Abstract
Sex hormones such as estrogen fluctuate across the female lifespan, with high levels during reproductive years and natural decline during the transition to menopause. Women's exposure to estrogen may influence their heightened risk of Alzheimer's disease (AD) relative to men, but little is known about how it affects normal brain aging. Recent findings from the UK Biobank demonstrate less apparent brain aging in women with a history of multiple childbirths, highlighting a potential link between sex‐hormone exposure and brain aging. We investigated endogenous and exogenous sex‐hormone exposure, genetic risk for AD, and neuroimaging‐derived biomarkers for brain aging in 16,854 middle to older‐aged women. The results showed that as opposed to parity, higher cumulative sex‐hormone exposure was associated with more evident brain aging, indicating that i) high levels of cumulative exposure to sex‐hormones may have adverse effects on the brain, and ii) beneficial effects of pregnancies on the female brain are not solely attributable to modulations in sex‐hormone exposure. In addition, for women using hormonal replacement therapy (HRT), starting treatment earlier was associated with less evident brain aging, but only in women with a genetic risk for AD. Genetic factors may thus contribute to how timing of HRT initiation influences women's brain aging trajectories.
Keywords: apolipoproteins E, estrogens, neuroimaging, parity, women's brain aging
In this study, we show that while parity is associated with less evident brain aging in middle and older aged women, higher cumulative sex‐hormone exposure may be associated with more apparent brain aging, indicating that beneficial effects of pregnancies are not solely attributable to modulations in sex‐hormone exposure. We also found that starting hormone replacement treatment earlier was associated with less apparent brain aging, but only in women with a genetic risk for AD.
1. INTRODUCTION
Women are at significantly greater risk of developing Alzheimer's disease (AD) or other types of dementia relative to men (Laws et al., 2018). The genotype‐related risk for developing AD is also modified by sex, with higher risk in female carriers of apolipoprotein E type 4 (APOE e4) compared to male carriers (Altmann et al., 2014). Emerging evidence suggests that APOE genotype may interact with effects of exogenous estrogen exposure (Yaffe, 2001; Srivastava et al., 1997; Stone et al., 1998), influencing dementia and AD risk (Yaffe et al., 2000). However, little is known about the influence of sex‐hormone exposure on normal brain aging trajectories. Changes in sex hormones such as estradiol are known to influence brain plasticity (Galea et al., 2014; Simerly, 2002), and in premenopausal women, magnetic resonance imaging (MRI) studies have indicated modulating effects of endogenous estrogen fluctuations on brain structure across the menstrual cycle (Barth et al., 2016) and during pregnancy (Hoekzema et al., 2017). While higher endogenous estrogen levels have been associated with larger brain volumes during women's reproductive years (Barth et al., 2016; Lisofsky et al., 2015), results from the Rotterdam Scan Study showed that in menopausal women, higher endogenous estrogen levels were associated with smaller brain volumes in specific areas (den Heijer et al., 2003). Negative effects of exogenous estrogen levels have also been reported, and findings from the Women's Health Initiative Memory Study showed that conjugated estrogen, both alone and in combination with progestin, was associated with greater atrophy among women aged 65 years and older (Resnick et al., 2009). In addition, conjugated estrogen administration has been linked to higher rates of ventricular expansion over 4 years in recently menopausal women (Kantarci et al., 2016). However, other neuroimaging studies suggest a protective effect of hormone replacement therapy (HRT) on gray matter (Erickson et al., 2005), as well as white matter and ventricle size (Ha et al., 2007). Furthermore, a recent meta‐analysis suggests protective effects of estrogen replacement therapy on the risk of onset and/or development of AD and Parkinson Disease in postmenopausal women (Song et al., 2020). Emerging evidence indicates that oral contraceptives (OC), another source of exogenous estrogen, affect aspects of brain structure and function in young adults (reviewed by [Pletzer & Kerschbaum, 2014]), but despite their widespread use (Christin‐Maitre, 2013), the impact of OCs on brain aging is unknown.
We recently showed lower brain age in parous compared to nulliparous women in the UK Biobank cohort (de Lange et al., 2019). In the present paper, we investigate the association between estimates of sex‐hormone exposure and brain aging beyond the effects of parity in 16,854 UK Biobank women (mean age 54.70 ± 7.29 years). Brain‐age prediction using machine learning and imaging‐derived measures of cortical thickness, cortical volume, and subcortical volume (Fischl et al., 2002; Glasser et al., 2016; Kaufmann et al., 2019; de Lange et al., 2019) was performed to estimate “brain age” (Franke & Gaser, 2019) for each participant. Brain age gap, calculated by subtracting chronological age from estimated brain age, was used as a measure of apparent brain aging (Cole et al., 2017; Franke & Gaser, 2019; Franke et al., 2019; Smith et al., 2019). Cumulative sex‐hormone exposure was estimated by an index of cumulative estrogen exposure (ICEE) (Smith et al., 1999), including age at menarche and menopause, time since menopause, body mass index (BMI), and duration of HRT use. Exogenous exposure was estimated by HRT and OC use. To examine the effect of APOE e4 genotype on HRT usage and brain aging, we performed follow‐up analyses including APOE e4 genotype interactions. As the “critical period hypothesis” states that HRT may be neuroprotective if it is initiated close to menopause (MacLennan et al., 2006; Gibbs & Gabor, 2003; Hodis et al., 2016), we further tested whether age at HRT initiation, both alone and in relation to age at menopause, was associated with apparent brain aging. In order to investigate the link between sex‐hormone exposure and brain aging beyond the effects of parity (de Lange et al., 2019), all analyses were corrected for number of childbirths.
2. MATERIALS AND METHODS
2.1. Sample
The sample was drawn from the UK Biobank (www.ukbiobank.ac.uk), and included 16,854 women. Sample demographics are provided in Tables 1, 2, 3. The data that support the findings of this study are available through the UK Biobank application procedure (https://www.ukbiobank.ac.uk/register‐apply/), scripts are available from the authors upon request.
TABLE 1.
Sample demographics
| N | 16,854 |
| Age range [years] | 40.2–70.3 |
| Age (mean ± SD) | 54.70 ± 7.29 |
| Ethnic background | W 97.39 – B 0.61 – M 0.52 – A 0.67 – C 0.33 – O 0.45 |
| Education | U 43.27 – A 13.97 – O 21.28 – C 4.14 – N 3.32 – P 5.75 – Noa 6.42 |
| Menopausal status | Yes 50.97% – No 30.29% – Not sure 21% – Prefer not to answer 0.08% |
Note: Ethnic background: W, white; B, black; M, mixed; A, Asian; C, Chinese; O, other. Educational qualification: U, university/college degree; A, A levels or equivalent; O, O levels/General Certificate of Secondary Education (GCSE) or equivalent; C, Certificate of Secondary Education (CSE) or equivalent; N, National Vocational Qualification (NVQ) or equivalent; P, professional qualification, for example, nursing/teaching; Noa, none of the above. For the categories, see http://biobank.ctsu.ox.ac.uk/crystal/coding.cgi?id=100305 and http://biobank.ctsu.ox.ac.uk/crystal/coding.cgi?id=1001. Menopausal status was based on responses to the question “Have you had your menopause?”. 10.50% answered “Not sure—had a hysterectomy,” 10.50% answered “Not sure—other reason”. N, sample size. http://biobank.ndph.ox.ac.uk/showcase/field.cgi?id=2724.
TABLE 2.
Sample demographics for hormone replacement therapy (HRT) users (n = 5,651) and non‐users (n = 11,172)
| HRT users | Non‐users | t | χ2 | p | N | |
|---|---|---|---|---|---|---|
| Age range (years) | 40.4–70.3 | 40.2–70.2 | 16,823 | |||
| Age (mean ± SD) | 59.3 ± 5.4 | 52.4 ± 7.00 | 965.4 | <.001 | 16,823 | |
| Number of births (years) | 1.8 ± 1.1 | 1.7 ± 1.2 | 144.1 | <.001 | 16,817 | |
| Age at first birth | 25.7 ± 4.8 | 27.5 ± 5.1 | 600.8 | <.001 | 13,270 | |
| BMI (kg/m2) | 27.5 ± 4.8 | 27.4 ± 4.8 | 730.5 | <.001 | 16,724 | |
| Age at menopause (years) | 48.5 ± 5.8 | 50.6 ± 3.9 | 951.17 | <.001 | 9,369 | |
| Menopause (yes) | 96.1% | 50.4% | 2,695.4 | <.001 | 14,179 | |
| Hysterectomy (yes) | 31.5% | 6.5% | 1,841.7 | <.001 | 16,797 | |
| Oophorectomy (yes) | 17.6% | 1.5% | 1,486.5 | <.001 | 16,712 | |
| Hypertension (yes) | 21.6% | 16.2% | 51.0 | <.001 | 12,029 |
Note: Mean ± SD/% for each variable in each of the groups; N, sample size; BMI, body mass index. χ2 refers to the Pearson's chi‐square test.
TABLE 3.
Sample demographics for apolipoprotein E type 4 (APOE e4) genotype carriers (n = 4,277) and non‐carriers (n = 11,655)
| Carriers | Non‐carriers | t | χ2 | p | N | |
|---|---|---|---|---|---|---|
| Age range (years) | 40.3–70.2 | 40.23–70.27 | 15,932 | |||
| Age (mean ± SD) | 54.4 ± 7.2 | 54.9 ± 7.3 | 942.4 | <.001 | 15,932 | |
| Number of births | 1.7 ± 1.2 | 1.7 ± 1.2 | 148.8 | <.001 | 15,924 | |
| Age at first birth (years) | 26.9 ± 5.1 | 26.9 ± 5.1 | 587.45 | <.001 | 12,583 | |
| BMI (kg/m2) | 27.5 ± 4.7 | 27.4 ± 4.8 | 712.18 | <.001 | 15,837 | |
| Age at menopause (years) | 49.7 ± 4.9 | 49.7 ± 5.0 | 937.8 | <.001 | 8,873 | |
| Estradiol (pmol/L) | 541.8 ± 439.1 | 551.5 ± 580.5 | 66.4 | <.001 | 4,346 | |
| Ever used HRT (yes) | 31.5% | 34.2% | 10.2 | <.001 | 15,904 | |
| Current HRT use (yes) | 24.7% | 21.5% | 5.5 | .02 | 4,977 | |
| Hypertension (yes) | 17.1% | 18.1% | 1.4 | .20 | 11,447 |
Note: Mean ± SD/% for each variable in each of the groups; N, sample size; BMI, body mass index; HRT, hormone replacement therapy. χ2 refers to the Pearson's chi‐square test.
2.2. Sex‐hormone exposure
Women who had missing data, or had responded “do not know” or “prefer not to answer” for any of the relevant variables, were excluded for each analysis. For relevant analyses, women with a reported age at menarche (n = 1) or age at OC start (n = 1) at 5 years were excluded. ICEE was approximated by including age at menarche and menopause, duration of HRT in years, BMI, and time since menopause in years. The variables were first standardized and then either added (duration HRT, age at menopause, BMI) to or subtracted (age at menarche, time since menopause) from the index depending on their impact on endogenous estrogen (Smith et al., 1999). After removing women with missing data, 8,878 women were included in the ICEE analyses involving the brain‐age model. For HRT, 11,139 never‐users and 5,546 users were included in the analyses. For OC, 2,213 never‐users and 14,615 users were included in the analyses. Women who had never used HRT/OC were coded 0, while current and former users were coded 1. Multiple regression analyses were run to investigate the association between each estimate of sex‐hormone exposure and brain age gap. All analyses were corrected for number of childbirths and age. In addition, hysterectomy and/or oophorectomy were included as covariates in the HRT models, and current HRT use, ever used HRT, and length since menopause in years were included a covariates in the APOE e4 status × circulating estradiol level models. To test whether other known confounders including education, BMI, hypertensive status, and age at first birth could influence the results, additional models including these variables were run. Hypertensive status (yes/no) was defined as systolic blood pressure ≥140 mmHg and diastolic blood pressure ≥90 mmHg, otherwise individuals were classified as non‐hypertensive (Warren et al., 2017). The statistical analyses were conducted using R, version 3.5.2, and Python 3, version 3.7.6.
2.3. Genotyping
For genotyping, we used the UK Biobank version 3 imputed data, which has undergone extensive quality control procedures as described by the UK Biobank genetics team (Bycroft et al., 2018). The APOE e genotype was approximated based on the two APOE e single‐nucleotide polymorphisms—rs7412 and rs429358 (Lyall et al., 2016). Further information on the genotyping process is available in the UK Biobank documentation (www.ukbiobank.ac.uk/scientists‐3/genetic‐data), including detailed technical documentation (genotyping_workflow.pdf). APOE e4 status was labeled carrier for e3/e4 and e4/e4 combinations, and non‐carrier for e2/e2, e2/e3, and e3/e3 combinations (Lyall et al., 2019). The homozygous e2/e4 allele combination was removed due to its ambiguity with e1/e3 (Wisdom et al., 2011).
2.4. Hormone assay
Serum blood samples were taken at the initial assessment visit (2006–2010; for details on estradiol availability see http://biobank.ctsu.ox.ac.uk/crystal/field.cgi?id=30800). Estradiol was analyzed at the UK Biobank's purpose‐build laboratory in Stockport, and measured by two step competitive analysis on a Unicel DXI 800 Access Immunoassay System (Beckman Coulter, UK, Ltd; analytical range: 73–17,621 pmol/L). Further information on the immunoassay and quality control steps is available in the UK Biobank documentation (serum_biochemistry.pdf). To investigate whether APOE e4 status interacted with circulating estradiol levels in menopausal women, a multiple linear regression was run including an APOE e4 status × circulating estradiol level interaction term. The model was corrected for current HRT use, ever used HRT, length since menopause, and number of births.
2.5. MRI Processing
A detailed overview of the data acquisition, protocol parameters, and image validation can be found in (Alfaro‐Almagro et al., 2018) and (Miller et al., 2016). Raw T1‐weighted MRI data for all participants were processed using a harmonized analysis pipeline, including automated surface‐based morphometry and subcortical segmentation as implemented in FreeSurfer 5.3 (Fischl et al., 2002). In line with recent large‐scale implementations (Kaufmann et al., 2019; de Lange et al., 2019), we utilized a fine‐grained cortical parcellation scheme (Glasser et al., 2016) to extract cortical thickness, area, and volume for 180 regions of interest per hemisphere, in addition to the classic set of subcortical and cortical summary statistics from FreeSurfer (Fischl et al., 2002). This yielded a total set of 1,118 structural brain imaging features (360/360/360/38 for cortical thickness/area/volume, as well as cerebellar/subcortical and cortical summary statistics, respectively). The MRI variables were residualized with respect to scanning site, ethnic background, intracranial volume, and Freesurfer‐derived Euler numbers (Rosen et al., 2018) using linear models. To remove outliers, participants with Euler numbers of SD ±4 were identified and excluded (n = 159). In addition, participants with SD ±4 on the global MRI measures mean cortical or subcortical gray matter volume were excluded (n = 79 and n = 13, respectively), yielding a total of 16,854 participants with T1‐weighted MRI data.
2.6. Principal component analysis (PCA)
A PCA was run with z‐transformed MRI variables z = (x − μ)/σ, where x is an MRI variable of mean μ and SD σ). The top 100 components were used in the subsequent analyses, explaining 56.48% of the total variance. As a cross check, the relationship between ICEE and brain age gap was re‐analyzed with 200 components, explaining 70.61% of the total variance. With 200 components included, the association between ICEE and brain age gap was β = 0.03, SE = 0.01, t = 2.42, p = .02. As the results were consistent, 100 components were chosen to reduce computational time.
2.7. Brain age prediction
The XGBRegressor model from XGBoost (https://xgboost.readthedocs.io/en/latest/python/index.html) was used to run the brain age prediction analysis with an algorithm that has been used in recent large‐scale brain age studies (de Lange et al., 2019; Kaufmann et al., 2019; Smith et al., 2019). Parameters were set to max depth = 3, number of estimators = 100, and learning rate = 0.1 (defaults). The predicted age based on the PCA components was estimated in a 10‐fold cross validation, assigning an estimated brain age value to each individual. Brain age gap was calculated using (estimated brain age—chronological age). Average RMSE and R2 were calculated from a 10‐fold cross validation with 10 repetitions per fold, and compared to null distributions calculated from 10,000 permutations. The results are shown in Figure 1.
FIGURE 1.
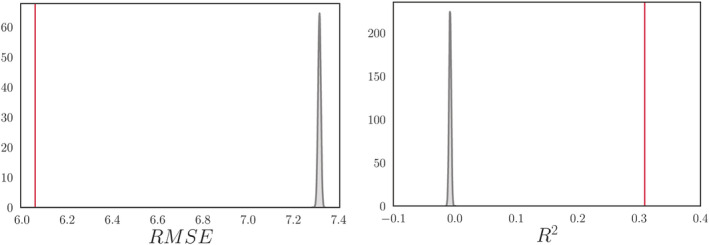
Average root mean square error (RMSE) and R 2 compared to null distributions. Left: The mean ± SD RMSE was 6.06 ± 0.09, based on a 10‐fold cross validation with 10 repetitions per fold (red vertical line). The null distribution calculated from 10,000 permutations is shown in gray, with a mean ± SD of 7.32 ± 0.006. The number of permuted results from the null distribution that exceeded the mean from the cross validation was 0 (p = 1.00 × 10−4). Right: The mean ± SD R2 for the brain age model was 0.31 ± 0.09 (red vertical line). The null distribution calculated from 10,000 permutations is shown in gray, with a mean ± SD of −0.007 ± 0.002 (p = 1.00 × 10−4)
3. RESULTS
The accuracy of the brain‐age prediction model is shown in Table 4. The associations between estimates of sex‐hormone exposure and apparent brain aging are summarized in Table 5 and Figure 2; p values are reported before and after false discover rate correction (pcorr) (Benjamini & Hochberg, 1995).
TABLE 4.
Number of magnetic resonance imaging (MRI) variables, root mean square error (RMSE), R2, mean absolute error (MAE), and the correlation between predicted and chronological age
| MRI variables | RMSE | R2 | MAE | Predicted age versus chronological age |
|---|---|---|---|---|
| 1,118 | 6.06 | 0.32 | 4.97 | r = 0.56, p = < .001, [0.55,0.57] |
Note: RMSE and MAE are reported in years. 95% confidence intervals are indicated in square brackets.
TABLE 5.
The associations between each estimate of sex‐hormone exposure and apparent brain aging
| variable | β | SE | t | p | pcorr |
|---|---|---|---|---|---|
| ICEE | 0.040 | 0.014 | 2.809 | .005 | .018* |
| HRT status | 0.169 | 0.055 | 3.073 | .002 | .015* |
| Age at HRT initiation | 0.022 | 0.009 | 2.509 | .012 | .028* |
| Duration of HRT usage | 0.004 | 0.005 | 0.787 | .432 | .503 |
| OC status | 0.017 | 0.066 | 0.250 | .802 | .802 |
Note: β, slope; ICEE, Index of cumulative estrogen exposure. Hormone replacement therapy (HRT) and oral contraceptive (OC) status = 0 for never‐users and 1 for current and former users. Each analysis included age and number of births as a covariates, in addition to specific covariates for each measure as detailed in Section 3. p values are reported before and after false discovery rate (FDR)‐correction (pcorr) (Benjamini & Hochberg, 1995). Corrected p values below 0.05 are marked with an asterisk.
FIGURE 2.
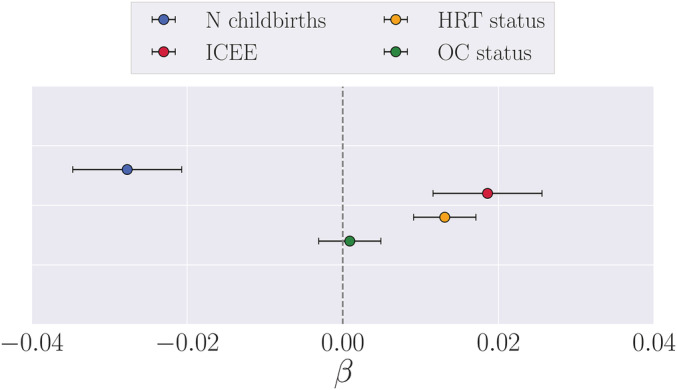
Associations between estimates of sex‐hormone exposure and apparent brain aging. ICEE, index of cumulative estrogen exposure. The points show the β values (slope) from separate multiple regression analyses with brain age gap (see Section 2.7) as dependent variable, and number of births (covariates: age and ICEE), ICEE (covariates: age and number of births), hormone replacement therapy (HRT) status (covariates: age, number of births, had hysterectomy and/or oophorectomy), and oral contraceptive (OC) status (covariates: age, number of births) as independent variables. To obtain a direct comparison of β values in the plot, all variables were standardized prior to performing the multiple regressions (subtracting the mean and dividing by the SD). HRT and OC status = 0 for never‐users and 1 for current and former users. The error bars represent the SE on the β
3.1. Index of cumulative estrogen exposure (ICEE)
A multiple linear regression showed a positive association between ICEE and apparent brain aging as shown in Table 5 and Figure 2, indicating that when correcting for number of previous childbirths and age, higher ICEE was linked to more apparent brain aging (n = 8,878). The inclusion of education, hypertensive status, and age at first birth as covariates yielded similar results (β = 0.045, SE = 0.019, t = 2.380, p = .017, pcorr = .030, n = 4,791). The associations between apparent brain aging and reproductive span, calculated as (age at menopause – age at menarche), as well as age at menarche and menopause separately, are provided in Data S1. Differential effects of surgical vs. natural menopause on brain aging were also examined (see Data S1).
3.2. Exogenous sex‐hormone exposure
A positive association was found between hormone replacement therapy (HRT) status and apparent brain aging in pre‐menopausal and menopausal women, with less evident brain aging in never‐users (n = 11,139) compared to users (n = 5,546 with 1,182 still using; covariates: number of births, had hysterectomy and/or oophorectomy, and age). When including age at first birth, education, BMI, and hypertensive status in the model, the results were similar (β = 0.168, SE = 0.074, t = 2.262, p = .024, pcorr = .033, never‐users = 6,236, n users = 2,971). No significant associations were found between Oral contraceptive (OC) status and apparent brain aging, as shown in Figure 2 and Table 5 (covariates: number of births and age; number of never‐users vs users was 2,213 vs 14,615. Three hundred and ninty eight women were still using OC).
Within the group of HRT users (n = 5,164), a positive relationship was found between age at HRT initiation and apparent brain aging as shown in Table 5, indicating less evident brain aging in women who started HRT treatment earlier (covariates: number of births, had hysterectomy and/or oophorectomy, and age). No significant association was found between duration of HRT use (age last used HRT − age started HRT) and apparent brain aging (covariates: number of births, age, had hysterectomy and/or oophorectomy, n = 5,164).
3.3. APOE e4 genotype and HRT initiation
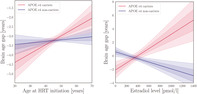
In HRT users, there was an effect of APOE e4 status × age started HRT on apparent brain aging, as shown in Table 6, with a trend‐level association after applying FDR correction (pcorr = .063). Follow‐up analyses showed that (a) the relationship between age at HRT initiation and apparent brain aging was confined to the carrier group (n = 1,227), as shown in Figure 3, and that (b) in menopausal APOE e4 carriers, age for HRT onset relative to age at menopause (age at menopause – age started HRT) was positively associated with apparent brain aging, indicating beneficial effects of HRT initiation before onset of menopause (β = − 0.078, SE = 0.023, t = − 3.441, p = 6.083 × 10−4, pcorr = .003, n = 826, covariates: number of births, age, had hysterectomy and/or oophorectomy). There was no dose‐dependent effect of the interaction age started HRT × carrier group (n with 1 e4 allele = 1,126, n with 2 e4 alleles = 101, β = 0.085, SE = 0.064, t = 1.317, p = .188, pcorr = .188).
TABLE 6.
The associations between apolipoprotein E type 4 (APOE e4) × age at hormone replacement therapy (HRT) initiation and APOE e4 × circulating estradiol, and main effects within carrier and non‐carriers
| β | SE | t | p | pcorr | |
|---|---|---|---|---|---|
| APOE e4 status and age at HRT initiation | |||||
| Interaction term | 0.038 | 0.018 | 2.076 | .038 | .063 |
| Main effect carriers | 0.042 | 0.018 | 2.349 | .019 | .047* |
| Main effect non‐carriers | 0.016 | 0.011 | 1.506 | .132 | .165 |
| APOE e4 status and circulating estradiol in menopausal women | |||||
| Interaction term | 0.008 | 0.002 | 3.711 | 2.47 × 10−4 | .001* |
| Main effect carriers | 0.006 | 0.002 | 3.194 | .002 | .005* |
| Main effect non‐carriers | −0.003 | 0.001 | −2.67 | .008 | .014* |
Note: β, slope. p values are reported before and after false discovery rate correction (pcorr) (Benjamini & Hochberg, 1995). Corrected p values below .05 are marked with an asterisk.
FIGURE 3.
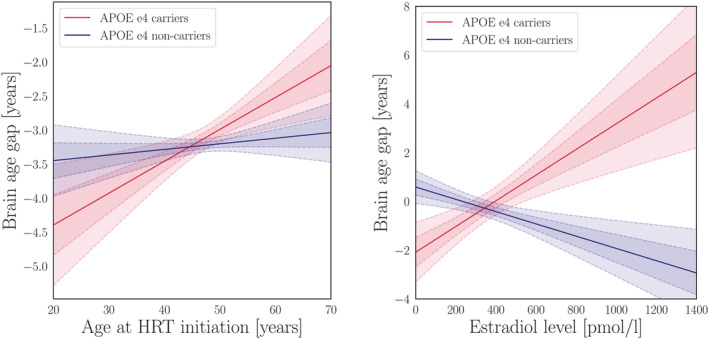
Apolipoprotein E (APOE) genotype interactions. Left plot: The lines show the association (β) between age started hormone replacement therapy (HRT) and apparent brain aging for the APOE e4 carriers (red) and non‐carriers (blue). The fitted values are corrected for the covariates in the model (age, number of births, had hysterectomy, and/or oophorectomy). Right plot: The lines show the association between estradiol levels and apparent brain aging for the APOE e4 carriers (red) and non‐carriers (blue). The fitted values are corrected for the covariates in the model (age, number of births, current HRT use, ever used HRT, length since menopause, age at first birth, and education). The shaded areas show the 68.3% (1 SD) and 95% (2 SD) confidence intervals for each fit
3.4. APOE e4 genotype and circulating estradiol levels
As shown in Figure 3, significant cross‐over interaction effects of APOE e4 status × circulating estradiol levels on apparent brain aging were found in menopausal women (β = 0.003, SE = 0.001, t = 2.270, p = .024, pcorr = .030, n = 539, covariates: current HRT use, ever used HRT, length since menopause, and number of births). When including age at first birth, education, hypertensive status, and BMI as covariates, the results showed a stronger effect of the interaction, as well as a negative main effect of estradiol levels on apparent brain aging (β = − 0.003, SE = 0.001, t = − 2.699, p = .007). Based on this finding, the subsequent follow‐up analyses were also corrected for education, age at birth, hypertensive status, and BMI. A positive main effect of estradiol levels on apparent brain aging was found in APOE e4 carriers (n = 101), while a negative main effect was found in non‐carriers (n = 310), as shown in Figure 3. No main association was found between APOE e4 status and apparent brain aging (β = 0.049, SE = 0.051, t = 0.954, p = .340, pcorr = .340, covariates: number of births and age; number of carriers vs non‐carriers = 4,276 vs 11,649).
The analyses were re‐run with corrections for (a) neurological and mental conditions, and (b) polygenic risk for Alzheimer's disease. The results are provided in Data S1. In brief, these corrections did not influence the results.
4. DISCUSSION
The results showed that as opposed to parity, higher cumulative and exogenous sex‐hormone exposure was associated with more evident brain aging. in vitro studies have shown that exposure to low concentrations of 17β‐estradiol promoted neuronal survival, whereas exposure to high concentrations was ineffective and led to increased cellular susceptibility to neurodegenerative insults (Chen et al., 2006). It has also been demonstrated that low‐dose estrogen replacements have anti‐inflammatory properties, whereas higher dosages show increases in inflammatory markers such as C‐reactive protein (Prestwood et al., 2004). In line with this, the current findings indicate that high levels of exposure to sex‐hormones may have adverse effects on women's brain aging.
While estrogen levels rise up to 300‐fold during pregnancy (Schock et al., 2016), they fall 100–1,000 fold postnatally (Nott et al., 1976), and parous women have shorter menstrual cycles and lower levels of estradiol than nulliparous women (Bernstein et al., 1985; Dorgan et al., 1995). Hormonal modulations contribute to maternal brain adaptations in pregnancy and postpartum (Galea et al., 2014; Kinsley & Lambert, 2008), but their long‐term effects on brain aging are not fully understood. The present results indicate that beneficial effects of pregnancies on brain aging may relate to factors beyond sex‐hormone fluctuations. Other pregnancy‐related mechanisms including immune regulations (Mor et al., 2011; Hillerer et al., 2014; Luppi, 2003) may have implications for inflammatory susceptibility later in life (Fox et al., 2018; Natri et al., 2019), subsequently impacting women's brain aging trajectories. For instance, one study found that higher cumulative time spent pregnant in first trimesters, but not third trimesters, conferred a protective effect against AD (Fox et al., 2018), indicating that immune processes such as the the proliferation of regulatory T cells (Kieffer et al., 2017), which is highest in the first trimester, could be more relevant for AD risk relative to estrogen exposure. In line with this, the “pregnancy compensation hypothesis” suggests that pregnancies involves long‐lasting, favorable regulations of the female immune system (Natri et al., 2019), which could underlie observed differences in apparent brain aging between parous and nulliparous women (de Lange et al., 2019).
The cessation of ovarian hormone function during menopause has been linked to altered inflammatory processes involving increases in cytokine levels, and changes in T cell biology (reviewed by [Mishra & Brinton, 2018]). For some, these processes may constitute a menopausal immune senescence that may increase the risk for AD (Fox et al., 2018; Wyss‐Coray & Rogers, 2012). The “critical period hypothesis” states that HRT may be neuroprotective if it is initiated near the time of cessation of ovarian function—approximately within 5 years of menopause (MacLennan et al., 2006; Gibbs & Gabor, 2003; Hodis et al., 2016). Our results lend further support to this hypothesis, as earlier age at HRT initiation, particularly before menopause, was associated with less evident brain aging. However, this relationship was present in APOE e4 carriers only, indicating that genetic factors may contribute to how timing of HRT initiation influences women's brain aging trajectories. Higher menopausal levels of estradiol were linked to more evident brain aging in carriers, while lower estradiol levels was linked to more evident brain aging in non‐carriers. In line with this, increased estradiol levels induced by estrogen therapy have been associated with reduced risk of developing AD in APOE e4 non‐carriers, but not in carriers (Yaffe et al., 2000; Manly et al., 2000).
To the best of our knowledge, the current work is the first comprehensive study of the associations between endogenous and exogenous hormone exposure, APOE genotype, and normal brain aging in a population‐based cohort. Large‐scale population‐based studies enable the identification of subtle effects that could go undetected in smaller samples, and are key to foster understanding of factors contributing to brain‐aging processes and risk for neurodegenerative disease. However, the cross‐sectional nature of the presented data does not enable causal inference, and longitudinal studies are required to fully understand how sex‐hormone exposure influences women's brain health across the lifespan. Furthermore, the current study lacks details on HRT and OC formulation, mode of delivery (e.g., oral or transdermal), and dosage. For instance, while HRT commonly consists of either combined hormone treatment (estrogen plus progestin) or unopposed estrogen treatment (estrogen alone), combined OC mostly contains ethinylestradiol and varying levels of progestins. These differences in compound compositions may affect brain aging trajectories differently (Savolainen‐Peltonen et al., 2019; Gleason et al., 2015), and more studies are needed to disentangle the effects of different HRT and OC treatment regimes on women's brain health later in life. The lack of information on breastfeeding, which is known to reduce cumulative exposure to endogenous estrogen (Bernstein, 2002), may also influence the precision of our ICEE approximation. Another major caveat is that the blood samples were taken at the initial visit, years prior to the imaging assessment. Although estradiol levels are relatively stable in menopausal women (Brinton et al., 2015), this time lag between assessments may have impacted our results on estradiol levels and brain aging, which should thus be interpreted with caution.
An issue with genetic aging studies is the bias towards survivors (Heffernan et al., 2016), which implies that the number of APOE e4 carriers could be lower in older‐age cohorts. In the present study, age was negatively associated with number of carriers (r = − 0.03, p = < .001,95 % CI = [−0.05, −0.02]), indicating potential survival‐bias in the sample. Further, estradiol levels were only available for a subset of women, and within this subset, a relatively high proportion (80%) had oestradiol values in the lower range. The UK Biobank notes that this reflects the menopausal status of the participants at recruitment (with 25% being premenopausal; see https://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/biomarker_issues.pdf), which is expected given the age range of the cohort. Based on these aspects, it should be noted that the presented results may not apply to populations beyond those represented in UK Biobank (Haworth et al., 2019).
In conclusion, our study provides evidence of an association between higher sex‐hormone exposure and more apparent brain aging, indicating that (a) high levels of exposure to sex‐hormones may have adverse effects on the brain, and (b) beneficial effects of pregnancies relate to factors beyond sex‐hormone fluctuations. Further, the influence of sex‐hormone exposure on the brain may be genotype‐specific, with more prominent effects of timing and dosage of hormone replacements in women with a genetic risk for AD. These findings represent an important contribution to the understudied field of female‐specific factors and women's brain aging (Galea et al., 2018), which may complement prospective longitudinal studies on women's brain health and epidemiological sex‐differences in AD.
CONFLICT OF INTEREST
All authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
A.‐M.G. de L., C.B., T.K., and L.T.W. designed the study; A.‐M.G. de L., C.B., T.K., I.I.M., and D.v.d.M. performed the data analysis; A.‐M.G. de L., C.B., T.K., I.A., and L.T.W. interpreted the data; A.‐M.G. de L., and C.B. drafted and finalized the manuscript. A.‐M.G. de L., and C.B. contributed equally to the work. I.A., L.T.W., T.K., I.I.M., and D.v.d.M. critically revised the first draft and approved the final manuscript.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available through the UK Biobank data access procedures (https://www.ukbiobank.ac.uk/researchers).
Supporting information
Data S1 Supporting Information
ACKNOWLEDGMENTS
This research was conducted using the UK Biobank under Application 27412, and received funding from the Research Council of Norway (286838, 273345, 249795, 276082, 250358), the South‐East Norway Regional Health Authority (2015073, 2019107) and the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (Grant agreement No. 802998). The permutation testing was performed using resources provided by UNINETT Sigma2—the National Infrastructure for High Performance Computing and Data Storage in Norway.
de Lange A‐MG, Barth C, Kaufmann T, et al. Women's brain aging: Effects of sex‐hormone exposure, pregnancies, and genetic risk for Alzheimer's disease. Hum Brain Mapp. 2020;41:5141–5150. 10.1002/hbm.25180
Ann‐Marie G. de Lange and Claudia Barth contributed equally to this study.
Funding information H2020 European Research Council, Grant/Award Number: 802998; Norges Forskningsråd, Grant/Award Numbers: 286838, 273345, 249795, 276082
Contributor Information
Ann‐Marie G. de Lange, Email: a.m.g.d.lange@psykologi.uio.no.
Claudia Barth, Email: claudia.barth@medisin.uio.no.
REFERENCES
- Alfaro‐Almagro, F. , Jenkinson, M. , Bangerter, N. K. , Andersson, J. L. , Griffanti, L. , Douaud, G. , et al. (2018). Image processing and quality control for the first 10,000 brain imaging datasets from UKbiobank. NeuroImage, 166, 400–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann, A. , Tian, L. , Henderson, V. W. , Greicius, M. D. , & Investigators, A. D. N. I. (2014). Sex modifies the apoe‐related risk of developing Alzheimer disease. Annals of Neurology, 75, 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth, C. , Steele, C. J. , Mueller, K. , Rekkas, V. P. , Arélin, K. , Pampel, A. , … Sacher, J. (2016). In‐vivo dynamics of the human hippocampus across the menstrual cycle. Scientific Reports, 6, 32833 10.1038/srep32833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57, 289–300. [Google Scholar]
- Bernstein, L. (2002). Epidemiology of endocrine‐related risk factors for breast cancer. Journal of Mammary Gland Biology and Neoplasia, 7, 3–15. [DOI] [PubMed] [Google Scholar]
- Bernstein, L. , Pike, M. C. , Ross, R. K. , Judd, H. L. , Brown, J. B. , & Henderson, B. E. (1985). Estrogen and sex hormone‐binding globulin levels in nulliparous and parous women. Journal of the National Cancer Institute, 74, 741–745. [PubMed] [Google Scholar]
- Brinton, R. D. , Yao, J. , Yin, F. , Mack, W. J. , & Cadenas, E. (2015). Perimenopause as a neurological transition state. Nature Reviews Endocrinology, 11, 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bycroft, C. , Freeman, C. , Petkova, D. , Band, G. , Elliott, L. T. , Sharp, K. , … Marchini, J. (2018). The UKbiobank resource with deep phenotyping and genomic data. Nature, 562, 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. , Nilsen, J. , & Brinton, R. D. (2006). Dose and temporal pattern of estrogen exposure determines neuroprotective outcome in hippocampal neurons: Therapeutic implications. Endocrinology, 147, 5303–5313. [DOI] [PubMed] [Google Scholar]
- Christin‐Maitre, S. (2013). History of oral contraceptive drugs and their use worldwide. Best Practice & Research Clinical Endocrinology & Metabolism, 27, 3–12. [DOI] [PubMed] [Google Scholar]
- Cole, J. H. , Poudel, R. P. , Tsagkrasoulis, D. , Caan, M. W. , Steves, C. , Spector, T. D. , & Montana, G. (2017). Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. NeuroImage, 163, 115–124. [DOI] [PubMed] [Google Scholar]
- Dorgan, J. F. , Reichman, M. E. , Judd, J. T. , Brown, C. , Longcope, C. , Schatzkin, A. , … Taylor, P. R. (1995). Relationships of age and reproductive characteristics with plasma estrogens and androgens in premenopausal women. Cancer Epidemiology and Prevention Biomarkers, 4, 381–386. [PubMed] [Google Scholar]
- Erickson, K. I. , Colcombe, S. J. , Raz, N. , Korol, D. L. , Scalf, P. , Webb, A. , … Kramer, A. F. (2005). Selective sparing of brain tissue in postmenopausal women receiving hormone replacement therapy. Neurobiology of Aging, 26, 1205–1213. [DOI] [PubMed] [Google Scholar]
- Fischl, B. , Salat, D. H. , Busa, E. , Albert, M. , Dieterich, M. , Haselgrove, C. , et al. (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron, 33, 341–355. [DOI] [PubMed] [Google Scholar]
- Fox, M. , Berzuini, C. , Knapp, L. A. , & Glynn, L. M. (2018). Women's pregnancy life history and Alzheimer's risk: Can immunoregulation explain the link? American Journal of Alzheimer's Disease & Other Dementias, 33, 516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke, K. , & Gaser, C. (2019). Ten years of brainage as a neuroimaging biomarker of brain aging: What insights have we gained? Frontiers in Neurology, 10, 789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke, K. , Ziegler, G. , Klöppel, S. , Gaser, C. , & Alzheimer's Disease Neuroimaging Initiative . (2010). Estimating the age of healthy subjects from t1‐weighted mri scans using kernel methods: Exploring the influence of various parameters. NeuroImage, 50(3), 883–892. [DOI] [PubMed] [Google Scholar]
- Galea, L. A. , Leuner, B. , & Slattery, D. A. (2014). Hippocampal plasticity during the peripartum period: Influence of sex steroids, stress and ageing. Journal of Neuroendocrinology, 26, 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea, L. A. , Qiu, W. , & Duarte‐Guterman, P. (2018). Beyond sex differences: Short and long‐term implications of motherhood on women's health. Current Opinion in Physiology, 6, 82–88. [Google Scholar]
- Gibbs, R. B. , & Gabor, R. (2003). Estrogen and cognition: Applying preclinical findings to clinical perspectives. Journal of Neuroscience Research, 74, 637–643. [DOI] [PubMed] [Google Scholar]
- Glasser, M. F. , Coalson, T. S. , Robinson, E. C. , Hacker, C. D. , Harwell, J. , Yacoub, E. , … van Essen, D. C. (2016). A multi‐modal parcellation of human cerebral cortex. Nature, 536, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason, C. E. , Dowling, N. M. , Wharton, W. , Manson, J. E. , Miller, V. M. , Atwood, C. S. , et al. (2015). Effects of hormone therapy on cognition and mood in recently postmenopausal women: Findings from the randomized, controlled keeps–cognitive and affective study. PLoS Medicine, 12(6), e1001833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha, D. M. , Xu, J. , & Janowsky, J. S. (2007). Preliminary evidence that long‐term estrogen use reduces white matter loss in aging. Neurobiology of Aging, 28, 1936–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth, S. , Mitchell, R. , Corbin, L. , Wade, K. H. , Dudding, T. , Budu‐Aggrey, A. , … J. Timpson, N. (2019). Apparent latent structure within the UKbiobank sample has implications for epidemiological analysis. Nature Communications, 10, 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan, A. L. , Chidgey, C. , Peng, P. , Masters, C. L. , & Roberts, B. R. (2016). The neurobiology and age‐related prevalence of the ε4 allele of apolipoprotein e in Alzheimer's disease cohorts. Journal of Molecular Neuroscience, 60, 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Heijer, T. , Geerlings, M. I. , Hofman, A. , de Jong, F. H. , Launer, L. J. , Pols, H. A. , & Breteler, M. M. (2003). Higher estrogen levels are not associated with larger hippocampi and better memory performance. Archives of Neurology, 60, 213–220. [DOI] [PubMed] [Google Scholar]
- Hillerer, K. M. , Jacobs, V. R. , Fischer, T. , & Aigner, L. (2014). The maternal brain: An organ with peripartal plasticity. Neural Plasticity, 2014, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodis, H. N. , Mack, W. J. , Henderson, V. W. , Shoupe, D. , Budoff, M. J. , Hwang‐Levine, J. , … ELITE Research Group . (2016). Vascular effects of early versus late postmenopausal treatment with estradiol. New England Journal of Medicine, 374, 1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema, E. , Barba‐Müller, E. , Pozzobon, C. , Picado, M. , Lucco, F. , García‐García, D. , … Vilarroya, O. (2017). Pregnancy leads to long‐lasting changes in human brain structure. Nature Neuroscience, 20, 287–296. [DOI] [PubMed] [Google Scholar]
- Kantarci, K. , Tosakulwong, N. , Lesnick, T. G. , Zuk, S. M. , Gunter, J. L. , Gleason, C. E. , … Miller, V. M. (2016). Effects of hormone therapy on brain structure: A randomized controlled trial. Neurology, 87, 887–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann, T. , van der Meer, D. , Doan, N. T. , Schwarz, E. , Lund, M. J. , Agartz, I. , et al. (2019). Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nature Neuroscience, 22, 1617–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer, T. E. , Faas, M. M. , Scherjon, S. A. , & Prins, J. R. (2017). Pregnancy persistently affects memory t cell populations. Journal of Reproductive Immunology, 119, 1–8. [DOI] [PubMed] [Google Scholar]
- Kinsley, C. H. , & Lambert, K. G. (2008). Reproduction‐induced neuroplasticity: Natural behavioural and neuronal alterations associated with the production and care of offspring. Journal of Neuroendocrinology, 20, 515–525. [DOI] [PubMed] [Google Scholar]
- de Lange, A.‐M. G. , Kaufmann, T. , van der Meer, D. , Maglanoc, L. A. , Alnæs, D. , Moberget, T. , … Westlye, L. T. (2019). Population‐based neuroimaging reveals traces of childbirth in the maternal brain. Proceedings of the National Academy of Sciences, 116, 22341–22346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laws, K. R. , Irvine, K. , & Gale, T. M. (2018). Sex differences in Alzheimer's disease. Current Opinion in Psychiatry, 31, 133–139. [DOI] [PubMed] [Google Scholar]
- Lisofsky, N. , Mårtensson, J. , Eckert, A. , Lindenberger, U. , Gallinat, J. , & Kühn, S. (2015). Hippocampal volume and functional connectivity changes during the female menstrual cycle. NeuroImage, 118, 154–162. [DOI] [PubMed] [Google Scholar]
- Luppi, P. (2003). How immune mechanisms are affected by pregnancy. Vaccine, 21, 3352–3357. [DOI] [PubMed] [Google Scholar]
- Lyall, D. M. , Cox, S. R. , Lyall, L. M. , Celis‐Morales, C. , Cullen, B. , Mackay, D. F. , et al. (2019). Association between apoe e4 and white matter hyperintensity volume, but not total brain volume or white matter integrity. Brain Imaging and Behavior, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall, D. M. , Ward, J. , Ritchie, S. J. , Davies, G. , Cullen, B. , Celis, C. , et al. (2016). Alzheimer disease genetic risk factor apoe e4 and cognitive abilities in 111,739 UKbiobank participants. Age and Ageing, 45, 511–517. [DOI] [PubMed] [Google Scholar]
- MacLennan, A. H. , Henderson, V. W. , Paine, B. J. , Mathias, J. , Ramsay, E. N. , Ryan, P. , … Taylor, A. W. (2006). Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: The remember pilot study. Menopause, 13, 28–36. [DOI] [PubMed] [Google Scholar]
- Manly, J. , Merchant, C. , Jacobs, D. , Small, S. , Bell, K. , Ferin, M. , & Mayeux, R. (2000). Endogenous estrogen levels and Alzheimer's disease among postmenopausal women. Neurology, 54, 833–837. [DOI] [PubMed] [Google Scholar]
- Miller, K. L. , Alfaro‐Almagro, F. , Bangerter, N. K. , Thomas, D. L. , Yacoub, E. , Xu, J. , et al. (2016). Multimodal population brain imaging in the UKbiobank prospective epidemiological study. Nature Neuroscience, 19, 1523–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra, A. , & Brinton, R. D. (2018). Inflammation: Bridging age, menopause and apoe ε4 genotype to Alzheimer's disease. Frontiers in Aging Neuroscience, 10, 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor, G. , Cardenas, I. , Abrahams, V. , & Guller, S. (2011). Inflammation and pregnancy: The role of the immune system at the implantation site. Annals of the new York Academy of Sciences, 1221, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natri, H. , Garcia, A. R. , Buetow, K. H. , Trumble, B. C. , & Wilson, M. A. (2019). The pregnancy pickle: Evolved immune compensation due to pregnancy underlies sex differences in human diseases. Trends in Genetics, 35, 478–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nott, P. , Franklin, M. , Armitage, C. , & Gelder, M. (1976). Hormonal changes and mood in the puerperium. The British Journal of Psychiatry, 128, 379–383. [DOI] [PubMed] [Google Scholar]
- Pletzer, B. A. , & Kerschbaum, H. H. (2014). 50 years of hormonal contraception–time to find out, what it does to our brain. Frontiers in Neuroscience, 8, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwood, K. M. , Unson, C. , Kulldorff, M. , & Cushman, M. (2004). The effect of different doses of micronized 17β‐estradiol on c‐reactive protein, interleukin‐6, and lipids in older women. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 59, M827–M832. [DOI] [PubMed] [Google Scholar]
- Resnick, S. M. , Espeland, M. A. , Jaramillo, S. A. , Hirsch, C. , Stefanick, M. L. , Murray, A. M. , … For the Women's Health Initiative Memory Study . (2009). Postmenopausal hormone therapy and regional brain volumes: The whims‐mri study. Neurology, 72, 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen, A. F. , Roalf, D. R. , Ruparel, K. , Blake, J. , Seelaus, K. , Villa, L. P. , et al. (2018). Quantitative assessment of structural image quality. NeuroImage, 169, 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen‐Peltonen, H. , Rahkola‐Soisalo, P. , Hoti, F. , Vattulainen, P. , Gissler, M. , Ylikorkala, O. , & Mikkola, T. S. (2019). Use of postmenopausal hormone therapy and risk of Alzheimer's disease in Finland: Nationwide case‐control study. Bmj, 364, l665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock, H. , Zeleniuch‐Jacquotte, A. , Lundin, E. , Grankvist, K. , Lakso, H.‐Å. , Idahl, A. , … Fortner, R. T. (2016). Hormone concentrations throughout uncomplicated pregnancies: A longitudinal study. BMC Pregnancy and Childbirth, 16, 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly, R. B. (2002). Wired for reproduction: Organization and development of sexually dimorphic circuits in the mammalian forebrain. Annual Review of Neuroscience, 25, 507–536. [DOI] [PubMed] [Google Scholar]
- Smith, C. , McCleary, C. , Murdock, G. , Wilshire, T. , Buckwalter, D. , Bretsky, P. , … Buckwalter, J. (1999). Lifelong estrogen exposure and cognitive performance in elderly women. Brain and Cognition, 39, 203–218. [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , Vidaurre, D. , Alfaro‐Almagro, F. , Nichols, T. E. , & Miller, K. L. (2019). Estimation of brain age delta from brain imaging. Neuroimage, 200, 528–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, Y.‐j. , Li, S.‐r. , Li, X.‐w. , Chen, X. , Wei, Z.‐x. , Liu, Q.‐s. , & Cheng, Y. (2020). The effect of estrogen replacement therapy on Alzheimer's disease and Parkinson's disease in postmenopausal women: A meta‐analysis. Frontiers in Neuroscience, 14, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava, R. A. K. , Srivastava, N. , Averna, M. , Lin, R. C. , Korach, K. S. , Lubahn, D. B. , & Schonfeld, G. (1997). Estrogen up‐regulates apolipoprotein e (apoe) gene expression by increasing apoe mRNA in the translating pool via the estrogen receptor α‐mediated pathway. Journal of Biological Chemistry, 272, 33360–33366. [DOI] [PubMed] [Google Scholar]
- Stone, D. J. , Rozovsky, I. , Morgan, T. E. , Anderson, C. P. , & Finch, C. E. (1998). Increased synaptic sprouting in response to estrogen via an apolipoprotein e‐dependent mechanism: Implications for Alzheimer's disease. Journal of Neuroscience, 18, 3180–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren, H. R. , Evangelou, E. , Cabrera, C. P. , Gao, H. , Ren, M. , Mifsud, B. , … UK Biobank CardioMetabolic Consortium BP working group . (2017). Genome‐wide association analysis identifies novel blood pressure loci and offers biological insights into cardiovascular risk. Nature Genetics, 49, 403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisdom, N. M. , Callahan, J. L. , & Hawkins, K. A. (2011). The effects of apolipoprotein e on non‐impaired cognitive functioning: A meta‐analysis. Neurobiology of Aging, 32, 63–74. [DOI] [PubMed] [Google Scholar]
- Wyss‐Coray, T. , & Rogers, J. (2012). Inflammation in Alzheimer disease ‐ A brief review of the basic science and clinical literature. Cold Spring Harbor Perspectives in Medicine, 2, a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe, K. (2001). Estrogens, selective estrogen receptor modulators, and dementia: What is the evidence? Annals of the new York Academy of Sciences, 949, 215–222. [DOI] [PubMed] [Google Scholar]
- Yaffe, K. , Haan, M. , Byers, A. , Tangen, C. , & Kuller, L. (2000). Estrogen use, apoe, and cognitive decline: Evidence of gene–environment interaction. Neurology, 54, 1949–1954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supporting Information
Data Availability Statement
The data that support the findings of this study are available through the UK Biobank data access procedures (https://www.ukbiobank.ac.uk/researchers).


