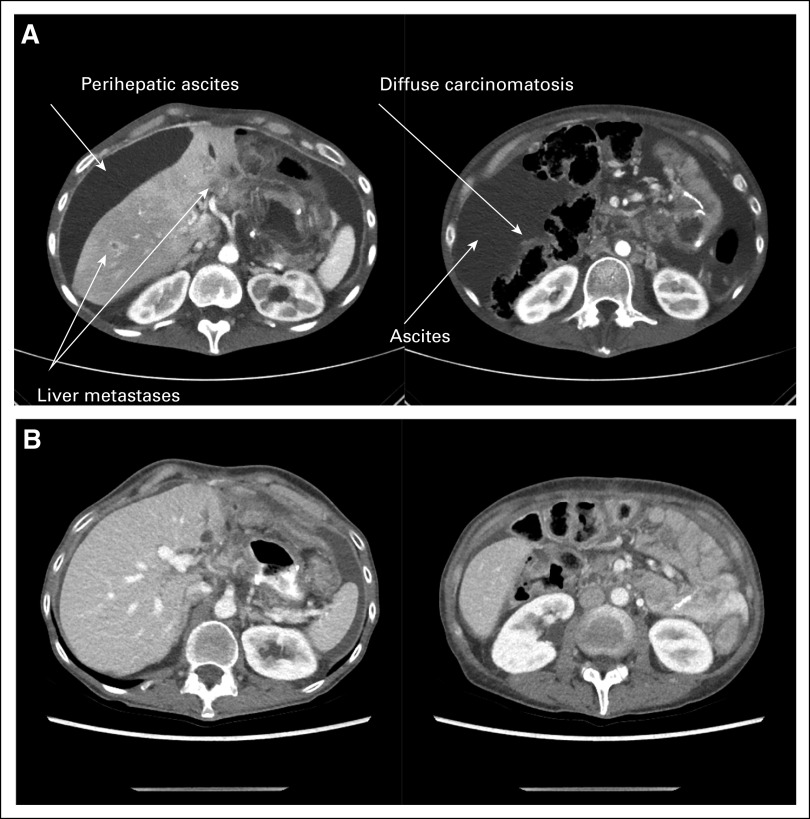
FIG 3.
Computed tomography image obtained at baseline on February 20, 2020, before therapy (A) and after four doses of cetuximab plus nivolumab (computed tomography image obtained on April 15, 2020; B), demonstrating complete resolution of carcinomatosis, ascites, and liver lesions.
She continued to receive the therapy every 2 weeks, with improvement of the cancer-related symptoms after one cycle of therapy. She tolerated treatment well, apart from a characteristic cetuximab rash on her chest and back and a few pustules on her face, controlled with topical therapies. She did not have any GI toxicities. At follow-up on April 15, 2020, after a total of four cycles of nivolumab/cetuximab, she had marked resolution of her cancer-related symptoms. Clinically, she had an improvement in appetite, energy, and no further need for paracentesis. Objectively, she had a weight gain of 10 pounds, improvement in serum albumin to 3.7 g/dL, normalization of carcinoembryonic antigen level from 8.2 to 3.0 ng/mL, and markedly decreased ctDNA levels after four cycles (Fig 2). At this time, a repeat CT scan demonstrated complete response of all metastatic foci, including hepatic, peritoneal, and periureteral masses (Fig 3). The patient continued to receive four more cycles with a restaging CT scan obtained on June 11, 2020, and then again with continued treatment up to CT and ctDNA assessments on July 14, 2020, which confirmed a continued clinical, serologic, and radiographic complete response. She continues to receive this therapy to date.
The investigators obtained informed consent to publish information and/or images from the patient.
DISCUSSION
Diffuse-type/signet ring morphologies comprise approximately 35% of all gastric cancers and are associated with aggressive biology, decreased responsiveness to standard therapies, and poor prognosis.4-6,30,31 This report illustrates a young woman who experienced rapid radiologic and clinical progression with symptomatic ascites and carcinomatosis following definitive treatment with multimodal therapy. Upon the expected and confirmed recurrence of peritoneal disease in November 2019, this case would historically have been associated with a poor prognosis.32-35
We and others have reported extraordinary responses with anti-EGFR therapy toward EGFR-amplified GEAs; however, the true response rate is difficult to assess because of relatively small cohorts.23,24 With late-line cetuximab monotherapy, two of three patients experienced objective responses, including one with a complete response, but with relatively quick progression because of various mechanisms of resistance.23 In addition, late-line anti-PD1 monotherapy in microsatellite stable, PD-L1 CPS ≥ 1–expressing tumors has objective response in only 13.3% of cases.25,36 It is established that patients with higher levels of PD-L1, such as CPS ≥ 10, experience higher rates of response, while reciprocally, those with lower-level CPS scores, such as our patient, have response rates even lower than 13%. Although recent literature has suggested that ARID1A alterations are associated with a favorable prognosis after immune checkpoint therapy, it is unknown if this contributed to our patient’s response, and this question should be further evaluated in larger sample sets.37,38
Recently, in HER2-amplified GEA, an additional strategy has emerged to treat with combination anti-HER2 therapy and ICIs.27-29,39 Margetuximab, an Fc-engineered monoclonal HER2 antibody designed to enhance antibody-dependent cellular cytotoxicity, in combination with pembrolizumab demonstrated safety and efficacy in a biomarker-enriched (HER2 3+/PD-L1 CPS ≥ 1) subpopulation of HER2-positive GEA. The synergistic antitumor activity is thought to be secondary to cross-talk between the innate (antibody-dependent cellular cytotoxicity) and adaptive (CD8-mediated) immune responses (Fig 4).28,40,41 Given the response rates observed with this combination, which are far higher than expected for either agent alone, a chemotherapy-free cohort A has been initiated within the MAHOGANY first-line study for this select biomarker group (ClinicalTrials.gov identifier: NCT04082364).42
FIG 4.
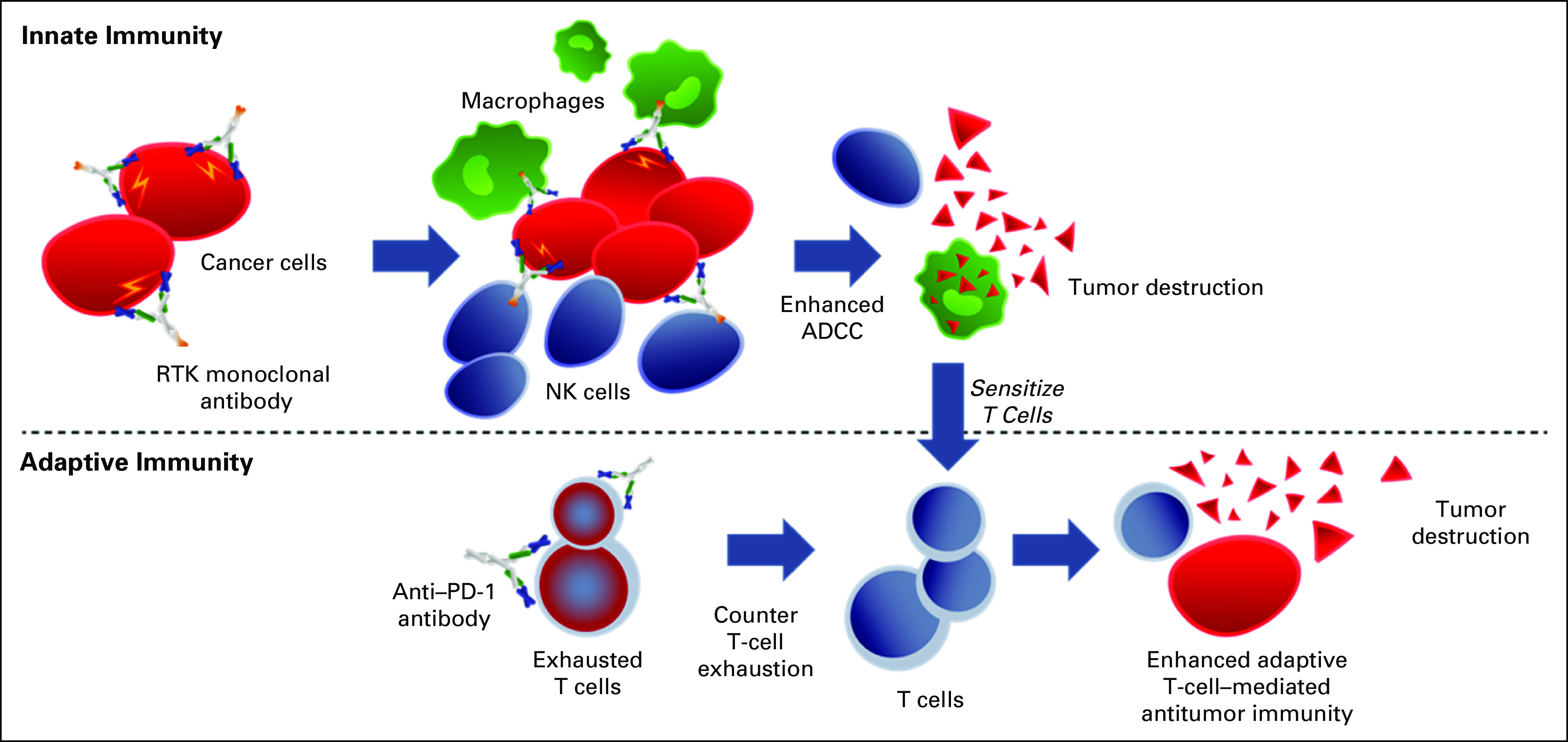
Strategy of combined anti–receptor tyrosine kinase (RTK) antibody to enhance innate antibody-dependent cell-mediated cytotoxicity (ADCC) along with anti–PD-1 antibody to enhance adaptive immunity. NK, natural killer.
As a consequence of this background, the chemorefractory nature of the tumor, and coupled with the molecular findings of EGFR DNA amplification, RNA overexpression, along with low-level PD-1 protein expression, we treated with a chemotherapy-free, dual-antibody strategy toward EGFR and PD-1. This resulted in a dramatic complete clinical, serological, and radiologic response. It should be noted that HER2 IHC was 3+ in the diagnostic and curative surgery samples, yet neither tissue-NGS nor fluorescence in situ hybridization confirmed gene amplification. In the palliative surgical sample, HER2 IHC and tissue-NGS were negative, as was ctDNA-NGS. Therefore, HER2 was not considered an important therapeutic target. Interestingly, there was presence of concurrent claudin fusion by RNA sequencing, which may be a future targetable option at the time of developed resistance to current therapy, if CLDN18.2 overexpression is confirmed by IHC.43,44
In conclusion, this case demonstrates tolerability and early efficacy of a novel combination of anti-EGFR and ICI antibodies in a poor prognosis, chemorefractory scenario. The strategy of dual–monoclonal antibody inhibition may play a role not only in HER2-positive/PD-L1–positive patients, but as demonstrated by this case, also in EGFR-positive/PD-L1–positive disease or other RTK-amplified subgroups such as MET and FGFR2, on the basis of similar hypotheses. In PANGEA, a recent phase IIa platform study for GEAs, the median overall survival was 15.7 months using a personalized treatment strategy of chemotherapy plus one matched monoclonal antibody sequentially through three lines of therapy in newly diagnosed GEA.45 This case of anti-EGFR plus anti–PD-1 combined antibody therapy lends support of dual inhibition of RTKs plus ICIs. A planned PANGEA-2 platform personalized treatment strategy will evaluate dual versus monotherapy monoclonal antibodies for patients with metastatic GEA.
SUPPORT
Supported by the following peer-reviewed grants: NIH K23 award (CA178203-01A1), UCCCC (University of Chicago Comprehensive Cancer Center) Award in Precision Oncology—CCSG (Cancer Center Support Grant) (P30CA014599), and UCCCC Ullman Scholar Award for Immunotherapy (D.V.T.C.).
AUTHOR CONTRIBUTIONS
Conception and design: Natalie Reizine, Yi-Hung Carol Y. H. Tan, Malcolm Bilimoria, Daniel V. T. Catenacci
Provision of study material or patients: Yan Wang
Collection and assembly of data: Natalie Reizine, Bryan Peterson, Stephanie Moya, Ernst Lengyel, Daniel V. T. Catenacci
Data analysis and interpretation: Natalie Reizine, Bryan Peterson, Yan Wang, Oliver S. Eng, Ernst Lengyel, Kiran Turaga, Daniel V. T. Catenacci
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Ernst Lengyel
Stock and Other Ownership Interests: Exelixis
Research Funding: AbbVie
Daniel V. T. Catenacci
Honoraria: Genentech, Eli Lilly, Amgen, Foundation Medicine, Taiho Pharmaceutical, Guardant Health, Merck, Bristol Myers Squibb, Gritstone Oncology, Five Prime Therapeutics, Astellas Pharma, Seattle Genetics, Tempus
Consulting or Advisory Role: Genentech, Amgen, Merck, Eli Lilly, Taiho Pharmaceutical, Bristol Myers Squibb, Astellas Pharma, Seattle Genetics
Speakers’ Bureau: Guardant Health, Foundation Medicine, Genentech, Eli Lilly, Merck, Tempus
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Sehdev A, Catenacci DV. Gastroesophageal cancer: Focus on epidemiology, classification, and staging. Discov Med. 2013;16:103–111. [PubMed] [Google Scholar]
- 3.Blot WJ, Devesa SS, Kneller RW, et al. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991;265:1287–1289. [PubMed] [Google Scholar]
- 4.Pernot S, Voron T, Perkins G, et al. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J Gastroenterol. 2015;21:11428–11438. doi: 10.3748/wjg.v21.i40.11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adachi Y, Yasuda K, Inomata M, et al. Pathology and prognosis of gastric carcinoma: Well versus poorly differentiated type. Cancer. 2000;89:1418–1424. [PubMed] [Google Scholar]
- 6.Kwon KJ, Shim KN, Song EM, et al. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer. 2014;17:43–53. doi: 10.1007/s10120-013-0234-1. [DOI] [PubMed] [Google Scholar]
- 7.Lin D, Khan U, Goetze TO, et al. Gastroesophageal junction adenocarcinoma: Is there an optimal management? Am Soc Clin Oncol Educ Book. 2019;39:e88–e95. doi: 10.1200/EDBK_236827. [DOI] [PubMed] [Google Scholar]
- 8.Pectasides E, Stachler MD, Derks S, et al. Genomic heterogeneity as a barrier to precision medicine in gastroesophageal adenocarcinoma. Cancer Discov. 2018;8:37–48. doi: 10.1158/2159-8290.CD-17-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woo J, Cohen SA, Grim JE. Targeted therapy in gastroesophageal cancers: Past, present and future. Gastroenterol Rep (Oxf) 2015;3:316–329. doi: 10.1093/gastro/gov052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyons TG, Ku GY. Systemic therapy for esophagogastric cancer: Targeted therapies. Linchuang Zhongliuxue Zazhi. 2017;6:48. doi: 10.21037/cco.2017.07.02. [DOI] [PubMed] [Google Scholar]
- 11.Maron SB, Catenacci DVT. Update on gastroesophageal adenocarcinoma targeted therapies. Hematol Oncol Clin North Am. 2017;31:511–527. doi: 10.1016/j.hoc.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catenacci DVT, Lomnicki S, Chase L, et al. Personalized ANtibodies for GastroEsophageal Adenocarcinoma (PANGEA): Primary efficacy analysis of the phase II platform trial (NCT02213289) J Clin Oncol. 2020;38(4_suppl; abstr 356) [Google Scholar]
- 13.Ali SM, Sanford EM, Klempner SJ, et al. Prospective comprehensive genomic profiling of advanced gastric carcinoma cases reveals frequent clinically relevant genomic alterations and new routes for targeted therapies. Oncologist. 2015;20:499–507. doi: 10.1634/theoncologist.2014-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong GS, Zhou J, Liu JB, et al. Targeting wild-type KRAS-amplified gastroesophageal cancer through combined MEK and SHP2 inhibition. Nat Med. 2018;24:968–977. doi: 10.1038/s41591-018-0022-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maron SB, Chase LM, Lomnicki S, et al. Circulating tumor DNA sequencing analysis of gastroesophageal adenocarcinoma. Clin Cancer Res. 2019;25:7098–7112. doi: 10.1158/1078-0432.CCR-19-1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato S, Okamura R, Baumgartner JM, et al. Analysis of circulating tumor DNA and clinical correlates in patients with esophageal, gastroesophageal junction, and gastric adenocarcinoma. Clin Cancer Res. 2018;24:6248–6256. doi: 10.1158/1078-0432.CCR-18-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lordick F, Luber B, Lorenzen S, et al. Cetuximab plus oxaliplatin/leucovorin/5-fluorouracil in first-line metastatic gastric cancer: A phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Br J Cancer. 2010;102:500–505. doi: 10.1038/sj.bjc.6605521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim C, Lee JL, Ryu MH, et al. A prospective phase II study of cetuximab in combination with XELOX (capecitabine and oxaliplatin) in patients with metastatic and/or recurrent advanced gastric cancer. Invest New Drugs. 2011;29:366–373. doi: 10.1007/s10637-009-9363-0. [DOI] [PubMed] [Google Scholar]
- 19.Pinto C, Di Fabio F, Barone C, et al. Phase II study of cetuximab in combination with cisplatin and docetaxel in patients with untreated advanced gastric or gastro-oesophageal junction adenocarcinoma (DOCETUX study) Br J Cancer. 2009;101:1261–1268. doi: 10.1038/sj.bjc.6605319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): A randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:490–499. doi: 10.1016/S1470-2045(13)70102-5. [DOI] [PubMed] [Google Scholar]
- 21.Dutton SJ, Ferry DR, Blazeby JM, et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): A phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol. 2014;15:894–904. doi: 10.1016/S1470-2045(14)70024-5. [DOI] [PubMed] [Google Scholar]
- 22.Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): A randomised, open-label phase 3 trial. Lancet Oncol. 2013;14:481–489. doi: 10.1016/S1470-2045(13)70096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maron SB, Alpert L, Kwak HA, et al. Targeted therapies for targeted populations: Anti-EGFR treatment for EGFR-amplified gastroesophageal adenocarcinoma. Cancer Discov. 2018;8:696–713. doi: 10.1158/2159-8290.CD-17-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petty RD, Dahle-Smith A, Stevenson DAJ, et al. Gefitinib and EGFR gene copy number aberrations in esophageal cancer. J Clin Oncol. 2017;35:2279–2287. doi: 10.1200/JCO.2016.70.3934. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joshi SS, Maron SB, Catenacci DV. Pembrolizumab for treatment of advanced gastric and gastroesophageal junction adenocarcinoma. Future Oncol. 2018;14:417–430. doi: 10.2217/fon-2017-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catenacci DVT, Kim SS, Gold PJ, et al. A phase 1b/2, open label, dose-escalation study of margetuximab (M) in combination with pembrolizumab (P) in patients with relapsed/refractory advanced HER2+ gastroesophageal (GEJ) junction or gastric (G) cancer. J Clin Oncol. 2017;35(4_suppl; abstr TPS219) [Google Scholar]
- 28.Catenacci DVT, Kang Y-K, Park H, et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22–05): A single-arm, phase 1b–2 trial. Lancet Oncol. 2020;21:1066–1076. doi: 10.1016/S1470-2045(20)30326-0. [DOI] [PubMed] [Google Scholar]
- 29.Janjigian YY, Maron SB, Chatila WK, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: An open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21:821–831. doi: 10.1016/S1470-2045(20)30169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ansari S, Gantuya B, Tuan VP, et al. Diffuse gastric cancer: A summary of analogous contributing factors for its molecular pathogenicity. Int J Mol Sci. 2018;19:2424. doi: 10.3390/ijms19082424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JY, Gong EJ, Chung EJ, et al. The characteristics and prognosis of diffuse-type early gastric cancer diagnosed during health check-ups. Gut Liver. 2017;11:807–812. doi: 10.5009/gnl17033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li C, Kim S, Lai JF, et al. Advanced gastric carcinoma with signet ring cell histology. Oncology. 2007;72:64–68. doi: 10.1159/000111096. [DOI] [PubMed] [Google Scholar]
- 33.Kim JP, Kim SC, Yang HK. Prognostic significance of signet ring cell carcinoma of the stomach. Surg Oncol. 1994;3:221–227. doi: 10.1016/0960-7404(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 34.Heger U, Blank S, Wiecha C, et al. Is preoperative chemotherapy followed by surgery the appropriate treatment for signet ring cell containing adenocarcinomas of the esophagogastric junction and stomach? Ann Surg Oncol. 2014;21:1739–1748. doi: 10.1245/s10434-013-3462-z. [DOI] [PubMed] [Google Scholar]
- 35.Yokota T, Kunii Y, Teshima S, et al. Signet ring cell carcinoma of the stomach: A clinicopathological comparison with the other histological types. Tohoku J Exp Med. 1998;186:121–130. doi: 10.1620/tjem.186.121. [DOI] [PubMed] [Google Scholar]
- 36.Chuang J, Chao J, Hendifar A, et al. Checkpoint inhibition in advanced gastroesophageal cancer: Clinical trial data, molecular subtyping, predictive biomarkers, and the potential of combination therapies. Transl Gastroenterol Hepatol. 2019;4:63. doi: 10.21037/tgh.2019.08.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okamura R, Kato S, Lee S, et al. ARID1A alterations function as a biomarker for longer progression-free survival after anti-PD-1/PD-L1 immunotherapy. J Immunother Cancer. 2020;8:e000438. doi: 10.1136/jitc-2019-000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen J, Ju Z, Zhao W, et al. ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat Med. 2018;24:556–562. doi: 10.1038/s41591-018-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janjigian YY, Chou JF, Simmons M, et al. First-line pembrolizumab (P), trastuzumab (T), capecitabine (C) and oxaliplatin (O) in HER2-positive metastatic esophagogastric adenocarcinoma (mEGA) J Clin Oncol. 2019;37(4_suppl; abstr 62) [Google Scholar]
- 40.Catenacci DVT, Park H, Lockhart AC, et al. Phase 1b/2 study of margetuximab (M) plus pembrolizumab (P) in advanced HER2+ gastroesophageal junction (GEJ) or gastric (G) adenocarcinoma (GEA) J Clin Oncol. 2018;36(4_suppl; abstr 140) [Google Scholar]
- 41.Nordstrom JL, Muth J, Erskine CL, et al. High frequency of HER2-specific immunity observed in patients (pts) with HER2+ cancers treated with margetuximab (M), an Fc-enhanced anti-HER2 monoclonal antibody (mAb) J Clin Oncol. 2019;37(15_suppl; abstr 1030) [Google Scholar]
- 42.Catenacci DVT, Rosales MK, Wigginton JM, et al. Margetuximab (M) combined with anti-PD-1 (MGA012) or anti-PD-1/LAG-3 (MGD013) +/- chemotherapy (CTX) in first-line therapy of advanced/metastatic HER2+ gastroesophageal junction (GEJ) or gastric cancer (GC) J Clin Oncol 382020. 4_suppl; abstr TPS468) [Google Scholar]
- 43.Al-Batran S-E, Schuler MH, Zvirbule Z, et al. FAST: An international, multicenter, randomized, phase II trial of epirubicin, oxaliplatin, and capecitabine (EOX) with or without IMAB362, a first-in-class anti-CLDN18.2 antibody, as first-line therapy in patients with advanced CLDN18.2+ gastric and gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol. 2016;34(18_suppl; abstr LBA4001) [Google Scholar]
- 44.Türeci O, Sahin U, Schulze-Bergkamen H, et al. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: The MONO study. Ann Oncol. 2019;30:1487–1495. doi: 10.1093/annonc/mdz199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Catenacci DVT, Peterson B, Chase L, et al. Personalized antibodies for gastroesophageal adenocarcinoma (PANGEA): Secondary and final primary efficacy analyses J Clin Oncol 382020. 15_suppl; abstr 4561) [Google Scholar]