Abstract
Immunohistochemistry is an integral component in the proper analysis of soft tissue tumours, and a simple panel of six markers is useful in practical triage: CD34, desmin, epithelial membrane antigen (EMA), keratin cocktail AE1/AE3, S100 protein and alpha smooth muscle actin (SMA). These markers frequently assist in the differential diagnosis of fibroblastic, myoid, nerve sheath and perineurial cell tumours, synovial and epithelioid sarcoma and others. However, they all are multispecific, so that one has to be cognizant of their distribution in normal and neoplastic tissues. Four additional useful markers for specific tumour types are discussed here: CD31 and ERG for vascular endothelial tumours, and KIT and DOG1/Ano-1 for gastrointestinal stromal tumours (GISTs). However, hardly any marker is totally monospecific for any one type of tumour. Furthermore, variably lineage-specific markers do not usually distinguish between benign and malignant proliferations, so that this distinction has to be made on histological grounds. Immunohistochemical evaluation is most useful, efficient and cost-effective when used in the context of careful histological evaluation by an experienced pathologist, aware of all diagnostic entities and their histological spectra. Additional diagnostic steps that must be considered in difficult cases include clinicoradiological correlation and additional sampling of remaining wet tissue, if possible.
Keywords: CD31, CD34, desmin, DOG1, EMA, ERG, immunohistochemistry, IT, keratins, S100 protein, sarcoma, SMA
Introduction
Diagnostic immunohistochemistry is a daily tool in the evaluation of soft tissue tumours. It is best used as a diagnostic adjunct following careful assessment of histopathology and formulation of differential diagnosis. The effective use of immunohistochemistry specifically assesses the differential diagnostic possibilities, and the use of a small ‘universal’ panel is suggested here: CD34, desmin, EMA, keratin cocktail AE1/AE3, S100 protein and SMA. The other four markers discussed below address two specific problems: endothelial cell proliferations (CD31 and ERG) and GISTs (KIT and DOG1/Ano-1).
Unthinking use of diagnostic immunohistochemistry out of histological context can lead to serious errors and is strongly discouraged. Although comprehensive diagnostic algorithms based on immunohistochemistry results alone have been suggested as diagnostic aids at various times, their general applicability in the diagnosis of soft tissue tumours has limitations. These are related to the multispecificity of most markers and antigenic complexity of many tumour types.
The widely used established markers have the advantage of extensive existing data. Some new markers are potentially more specific to certain tumour types. However, it is necessary to be careful with diagnostic conclusions based on the newly introduced markers, as data may be scant with overly optimistic impression on specificity. The more an immunohistochemical marker is used, the more readily the findings can be predicted and potentially applied in a more targeted and economical immunohistochemical evaluation. The best combination for diagnostic immunohistochemistry of soft tissue tumours is a pathologist extensively knowledgeable of all diagnostic entities and their variants and of the distribution of antigens in normal and neoplastic tissues. Widespread automation of immunohistochemistry facilitates reproducibility.
The first section of this review contains outlines of the 10 markers (six core markers and four supplemental markers), and the second section discusses briefly selected tumour type-specific applications, with mentions of diagnostically critical supplemental markers. Finally, technical considerations are discussed briefly.
Ten key markers for the diagnosis of soft tissue tumours
CD34
CD34 is a transmembrane glycoprotein containing sialomucin elements. It is believed to be important in the regulation of cell recognition and trafficking. CD 34 was originally recognized as an antigen expressed in haematopoietic stem cells, and remains one of the key markers in their characterization.1 As a membrane antigen, CD34 is expressed typically in a distinct membrane pattern, which is more obvious in cells with epithelioid morphology. Clone QBEND/10, which performs well in formalin-fixed and paraffin-embedded tissue following heat-induced epitope retrieval, is offered by many major immunohistochemistry vendors.
CD34 is expressed in vascular endothelial cells, but often shows a weaker expression in lymphatic endothelia. Originally reported specifically in periadnexal and perivascular dermal fibroblasts,2 CD34 is also expressed in other soft tissue fibroblasts and septal and stromal elements in various visceral locations. CD34 is a multispecific marker, which is nevertheless highly useful in soft tissue tumours. CD34 shows limited expression in epithelia, but is present in hair shaft outer root cells and their neoplastic derivatives.3
CD34 is a marker of many types of fibroblastic tumours, such as dermatofibrosarcoma protuberans,4 contrasting with dermatofibroma/benign cutaneous fibrous histiocytoma (Table 1).5 Solitary fibrous tumour is also CD34-positive6 but, similar to dermatofibrosarcoma, the corresponding fibrosarcomatous or ‘de-differentiated’ forms may show low or no expression. A variety of other, mostly morphologically highly distinctive fibroblastic tumours, are also CD34-positive, so that CD34-positivity alone does not define any fibroblastic tumour type. Among these are the benign superficial acral fibromyxoma,7 and malignant tumours such as myxofibrosarcoma and de-differentiated liposarcoma, which are variably positive. CD34 is expressed in many lipomatous tumours, and particularly strongly in the spindle cell component in spindle cell and pleomorphic lipoma.8
Table 1.
Applications for CD34 in immunohistochemistry in soft tissue tumors shown by pairs of tumors with potential histological resemblance but contrasting patterns on CD34 immunoreactivity
| Dermatofibrosarcoma protuberans (DFSP) | Fibrous histiocytoma/dermatofibroma, neurothekeoma |
| Immunoreactivity may be lost or reduced in fibrosarcomatous transformation of DFSP | Tumor periphery in fibrous histiocytoma may have positivity but central areas are negative. Rare atypical variants are positive |
| Solitary fibrous tumor | Monophasic synovial sarcoma, desmoid fibromatosis, smooth muscle tumors |
| Malignant variants may show reduced expression | Minority of leiomyosarcomas can be CD34-positive |
| Kaposi sarcoma | Spindle cell hemangioma (spindle cells) |
| Spindle cell lipoma, usual and myxoid variant | Myxoid liposarcoma |
| Neurofibroma | Schwannoma |
| Fibroblastic CD34-positive component usually prominent and often seen in a net-like pattern | CD34-positive fibroblastic component scant and usually limited to capsular and degenerative areas |
| Epithelioid sarcoma (50%) | Metastatic carcinoma (<1%) |
In vascular endothelial tumours, CD34 highlights the endothelial component of benign angiomas. Its expression is variable (50–60%) in malignant endothelial neoplasms, such as epithelioid haemangioendothelioma and angiosarcoma; however, CD34 is expressed consistently in Kaposi sarcoma.1
Approximately 50% of epithelioid sarcomas show membranous positivity for CD34, whereas positivity in carcinomas and other epithelial or keratin-positive neoplasms, such as synovial sarcoma, is very rare.9,10 Table 1 lists diagnostic applications of CD34 immunohistochemistry, showing tumours with contrasting patterns of immunoreactivity.
DESMIN
Desmin is the intermediate (10 nm diameter) filament protein of muscle cells and is expressed typically in skeletal muscle and most smooth muscle cells, except some vascular smooth muscle. When judging desmin positivity in tumours, its presence in entrapped reactive smooth or skeletal muscle cells must be carefully excluded. A commonly used clone is D33, with excellent performance in formalin-fixed tissue following heat-induced epitope retrieval. Desmin is also expressed in a subset of interstitial reticulum cells of lymph nodes and the so-called myoid cells in thymus.
Desmin is useful in the diagnosis of skeletal muscle and smooth muscle tumours. In some cases, desmin immunohistochemistry can appropriately alert one to an unusual rhabdomyosarcoma, which has to be evaluated further with additional, more lineage-specific markers such as myogenin and MyoD1 (Figure 1). Similarly, h-caldesmon is another useful marker to support smooth muscle differentiation. Desmin can also be present in myofibroblasts, and therefore myofibroblastic tumours such as desmoid fibromatosis can be focally positive.11
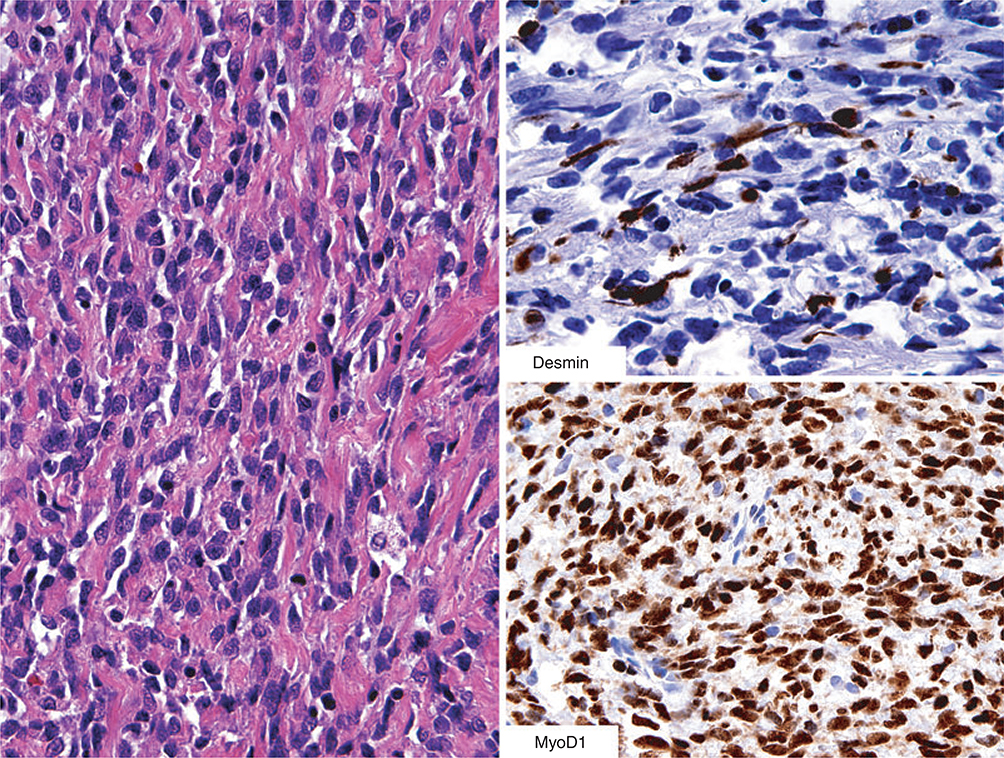
Figure 1.
This spindle cell sarcoma with a fibrosarcoma-like appearance does not give any hint for rhabdomyosarcomatous differentiation. However, desmin positivity should lead to further studies for MyoD1 (or myogenin) to explore the possibility of spindle cell rhabdomyosarcoma – indeed, this diagnosis was confirmed here based on strong nuclear MyoD1-positivity.
Notably, desmin is expressed strongly in a number other tumours, none of which can be considered skeletal or smooth muscle tumours. The most important examples include desmoplastic small round cell tumour, which often contains desmin-positive perinuclear dots.12,13 Even Ewing sarcoma can be occasionally desmin-positive. In aggressive angiomyxoma, desmin immunostain highlights the cytoplasm of dendriticshaped tumour cells, as well as possible perivascular smooth muscle elements.14 Cellular angiofibroma in the pelvic/inguinal region is variably desmin-positive, and approximately half of angiomatoid fibrous histiocytomas contain desmin-positive tumour cells.15,16 Tenosynovial giant cell tumours often contain scattered desmin-positive dendrite-shaped cells, which can help to identify these tumours when seen at unusual locations.17
EPITHELIAL MEMBRANE ANTIGEN (EMA, MUC1)
EMA is a glycoprotein also known as MUC1 apoprotein. It is expressed in a variety of ductal, secretory and other epithelial cells, often in a luminal membrane pattern. In addition, EMA is expressed in meningothelial and perineurial cell membranes and in subpopulations of plasma cells. The monoclonal antibody clone E29 is used commonly to detect EMA/MUC1 in routinely processed tissue, and performs well following heat-induced epitope retrieval.18
EMA is useful in detecting epithelial differentiation in soft tissue tumours, and in such tumours it is typically detectable in synovial sarcoma, epithelioid sarcoma, myoepithelioma and many, but not all, metastatic carcinomas. Notably, some other sarcomas, such as leiomyosarcoma and rarely angiosarcomas, can also be positive, and this has to be considered in the differential diagnosis.
EMA is also useful in the diagnosis of meningiomas and perineuriomas, most of which show at least focal positivity.19,20 Specific variants of perineuriomas have been discovered based on EMA positivity, supplementary perineurial cell markers (claudin-1, GLUT-1) or ultrastructural analysis. Among these variants are sclerosing perineurioma of fingers21 and spindle cell perineuriomas of soft tissue in general.22–24 Most perineuriomas are identified on the basis of slender or slightly epithelioid spindle cells in lamellar organization showing variable positivity for EMA (Figure 2).
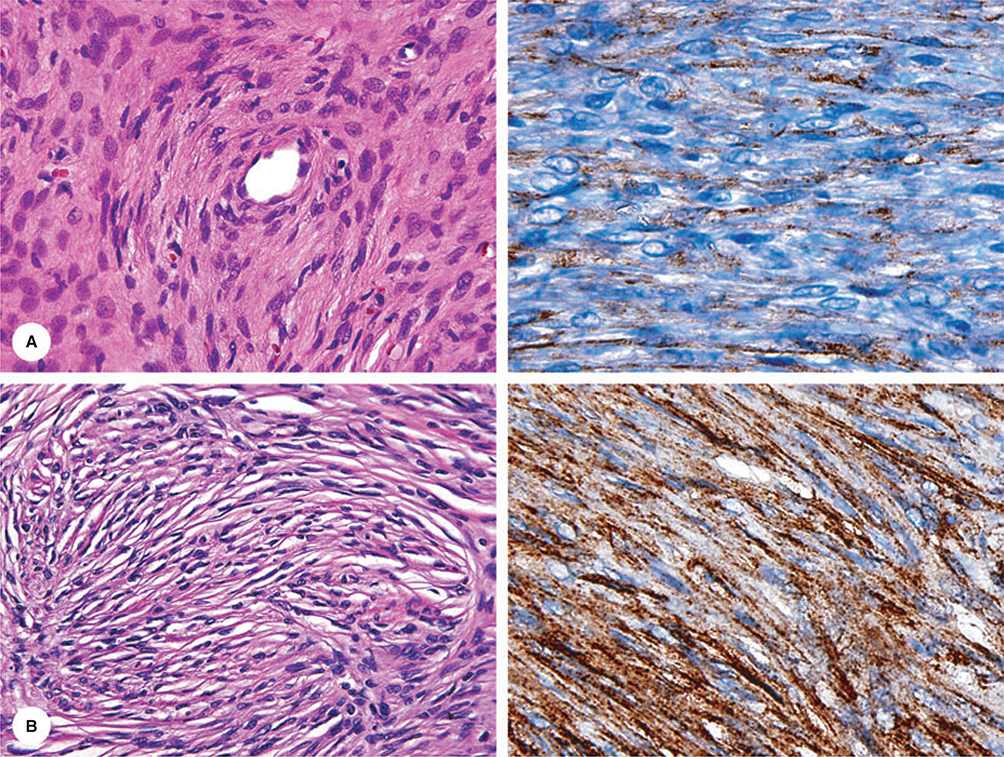
Figure 2.
Immunohistochemical documentation of two perineuriomas. Both cases demonstrate EMA-positivity in the lamellar spindle cells.
It is not known whether EMA positivity, often detected in low-grade fibromyxoid sarcoma,25 is due to real expression of MUC1 or possible cross-reaction of the MUC1 antibodies with MUC4, another EMA-family protein, which is expressed highly in low-grade fibromyxoid sarcoma.26
KERATINS
Keratin expression in soft tissue tumours is summarized in Table 2. Keratins are a complex family of cytoskeletal intermediate filament proteins expressed typically in various epithelia in a differentiation-dependent manner. The following summary concerns 20 epithelial or ‘soft’ keratins; the ‘hard’ keratins expressed in hair and nails are not discussed here.
Table 2.
Summary of keratin expression in soft tissue tumors. Data based on Chapters 3, 27, 28 and 29 Miettinen M (editor): Modern Soft Tissue Pathology
| Synovial sarcoma, biphasic | Complex pattern of keratins, including extensive reactivity for K7, K8, K18 and K19 and potential focal immunoreactivity for K13, K14, K17, and K20 |
| Synovial sarcoma, monophasic | Scattered or clustered cells positive for K7, K8, K18, and K19 |
| Other keratins generally absent | |
| Synovial sarcoma, poorly differentiated (high mitotic rate fibrosarcoma or Ewing sarcoma-like) | Keratins may be focally present or absent |
| Molecular genetic studies may be required for confirmation | |
| Epithelioid sarcoma | K8, K18, K19 generally positive. K7 can be focally present, high other keratins typically absent |
| Mixed tumor myoepithelioma | AE1/AE3 positivity detected in most cases, individual keratin types not characterized in detail |
| Angiosarcoma, epithelioid hemangioendothelioma | Up to one third of cases positive for AE1/AE3 and K7 and K8 |
| K18 may be more widespread | |
| Smooth muscle tumors | AE1/AE3 positivity common in uterine leiomyomas and Present in 30% of leiomyosarcomas. These mainly contain K7, K8, and K18 |
| Undifferentiated sarcomas (MFH) | Up to one third contain AE1/AE3-positive tumor cells |
| Rhabdomyosarcoma | More common in alveolar rhabdomyosarcoma |
| Inflammatory myofibroblastic tumor | AE1/AE3 positivity common but not invariable |
| Schwannoma (retroperitoneal) | AE1/AE3 positivity common, probably due to cross reaction of the antibody with GFAP |
| Merkel cell carcinoma | Positive with keratin cocktail AE1/AE3 and K20-specific antibodies. |
| Sarcomatoid squamous carcinoma | Usually positive for AE1/AE3 and also K5/6 recognizing antibodies |
| Other sarcomatoid carcinomas | Variable, but keratin positivity is usually present |
| Epithelioid mesothelioma | K7, K8, K18, K19, variable K5/6, often only focal |
| Sarcomatoid mesothelioma | K7, K8, K18, K19, K5/6 usually absent |
Diagnostically more important in soft tissue tumours are simple epithelial (‘low molecular weight’) keratins, based on their wider expression profile in different epithelial cells. However, on balance, these keratins may have increased presence in mesenchymal cells so that careful judgement and possibly additional markers are needed to determine whether or not their expression signifies epithelial differentiation.
Keratin proteins present in regular epithelia (‘soft keratins’) are encoded by two multigene families, with the individual names abbreviated as KRT, followed by a number. The classic keratin catalogue of Moll et al. classifies denatured and urea-depolymerized keratins by molecular masses and isolelectric points by two-dimensional gel electrophoresis.27 Type 2 (or B for basic) keratins with more basic isoelectric points are numbered 1–8 from the highest to lowest molecular weight. Of these, numbers 7 and 8 are more important in soft tissue tumours.
Type 1 (or A for acidic) keratins have lower (more acidic) isoelectric points and are numbered 9–18, from the highest to lowest molecular weight. Keratin 20, a later addition to the Moll et al. catalogue, also belongs to this family. Most important in soft tissue tumours are lower molecular weight keratins of this group, keratins 18 and 19.27–29
Although type-specific antibodies are available for nearly all individual keratins, multispecific keratin antibodies such as AE1 (recognizing keratins 9–17) and AE3 (recognizing keratins 1–8) have wide practical use, often as a cocktail containing both antibodies. Note that even the AE1/AE3 cocktail does not detect all keratins, and in particular has a ‘blind spot’ in not recognizing keratin 18. Therefore, alternative multispecific or K18-specific antibodies have to be used to detect keratin 18.
The lower molecular weight keratins (7, 8, 18 and 19) are expressed generally in non-stratified (simple epithelia), and the higher molecular weight keratins (5, 6, 13, 14) are present in stratified epithelia and basal cells of complex glandular epithelia (such as respiratory epithelia). Selective mesenchymal expression of keratins includes the presence of keratins 7, 8 and 18 in some smooth muscle cells (myometrium, vascular and some parenchymal smooth muscle) and expression of keratins 7 and 18 in some vascular endothelial cells.29
Keratin 20 has a very characteristic distribution, being present in intestinal epithelia, the uppermost layer of urothelia (the umbrella cells) and in Merkel cells. In soft tissue tumours, K20 is practically important as a marker for Merkel cell carcinoma.30,31
Soft tissue tumours with epithelial differentiation include synovial sarcoma and epithelioid sarcoma, and these tumours generally express simple epithelial keratins K8, K18 and K19. In addition, keratin 7 is also expressed in synovial sarcoma but usually not in epithelioid sarcoma. Even the high molecular weight keratins typical of stratified epithelia and keratin 20 can be focally present in biphasic synovial sarcoma.32
Mixed tumours or myoepitheliomas of soft tissue most commonly contain keratin-positive elements, as detected with the AE1/AE3 keratin cocktail, but keratin subtypes have not been analysed in detail.33
As might be expected from normal tissue distribution, keratins (especially K7 and K18) are also present in some vascular endothelial neoplasms (especially haemangiomas and some angiosarcomas) and in some smooth muscle tumours. In addition, K8 is expressed in some angiosarcomas.34,35 Additional sarcomas with keratin expression include desmoplastic small round cell tumour (typically extensive) and Ewing sarcoma (almost always focal, if present).
Experimental studies showed that virally transformed cultured fibroblasts can also express keratins, especially K8 and K18, and this forms a logical scientific basis for the observation that various sarcomas with non-epithelial differentiation can also show variable, usually limited keratin expression.36,37 For example, myxofibrosarcomas and related tumours somewhat frequently contain keratin-positive tumour cells (Figure 3).
Figure 3.
Immunohistochemical findings in myxofibrosarcoma (upper row) and acral myxoinflammatory fibrosarcoma (lower row). Both tumours are focally positive with keratin cocktail AE1/AE3.
A specific problem related to AE3 antibody is cross-reactivity in the brain, possibly via its cross-reaction with GFAP.38 This may be the reason why schwannomas (especially spinal and retroperitoneal lesions) can also be AE1/AE3-positive.39
ALPHA SMOOTH MUSCLE ACTIN (SMA)
While all cells contain actin microfilaments as part of their cytoskeleton, SMA has some specificity for smooth muscle cells. It is also expressed in the smooth muscle-related pericytes and glomus cells, and in myofibroblasts and myoepithelial cells.
Applications of SMA immunohistochemistry in soft tissue tumours are listed in Table 3. SMA can be utilized as marker to diagnose myofibroblastic, smooth muscle and related tumours. In order to observe the true tumour cell phenotype, one has to distinguish reactivity in tumour cells from that in entrapped non-neoplastic cells, and this can be tricky at times.
Table 3.
Applications of alpha smooth muscle actin (SMA) in the diagnosis of soft tissue tumors and findings that should be taken into account in differential diagnosis
| Benign myofibroblastic lesions (strongly positive) | Nodular fasciitis (typical, some are only weakly positive of even negative), fibroma of tendon sheath |
| Reactive myofibroblastic proliferations (typically) | |
| Myofibroblastic lesions (focally positive) | Desmoid fibromatosis, other fibromatoses |
| Inflammatory myofibro-blastic tumor | Variably positive (negative for extensively positive) |
| Leiomyoma/leiomyosarcoma | Virtually all cases are positive. If tumor is SMA negative, this is a reason to doubt a diagnosis of smooth muscle tumor |
| Gastrointestinal stromal tumor (GIST) | Approximately 1/3 of GISTs are variably SMA-positive, some strongly, as seen for smooth muscle tumors |
| Vascular tumors | Benign angiomas show retained pericytes |
| Vasoformations in malignant vascular tumors typically lack pericytes | |
| Myoepithelial cells/lesions | Normal myopeithelial cells are positive but myoepithelial neoplasms only focally positive or totally negative |
Among the myofibroblastic tumours, nodular fasciitis and fibroma of tendon sheath are strongly positive, a fact that should not lead to confusion with smooth muscle tumours, such as leiomyosarcoma. SMA is expressed in 30% of GISTs, so that other markers such as KIT also have to be used. In addition, SMA can be applied in detection of pericytic cell populations in vascular tumours and myoepithelial cell differentiation in neoplasms. However, in soft tissue myoepithelioma this is rarely fruitful.40
S100 PROTEIN
S100 protein, so named based on its 100% solubility to neutral ammonium sulphate, was isolated originally from brain tissue and subsequently shown to be a good marker for Schwann cells, melanocytes, glial cells and some neurones of the brain. In addition, S100 protein is also expressed in cutaneous Langerhans cells, their derivatives interdigitating reticulum cells of lymphoid tissue, adipocytes, cartilage and some myoepithelial cells.40,41
Although S100 protein is actually encoded by a multigene family with various subunits, polyclonal multispecific antibodies are typically used and analysis with subunit-specific antibodies is not widely used in clinical practice. Despite its multispecificity, S100 protein is a very useful marker in the evaluation of soft tissue tumours. Polyclonal antibodies are still the most commonly used, often with no epitope retrieval. The polyclonal antibody requires careful titration of the dilution to obtain the maximal possible detection sensitivity and specificity. S100-positive dendritic antigen-presenting cells are a ubiquitous internal control, especially useful when evaluating tumours.
S100 protein is useful in detecting Schwann cell differentiation in nerve sheath tumours and highlighting normal Langerhans cells in skin and interdigitating reticulum cells in lymphoid tissue, as well as Langerhans cell histiocytosis and related lesions, and also the large histiocyte-like cells typical of Rosai–Dorfman disease.
Metastatic melanoma is typically strongly positive for S100 protein and is perhaps the most common S100-positive malignant soft tissue tumour. In fact, malignant peripheral nerve sheath tumours (MPNST) are more commonly S100 protein-negative than positive, although they may contain S100 protein-positive residual Schwann cells of the pre-existing neurofibroma. S100 protein-only phenotype of metastatic melanoma (without more melanoma-specific markers such as HMB45 and MelanA) may be difficult to differentiate from S100 protein-positive MPNST. Nodal tumour location and the history of melanoma would point towards metastatic melanoma, although it should be remembered that the melanoma-related clear cell sarcoma of soft parts may also metastasize to lymph nodes; the distinction of melanoma from clear cell sarcoma is aided by genetics, as the latter contains EWSR1 gene rearrangements, in contrast to melanoma. The pre-existing neurofibroma component within the tumour is practically the only definitive evidence for MPNST over other neuroectodermal malignancy. In some cases, the distinction may be impossible and then the descriptive diagnosis of ‘malignant neuroectodermal neoplasm’ (including melanoma, MPNST and possibly also clear cell sarcoma) is appropriate.
S100 protein is also expressed in cartilage cells and has some value in identification of cartilaginous components in various tumours. It may also be useful in pinpointing atypical adipocytes and lipoblasts in the diagnosis of atypical lipomatous tumours and liposarcomas.
The Sox10 transcription factor is emerging as a new marker for schwannian and melanocytic tumours, although the data are still somewhat limited.42
CD31
Also known as PECAM-1 (platelet-endothelial cell adhesion molecule-1), CD31 is a cell membrane protein expressed in endothelial cells, platelets and some primitive haematopoietic (myeloid) cells.43
CD31 has been the gold standard as a marker for endothelial differentiation in the recognition of vascular endothelial tumours. Although benign angiomas can usually be diagnosed histologically, the diagnosis of malignant vascular tumours – epithelioid haemangioendothelioma, angiosarcomas and Kaposi sarcoma – is aided by demonstration of CD31, which is expressed almost universally in this group.44–46 The use of additional endothelial markers such as ERG (see below) is beneficial as a double confirmation for any malignant endothelial tumour, especially in poorly differentiated tumours. HHV-8 offers a specific confirmation for Kaposi sarcoma.
In addition, CD31 is expressed in some other mature haematopoietic cells, especially some plasma cells and histiocytes. The latter-mentioned immunoreactivities are pitfalls in diagnostic use, potentially causing overdiagnosis of endothelial differentiation.47 Another pitfall is the presence of CD31 in platelets and thrombi from where the antigen can be adsorbed onto the surface of tumour cells, potentially simulating antigen expression.
ERG
The protein encoded by the ERG gene (abbreviated from ETS-related gene) belongs to the ETS (erythroblastosis family) transcription factors, many of which are expressed constitutively in endothelial cells. Because ERG expression is generally retained, even in malignant endothelial cells, ERG is a good diagnostic marker for angiosarcoma and other malignant vascular tumours.48 Illustrative case reports and small series have supported the diagnostic value of ERG in the differential diagnosis of cutaneous and soft tissue tumours of endothelial origin.49,50 A monoclonal antibody generated by the Center of Prostate Disease Research and now made commercially available is an excellent diagnostic reagent.51 Other antibodies are also available, but some might cross-react with other ETS family transcription factors and thus lack specificity.52
Natural expression of ERG in non-endothelial cells also includes a subset of myeloid precursors and a majority of blastic extramedullary myeloid tumours (myeloid sarcomas). Therefore, additional markers, such as myeloperoxidase and pan-leucocyte or other widely expressed antigens such as CD45 (LCA) or CD43 should be used to distinguish these tumours from poorly differentiated angiosarcomas.48 Recently, ERG has also been commonly detected in epithelioid sarcoma, which is a diagnostic pitfall when using ERG as an endothelial cell marker.53
In addition, ERG expression can be induced by ERG-activating translocations in certain sarcomas, most importantly a small subset of Ewing sarcomas (10%).54 ERG is also involved in rare variant myxoid liposarcoma translocations and therefore might be expressed occasionally in these tumours.
ERG-activating translocations are common in prostate carcinoma and have been estimated to occur in 40–50% of cases. They result in immunohistochemical ERG positivity, which does not seem to occur otherwise in carcinomas. Therefore, immunohistochemical evaluation of ERG may be a useful test to evaluate the possible prostatic origin of metastatic carcinomas.48,51,55
KIT
KIT receptor tyrosine kinase is a key cell signalling molecule, also referred to as CD117. It becomes normally activated (phosphorylated) when binding of its ligand stem cell factor links together two KIT molecules which then cross-phosphorylate each other’s KIT tyrosine kinase domain, making it ready to phosphorylate downstream targets in the KIT signal transduction cascade. Activation of the KIT signalling pathway typically promotes cellular growth over apoptosis. A classical example of pathological KIT activation by gain-of-function mutation occurs in GISTs, most of which have these mutations.56–60
KIT-dependent and immunohistochemically KIT-expressing cells include haematopoietic stem cells, mast cells, germ cells, melanocytes, certain epithelia (especially in skin adnexa) and Cajal cells of the gastrointestinal tract.61,62
The main application of KIT detection by immunohistochemistry is identification of GIST, but rare subsets of other soft tissue tumours can be positive. These include Ewing sarcoma and angiosarcoma, and extraskeletal myxoid chondrosarcoma has also been reported to be occasionally positive. Neovascular endothelia in various tumours can also be KIT-positive, which should not be confused with tumour cell immunophenotypes.63,64 The search for treatment targets for KIT inhibitors by immunohistochemical KIT-positivity only is no longer considered a valid idea.
GIST always needs to be recognized specifically, as these tumours can have specific oncological treatment with KIT–tyrosine kinase inhibitors such imatinib and the expanding family of newer inhibitors. GIST has to be included in the differential diagnosis not only for gastrointestinal but also for any intraabdominal or hepatic mesenchymal tumours. In our experience, GISTs are more common than true leiomyosarcomas in the retroperitoneum. Furthermore, rare peripheral soft tissue or skin metastases can be diagnostic pitfalls.
The most practical way to evaluate GIST is immunohistochemistry for KIT, which captures >95% of GISTs. A small subset of GIST that especially include some PDGFRA mutant gastric GISTs can be KIT-negative or only focally positive (Figure 4). However, these GISTs are typically positive for DOG1/Ano-1 (see below).
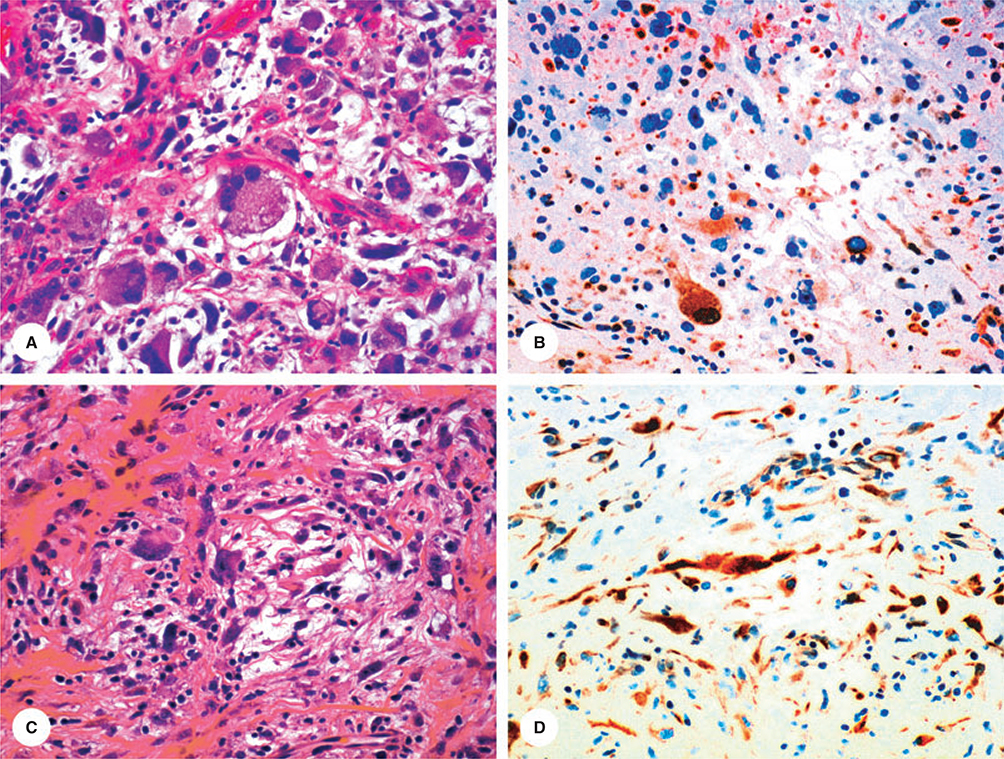
Figure 4.
Examples of different patterns of KIT-positivity in gastrointestinal stromal tumours. Reproduced from Miettinen.40
DOG1/ANO - 1
DOG1 (discovered on GIST), also known as Anoctamin-1 (Ano-1) is a calcium-activated chloride channel protein expressed strongly in the gastrointestinal Cajal cells and GISTs. This gene product was named following discoveryin GIST in expression arrays, and was also discovered independently and alternatively named as a transmembrane protein 16A (TMEM16A), overexpressed in oral (squamous cell) carcinoma 2 (ORAOV2) and tumour-amplified and overexpressed sequence 1 (TAOS1).65–67
DOG1/Ano-1 has been recognized as a valuable alternative marker after KIT. It is especially useful in KIT-negative gastric GISTs, many of which are PDG-FRA-mutant tumours: such mutants still appear strongly positive for DOG1.68,69
Relatively few non-GIST mesenchymal tumours are positive for DOG1. Such tumours include a minority of benign and malignant smooth muscle tumours and synovial sarcomas (DOG1 positivity in all these categories is rare). In addition, endothelial cell labelling is also possible. However, DOG1 is expressed commonly in gastrointestinal carcinomas, especially oesophageal (and other) squamous cell carcinomas and various adenocarcinomas. Normal gastric mucosa can also be positive.70,71 A recent study suggested that DOG1 is useful in the diagnosis of chondroblastoma.72
Discussion of selected entities
SMOOTH MUSCLE AND RELATED TUMOURS
Smooth muscle tumours, both benign and malignant, are generally recognizable histologically by their variable likeness to smooth muscle cells. They are often arranged in intersecting fascicles and composed of spindled or rarely epithelioid cells with eosinophilic cytoplasm and blunt-ended nuclei. Undifferentiated or ‘de-differentiated’ smooth muscle tumours are immunohistochemically challenging, as they may lack the antigens typical of differentiated smooth muscle cells. A more fruitful approach could be additional sampling to find differentiated elements that would allow a specific diagnosis.
Immunohistochemically differentiated smooth muscle and smooth muscle tumours are almost invariably alpha SMA-positive, so that if this marker is absent one should hesitate in diagnosing a smooth muscle tumour. However, SMA positivity alone is not sufficient for the diagnosis of smooth muscle differentiation, as positive tumours include many other entities. Among these are myofibroblastic lesions, such as nodular fasciitis and sarcomas with myofibroblastic differentiation. Also, 30% of GISTs are SMA-positive, in many cases extensively.
Desmin positivity is detectable in a great majority of smooth muscle tumours. Benign smooth muscle tumours are generally uniformly positive, but leiomyosarcomas vary. In all, 70–80% of cases show some positivity, but this can vary from focal to extensive.
Supplementary markers for smooth muscle differentiation include smooth muscle myosin73 and h-caldesmon.74,75 Both are generally absent in myofibroblastic tumours. However, in our experience both are present in at least 30–50% of GISTs, which has to be considered in a the differential diagnosis.
PEComa is a smooth muscle-related tumour. It has variable expression of smooth muscle markers and additional expression of HMB45 (gp 120) and potentially MelanA. Furthermore, oestrogen receptor is frequently present. Occurrence is most common in the abdominal cavity, especially in the uterus and retroperitoneum.76
SKELETAL MUSCLE TUMOURS
Skeletal muscle differentiation is a defining feature of rhabdomyosarcoma, but it has to be remembered that rhabdomyosarcoma-like components occur in many other tumours, so that rhabdomyosarcomatous differentiation is not synonymous with rhabdomyosarcoma. This is especially true for tumours in adult patients. Among the most common non-rhabdomyosarcoma tumours with rhabdomyosarcoma-like differentiation are gynaecological carcinosarcoma (malignant mixed mullerian tumour), de-differentiated liposarcoma and MPNST with rhabdomyosarcomatous differentiation (malignant Triton tumour). In rare cases even some carcinomas, such as Merkel cell carcinoma, have been reported to acquire rhabdomyosarcomatous elements. Heterologous rhabdomyosarcomatous differentiation has also been reported in imatinib-treated GISTs.77 From this it follows that the diagnosis of rhabdomyosarcoma (especially in an adult patient) can hardly be ever made without extensive sampling and clinical correlation.
Desmin-positive sarcomas, especially when SMA-negative, should be studied further to rule out rhabdomyosarcoma. The best markers are myogenic determination factors (MyoD1, myogenin), with positive tumours showing nuclear positivity. Although nuclear myogenin or MyoD1 indicates rhabdomyosarcomatous differentiation, regenerative skeletal muscle nuclei can also be positive, which is a diagnostic pitfall.78
VASCULAR ENDOTHELIAL TUMOURS
Epithelioid haemangioendothelioma and angiosarcoma are the main groups of malignant vascular endothelial neoplasms. They all display an endothelial phenotype.
Nearly all malignant vascular endothelial tumours are immunohistochemically positive for CD31 and ERG with nuclear staining. However, CD34 is only variably expressed in malignant vascular tumours (approximately 50% positive) and is therefore less useful in their diagnosis.
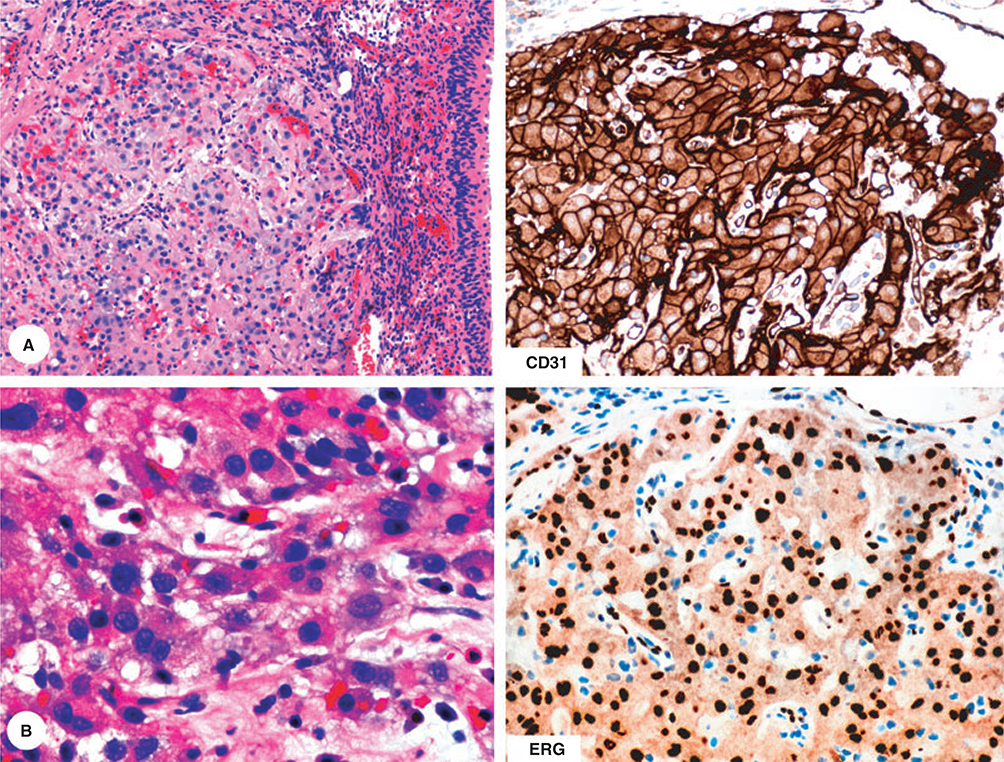
Endothelial markers assist greatly in the diagnosis of epithelioid haemangioendothelioma and poorly differentiated angiosarcoma, as these tumours can otherwise be easily misdiagnosed as primary or metastatic carcinoma or even mesothelioma, depending on the site of occurrence. For example, epithelioid haemangioendothelioma in the urinary bladder can be easily confused with urothelial carcinoma or possibly paraganglioma (Figure 5).
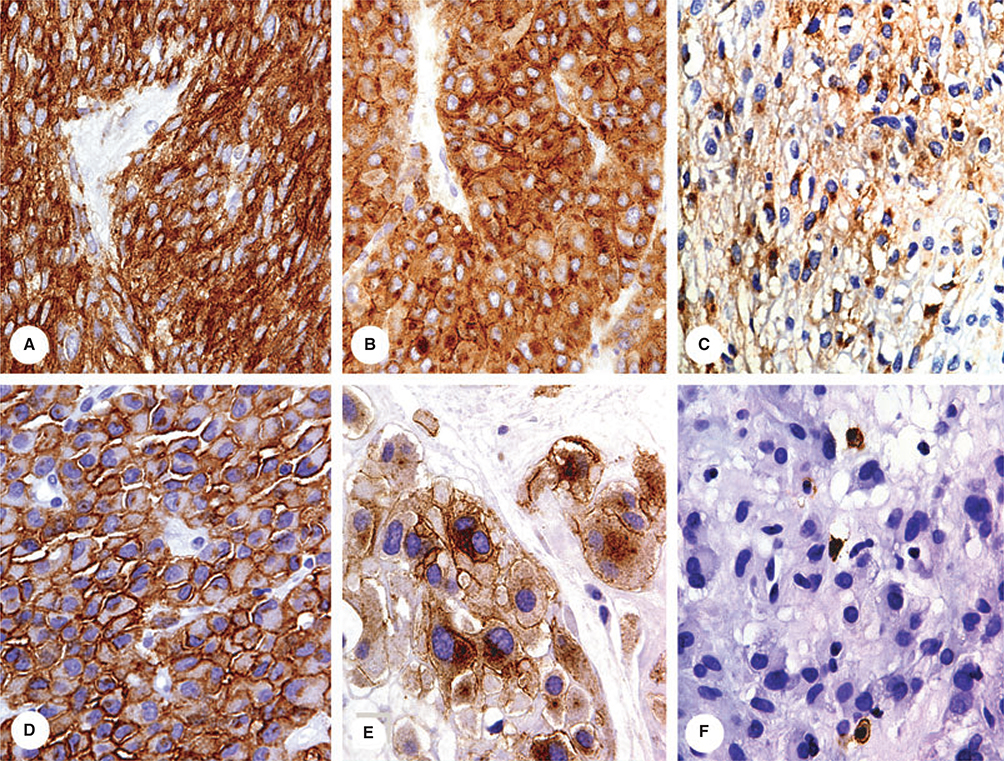
Figure 5.
This epithelioid tumour with somewhat organoid growth pattern and involving urinary bladder has a histological resemblance either with urothelial carcinoma or paraganglioma. Immunohistochemical studies show positivity for two endothelial markers and establish the diagnosis of epithelioid haemangioendothelioma.
Those angiosarcomas that do not contain histologically obvious vasoformation are extremely difficult to identify. In some cases, angiosarcoma can even resemble lymphoma in being composed of uniform round to ovoid cells. Both ERG and CD31 highlight the neoplastic cells which, in addition, contain other less specific endothelial markers, such as claudin-5 and Prox1.
BENIGN NERVE SHEATH TUMOURS
Three categories of nerve sheath tumours are discussed here: schwannoma, neurofibroma and perineurioma. In this study, granular cell tumour is mentioned only as an S100 protein-positive Schwann cell-related tumour.
Schwannoma is a purely schwannian tumour, and its immunohistochemical profile reflects this. Schwannomas typically show S100 protein positivity in all tumour cells, whereas the CD34-positive fibroblastic component is absent or scant and essentially present only in pericapsular or degenerative areas.
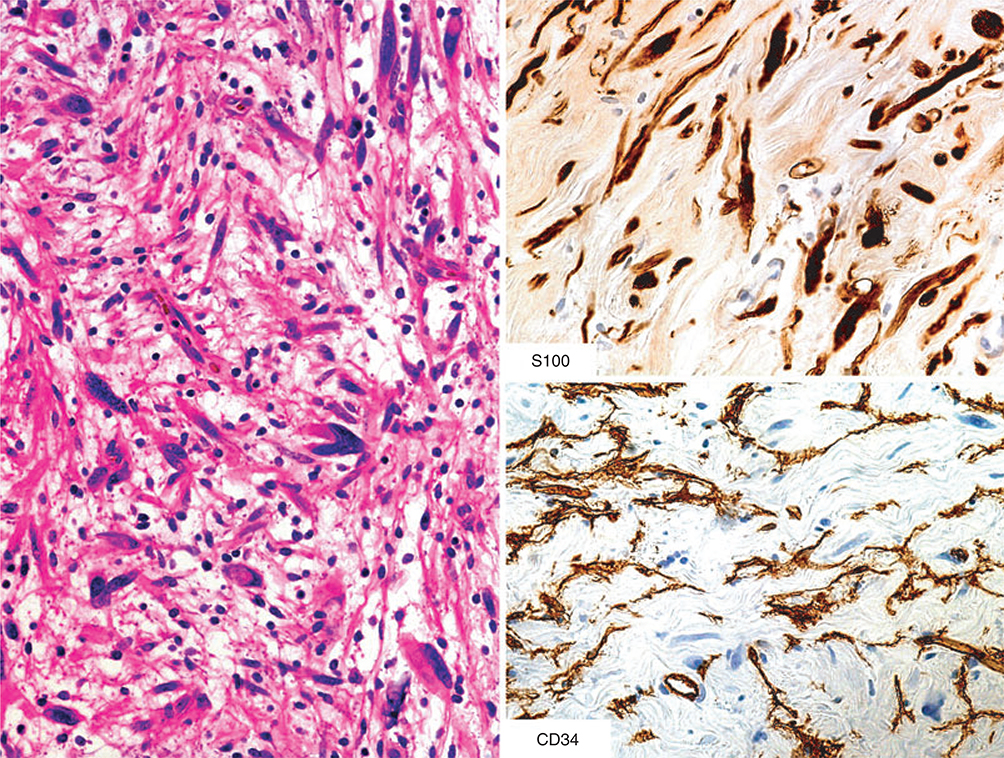
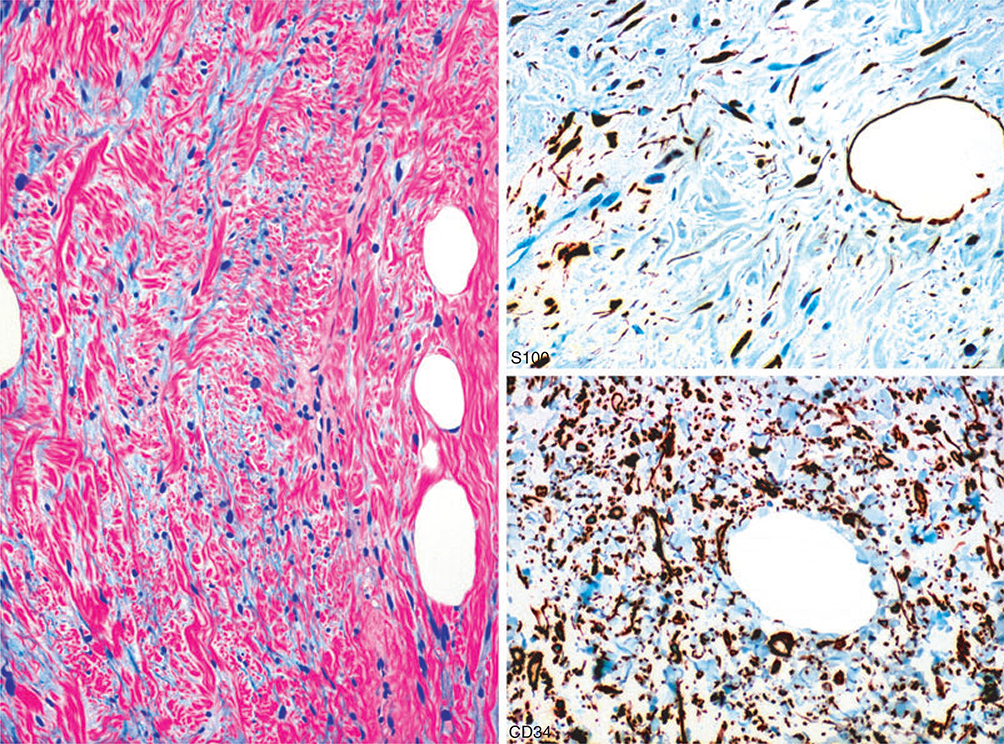
In contrast to schwannoma, neurofibromas have a heterogeneous cellular composition, and in addition to S100-positive Schwann cells also contain CD34-positive fibroblasts (Figure 6). Observation of such dual populations by immunohistochemistry can be helpful in distinguishing schwannomas and neurofibromas.79,80 However, hybrid tumours, such as the schwannoma–neurofibroma hybrids reported recently in neurofibromatosis type 2 (NF2) patients, have to be considered.81 Also, other tumours such as spindle cell lipoma can simulate neurofibroma by showing CD34-positive and S100 protein-positive populations (Figure 7).
Figure 6.
Intraneural neurofibroma with focal nuclear atypia, containing an S100 protein-positive Schwann cell component and CD34-positive fibroblasts.
Figure 7.
This tumour also contains S100- and CD34-positive cells, but the histological appearance, with fat cell content, and the dominance of the CD34-positive cell population over the S100 protein-positive component, supports the diagnosis of spindle cell lipoma.
Perineuriomas are a histologically heterogeneous category. However, they are often distinctive in their resemblance to meningioma, whether being composed of epithelioid cells in a trabecular arrangement (sclerosing perineurioma) or spindle cells with lamellar organization. EMA is a good screening marker, but the addition of GLUT-1 and claudin-5 can be useful. However, none of these markers is totally specific for perineurial cells. In general, malignant perineurioma is difficult to define, as all perineurial cell markers can be expressed in a variety of sarcomas.
MALIGNANT PERIPHERAL NERVE SHEATH TUMOUR (MPNST)
In general, MPNST is a diagnosis tied to a clinical context: occurrence from a neurofibroma or within a nerve trunk, often in the context of neurofibromatosis type 1 (NF1). Histologically, most examples are composed of spindle cells with an overall fibrosarcoma-like appearance.
In most cases MPNST has a non-specific immunohistochemical profile, as the tumour cells often do not contain S100 protein. However, the latter highlights the schwannian component in residual neurofibroma frequently intermixed in an MPNST lesion, and this can be diagnostically useful.82 As an exception, epithelioid MPNST is an unusual variant, which is typically strongly positive for S100 protein. Also, immunostains for desmin or myogenic determination markers can highlight skeletal muscle differentiation seen in some MPNSTs.
UNDIFFERENTIATED PLEOMORPHIC SARCOMA (MFH)
By definition, undifferentiated pleomorphic sarcoma is a diagnosis by exclusion, and no markers exist for its specific verification. Most importantly, other pleomorphic neoplasms, including variants of carcinomas, melanomas and specific sarcomas, have to be ruled out. In general, strong S100 protein positivity must raise strong suspicion of a melanoma (primary or metastatic), noting that malignant epithelioid nerve sheath tumours are also typically S100 protein-positive. Conversely, widespread positivity for keratins more probably suggests metastatic carcinoma or mesothelioma. However, isolated or scattered keratin-positive cells occur relatively frequently in undifferentiated sarcomas and myxofibrosarcomas.
SYNOVIAL SARCOMA
Synovial sarcoma is a morphologically characteristic sarcoma of uncertain lineage. It typically contains epithelial and mesenchymal-like elements, including what appears morphologically to be epithelio–mesenchymal transition. The most common variant is the spindle cell monophasic variant, composed of relatively uniform spindle cells, often arranged in distinct fascicles, variably interspersed by collagenous matrix and calcification. A haemangiopericytoma-like vascular pattern is also often present and characteristic. The classic biphasic morphology with glands is detected in only 20–30% of cases.
The epithelial component of biphasic synovial sarcoma is typically positive for keratin cocktails and keratins 7, 8, 18 and 19, and also focally for keratin 20 and higher molecular weight keratins. EMA positivity is observed typically in a luminal or cytoplasmic pattern. In many cases, non-glandular components are also revealed as part of the epithelial component, based on strong keratin and EMA immunoreactivity.
Monophasic spindle cell synovial sarcoma also contains small numbers of scattered or clustered keratin (K7, K8, K18, K19)-positive cells. EMA positivity is often seen in greater cell numbers than keratins. In order not to overdiagnose synovial sarcoma immunohistochemically, one has to carefully exclude keratin-positive normal cell elements such as endothelial cells.
Poorly differentiated synovial sarcoma, which can be histologically high-grade fibrosarcoma-like or have a round cell pattern resembling Ewing sarcoma and other round cell tumours, can be difficult to diagnose immunohistochemically as it may contain few or no keratin and EMA-positive cells. The diagnosis as synovial sarcoma rests on demonstration of better-differentiated areas by additional sampling, or by molecular genetic studies to document synovial sarcoma gene fusion.
In addition to positivity for epithelial markers, the absence of other markers could also be useful in synovial sarcoma. These tumours are almost never positive for CD34, helping to differentiate them from other tumours with a haemangiopericytomatous pattern, such as solitary fibrous tumour.83
An alternative marker for synovial sarcoma is TLE1 protein. However, it is not specific for synovial sarcoma, but occurs in solitary fibrous tumour and MPNST and even in mesothelioma, limiting its diagnostic value.84–86
Technical considerations
Technical optimization of immunohistochemistry is now performed predominantly using various automated systems, which allows for greater reproducibility. A key part of the optimization is epitope (antigen) retrieval. This is based predominantly on heat-induced retrieval using generic or proprietary, platform-based buffers. Some automated systems contain integrated epitope retrieval, whereas in others epitope retrieval is performed manually. Enzyme digestion is an alternative epitope retrieval method now used for a minority of antigens.
We generally favour heat-induced retrieval because the performance characteristics are independent of fixation time. In contrast, optimization of enzyme digestion can be dependent upon fixation time, which makes optimization more difficult. Thus, tissues that have undergone very short fixation tend to be overdigested, and those fixed for an excessive time underdigested. Both events can hamper optimal antigen detection, and the former may be deleterious for tissue integrity.
Markers not recommended in clinical practice are listed in Table 4. These markers either lack diagnostic specificity, or available reagents are unsatisfactory.
Table 4.
Markers not recommended for immunohistochemistry of soft tissue tumors
| Alpha-1-antrypsin | Once considered useful in the diagnosis of “fibrohistiocytic” tumors these markers are recognized having widespread distribution, especially in malignant tumors and therefore, they are not useful in lineage determination |
| Alpha-1-antichymotrypsin | |
| Bcl2 | Present in diverse types of soft tissue tumors and lacks any lineage-specificity |
| Myoglobin | Available antibodies have specificity problems and frequently lead to incorrect conclusion of skeletal muscle phenotype |
| Vimentin | Present in most mesenchymal cells and Generally useless for specific diagnosis |
Conclusion
The application of a limited immunohistochemical panel containing six markers – CD34, desmin, EMA, keratin cocktail, S100 protein and SMA – can be very useful in the diagnosis of soft tissue tumours. However, this panel must be supplemented with well-selected additional markers. Furthermore, diagnosis of difficult tumours has to always begin with meticulous histological analysis, and immunohistochemistry should be used as an adjunct tool in the context of morphology.
Acknowledgements
This work was supported as a part of NCI’s intramural research programme. The author reports no conflicts of interest regarding the contents of this paper.
References
- 1.Van de Rijn M, Rouse RV. CD34 – a review. Appl. Immunohistochem. 1994; 2; 71–80. [Google Scholar]
- 2.Nickoloff BJ. The human progenitor cell antigen (CD34) islocalized on endothelial cells, dermal dendritic cells, and perifollicular cells in formalin-fixed normal skin, and on proliferating endothelial cells and stromal spindle-shaped cells in Kaposi’s sarcoma. Arch. Dermatol. 1991; 127; 523–529. [PubMed] [Google Scholar]
- 3.Poblet E, Jimenez-Acosta F, Rocamora A. QBEND/10 (anti-CD34 antibody) in external root sheath cells and follicular tumors. J. Cutan. Pathol. 1994; 21; 224–228. [DOI] [PubMed] [Google Scholar]
- 4.Aiba S, Tabata N, Ishii H, Ootani H, Tagami H. Dermatofibrosarcoma protuberans is a unique fibrohistiocytic tumour expressing CD34. Br. J. Dermatol. 1992; 127; 79–84. [DOI] [PubMed] [Google Scholar]
- 5.van de Rijn M, Lombard CM, Rouse RV. Expression of CD34 bysolitary fibrous tumors of the pleura, mediastinum, and lung. Am. J. Surg. Pathol. 1994; 18; 814–820. [DOI] [PubMed] [Google Scholar]
- 6.Kutzner H Expression of the human progenitor cell antigenCD34 (HPCA-1) distinguishes dermatofibrosarcoma protuberans from fibrous histiocytoma in formalin-fixed, paraffin-embedded tissue. J. Am. Acad. Dermatol. 1993; 28; 613–617. [DOI] [PubMed] [Google Scholar]
- 7.Fetsch JF, Laskin WB, Miettinen M. Superficial acral acral fibromyxoma. A clinicopathologic study of 30 cases. Hum. Pathol. 2001; 32; 704–714. [DOI] [PubMed] [Google Scholar]
- 8.Suster S, Fisher C. Immunoreactivity for the human hematopoietic progenitor cell antigen (CD34) in lipomatous tumors. Am. J. Surg. Pathol. 1997; 21; 195–200. [DOI] [PubMed] [Google Scholar]
- 9.Traweek ST, Kandalaft PL, Mehta P, Battifora H. The humanhematopoietic progenitor cell antigen (CD34) in vascular neoplasia. Am. J. Clin. Pathol. 1991; 96; 25–31. [DOI] [PubMed] [Google Scholar]
- 10.Miettinen M, Fanburg-Smith JC, Virolainen M, Shmookler BM,Fetsch JF. Epithelioid sarcoma: an immunohistochemical analysis of 112 classical and variant cases and a discussion of the differential diagnosis. Hum. Pathol. 1999; 30; 934–942. [DOI] [PubMed] [Google Scholar]
- 11.Truong LD, Rangdaeng S, Cagle P, Ro JY, Hawkins H, FontRL. The diagnostic utility of desmin. A study of 584 cases and review of the literature. Am. J. Clin. Pathol. 1990; 93; 305–314. [DOI] [PubMed] [Google Scholar]
- 12.Gerald WL, Miller HK, Battifora H, Miettinen M, Silva EG, Rosai J. Intra-abdominal desmoplastic small round-cell tumor. Report of 19 cases of a distinctive type of high-grade polyphenotypic malignancy affecting young individuals. Am. J. Surg. Pathol. 1991; 15; 499–513. [PubMed] [Google Scholar]
- 13.Ordóñez NG. Desmoplastic small round cell tumor: II: an ultrastructural and immunohistochemical study with emphasis on new immunohistochemical markers. Am. J. Surg. Pathol. 1998; 22; 1314–1327. [DOI] [PubMed] [Google Scholar]
- 14.Fletcher CD. Angiomatoid ‘malignant fibrous histiocytoma’: an immunohistochemical study indicative of myoid differentiation. Hum. Pathol. 1991; 22; 563–568. [DOI] [PubMed] [Google Scholar]
- 15.Fanburg-Smith JC, Miettinen M. Angiomatoid ‘malignant’ fibrous histiocytoma: a clinicopathologic study of 158 cases and further exploration of the myoid phenotype. Hum. Pathol. 1999; 30; 1336–1343. [DOI] [PubMed] [Google Scholar]
- 16.Fetsch JF, Laskin WB, Lefkowitz M, Kindblom LG, Meis-Kindblom JM. Aggressive angiomyxoma: a clinicopathologic study of 29 female patients. Cancer 1996; 78; 79–90. [DOI] [PubMed] [Google Scholar]
- 17.Folpe AL, Weiss SW, Fletcher CD, Gown AM. Tenosynovial giant cell tumors: evidence for a desmin-positive dendritic cell subpopulation. Mod. Pathol. 1998; 11; 939–944. [PubMed] [Google Scholar]
- 18.Ordóñez NG. Broad-spectrum immunohistochemical epithelial markers: a review. Hum. Pathol. 2013; 44; 1195–1215. [DOI] [PubMed] [Google Scholar]
- 19.Theaker JM, Gatter KC, Esiri MM, Fleming KA. Epithelial membrane antigen and cytokeratin expression by meningiomas: an immunohistological study. J. Clin. Pathol. 1986; 39; 435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perentes E, Nakagawa Y, Ross GW, Stanton C, Rubinstein LJ. Expression of epithelial membrane antigen in perineurial cells and their derivatives. An immunohistochemical study with multiple markers. Acta Neuropathol. 1987; 75; 160–165. [DOI] [PubMed] [Google Scholar]
- 21.Fetsch JF, Miettinen M. Sclerosing perineurioma: a clinicopathologic study of 19 cases of a distinctive soft tissue lesion with a predilection for the fingers and palms of young adults. Am. J. Surg. Pathol. 1997; 21; 1433–1442. [DOI] [PubMed] [Google Scholar]
- 22.Hornick JL, Fletcher CD. Soft tissue perineurioma: clinicopathologic analysis of 81 cases including those with atypical histologic features [Review]. Am. J. Surg. Pathol. 2005; 29; 845–858. [DOI] [PubMed] [Google Scholar]
- 23.Folpe AL, Billings SD, McKenney JK, Walsh SV, Nusrat A, Weiss SW. Expression of claudin-1, a recently described tight junction-associated protein, distinguishes soft tissue perineurioma from potential mimics. Am. J. Surg. Pathol. 2002; 26; 1620–1626. [DOI] [PubMed] [Google Scholar]
- 24.Hornick JL, Bundock EA, Fletcher CD. Hybrid schwannoma/perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am. J. Surg. Pathol. 2009; 33; 1554–1561. [DOI] [PubMed] [Google Scholar]
- 25.Billings SD, Giblen G, Fanburg-Smith JC. Superficial low-grade fibromyxoid sarcoma (Evans tumor): a clinicopathologic analysis of 19 cases with a unique observation in the pediatric population. Am. J. Surg. Pathol. 2005; 29; 204–210. [DOI] [PubMed] [Google Scholar]
- 26.Doyle LA, Möller E, Dal Cin P, Fletcher CD, Mertens F, Hornick€ JL. MUC4 is a highly sensitive and specific marker for low-grade fibromyxoid sarcoma. Am. J. Surg. Pathol. 2011; 35; 733–741. [DOI] [PubMed] [Google Scholar]
- 27.Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982; 31; 11–24. [DOI] [PubMed] [Google Scholar]
- 28.Cooper D, Schermer A, Sun TT. Classification of human epithelia and their neoplasms using monoclonal antibodies to keratins: strategies, applications, and limitations. Lab. Invest. 1985; 52; 243–256. [PubMed] [Google Scholar]
- 29.Miettinen M Keratin immunohistochemistry: update of applications and pitfalls. Pathol. Annu. 1993; 28; 113–143. [PubMed] [Google Scholar]
- 30.Moll R, Löwe A, Laufer J, Franke WW. Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies. Am. J. Pathol. 1992; 140; 427–447. [PMC free article] [PubMed] [Google Scholar]
- 31.Keratin Miettinen M. 20: immunohistochemical marker for gastrointestinal, urothelial, and Merkel cell carcinomas. Mod. Pathol. 1995; 8; 384–388. [PubMed] [Google Scholar]
- 32.Miettinen M, Limon J, Niezabitowski A, Lasota J. Patterns of keratin polypeptides in 110 biphasic, monophasic, and poorly differentiated synovial sarcomas. Virchows Arch. 2000; 437; 275–283. [DOI] [PubMed] [Google Scholar]
- 33.Hornick JL, Fletcher CD. Myoepithelial tumors of soft tissue: a clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. Am. J. Surg. Pathol. 2003; 27; 1183–1196. [DOI] [PubMed] [Google Scholar]
- 34.Eusebi V, Carcangiu ML, Dina R, Rosai J. Keratin-positive epithelioid angio-sarcoma of thyroid. A report of four cases. Am. J. Surg. Pathol. 1990; 14; 737–747. [DOI] [PubMed] [Google Scholar]
- 35.Miettinen M, Fetsch JF. Distribution of keratins in normal endothelial cells and a spectrum of vascular tumors: implications in tumor diagnosis. Hum. Pathol. 2000; 31; 1062–1067. [DOI] [PubMed] [Google Scholar]
- 36.von Koskull H, Virtanen I. Induction of cytokeratin expression in human mesenchymal cells. J. Cell. Physiol. 1987; 133; 321–329. [DOI] [PubMed] [Google Scholar]
- 37.Knapp AC, Franke WW. Spontaneous losses of control of cytokeratin gene expression in transformed, non-epithelial human cells occurring at different levels of regulation. Cell 1989; 59; 67–79. [DOI] [PubMed] [Google Scholar]
- 38.Kriho VK, Yang HY, Moskal JR, Skalli O. Keratin expression inastrocytomas: an immunofluorescent and biochemical reassessment. Virchows Arch. 1997; 431; 39–47. [DOI] [PubMed] [Google Scholar]
- 39.Fanburg-Smith JC, Majidi M, Miettinen M. Keratin expressionin schwannoma; a study of 115 retroperitoneal and 22 peripheral schwannomas. Mod. Pathol. 2006; 19; 115–121. [DOI] [PubMed] [Google Scholar]
- 40.Miettinen M Immunohistochemistry of soft tissue tumors In Miettinen M ed. Modern soft tissue pathology. Cambridge/New York: Cambridge University Press, 2010; 52–53. [Google Scholar]
- 41.Nakajima T, Watanabe S, Sato Y, Kameya T, Hirota T, Shimosato Y. An immunoperoxidase study of S-100 protein distribution in normal and neoplastic tissues. Am. J. Surg. Pathol. 1982; 6; 715–727. [DOI] [PubMed] [Google Scholar]
- 42.Karamchandani JR, Nielsen TO, van de Rijn M, West RB.Sox10 and S100 in the diagnosis of soft-tissue neoplasms. Appl. Immunohistochem. Mol. Morphol. 2012; 20; 445–450. [DOI] [PubMed] [Google Scholar]
- 43.Newman PJ, Berndt MC, Gorski J et al. PECAM-1 (CD31) cloning and relation to adhesion molecules of the immunoglobulin gene superfamily. Science 1990; 47; 1219–1222. [DOI] [PubMed] [Google Scholar]
- 44.Kuzu I, Bicknell R, Harris AL, Jones M, Gatter KC, Mason DY. Heterogeneity of vascular endothelial cells with relevance to diagnosis of vascular tumours. J. Clin. Pathol. 1992; 45; 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Young BR, Wick MR, Fitzgibbon JF, Sirgi KE, Swanson PE.CD31: an immunospecific marker for endothelial differentiation in human neoplasms. Appl. Immunohistochem. 1993; 1; 97–100. [Google Scholar]
- 46.Miettinen M, Lindenmayer AE, Chaubal A. Endothelial cell markers CD31, CD34, and BNH9 antibody to H- and Y-antigens – evaluation of their specificity and sensitivity in the diagnosis of vascular tumors and comparison with von Willebrand’s factor. Mod. Pathol. 1994; 7; 82–90. [PubMed] [Google Scholar]
- 47.McKenney JK, Weiss SW, Folpe AL. CD31 expression in intratumoral macrophages: a potential diagnostic pitfall. Am. J. Surg. Pathol. 2001; 25; 1167–1173. [DOI] [PubMed] [Google Scholar]
- 48.Miettinen M, Wang ZF, Paetau A et al. ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma. Am. J. Surg. Pathol. 2011; 35; 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McKay KM, Doyle LA, Lazar AJ, Hornick JL. Expression of ERG,an ETS family transcription factor, distinguishes cutaneous angiosarcoma from histological mimics. Histopathology 2012; 61; 989–991. [DOI] [PubMed] [Google Scholar]
- 50.Agaimy A, Kirsche H, Semrau S, Iro H, Hartmann A. Cytokeratin-positive epithelioid angiosarcoma presenting in the tonsil: a diagnostic challenge. Hum. Pathol. 2012; 43; 1142–1147. [DOI] [PubMed] [Google Scholar]
- 51.Furusato B, Gao CL, Ravindranath L et al. Mapping of TMPRSS2–ERG fusions in the context of multi-focal prostate cancer. Mod. Pathol. 2008; 21; 67–75. [DOI] [PubMed] [Google Scholar]
- 52.Yaskiv O, Rubin BP, He H, Falzarano S, Magi-Galluzzi C, ZhouM. ERG protein expression in human tumors detected with a rabbit monoclonal antibody. Am. J. Clin. Pathol. 2012; 138; 803–810. [DOI] [PubMed] [Google Scholar]
- 53.Miettinen M, Wang Z, Sarlomo-Rikala M, Abdullaev Z, PackSD, Fetsch JF. ERG expression in epithelioid sarcoma: a diagnostic pitfall. Am. J. Surg. Pathol. 2013; 37; 1580–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang WL, Patel NR, Caragea M et al. Expression of ERG, an ETS family transcription factor, identifies ERG-rearranged Ewing sarcoma. Mod. Pathol. 2012; 25; 1378–1383. [DOI] [PubMed] [Google Scholar]
- 55.Verdu M, Trias I, Roman R et al. ERG expression and prostatic adenocarcinoma. Virchows Arch. 2013; 462; 639–644. [DOI] [PubMed] [Google Scholar]
- 56.Besmer P, Murphy JE, George PC et al. A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nature 1986; 320; 415–421. [DOI] [PubMed] [Google Scholar]
- 57.Hirota S, Isozaki K, Moriyama Y et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998; 279; 577–580. [DOI] [PubMed] [Google Scholar]
- 58.Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM.Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am. J. Pathol. 1998; 152; 1259–1269. [PMC free article] [PubMed] [Google Scholar]
- 59.Sarlomo-Rikala M, Kovatich AJ, Barusevicius A, Miettinen M.CD117: a sensitive marker for gastrointestinal stromal tumors that is more specific than CD34. Mod. Pathol. 1998; 11; 728–734. [PubMed] [Google Scholar]
- 60.Miettinen M, Lasota J. KIT (CD117): a review on expression innormal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl. Immunohistochem. Mol. Morphol. 2005; 13; 205–220. [DOI] [PubMed] [Google Scholar]
- 61.Tsuura Y, Hiraki H, Watanabe K et al. Preferential localization of c-kit product in tissue mast cells, basal cells of skin, epithelial cells of breast, small cell lung carcinoma and seminoma/dysgerminoma in human: immunohistochemical study on formalin-fixed, paraffin-embedded tissues. Virchows Arch. 1994; 424; 135–141. [DOI] [PubMed] [Google Scholar]
- 62.Lammie A, Drobnjak M, Gerald W, Saad A, Cote R, CordonCardo C. Expression of c-kit and kit ligand proteins in normal human tissues. J. Histochem. Cytochem. 1994; 42; 1417–1425. [DOI] [PubMed] [Google Scholar]
- 63.Hornick JL, Fletcher CD. Immunohistochemical staining forKIT (CD117) in soft tissue sarcomas is very limited in distribution. Am. J. Clin. Pathol. 2002; 117; 188–193. [DOI] [PubMed] [Google Scholar]
- 64.Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod. Pathol. 2000; 13; 1134–1142. [DOI] [PubMed] [Google Scholar]
- 65.West RB, Corless CL, Chen X et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am. J. Pathol. 2004; 165; 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Espinosa I, Lee CH, Kim MK et al. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am. J. Surg. Pathol. 2008; 32; 210–218. [DOI] [PubMed] [Google Scholar]
- 67.Yang YD, Cho H, Koo JY et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 2008; 455; 1210–1215. [DOI] [PubMed] [Google Scholar]
- 68.Miettinen M, Wang ZF, Lasota J. DOG1 antibody in the differential diagnosis of gastrointestinal stromal tumors: a study of 1840 cases. Am. J. Surg. Pathol. 2009; 33; 1401–1408. [DOI] [PubMed] [Google Scholar]
- 69.Novelli M, Rossi S, Rodriguez-Justo M et al. DOG1 and CD117 are the antibodies of choice in the diagnosis of gastrointestinal stromal tumours. Histopathology 2010; 57; 259–270. [DOI] [PubMed] [Google Scholar]
- 70.Sah SP, McCluggage WG. DOG1 immunoreactivity in uterine leiomyosarcomas. J. Clin. Pathol. 2013; 66; 40–43. [DOI] [PubMed] [Google Scholar]
- 71.Hemminger J, Iwenofu OH. Discovered on gastrointestinal stromal tumours 1 (DOG1) expression in non-gastrointestinal stromal tumour (GIST) neoplasms. Histopathology 2012; 61; 170–177. [DOI] [PubMed] [Google Scholar]
- 72.Akpalo H, Lange C, Zustin J. Discovered on gastrointestinal stromal tumour 1 (DOG1): a useful immunohistochemical marker for diagnosing chondroblastoma. Histopathology 2012; 60; 1099–1106. [DOI] [PubMed] [Google Scholar]
- 73.Perez-Montiel MD, Plaza JA, Dominguez-Malagon H, Suster S. Differential expression of smooth muscle myosin, SMA, h-caldesmon, and calponin in the diagnosis of myofibroblastic and smooth muscle lesions of skin and soft tissue. Am. J. Dermatopathol. 2006; 28; 105–111. [DOI] [PubMed] [Google Scholar]
- 74.Watanabe K, Kusakabe T, Hoshi N, Saito A, Suzuki T. h-Caldesmon in leiomyosarcoma and tumors with smooth muscle cell-like differentiation: its specific expression in the smooth muscle cell tumor. Hum. Pathol. 1999; 30; 392–396. [DOI] [PubMed] [Google Scholar]
- 75.Miettinen M, Sarlomo-Rikala M, Kovatich AJ, Lasota J. Calponin and h-caldesmon in soft tissue tumors: consistent h-caldesmon immunoreactivity in gastrointestinal stromal tumors indicates traits of smooth muscle differentiation. Mod. Pathol. 1999; 12; 756–762. [PubMed] [Google Scholar]
- 76.Folpe AL, Kwiatkowski DJ. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum. Pathol. 2010; 41; 1–15. [DOI] [PubMed] [Google Scholar]
- 77.Eusebi V, Damiani S, Pasquinelli G, Lorenzini P, Reuter VE, Rosai J. Small cell neuroendocrine carcinoma with skeletal muscle differentiation: report of three cases. Am. J. Surg. Pathol. 2000; 24; 223–230. [DOI] [PubMed] [Google Scholar]
- 78.Cessna MH, Zhou H, Perkins SL et al. Are myogenin and myoD1 expression specific for rhabdomyosarcoma? A study of 150 cases, with emphasis on spindle cell mimics. Am. J. Surg. Pathol. 2001; 25; 1150–1157. [DOI] [PubMed] [Google Scholar]
- 79.Weiss SW, Nickoloff BJ. CD-34 is expressed by a distinctive cell population in peripheral nerve, nerve sheath tumors, and related lesions. Am. J. Surg. Pathol. 1993; 17; 1039–1045. [DOI] [PubMed] [Google Scholar]
- 80.Chaubal A, Paetau A, Zoltick P, Miettinen M. CD34 immunoreactivity in nervous system tumors. Acta Neuropathol. 1994; 88; 454–458. [DOI] [PubMed] [Google Scholar]
- 81.Harder A, Wesemann M, Hagel C et al. Hybrid neurofibroma/schwannoma is overrepresented among schwannomatosis and neurofibromatosis patients. Am. J. Surg. Pathol. 2012; 36; 702–709. [DOI] [PubMed] [Google Scholar]
- 82.Zhou H, Coffin CM, Perkins SL, Tripp SR, Liew M, Viskochil DH. Malignant peripheral nerve sheath tumor: a comparison of grade, immunophenotype, and cell cycle/growth activation marker expression in sporadic and neurofibromatosis 1-related lesions. Am. J. Surg. Pathol. 2003; 27; 1337–1345. [DOI] [PubMed] [Google Scholar]
- 83.Pelmus M, Guillou L, Hostein I, Sierankowski G, Lussan C,Coindre JM. Monophasic fibrous and poorly differentiated synovial sarcoma: immunohistochemical reassessment of 60 t (X;18)(SYT–SSX)-positive cases. Am. J. Surg. Pathol. 2002; 26; 1434–1440. [DOI] [PubMed] [Google Scholar]
- 84.Terry J, Saito T, Subramanian S et al. TLE1 as a diagnostic immunohisto-chemical marker for synovial sarcoma emerging from gene expression profiling studies. Am. J. Surg. Pathol. 2007; 31; 240–246. [DOI] [PubMed] [Google Scholar]
- 85.Knösel T, Heretsch S, Altendorf-Hofmann A et al. TLE1 is a robust diagnostic biomarker for synovial sarcomas and correlates with t(X;18): analysis of 319 cases. Eur. J. Cancer 2010; 46; 1170–1176. [DOI] [PubMed] [Google Scholar]
- 86.Matsuyama A, Hisaoka M, Iwasaki M, Iwashita M, Hisanaga S, Hashimoto H. TLE1 expression in malignant mesothelioma. Virchows Arch. 2010; 457; 577–583. [DOI] [PubMed] [Google Scholar]