Abstract
INTRODUCTION:
The increase in the prevalence of multidrug-resistant Acinetobacter baumannii infections in hospital settings has rapidly emerged worldwide as a serious health problem.
METHODS:
This review synthetizes the epidemiology of multidrug-resistant A. baumannii, highlighting resistance mechanisms.
CONCLUSIONS:
Understanding the genetic mechanisms of resistance as well as the associated risk factors is critical to develop and implement adequate measures to control and prevent acquisition of nosocomial infections, especially in an intensive care unit setting.
Keywords: Risk factors, Multidrug-resistant, ICU
METHODS
A comprehensive search of the literature was performed using PubMed, ScienceDirect, and Web of Science. The search was restricted to original articles published in English related to risk factors, epidemiology, and multidrug-resistant A. baumannii (MDR-Ab). The key words used were (Acinetobacter baumannii OR A. baumannii) AND infection AND (multidrug-resistant OR MDR) AND (ICU), or (Acinetobacter baumannii OR A. baumannii) AND risk factors AND epidemiology. Case reports or conference abstracts were excluded. Two independent investigators searched the electronic databases using an identical method. The full texts of articles were reviewed by two independent reviewers to determine whether they met the eligibility criteria for inclusion. References in the included articles were reviewed to explore additional papers.
ACINETOBACTER BAUMANNII CONTEXT
Acinetobacter spp. is a pathogen that belongs to the Moraxellaceae family, which consists of 59 different species 1 , 2 . In this family, Acinetobacter spp. is the fifth most frequently isolated microorganism, distributed across five continents, among the gram-negative bacteria involved in nosocomial infections 3 . It is known that the species Acinetobacter baumannii is an opportunistic pathogen with clinical relevance 3 - 6 . The most frequent clinical manifestations are pneumonia associated with mechanical ventilation, bloodstream infections, urinary tract infections, and bacteremia associated with long periods of device use, meningitis, eye infections, intra-abdominal infections, surgical sites, the respiratory tract, and the gastrointestinal tract 7 , 8 . Nonetheless, this pathogen can survive in the intensive care unit (ICU) environment for up to four weeks due to its capacity to produce biofilms and thus contaminates patients admitted later 9 . Lipopolysaccharides (LPS), vesicles and proteins, polysaccharide capsules, phospholipases, proteases, outer membrane porins, and iron uptake systems are the most important factors for A. baumannii resistance 10 .
MDR-Ab is considered a hospital-acquired infection, which has been rapidly increasing worldwide due to the fitness effect of its resistance mutations 3 . The exacerbated and undue use of antibiotics associated with ineffective hospital interventions are related to the spread of MDR and consequently reduce treatment options. The World Health Organization (WHO) published in early 2017 a list of priorities for research into the development of active antibiotics against MDR and extensively resistant bacteria, which put A. baumannii first in the list of critical situations around the world 11 . It was estimated that multidrug-resistant A. baumannii can cost $33,510 to $129,917 per infection 12 . Moreover, patients with bacteremia can be related to high mortality rates due to multidrug-resistant A. baumannii (56.2%), when compared to A. baumannii strains with no multidrug resistance (4.7%) 13 . An average of 10.6% of patients die as a result of infections caused by MDR-Ab 12 .
OVERVIEW OF A. BAUMANNII ANTIBIOTIC RESISTANCE
The key resistance mechanisms of A. baumannii are the low permeability of the outer membrane, alteration in antibiotic binding sites, and mutations, which can cause upregulation or downregulation of efflux system activity 4 , 10 . Among these mechanisms, alteration of bacterial membrane permeability by the outer membrane proteins (OMPs) is associated with the loss or reduced expression of porins 8 . This group is represented by OmpA, OprD, and CarO proteins 14 . The OccD1 (OprD) channel of the Pseudomonas aeruginosa species plays an important role in the uptake of molecules such as imipenem and meropenem. This OM channel is closely related to the OM family in A. baumannii and is the largest pore described amongst Occ proteins with efficient in vitro uptake responsible for transporting small molecules, presenting a huge potential for future antibiotic design 15 .
The efflux system expels toxic compounds to the extracellular environment. Within it, five families of systems have been described in A. baumannii, such as the major facilitator super family (MFS), ATP binding cassette (ABC), resistance nodulation division (RND), small multidrug resistance family 1 (SMR), multidrug and toxic compound extrusion (MATE), and drug/metabolite transporter (DMT) 16 . The RND family is well characterized and is represented by the AdeABC, AdeIJK, and AdeFGH efflux system 17 . Mutations can influence the expression of the efflux system, resulting in increased cases of clinical infections. A study highlighted resistance to aminoglicosides, tetracyclines, chloramphenicol, fluoroquinolones, some beta-lactams, and tigecycline related to mutations on the chromosome or plasmids 18 . The efflux systems CraA, AmvA/AedF, Tet(A), and Tet(B) of the MFS system are known to have a drug-specific substrate profile, and are involved in chloramphenicol, erythromycin, chlorhexidine, and tetracycline resistance 19 , 20 . The expression of Acel protein is strictly related to chlorhexidine transportation and the AbeM gene (a member of the MATE family), which confers resistance to fluoroquinolones through the H+ antiport 20 , 21 . Quinolone resistance can be related to the AbaQ gene, which belongs to the MFS transporter and has its N- and C- ends located in the cytoplasm, which confers its characteristic as a drug H+ antiporter-1 (DHA1). AbaQ knockout in A. baumannii confirmed its involvement with quinolone susceptibility, resulting in decreased susceptibility caused by active efflux transportation 22 .
It is known that the fluoroquinolone resistance mechanism is mainly encoded by mutations in DNA gyrase (gyrA, gyrB genes) and topoisomerase IV (parC, parE ), with gyrB and parE mutated at a lower frequency. These mutations are sequential, as primary mutations in gyrA81 are followed by mutations in parC88 and parC84 in A. baumannii. However, a study described strains carrying mutations in only the parC gene, revealing the involvement of other resistance mechanisms for fluoroquinolone 23 - 24 .
One of the main mechanisms of resistance to beta-lactam antibiotics is associated with changes in the structure or expression profile of penicillin binding proteins (PBPs) 25 . PBPs are transglycosylases, transpeptidases, and carboxypeptidases, enzymes located in the plasma membrane, and are involved in the synthesis of peptidoglycan, an essential component of the bacterial cell wall. Once a PBP is acylated by a beta-lactam antibiotic, it is unable to catalyze hydrolysis of the covalent acyl-enzyme intermediate and is inactivated. Peptidoglycan transpeptidation cannot occur; thus, the cell wall is weakened 25 .
PBPs are divided into high molecular mass (HMM) and low molecular mass (LMM). The first is responsible for insertion into the cell wall, which, depending on the structure and catalytic activity of the N-terminal domain, can be classified as class A or B 26 . Therefore, changes in PBP expression lead to decreased susceptibility to these antimicrobial agents, favoring the occurrence of beta-lactam-resistant strains 27 . Due to the lack of interaction that occurs in the connection between beta-lactams and PBPs, the susceptibility of A. baumannii strains to beta-lactams has been observed 27 - 29 .
Mutations can occur and modify the binding of antibiotics, inactivating some lipids, such as lipid A 30 . Polymyxins interact with lipid A through the addition of phosphoethanolamine (PEtn), resulting in displacement of cations Mg2+ and Ca 2+, which destabilizes the membrane. These molecules are mediated by the pmrCAB operon 31 - 33 . Alterations in the pmrA-pmrB two-component system, which is also involved in lipid A biosynthesis, upregulate pmrC, influencing the synthesis of PEtn. It is known that LPS is synthesized through the lpx pathway; mutations in lpxA, lpxC, and lpxD genes lead to deficiency in LPS production and its complete loss, conferring the colistin resistance phenotype 34 - 35 . Colistin resistance can be chromosomal or plasmid-encoded, carrying the mcr gene (mcr-1 to mcr-5) 36 - 37 .
Carbapenemases, belonging to class A of Ambler (1980) and to group 2 of Bush and Jacob (2010) are considered one of the most versatile enzymatic families among β-lactamases, since they are able to hydrolyze most β-lactam antibiotics, such as carbapenems, penicillins, cephalosporins, and monobactams, in addition to being resistant against some commercial β-lactamase inhibitors 35 - 38 . Enzymes such as KPC-2, KPC-3, KPC-4, and KPC-10 39 , as well as GES-11, GES-12, and GES-14 40 , have already been described in A. baumannii 38 .
Metallo-β-lactamases belong to class B of Ambler (1980) and group 3 of Bush and Jacoby (2010). They confer resistance against penicillins, cephalosporins, and carbapenems, and are inhibited by β-lactamase inhibitors (clavulanic acid, sulbactam, and tazobactam). The enzymes representing this family are VIM-1 and NDM-1, commonly related to penicillin hydrolysis 39 - 43 . Class C of Ambler (1980), group 1 of Bush and Jacob (2010), is represented by chromosomal cephalosporinases (AmpC), which hydrolyze penicillins, and cephalosporins at a low level. When the insertion element ISAba1 or ISAba125 is inserted upstream of the bla AmpC gene, it is overexpressed, resulting in resistance to extended-spectrum cephalosporins as upstream ISAba induces strong promoter sequences 44 - 45 .
Oxacillinases belong to class D of Ambler (1980) and group 2 of Bush and Jacob (2010) and are encoded by the bla OXA genes. These proteins hydrolyze carbapenems and penicillins at a low level and has weak hydrolysis of second and third generation cephalosporins 44 . Oxacillinases have been reported in clinical isolates of A. baumannii associated with hospital outbreaks 46 . Six subgroups of Class D carbapenem-hydrolyzing enzymes (CHDLs), including OXA-23, OXA-24, OXA-51, OXA-58, OXA-143, and OXA-235, were identified 47 . These enzymatic groups hydrolyze penicillins at a high level and carbapenems at a low level. However, the presence of insertion sequence (IS) is considered a strong promoter for the increase of oxacillin expression and dissemination 48 . It was reported that the ISAba1/bla OXA-23 or ISAba1/bla OXA-51 combination amplified resistance to carbapenems 49 .
Aminoglycosides bind to 16S rRNA in the 30S ribosomal subunits and inhibit protein synthesis. Resistance is mediated by aminoglycoside-modifying enzymes (AMEs), such as acetyltransferases (AAC), adenyltransferases (ANT), and phosphotransferases (APH), which are found on mobile elements such as transposons and plasmids. AAC enzymes are responsible for modifying amino groups, while the ANT and APH enzymes act on hydroxyl groups, breaking bonds and inactivating the antibiotic molecule 10 . Methylase production (armA, rmtA, rmtB, rmtC, rmtD) decreases the affinity of the aminoglycosides for 30S ribosomal subunits 50 . A study with carbapenem-resistant (CR) A. baumannii identified 97.2% of the isolates carrying the aph(3´)-VI gene, with the majority found in 4 different clusters (A, B, C, and E), conferring resistance to amikacin, and group D, harboring AME genes (aac(6´)-Ib, aac(3)-Ia, and aph(3´)-Ia), responsible for gentamicin resistance and intermediate resistance to amikacin 51 , 52 . The presence of methylase armA coexisting with bla OXA-23 in MDR A. baumannii has been previously described and identified in quinolone-resistant A. baumannii 53 , 54 .
In addition to the multiple mechanisms of resistance, A. baumannii can acquire resistance genes through mobile genetic elements. Mobile elements, such as IS, transposons, genomic islands, integrons, and plasmids, are related to variations in the insertion site and carry strong transcriptional promoters that are abundantly synthesized 55 , 56 . Multiple A. baumannii plasmids have been reported: pA297-1, carrying gentamicin, kanamycin, and tobramycin resistance genes; pA297-3, carrying sulfonamide and streptomycin resistance genes; and pAb-G7-2, carrying an amikacin resistance gene 57 , 58 .
Transposons, such as Tn2006, Tn2007, and Tn2008, increase the spread of resistance genes and may present integrons, which were captured and express exogenous resistance genes 40 , 48 , 59 . Thus, integrons are composed of gene cassettes, and classes 1 and 2 are commonly found in A. baumannii clinical isolates 60 - 62 . As previously stated, insertion sequences act as strong promoters that increase the resistance levels of OXA carbapenemases in A. baumannii isolates 47 , 59 , 63 . Insertion sequence Acinetobacter baumannii (ISAba) can be located upstream of the resistant gene, overexpressing genes such as AmpC and OXA-51, which increases cephalosporin resistance 64 , 65 . Resistance to colistin in A. baumannii clinical isolates was related to the presence of the ISAba125 at the 3' end of the hns gene, disrupting the normal expression of a transcriptional gene regulator 66 .
RISK FACTORS RELATED TO A. BAUMANNII
Risk factors are directly related to increased susceptibility in hospitalized patients who develop some type of infectious disease involving bacterial resistance, consequently resulting in mortality in nosocomial environments. Investigation of the risk factors associated with A. baumannii infection/colonization contributes to the prevention and control of bacterial resistance, reducing the impact of A. baumannii isolates 67 (Table 1 and Table 2). The prevalence of A. baumannii infection and colonization is higher in ICUs, since patients with severe clinical conditions are hospitalized in such wards. In addition, these patients have compromised immune systems due to the presence of comorbidities, altered nutritional status, prolonged hospitalization, invasive procedures, immunosuppressive drugs, and broad-spectrum antibiotics 67 , 68 .
TABLE 1: Risk factors associated with infection and colonization caused by A. baumannii in adult ICUs.
| Study | Place of Study | Study Period | No. of Patients | Cases | Controls | Risk Factors | P-value |
|---|---|---|---|---|---|---|---|
| JANG et al., 2009 | Taiwan | 1997-2006 | 154 | 77 patients with AB bloodstream infection. | 77 patients with bloodstream infection without AB. | Use of central venous catheter, mechanical ventilation, colonization by AB, respiratory failure, cardiovascular failure. | P < 0.05 |
| YE et al., 2010 | Germany | 2001-2005 | 209 | 49 patients with MDRAB. | 160 patients with CSAB. | Previous use of antibiotics, use of mechanical ventilation, > 60 years, length of hospital stay. | P < 0.05 |
| ROCHA et al., 2008 | Brazil | 2005-2006 | 275 | 84 patients with PAVM. | 191 patients without PAVM. | Stay > 7 days in hospital, use of corticoids, invasive procedures, use of central venous catheter, and tracheostomy. | P < 0.05 |
| BROTFAIN et al, 2016 | Israel | 2005-2011 | 129 | 46 patients with pneumonia and positive sputum culture for MDRAB 72 h after MV onset and bacteremia. | 83 patients with pneumonia and positive sputum culture for MDRAB 72 h after the onset of MV, without developing bacteremia. | Hospitalization > 3 days in the ICU, advanced age, and recent bacteremia. | P < 0.05 |
| BLANCO et al., 2017 | United States | 2005-2009 | 101 | 90 patients with MDRAB. | 11 patients with CSAB. | Advanced age, previous hospitalization, heart failure, paralysis, HIV-AIDS, and rheumatoid arthritis. | P < 0.05 |
| ELLIS et al., 2015 | United States | 2006-2012 | 671 | 302 patients with infection caused by MDRAB. | 369 patients with infection caused by CSAB. | Length of hospital stay, transfer from another hospital, previous use of antibiotics | P < 0.25 |
| HENIG et al., 2015 | Israel | 2007-2012 | 2380 | 1190 patients with CRAB. | 1190 patients without AB. | Chemotherapy, organ transplant, chronic diseases, invasive procedures, recent bacteremia, tumor, hematological diseases, and recurrent hospitalizations. | P < 0.05 |
| JUNG et al., 2010 | South Korea | 2008-2009 | 200 | 108 patients with bacteremia caused by AB. | 92 patients without bacteremia. | Respiratory failure, mechanical ventilation, tracheal tube, central venous catheter, bacteremia caused by other microorganisms, previous use of antibiotics. | P < 0.05 |
| NUTMAN et al., 2014 | Israel | 2008-2011 | 172 | 83 patients with bacteremia who died within 14 days. | 89 patients with bacteremia who survived after 14 days. | Disease severity and surgical procedure. | P ≤ 0.10 |
| CHUSRI et al., 2015 | Thailand | 2010-2011 | 394 | 139 patients with CRAB. | 197 patients without AB and 58 patients with CSAB. | Use of fluoroquinolones, broad spectrum cephalosporins, and carbapenems > 3 days. | P < 0.05 |
| MOGHNIEH et al., 2016 | Lebanon | 2012-2013 | 257 | 40 patients with AB. | 217 patients without AB. | Use of urinary catheter, ICU contact pressure, gastrectomy tube, and carbapenem use. | P < 0.05 |
| GUO et al., 2016 | China | 2012-2015 | 87 | 64 patients with bloodstream infection by MDRAB. | 23 patients with bloodstream infection by CSAB. | Pneumonia, drain use, ICU stay> 7 days, and use of mechanical ventilation. | P < 0.05 |
AB: A. baumannii; MDRAB: multidrug-resistant A. baumannii; PAVM: pneumonia associated with mechanical ventilation; MV: mechanical ventilation; CRAB: carbapenem-resistant A. baumannii; CSAB: carbapenem-susceptible A. baumannii; ICU: intensive care unit.
TABLE 2: Risk factors associated with infection and colonization caused by A. baumannii in pediatric and neonatal ICUs.
| Study | Place of study | Study period | No. of patients | Cases | Controls | Risk factors | P-value |
|---|---|---|---|---|---|---|---|
| BRITO et al., 2010 | Brazil | 2001-2002 | 33 | 11 patients with infectious conditions caused by AB. | 22 patients without infectious conditions caused by AB. | Birth weight <2500 grams, respiratory syndromes, parental feeding, re-intubation, carbapenem use, and mechanical ventilation. | P < 0.05 |
| DENG et al., 2011 | China | 2002-2008 | 349 | 117 patients with PAVM caused by AB. | 232 patients without PAVM caused by AB. | Use of mechanical ventilation> 7 days. | P < 0.01 |
| HSU et al., 2014 | Taiwan | 2004-2010 | 248 | 37 patients with bacteremia caused by AB. | 74 patients without bacteremia and 137 patients with bacteremia caused by Escherichia coli or Klebsiella spp. | Cholestasis, gestational age < 29 weeks. | P < 0.05 |
| LEE et al., 2017 | China | 2004-2014 | 40 | 37 patients with AB susceptible to imipenem | 3 patients with AB resistant to imipenem | Prematurity, low birth weight (70% < 1500 g), prolonged intubation, percutaneous use of central venous catheter, inappropriate initial therapy, infection within the first 10 days of life, use of imipenem for up to 5 days, and high frequency oscillation ventilation. | P < 0.05 |
| PUNPANICH et al., 2012 | Thailand | 2005-2010 | 176 | 91 patients with bacteremia caused by CRAB. | 85 patients with bacteremia caused by CSAB. | Prematurity, use of mechanical ventilation, previous exposure to carbapenems. | P < 0.05 |
| HOSOGLU et al., 2012 | Turkey | 2006-2007 | 192 | 64 patients with AB sepsis. | 128 patients with blood samples without AB. | Stay in the ICU> 7 days, re-intubation. | P < 0.001 |
| De OLIVEIRA COSTA et al., 2015 | Brazil | 2009-2012 | 101 | 47 patients with infection caused by BGN. | 54 patients without infection caused by BGN. | Hematologic diseases, neutropenia > 3 days, previous use of antibiotics, previous hospitalization, stay in the ICU > 3 days. | P < 0.05 |
| THATRIMONTRICHAI et al., 2013 | Thailand | 2009-2014 | 101 | 63 patients with CRAB pneumonia and 13 patients with CSAB. | 25 patients with pneumonia without bacterial growth or caused by other microorganisms. | Weight of newborns, previous use of cephalosporins, surfactant replacement therapy, re-intubation, umbilical artery catheterization. | P < 0.05 |
| REDDY et al., 2015 | South Africa | 2010 | 388 | 194 patients with blood culture or respiratory sample positive for AB. | 194 patients with blood culture or negative respiratory sample for AB. | Mechanical ventilation and traumatic brain injury. | P < 0.05 |
| ZARRILLI et al., 2012 | Italy | 2010-2011 | 161 | 22 patients with AB. | 139 patients without AB in the first 48 h. | Use of mechanical ventilation and central venous catheter. | |
| TRAN et al., 2015 | Vietnam | 2010-2011 | 2555 | 69 patients with sepsis caused by AB. | 2486 patients without sepsis caused by AB. | Maternal infection, gestational age, central catheter, surgical procedure, and blood transfusion. | P < 0.05 |
| KUMAR et al., 2014 | India | 2010-2012 | 65 | 33 patients with CRAB bloodstream infection. | 32 patients without CSAB bloodstream infection. | Previous use of antibiotics, hospitalization > 7 days, use of mechanical ventilation > 7 days. | P < 0.05 |
| WEI et al., 2014 | Taiwan | 2010-2013 | 59 | 12 deaths due to sepsis caused by MDRAB. | 47 deaths due to sepsis caused by other microorganisms. | Prolonged intubation, mechanical ventilation, peripheral central venous catheter, umbilical catheter, total parental nutrition, ICU stay > 7 days, surgical procedure, and bronchopulmonary dysplasia. | P < 0.05 |
| MACIEL et al., 2017 | Brazil | 2013-2015 | 21 | 21 patients with AB colonization without clinical manifestation. | 17 patients without sepsis. | Low birth weight, prematurity, hospitalization time, previous exposure to beta-lactams, use of peripheral access, and respiratory syndromes. | P < 0.05 |
AB: A. baumannii; PAVM: pneumonia associated with mechanical ventilation; CRAB: carbapenem-resistant A. baumannii; CSAB: carbapenem-susceptible A. baumannii; BGN: gram-negative bacillus; MDRAB: multidrug-resistant A. baumannii; ICU: intensive care unit.
Skin colonization, length of hospital stays > 7 days, use of corticosteroids, and invasive procedures such as central venous catheter or tracheostomy, were the main risk factors related to the development of pneumonia associated with mechanical ventilation by MDR A. baumannii in hospitalized patients (Table 1) 69 , 70 . Risk factors such as use of urinary catheters for more than 6 days, ICU contact pressure > 4 days, presence of gastrectomy tubes, chemotherapy, organ transplantation, chronic diseases, invasive procedures, recent bacteremia, tumors, hematological diseases, recurrent hospitalizations, hospitalization time > 7 days, transfer from another hospital, and previous use of carbapenems or broad-spectrum cephalosporins were related to acquisition of MDR A. baumannii infection in adult patients hospitalized in the ICU 69 , 71 . Isolation of MDR A. baumannii after medical ICU (MICU) admission was related to a greater likelihood of the patient being older 72 . Previous hospitalization was associated with the isolation of A. baumannii after admission to the surgical ICU (SICU). Positive colonization in SICU was strongly correlated with heart failure, paralysis, human immunodeficiency virus infection and acquired immune deficiency syndrome (HIV-AIDS), and rheumatoid arthritis 73 .
Bloodstream infections by A. baumannii are frequent in ICUs and have been associated with central venous catheters, mechanical ventilation, pneumonia, drain use, and respiratory and cardiovascular failure 74 . The risk of bacteremia caused by A. baumannii was associated with respiratory failure, mechanical ventilation, endotracheal tubes, central venous catheters, surgical procedures, and previous use of antibiotics 75 , 76 .
Newborns are considered susceptible to A. baumannii colonization and infections, since they have immature immune systems. The risk is greater for newborns if they are also preterm (< 28 weeks) and underweight (< 2,500 g) 76 , 77 . Birth weight < 2500 grams, respiratory syndromes, parental feeding, re-intubation, carbapenem use, mechanical ventilation, hematologic diseases, neutropenia > 3 days, previous use of broad-spectrum antibiotics, use of invasive devices, immunosuppressants, corticosteroids, previous hospitalization, and ICU stay > 3 days were considered risk factors for the acquisition of A. baumannii infections in the neonatal ICU (Table 2) 78 - 80 .
Bloodstream infections caused by A. baumannii in neonates were related to the use of mechanical ventilation, and additionally to the presence of traumatic brain injury, previous use of antibiotics, hospitalization > 7 days, and use of mechanical ventilation > 7 days 81 - 83 . The weight of newborns (1000-1499 g), previous use of cephalosporins, surfactant replacement therapy, re-intubation, and umbilical artery catheterization were also indicated as risk factors for the development of neonatal pneumonia caused by carbapenem-resistant A. baumannii 84 . Maternal infection, gestational age among 26 to 36 weeks, use of central venous catheters, surgical procedures, blood transfusions, prolonged intubation, use of mechanical ventilation, central peripheral venous catheters, umbilical catheters, total parental nutrition, ICU stay > 7 days, surgical procedures, and bronchopulmonary dysplasia were described as risk factors for sepsis by A. baumannii 77 , 85 . Cholestasis, gestational age < 29 weeks, prematurity, low birth weight (70% < 1500 g), prolonged intubation, central venous catheters, use of imipenem for up to 5 days, mechanical ventilation, and prior carbapenem exposure are related to A. baumannii bacteremia in neonates 10 , 86 , 87 . Similar results were reported for colonization in neonates 88 . These studies pinpoint persistent endemic isolates in hospitals, highlighting the need to implement efficient control measures and prevent outbreaks.
Seasonality of A. baumannii infection is another risk factor that should be taken into consideration. A systematic review compiled studies showing 57.1% (12/21) of A. baumannii infections occurred in warmer seasons. The hypothesis for this was that it was due to enhanced lipid A moiety regulation, which was responsible for the virulence; it was also reported there was biofilm formation and a higher flow of people entering the hospital facility (carriers, patients, healthcare workers, and sanitation workers) in warmer months. This study highlights the importance of correlating different factors of A. baumannii adaptability in the ambient environment to implement preventive measures for seasonal peaks of infection 89 .
Information related to colonization pressure (CP) is important for mediating risk factors. CP is a tool to measure the proportion of A. baumannii reservoirs within a health care facility. For A. baumannii surveillance, CP can help enhance patient screening and determine infection control measures 90 , 91 .
MOLECULAR EPIDEMIOLOGY OF A. BAUMANNII IN BRAZIL
In Brazil, the first outbreak associated with OXA-23-producing A. baumannii isolates was in 1999 92 . Subsequently, different outbreaks were reported 93 . A. baumannii dissemination in different Brazilian hospitals was associated with bla OXA-51 and bla OXA-23 genes and highlighted the prevalence of ISAba1/OXA-23 and ISAba1/OXA-51 genetic profiles 94 . Isolates carrying the bla OXA-51, bla OXA-58, and bla OXA-23 genes, and ISAba1 upstream of OXA-51 and OXA-23 were found in different ICUs, indicating an outbreak of cross-contamination among patients, equipment, or medical staff 94 . The bla OXA-58 and bla OXA-65 genes with the upstream ISAba1 sequence for both genes have been reported. The bla OXA-58 gene is prevalent in Argentina, indicating a possible spread from the border with Rio Grande do Sul 95 . In addition, two genotypes of OXA-23-producing A. baumannii were present at 8 hospitals in the same city, suggesting the spread of isolates in these environments 93 . The sequence type (ST) 156, ST25, and ST160 were identified in a Brazilian hospital 96 . Cephalosporin-resistant A. baumannii and producers of extended-spectrum beta-lactamases (ESBL) were identified in a neonatal intensive care unit (NICU), causing septicemia in hospitalized neonates (Table 3) 5 . A study in neonates described most isolates as belonging to ST1 and had ISAba1 upstream of the bla OXA-51 and bla OXA-23 genes 88 .
TABLE 3: Outbreaks of Acinetobacter baumannii in Brazil.
| Study | Place of study | Year of outbreak | Place of outbreak | No. of patients | Antibiotic Resistance | Reported genes |
|---|---|---|---|---|---|---|
| DALIA-COSTA et al., 2003 | Curitiba | 1999 | Ward | 8 | IPM, MEM, CIP, and AMG | bla OXA-23 |
| BRITO et al., 2005 | Uberlândia | 2005 | NICU | 11 | GEN, CIP, CAZ, FEP, and ATM | - |
| TAKAGI et al., 2009 | São Paulo | 2005-2006 | ICU | 8 | PIP, TZP, CAZ, CTX, ATM, IPM, MEM, CIP, AMK, GEN, and SXT | bla OXA-51 |
| MARTINS et al., 2009 | Porto Alegre | 2007 | DHW | 53 | CIP, GEN, TZP, and SXT | bla OXA-51, bla OXA-23 |
| GUSATTI et al., 2012 | Porto Alegre | 2007 | Ward | 74 | IPM, MEM, AMK, CIP, GEN, CET, AMA, SXT, and TIM | bla OXA-51, bla OXA-58, bla OXA-65, ISAba1/OXA-51 |
| PAGANO et al., 2015 | Porto Alegre | 2011 | DHW | 122 | FEP, CIP, CAZ, AMA, AMK, PMB, IMP and MEM | bla OXA-23 |
| CASTILHO et al., 2017 | Goiás | 2010 | ICU | 64 | AMA, FEP, AMK, PMB, and TGC | ISAba1/OXA-23 and ISAba1/OXA-51, bla OXA-51, bla OXA-23, bla OXA-58 |
| MACIEL et al., 2017 | Dourados | 2013-2015 | NICU | 21 | AMA, TZP, CAZ, CRO, FEP, GEN, AMK, CIP, and TGC. | ISAba1/OXA-23 and ISAba1/OXA-51 |
ICU: intensive care unit; NICU: neonatal intensive care unit; DHW: different hospital wards; IPM: imipenem; MEM: meropenem; CIP: ciprofloxacin; AMG: aminoglycoside; GEN: gentamicin; CAZ: ceftazidime; FEP: cefepime; ATM: aztreonam; TZP: piperacillin/tazobactam; AMK: amikacin; SXT: trimethoprim/sulfamethoxazole; CET: cephalothin; TIM: ticarcillin/clavulanic acid; PMB: polymyxin B; TGC: tigecycline; CRO: ceftriaxone; AMA: ampicillin/sulbactam; CTX: cefotaxime; PIP: piperacillin.
A study in Recife, Brazil described isolates belonging to ST1, ST15, ST25, ST79, ST113, and ST881 (related to ST1). Among them, ST79 and ST113 were found to be more virulent and presented resistance genes. ST113 and ST15 were commonly found in all 5 hospitals of the study, while ST79 was found in 4 hospitals and ST1 in 3 hospitals. Among the CCs circulating between hospitals, Leal et al. described CC1, CC15, and CC113, which are globally spread types, and CC79, which is found in South America, North America, and Europe 97 .
A study carried out in nine hospitals in South America identified A. baumannii clinical isolates presenting bla OXA-51, bla OXA-23, bla OXA-72, bla OXA-132, bla OXA-65, bla OXA-69, and bla OXA-64 genes. Multilocus sequence type (MLST) analysis identified ST79, ST25, and ST15 98 . The two major clonal complexes (CC) found in bla OXA-23 multidrug-resistant A. baumannii are CC15 and CC79, and CC15 has already been described in 9 Brazilian states 77 . In addition, ST15 was described in other countries, such as Argentina and Turkey, and ST79 was described in the United States, Canada, and Spain 99 . Of the clonal profiles identified, ST15 and ST79 were described in several countries, indicating their spread among hospitals around the world and high mortality rates 100 .
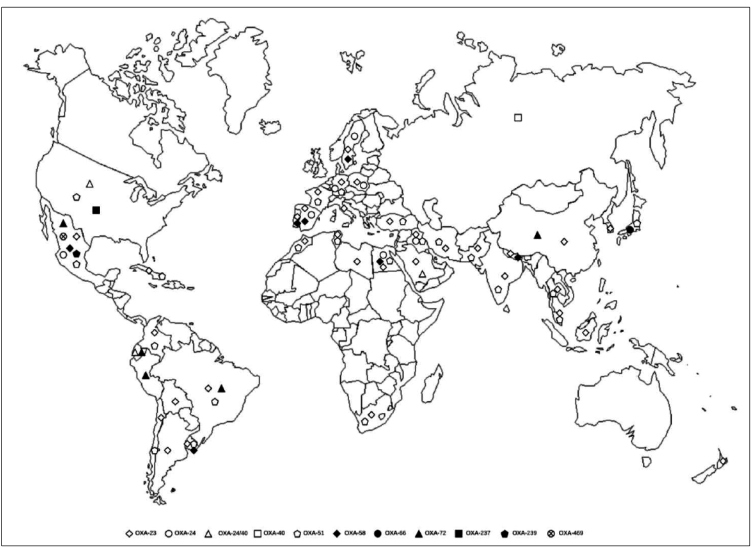
The Antimicrobial Surveillance Program (SENTRY) evaluated the prevalence of Acinetobacter spp. and other gram-negative bacilli isolated from Latin American (Argentina, Brazil, Chile, and Mexico) medical centers from 2008 to 2010. In this period, 5,704 gram-negative bacilli were isolated and 845 (17.7%) were classified as Acinetobacter spp. This microorganism was responsible for 7.2% of the 6,035 bloodstream infections, 7% of the 1,442 pneumonia cases, and 9.9% of the 1,531 skin and soft tissue infections. The oxacillinases found in this study were OXA-23 and OXA-24 in Argentina, OXA-23 in Brazil, OXA-58 in Chile, and OXA-24 in Mexico 101 . Figure 1 shows a map representing the description of the resistant gene OXA in the last eight years 3 , 102 - 145 .
FIGURE 1: Geographic distribution of OXA enzymes in the last seven years.
MOLECULAR EPIDEMIOLOGY OF A. BAUMANNII IN THE WORLD
In France, 110 A. baumannii clinical strains were isolated between 2010 and 2011. Of these, 90 isolates harbored bla OXA-23, 12 bla OXA-24, and 8 bla OXA-58. One of the isolates simultaneously displayed bla OXA-23 and bla PER-1, and 2 isolates possessed bla OXA-23 and bla OXA-58. Pulsed-field gel electrophoresis (PFGE) analysis showed 30 clusters and MLST revealed 11 STs (ST115, ST1, ST2, ST10, ST20, ST25, ST79, ST85, ST107, ST108, and ST125) 43 . A study conducted in China evaluated 57 clinical isolates of carbapenem-resistant A. baumannii that were positive for the bla OXA-23/ISAba1 and bla OXA-51 genes, harboring ST75 and ST137 145 . In addition, a Chinese hospital identified transposons Tn2006, Tn2007, and Tn2008 in 59 clinical isolates of OXA-23-producing A. baumannii 146 .
In Saudi Arabia, 107 A. baumannii clinical isolates were identified, of which 75 harbored the genes bla TEM and bla CTX-M (n = 86), bla OXA-51 (n = 100), and bla OXA-23 (n = 97). MLST analysis identified ST195, ST557, ST208, ST499, ST218, ST231, ST222, and ST286, all belonging to CC2, except ST231 147 . In the United States, in 2008 and 2009, 65 A. baumannii clinical isolates producing bla OXA-51/ISAba1 were found in different hospitals, harboring bla OXA-23 (65/65) and bla OXA-40 genes (09/65). PFGE analysis indicated 24 clusters, whereas MLST identified ST1, ST2, ST77, ST79, ST123, ST124, CC1, and CC2 148 . A total of 149 clinical isolates of A. baumannii, containing bla OXA-58 (n = 31), bla OXA-58/ISAba3 (n = 14), and bla OXA-72 (n = 18) were isolated from different hospitals in Egypt. These presented as 54 clusters by PFGE and ST763, ST777, ST369, ST762, and ST229 were identified 149 .
In South Africa, 94 clinical isolates of A. baumannii were found in different hospitals; 93 carried the bla OXA-51 gene and 72 the bla OXA-23. PFGE analysis grouped the isolates into 4 clusters with 5 STs (ST106, ST258, ST339, ST502, ST758, ST848), in which ST258 and ST758 corresponded to the international clone I, and ST502 and ST848 to the international clone II 150 . In India, 100 A. baumannii strains showed high genetic variability. MLST identified ST110, ST108, ST194, ST14, ST146, ST69, ST188, ST386, ST387, ST388, ST389, ST390, and ST391 151 . A total of 160 A. baumannii clinical isolates were identified in Vietnam, of which 119 were MDR or extensively resistant, presenting a high level of resistance against third- and fourth-generation cephalosporins. Of these, 128 isolates harbored the bla OXA-51 and bla OXA-23 genes associated with the ISAba1 element. MLST analysis identified 16 STs from 23 isolates, confirmed new STs, and some isolates belonged to ST136 152 .
In Malaysia, 162 clinical isolates of MDR A. baumannii were identified, of which 128 were resistant to carbapenems. The bla OXA-23, bla OXA-IMP, and bla OXA-ADC genes were identified, and ISAba1, upstream of the bla OXA-23 and bla OXA-ADC genes, was also found. Point mutations in gyrA (Ser83Leu) and parC (Ser80Leu), which provide resistance to ciprofloxacin, were also identified in the isolates. MLST identified two predominant STs (ST195 and ST208) 104 .
Molecular typing of A. baumannii provides a better understanding of the epidemiology of outbreaks and identification of cross-transmission, as well as assisting in the monitoring and control of nosocomial infections 17 , 153 . Thus, several methods have been used to study the molecular epidemiology of A. baumannii and analyze the mechanisms involved in the resistance of this microorganism.
CONCLUSION
The increase in healthcare-associated infection (HAI) rates connected to A. baumannii antimicrobial resistance has become a major public health challenge worldwide. A. baumannii possesses several resistance mechanisms. However, hydrolysis by OXA-type carbapenemases and metallo-β-lactamases are considered the most prevalent mechanisms conferring resistance to most beta-lactam antibiotics and reduce therapeutic options. This study highlights the occurrence of outbreaks in hospital settings, especially in ICUs, which are commonly related to prolonged hospital stays and invasive procedures. Thus, epidemiological studies are important for monitoring the occurrence of A. baumannii clinical isolates and may assist in the implementation of appropriate measures, contributing to the control of hospital infections.
ACKNOWLEDGMENTS
We are grateful to the Universidade Federal da Grande Dourados (UFGD) and the research group in Molecular Biology of Microorganisms of this institution for their support.
Footnotes
Financial Support: Mariana Neri Lucas Kurihara and Romário Oliveira de Sales received a scholarship from National Council for Science and Technological Development (CNPq), Késia Esther da Silva and Wirlaine Glauce Maciel from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The sponsors had no role in the collection, analysis and interpretation of data or the writing of the manuscript.
REFERENCES
- 1.Rossau R, Van Landschoot A, Gillis M, De Ley J. Taxonomy of Moraxellaceae fam. nov., a new bacterial family to accommodate the genera Moraxella, Acinetobacter, and Psychrobacter and related organisms. Int J Syst Bacteriol. 1991;41(2):310–329. [Google Scholar]
- 2.Euzeby JP. List of bacterial names with standing in nomenclature: a folder available on the internet. Int J Syst Bacteriol. 1997;47:590–592. doi: 10.1099/00207713-47-2-590. [DOI] [PubMed] [Google Scholar]
- 3.Vincent J-L, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 4.Peleg AY, Seifert H, Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008;21(3):538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy-Blitchtein S, Roca I, Plasencia-Rebata S, Vicente-Taboada W, Velásquez-Pomar J, Muñoz L, et al. Emergence and spread of carbapenem-resistant Acinetobacter baumannii international clones II and III in Lima, Peru article. Emerg Microbes Infect. 2018;7(1):119–128. doi: 10.1038/s41426-018-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo H, Qin J, Xiang J. Surveillance for and susceptibility of Acinetobacter baumannii in a large hospital and burn center in Shanghai, China, 2007-2013. Am J Infect Control. 2016;44(12):1718–1719. doi: 10.1016/j.ajic.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Nasr P. Genetics, epidemiology, and clinical manifestations of multidrug-resistant Acinetobacter baumannii. J Hosp Infect. 2020;104(1):4–11. doi: 10.1016/j.jhin.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Doi Y, Murray GL, Peleg AY. Acinetobacter baumannii: Evolution of antimicrobial resistance-treatment options. Semin Respir Crit Care Med. 2015;36(1):85–98. doi: 10.1055/s-0034-1398388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pakharukova N, Tuittila M, Paavilainen S, Malmi H, Parilova O, Teneberg S. Structural basis for Acinetobacter baumannii biofilm formation. Proc Natl Acad Sci U S A. 2018;115(21):5558–5563. doi: 10.1073/pnas.1800961115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HY, Hsu SY, Hsu JF, Chen CL, Wang YH, Chiu CH. Risk factors and molecular epidemiology of Acinetobacter baumannii bacteremia in neonates. J Microbiol Immunol Infect. 2018;51(3):367–376. doi: 10.1016/j.jmii.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization (WHO) Antibacterial Agents in preclinical development. Geneva: WHO; 2019. 20 p [Google Scholar]
- 12.Zhou H, Yao Y, Zhu B, Ren D, Yang Q, Fu Y, et al. Risk factors for acquisition and mortality of multidrug-resistant Acinetobacter baumannii bacteremia. Medicine. 2019;98(13):e14937. doi: 10.1097/MD.0000000000014937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gramatniece A, Silamikelis I, Zahare I, Urtans V, Zahare I, Dimina E, et al. Control of Acinetobacter baumannii outbreak in the neonatal intensive care unit in Latvia: whole-genome sequencing powered investigation and closure of the ward. Antimicrob Resist Infect Control. 2019;8:84–84. doi: 10.1186/s13756-019-0537-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smani Y, Fab̀rega A, Roca I, Sańchez-Encinales V, Vila J, Pachón J. Role of OmpA in the multidrug resistance phenotype of Acinetobacter baumannii. Antimicrob Agents Chemother. 2014;58(3):1806–1808. doi: 10.1128/AAC.02101-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zahn M, Bhamidimarri SP, Baslé A, Winterhalter M, Van den Berg B. Structural Insights into Outer Membrane Permeability of Acinetobacter baumannii. Struct. 2016;24(2):221–231. doi: 10.1016/j.str.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Coyne S, Courvalin P, Périchon B. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob Agents Chemother. 2011;55(3):947–953. doi: 10.1128/AAC.01388-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West AH, Stock AM. Histidine kinases and response regulator proteins in two-component signaling systems. Trends in Biochemical Sciences. 2001;26(6):369–376. doi: 10.1016/s0968-0004(01)01852-7. [DOI] [PubMed] [Google Scholar]
- 18.Wieczorek P, Sacha PHT, Zórawski M, Krawczyk M, Tryniszewska E. Multidrug resistant Acinetobacter baumannii - The role of AdeABC (RND family) efflux pump in resistance to antibiotics. Folia Histochem Cytobiol. 2008;46(3):257–267. doi: 10.2478/v10042-008-0056-x. [DOI] [PubMed] [Google Scholar]
- 19.Roca I, Marti S, Espinal P, Martínez P, Gibert I, Vila J. CraA, a major facilitator superfamily efflux pump associated with chloramphenicol resistance in Acinetobacter baumannii. Antimicrob Agents Chemother. 2009;53(9):4013–4014. doi: 10.1128/AAC.00584-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassan KA, Jackson SM, Penesyan A, Patching SG, Tetu SG, Eijkelkamp BA, et al. Transcriptomic and biochemical analyses identify a family of chlorhexidine efflux proteins. Proc Natl Acad Sci U S A. 2013;110(50):20254–20259. doi: 10.1073/pnas.1317052110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su XZ, Chen J, Mizushima T, Kuroda T, Tsuchiya T. AbeM, an H+-coupled Acinetobacter baumannii multidrug efflux pump belonging to the MATE family of transporters. Antimicrob Agents Chemother. 2005;49(10):4362–4364. doi: 10.1128/AAC.49.10.4362-4364.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pérez-Varela M, Corral J, Aranda J, Barbé J. Functional Characterization of AbaQ, a Novel Efflux Pump Mediating Quinolone Resistance in Acinetobacter baumannii. Antimicrob Agents Chemother. 2018;62(9):e00906-18. doi: 10.1128/AAC.00906-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostrer L, Khodursky RF, Johnson JR, Hiasa H, Khodursky A. Analysis of Mutational Patterns in Quinolone Resistance-Determining Regions of GyrA and ParC of Clinical Isolates. Int J Antimicrob Agents. 2018;(18):30366–30362. doi: 10.1016/j.ijantimicag.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Zaki MES, Abou ElKheir N, Mofreh M. Molecular Study of Quinolone Resistance Determining Regions of gyrA Gene and parC Genes in Clinical Isolates of Acinetobacter baumannii Resistant to Fluoroquinolone. Open Microbiol J. 2018;12:116–122. doi: 10.2174/1874285801812010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papp-Wallace KM, Senkfor B, Gatta J, Chai W, Taracila MA, Shanmugasundaram V, et al. Early Insights into the Interactions of Different β-Lactam Antibiotics and β-Lactamase Inhibitors against Soluble Forms of Acinetobacter baumannii PBP1a and Acinetobacter sp. PBP3. Antimicrob Agents Chemother. 2012;56(11):5687–5692. doi: 10.1128/AAC.01027-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol Rev. 2008;32(2):234–258. doi: 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- 27.Cayô R, Rodríguez M-C, Espinal P, Fernández-Cuenca F, Ocampo-Sosa AA, Pascual Á, et al. Analysis of Genes Encoding Penicillin-Binding Proteins in Clinical Isolates of Acinetobacter baumannii. Antimicrob Agents Chemother. 2011;55(12):5907–5913. doi: 10.1128/AAC.00459-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez-Cuenca F, Martínez-Martínez L, Conejo MC, Ayala JA, Perea EJ, Pascual A. Relationship between beta-lactamase production, outer membrane protein and penicillin-binding protein profiles on the activity of carbapenems against clinical isolates of Acinetobacter baumannii. J Antimicrob Chemother. 2003;51(3):565–574. doi: 10.1093/jac/dkg097. [DOI] [PubMed] [Google Scholar]
- 29.Hawkey J, Ascher DB, Judd LM, Wick RR, Kostoulias X, Cleland H, et al. Evolution of carbapenem resistance in Acinetobacter baumannii during a prolonged infection. Microb.Genomics. 2018;4:e000165. doi: 10.1099/mgen.0.000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boll JM, Crofts AA, Peters K, Cattoir V, Vollmer W, Davies BW, et al. A penicillin-binding protein inhibits selection of colistin-resistant, lipooligosaccharide-deficient Acinetobacter baumannii. Proc Natl Acad Sci U S A. 2016;113(41):E6228-37. doi: 10.1073/pnas.1611594113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moffatt JH, Harper M, Boyce JD. Mechanisms of Polymyxin Resistance. In: J Li, R L Nation, K S Kaye., editors. Polymyxin Antibiotics: From Laboratory Bench to Bedside. 1st ed. Springer; 2019. pp. 55–71. [DOI] [PubMed] [Google Scholar]
- 32.Garnacho-Montero J, Timsit J-F. Managing Acinetobacter baumannii infections. Curr Opin Infect Dis. 2018;32:69–76. doi: 10.1097/QCO.0000000000000518. [DOI] [PubMed] [Google Scholar]
- 33.Dortet L, Potron A, Bonnin RA, Plesiat P, Naas T, Filloux A, et al. Rapid detection of colistin resistance in Acinetobacter baumannii using MALDI-TOF-based lipidomics on intact bacteria. Scientific Reports. 2018;8(1) doi: 10.1038/s41598-018-35041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beceiro A, Llobet E, Aranda J, Bengoechea JA, Doumith M, Hornsey M, et al. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob Agents Chemother. 2011;55(7):3370–3379. doi: 10.1128/AAC.00079-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rawat D, Nair D. Extended-spectrum β-lactamases in Gram Negative Bacteria. J Glob Infect Dis. 2010;2(3):263‐74–263‐74. doi: 10.4103/0974-777X.68531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Da Silva GJ, Domingues S. Interplay between Colistin Resistance, Virulence and Fitness in Acinetobacter baumannii. Antibiot. 2017;6:28–28. doi: 10.3390/antibiotics6040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hameed F, Khan MA, Muhammad H, Sarwar T, Bilal H, Rehman TU. Plasmid-mediated mcr-1 gene in Acinetobacter baumannii and Pseudomonas aeruginosa: first report from Pakistan. Rev Soc Bras Med Trop. 2019;52:e20190237. doi: 10.1590/0037-8682-0237-2019. [DOI] [PubMed] [Google Scholar]
- 38.Queenan AM, Bush K. Carbapenemases: The versatile β-lactamases. Clin Microbiol Rev. 2007;20(3):440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robledo IE, Aquino EE, Santé MI, Santana JL, Otero DM, Léon CF, et al. Detection of KPC in Acinetobacter spp. in Puerto Rico. Antimicrob Agents Chemother. 2010;54(3):1354–1357. doi: 10.1128/AAC.00899-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bogaerts P, Naas T, El Garch F, Cuzon G, Deplano A, Delaire T, et al. GES extended-spectrum β-lactamases in Acinetobacter baumannii isolates in Belgium. Antimicrob Agents Chemother. 2010;54(11):4872–4878. doi: 10.1128/AAC.00871-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bush K, Jacoby GA, Medeiros AA. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39(6):1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poirel L, Nordmann P. Carbapenem resistance in Acinetobacter baumannii: Mechanisms and epidemiology. Clin Microbiol Infect. 2006;12(9):826–836. doi: 10.1111/j.1469-0691.2006.01456.x. [DOI] [PubMed] [Google Scholar]
- 43.Jeannot K, Diancourt L, Vaux S, Thouverez M, Ribeiro A, Coignard B, et al. Molecular epidemiology of carbapenem non-susceptible Acinetobacter baumannii in France. PLoS One. 2014;9(12):e115452. doi: 10.1371/journal.pone.0115452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bush K, Jacoby GA. Updated functional classification of beta-lactamases. Antimicrob Agents Chemother. 2010;54(3):969–976. doi: 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karah N, Jolley KA, Hall RM, Uhlin BE. Database for the ampC alleles in Acinetobacter baumannii. PLoS One. 2017;12(5):e0176695. doi: 10.1371/journal.pone.0176695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ning NZ, Liu X, Bao CM, Chen SM, Cui EB, Zhang JL, et al. Molecular epidemiology of bla OXA-23-producing carbapenem-resistant Acinetobacter baumannii in a single institution over a 65-month period in north China. BMC Infect Dis. 2017;17(1):14–14. doi: 10.1186/s12879-016-2110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamolvit W, Sidjabat HE, Paterson DL. Molecular Epidemiology and Mechanisms of Carbapenem Resistance of Acinetobacter spp. in Asia and Oceania. Microb Drug Resist. 2015;21(4):424–434. doi: 10.1089/mdr.2014.0234. [DOI] [PubMed] [Google Scholar]
- 48.Wang D, Yan D, Hou W, Zeng X, Qi Y, Chen J. Characterization of blaOxA-23 gene regions in isolates of Acinetobacter baumannii. J Microbiol Immunol Infect. 2015;48(3):284–290. doi: 10.1016/j.jmii.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Martínez P, Mattar S. Imipenem-resistant Acinetobacter baumannii carrying the ISABA1-BLAOXA-23, 51 and ISABA1-BLAADC-7 genes in Monteria, Colombia. Brazilian J Microbiol. 2012;43(4):1274–1280. doi: 10.1590/S1517-83822012000400006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bakour S, Alsharapy SA, Touati A, Rolain J-M. Characterization of Acinetobacter baumannii clinical isolates carrying bla(OXA-23) carbapenemase and 16S rRNA methylase armA genes in Yemen. Microb Drug Resist. 2014;20(6):604–609. doi: 10.1089/mdr.2014.0018. [DOI] [PubMed] [Google Scholar]
- 51.Polotto M, Casella T, Tolentino FM, Mataruco MM, Porto NKM, Binhardi MFB, et al. Investigation of carbapenemases and aminoglycoside modifying enzymes of Acinetobacter baumannii isolates recovered from patients admitted to intensive care units in a tertiary-care hospital in Brazil. Rev Soc Bras Med Trop. 2020;53:e20190044. doi: 10.1590/0037-8682-0094-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Magnet S, Blanchard JS. Molecular insights into aminoglycoside action and resistance. Chem Rev. 2005;105:477–498. doi: 10.1021/cr0301088. [DOI] [PubMed] [Google Scholar]
- 53.Hong SB, Shin KS, Ha J, Han K. Co-existence of blaOXA-23 and armA in multidrug-resistant Acinetobacter baumannii isolated from a hospital in South Korea. Pt_6J Med Microb. 2013;62:836–844. doi: 10.1099/jmm.0.055384-0. [DOI] [PubMed] [Google Scholar]
- 54.Cho YJ, Moon DC, Jin JS, Choi CH, Lee YC, Lee JC. Genetic basis of resistance to aminoglycosides in Acinetobacter spp. and spread of armA in Acinetobacter baumannii sequence group 1 in Korean hospitals. Diagn Microb Infect Dis. 2009;64(2):185–190. doi: 10.1016/j.diagmicrobio.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 55.Kobs VC, Ferreira JA, Bobrowicz TA, Ferreira LE, Deglmann RC, Westphal GA, et al. The role of the genetic elements blaoxa and ISAba1 in the Acinetobacter calcoaceticus-Acinetobacter baumannii complex in carbapenem resistance in the hospital setting. Rev Soc Bras Med Trop. 2016;49(4):433–440. doi: 10.1590/0037-8682-0002-2016. [DOI] [PubMed] [Google Scholar]
- 56.Héritier C, Poirel L, Lambert T, Nordmann P. Contribution of acquired carbapenem-hydrolyzing oxacillinases to carbapenem resistance in Acinetobacter baumannii. Antimicrob Agents Chemother. 2005;49(8):3198–3202. doi: 10.1128/AAC.49.8.3198-3202.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamidian M, Ambrose SJ, Hall RM. A large conjugative Acinetobacter baumannii plasmid carrying the sul2 sulphonamide and strAB streptomycin resistance genes. Plasmid. 2016;87(88):43–50. doi: 10.1016/j.plasmid.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 58.Hamidian M, Hall RM. pACICU2 is a conjugative plasmid of Acinetobacter carrying the aminoglycoside resistance transposon TnaphA6. J Antimicrob Chemother. 2014;69(4):1146–1148. doi: 10.1093/jac/dkt488. [DOI] [PubMed] [Google Scholar]
- 59.Nigro S, Hall RM. Distribution of the blaOXA-23-containing transposons Tn2006 and Tn2008 in Australian carbapenem-resistant Acinetobacter baumannii isolates. J Antimicrob Chemother. 2015;70(8):2409–2411. doi: 10.1093/jac/dkv102. [DOI] [PubMed] [Google Scholar]
- 60.Zong Z, Zhang X. BlaNDM-1-carrying Acinetobacter johnsonii detected in hospital sewage. J Antimicrob Chemother. 2013;68(5):1007–1010. doi: 10.1093/jac/dks505. [DOI] [PubMed] [Google Scholar]
- 61.Najar Peerayeh S, Karmostaji A. Molecular Identification of Resistance Determinants, Integrons and Genetic Relatedness of Extensively Drug Resistant Acinetobacter baumannii Isolated From Hospitals in Tehran, Iran. Jundishapur J Microbiol. 2015;8(7):e27021. doi: 10.5812/jjm.27021v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Azizi O, Shakibaie MR, Badmasti F, Modarresi F, Ramazanzadeh R, Mansouri S, et al. Class 1 integrons in non-clonal multidrugresistant Acinetobacter baumannii from Iran, description of the new blaIMP-55 allele in in1243. J Med Microbiol. 2016;65(9):928–936. doi: 10.1099/jmm.0.000315. [DOI] [PubMed] [Google Scholar]
- 63.Khorsi K, Messai Y, Hamidi M, Ammari H, Bakour R. High prevalence of multidrug-resistance in Acinetobacter baumannii and dissemination of carbapenemase-encoding genes blaOXA-23-like, blaOXA-24-like and blaNDM-1 in Algiers hospitals. Asian Pac J Trop Med. 2015;8(6):438–446. doi: 10.1016/j.apjtm.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 64.Mugnier PD, Poirel L, Naas T, Nordmann P. Worldwide dissemination of the blaOXA-23 Carbapenemase gene of Acinetobacter baumannii. Emerg Infect Dis. 2010;16(1):35–40. doi: 10.3201/eid1601.090852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Figueiredo DQ, Castro LFS, Santos KRN, Teixeira LM, De Mondlno SSB. Detecção de metalo-beta-lactamases em amostras hospitalares de Pseudomonas aeruginosa e Acinetobacter baumannii. J Bras Patol Med Lab. 2009;45(3):177–184. [Google Scholar]
- 66.Lucas DD, Crane B, Wright A, Han ML, Moffatt J, Bulach D, et al. Emergence of high-level colistin resistance in an Acinetobacter baumannii clinical isolate mediated by inactivation of the global regulator H-NS. Antimicrob Agents Chemother. 2018;62(7) doi: 10.1128/AAC.02442-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Henig O, Weber G, Hoshen MB, Paul M, German L, Neuberger A, et al. Risk factors for and impact of carbapenem-resistant Acinetobacter baumannii colonization and infection: matched case-control study. Eur J Clin Microbiol Infect Dis. 2015;34(10):2063–2068. doi: 10.1007/s10096-015-2452-4. [DOI] [PubMed] [Google Scholar]
- 68.Chusri S, Silpapojakul K, McNeil E, Singkhamanan K, Chongsuvivatwong V. Impact of antibiotic exposure on occurrence of nosocomial carbapenem-resistant Acinetobacter baumannii infection: a case control study. J Infect Chemother. 2015;21(2):90–95. doi: 10.1016/j.jiac.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 69.Da Rocha LDA, Vilela CAP, Cezário RC, Almeida AB, Gontijo P., Filho Ventilator-associated pneumonia in an adult clinical-surgical intensive care unit of a Brazilian university hospital: incidence, risk factors, etiology, and antibiotic resistance. Braz J Infect Dis. 2008;12(1):80–85. doi: 10.1590/s1413-86702008000100017. [DOI] [PubMed] [Google Scholar]
- 70.Brotfain E, Borer A, Koyfman L, Saidel-Odes L, Frenkel A, Gruenbaum SE, et al. Multidrug Resistance Acinetobacter Bacteremia Secondary to Ventilator-Associated Pneumonia: Risk Factors and Outcome. J Intensive Care Med. 2017;32(9):528–534. doi: 10.1177/0885066616632193. [DOI] [PubMed] [Google Scholar]
- 71.Ellis D, Cohen B, Liu J, Larson E. Risk factors for hospital-acquired antimicrobial-resistant infection caused by Acinetobacter baumannii. Antimicrob Resist Infect Control. 2015;4:40–40. doi: 10.1186/s13756-015-0083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moghnieh R, Siblani L, Ghadban D, El Mchad H, Zeineddine R, Abdallah D, et al. Extensively drug-resistant Acinetobacter baumannii in a Lebanese intensive care unit: risk factors for acquisition and determination of a colonization score. J Hosp Infect. 2016;92(1):47–53. doi: 10.1016/j.jhin.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 73.Blanco N, Harris AD, Rock C, Johnson JK, Pineles L, Bonomo RA, et al. Risk factors and outcomes associated with multidrug- resistant Acinetobacter baumannii upon intensive care unit admission. Antimicrob Agents Chemother. 2018;62(1):e01631-17. doi: 10.1128/AAC.01631-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo N, Xue W, Tang D, Ding J, Zhao B. Risk factors and outcomes of hospitalized patients with blood infections caused by multidrug-resistant Acinetobacter baumannii complex in a hospital of Northern China. Am J Infect Control. 2016;44(4):e37-9. doi: 10.1016/j.ajic.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 75.Nutman A, Glick R, Temkin E, Hoshen M, Edgar R, Braun T, et al. A case-control study to identify predictors of 14-day mortality following carbapenem-resistant Acinetobacter baumannii bacteraemia. Clin Microbiol Infect. 2014;20(12):1028–1034. doi: 10.1111/1469-0691.12716. [DOI] [PubMed] [Google Scholar]
- 76.Romanellia RMC, Anchieta LM, Mourão MVA, Campos FA, Loyola FC, Mourão PHO, et al. J Pediatr. 2. Vol. 89. Rio J: 2013. Risk factors and lethality of laboratory-confirmed bloodstream infection caused by non-skin contaminant pathogens in neonates; pp. 189–196. [DOI] [PubMed] [Google Scholar]
- 77.Wei H-M, Hsu Y-L, Lin H-C, Hsieh T-H, Yen T-Y, Lin H-C, et al. Multidrug-resistant Acinetobacter baumannii infection among neonates in a neonatal intensive care unit at a medical center in central Taiwan. J Micro Immun Infect. 2014;48(5):531–539. doi: 10.1016/j.jmii.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 78.Hosoglu S, Hascuhadar M, Yasar E, Uslu S, Aldudak B. Control of an Acinetobacter baumannii outbreak in a neonatal ICU without suspension of service: A devastating outbreak in Diyarbakir, Turkey. Infection. 2012;40(1):11–18. doi: 10.1007/s15010-011-0180-y. [DOI] [PubMed] [Google Scholar]
- 79.Zarrilli R, Di Popolo A, Bagattini M, Giannouli M, Martino D, Barchitta M, et al. Clonal spread and patient risk factors for acquisition of extensively drug-resistant Acinetobacter baumannii in a neonatal intensive care unit in Italy. J Hosp Infect. 2012;82(4):260–265. doi: 10.1016/j.jhin.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 80.De Oliveira CP, Atta EH, Da Silva ARA. J Pediatr. 5. Vol. 91. Rio J: 2015. Infection with multidrug-resistant gram-negative bacteria in a pediatric oncology intensive care unit: Risk factors and outcomes; pp. 435–441. [DOI] [PubMed] [Google Scholar]
- 81.Deng C, Li X, Zou Y, Wang J, Wang J, Namba F, et al. Risk factors and pathogen profile of ventilator-associated pneumonia in a neonatal intensive care unit in China. Pediatr Int. 2011;53(3):332–337. doi: 10.1111/j.1442-200X.2011.03382.x. [DOI] [PubMed] [Google Scholar]
- 82.Reddy D, Morrow BM, Argent AC. Acinetobacter baumannii infections in a South African paediatric intensive care unit. J Trop Pediatr. 2015;61(3):182–187. doi: 10.1093/tropej/fmv017. [DOI] [PubMed] [Google Scholar]
- 83.Kumar A, Randhawa VS, Nirupam N, Rai Y, Saili A. Risk factors for carbapenem-resistant Acinetobacter baumanii blood stream infections in a neonatal intensive care unit, Delhi, India. J Infect Dev Ctries. 2014;8(8):1049–1054. doi: 10.3855/jidc.4248. [DOI] [PubMed] [Google Scholar]
- 84.Thatrimontrichai A, Apisarnthanarak A, Chanvitan P, Janjindamai W, Dissaneevate S, Maneenil G. Risk factors and outcomes of carbapenem-resistant Acinetobacter baumannii bacteremia in neonatal intensive care unit: a case-case-control study. Pediatr Infect Dis J. 2013;32(2):140–145. doi: 10.1097/INF.0b013e318270b108. [DOI] [PubMed] [Google Scholar]
- 85.Tran HT, Doyle LW, Lee KJ, Dang NM, Graham SM. A high burden of late-onset sepsis among newborns admitted to the largest neonatal unit in central Vietnam. J Perinatol. 2015;35(10):846–851. doi: 10.1038/jp.2015.78. [DOI] [PubMed] [Google Scholar]
- 86.Hsu JF, Chu SM, Lien R, Chiu CH, Chiang MC, Fu RH, et al. Case-control analysis of endemic Acinetobacter baumannii bacteremia in the neonatal intensive care unit. Am J Infect Control. 2014;42(1):23–27. doi: 10.1016/j.ajic.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 87.Punpanich W, Nithitamsakun N, Treeratweeraphong V, Suntarattiwong P. Risk factors for carbapenem non-susceptibility and mortality in Acinetobacter baumannii bacteremia in children. Int J Infect Dis. 2012;16(11):811–815. doi: 10.1016/j.ijid.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 88.Maciel WG, da Silva KE, Croda J, Cayô R, Ramos AC, de Sales RO, et al. Clonal spread of carbapenem-resistant Acinetobacter baumannii in a neonatal intensive care unit. J Hosp Infect. 2017;98(3):300–304. doi: 10.1016/j.jhin.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 89.Kritsotakis EI, Kozhageldiyeva AG. A systematic review of the global seasonality of infections caused by Acinetobacter species in hospitalized patients. Clin Microb Infect. 2019;26(5):553–562. doi: 10.1016/j.cmi.2019.09.020. [DOI] [PubMed] [Google Scholar]
- 90.Williams VR, Callery S, Vearncombe M, Simor AE. The role of colonization pressure in nosocomial transmission of methicillin-resistant Staphylococcus aureus. Am J Infect Control. 2009;37(2):106–110. doi: 10.1016/j.ajic.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 91.Castelo-Branco FCM, Moreira FF, da Paz LG. Colonization pressure and risk factors for acquisition of imipenem-resistant Acinetobacter baumannii in a medical surgical intensive care unit in Brazil. Am J Infect Control. 2013;41(3):263–265. doi: 10.1016/j.ajic.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 92.Dalia-Costa LM, Coelho JM, Souza HAPHM, Castro MES, Stier CJN, Bragagnolo KL, et al. Outbreak of carbapenem-resistant Acinetobacter baumannii producing the OXA-23 enzyme in Curitiba, Brazil. J Clin Microbiol. 2003;41(7):3403–3406. doi: 10.1128/JCM.41.7.3403-3406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carvalho KR, Carvalho-Assef APDA, Peirano G, Santos LCG dos, Pereira MJF, Asensi MD. Dissemination of multidrug-resistant Acinetobacter baumannii genotypes carrying blaOXA-23 collected from hospitals in Rio de Janeiro, Brazil. Int J Antimicrob Agents. 2009;34(1):25–28. doi: 10.1016/j.ijantimicag.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 94.Castilho SRA, Godoy CSDM, Guilarde AO, Cardoso JL, André MCP, Junqueira-Kipnis AP, et al. Acinetobacter baumannii strains isolated from patients in intensive care units in Goiânia, Brazil: Molecular and drug susceptibility profiles. PLoS One. 2017;12(5):e0176790. doi: 10.1371/journal.pone.0176790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.de Souza Gusatti C, Bertholdo LM, Otton LM, Marchetti DP, Ferreira AE, Corção G. First occurrence of bla OXA-58 in Acinetobacter baumannii isolated from a clinical sample in Southern Brazil. Braz J Microbiol. 2012;43(1):243–246. doi: 10.1590/S1517-838220120001000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martins N, Martins IS, de Freitas WV, de Matos JA, Girão VBDC, Coelho-Souza T, et al. Imported and Intensive Care Unit-Born Acinetobacter baumannii Clonal Complexes: One-Year Prospective Cohort Study in Intensive Care Patients. Microb Drug Resist. 2013;19(3):216–223. doi: 10.1089/mdr.2012.0174. [DOI] [PubMed] [Google Scholar]
- 97.Leal NC, Campos TL, Rezende AM, Docena C, Mendes-Marques CL, de Sá Cavalcanti FL, et al. Comparative Genomics of Acinetobacter baumannii Clinical Strains From Brazil Reveals Polyclonal Dissemination and Selective Exchange of Mobile Genetic Elements Associated With Resistance Genes. Front Microbiol. 2020;11:1176–1176. doi: 10.3389/fmicb.2020.01176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rodríguez CH, Balderrama Yarhui N, Nastro M, Nuñez Quezada T, Castro Cañarte G, Ventura RM, et al. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii in South America. J Med Microbiol. 2016;65(10):1088–1091. doi: 10.1099/jmm.0.000328. [DOI] [PubMed] [Google Scholar]
- 99.MLST Pasteur Acinetobacter baumannii, MLST (Pasteur) database. 2016. [2020 Oct 15]. Available from: https://pubmlst.org/bigsdb?db=pubmlst_abaumannii_pasteur_seqdef.
- 100.Chagas TPG, Carvalho KR, de Oliveira Santos IC, Carvalho-Assef APDA, Asensi MD. Characterization of carbapenem-resistant Acinetobacter baumannii in Brazil (2008-2011): Countrywide spread of OXA-23-producing clones (CC15 and CC79) Diagn Microbiol Infect Dis. 2014;79(4):468–472. doi: 10.1016/j.diagmicrobio.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 101.Gales AC, Castanheira M, Jones RN, Sader HS. Antimicrobial resistance among Gram-negative bacilli isolated from Latin America: Results from SENTRY Antimicrobial Surveillance Program (Latin America, 2008-2010) Diagn Microbiol Infect Dis. 2012;73(4):354–360. doi: 10.1016/j.diagmicrobio.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 102.Almaghrabi MK, Joseph MRP, Assiry MM, Hamid ME. Multidrug-Resistant Acinetobacter baumannii: An Emerging Health Threat in Aseer Region, Kingdom of Saudi Arabia. Can J Infect Dis Med Microbiol. 2018;2018:1–4. doi: 10.1155/2018/9182747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Teo J, Lim TP, Hsu LY, Tan TY, Sasikala S, Hon PY, et al. Extensively drug-resistant Acinetobacter baumannii in a Thai hospital: A molecular epidemiologic analysis and identification of bactericidal Polymyxin B-based combinations. Antimicrob Resist Infect Control. 2015;4(1):2–2. doi: 10.1186/s13756-015-0043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Biglari S, Hanafiah A, Mohd Puzi S, Ramli R, Rahman M, Lopes BS. Antimicrobial Resistance Mechanisms and Genetic Diversity of Multidrug-Resistant Acinetobacter baumannii Isolated from a Teaching Hospital in Malaysia. Microb Drug Resist. 2017;23(5):545–555. doi: 10.1089/mdr.2016.0130. [DOI] [PubMed] [Google Scholar]
- 105.Camargo CH, Tiba MR, Saes MR, De Vasconcellos FM, Dos Santos LF, Romero EC, et al. Population structure analysis of carbapenem-resistant Acinetobacter baumannii clinical isolates from Brazil reveals predominance of clonal complexes 1, 15, and 79. Antimicrob Agents Chemother. 2016;60(4):2545–2547. doi: 10.1128/AAC.02186-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Royer S, Amaral de Campos P, Araújo BF, Ferreira ML, Gonçalves IR, William da Fonseca Batistão D, et al. Molecular characterization and clonal dynamics of nosocomial blaOXA-23producing XDR Acinetobacter baumannii. PLoS One. 2018;13(6):e0198643. doi: 10.1371/journal.pone.0198643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Asai S, Umezawa K, Iwashita H, Ohshima T, Ohashi M, Sasaki M, et al. An outbreak of blaOXA-51-like- and blaOXA-66- positive Acinetobacter baumannii ST208 in the emergency intensive care unit. Pt 11J Med Microbiol. 2014;63:1517–1523. doi: 10.1099/jmm.0.077503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Correa A, Del Campo R, Escandón-Vargas K, Perenguez M, Rodríguez-Banõs M, Hernández-Gómez C, et al. Distinct Genetic Diversity of Carbapenem-Resistant Acinetobacter baumannii from Colombian Hospitals. Microb Drug Resist. 2018;24(1):48–54. doi: 10.1089/mdr.2016.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Uzunoglu E, Direkel S, Kocbiyik M, Uludag SK, Cicek AC. Co-existance of isaba1/blaoxa-51/23 is increasing in carbapenem rersistant Acinetobacter baumannii isolates in Turkey. Acta Medica Mediterr. 2017;33(6):1001–1001. [Google Scholar]
- 110.Jaidane N, Naas T, Mansour W, Ben Radhia B, Jerbi S, Boujaafar N, et al. Genomic analysis of in vivo acquired resistance to colistin and rifampicin in Acinetobacter baumannii. Int J Antimicrob Agents. 2018;51(2):266–269. doi: 10.1016/j.ijantimicag.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 111.El Kettani A, Maaloum F, Diawara I, Katfy K, Harrar N, Zerouali K, et al. Prevalence of Acinetobacter baumannii bacteremia in intensive care units of ibn rochd university hospital, Casablanca. Iran J Microbiol. 2017;9(6):318–323. [PMC free article] [PubMed] [Google Scholar]
- 112.Dahdouh E, Gómez-Gil R, Pacho S, Mingorance J, Daoud Z, Suárez M. Clonality, virulence determinants, and profiles of resistance of clinical Acinetobacter baumannii isolates obtained from a Spanish hospital. PLoS One. 2017;12(4):e0176824. doi: 10.1371/journal.pone.0176824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lowe M, Ehlers MM, Ismail F, Peirano G, Becker PJ, Pitout JDD, et al. Acinetobacter baumannii: Epidemiological and beta-lactamase data from two tertiary academic hospitals in Tshwane, South Africa. Front Microbiol. 2018;9:1280–1280. doi: 10.3389/fmicb.2018.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mathlouthi N, Ben lamine Y, Somai R, Bouhalila-Besbes S, Bakour S, Rolain J-M, et al. Incidence of OXA-23 and OXA-58 Carbapenemases Coexpressed in Clinical Isolates of Acinetobacter baumannii in Tunisia. Microb Drug Resist. 2017;24(2):136–141. doi: 10.1089/mdr.2016.0306. [DOI] [PubMed] [Google Scholar]
- 115.Chmielarczyk A, Pilarczyk-Żurek M, Kamińska W, Pobiega M, Romaniszyn D, Ziółkowski G, et al. Molecular Epidemiology and Drug Resistance of Acinetobacter baumannii Isolated from Hospitals in Southern Poland: ICU as a Risk Factor for XDR Strains. Microb Drug Resist. 2016;22(4):328–335. doi: 10.1089/mdr.2015.0224. [DOI] [PubMed] [Google Scholar]
- 116.Katchanov J, Asar L, Klupp EM, Both A, Rothe C, König C, et al. Carbapenem-resistant Gram-negative pathogens in a German university medical center: Prevalence, clinical implications and the role of novel β-lactam/β-lactamase inhibitor combinations. PLoS One. 2018;13(4):e0195757. doi: 10.1371/journal.pone.0195757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hujer AM, Higgins PG, Rudin SD, Buser GL, Marshall SH, Xanthopoulou K, et al. Nosocomial outbreak of extensively drug-resistant Acinetobacter baumannii isolates containing blaOXA-237 carried on a plasmid. Antimicrob Agents Chemother. 2017;61(11) doi: 10.1128/AAC.00797-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sheck EA, Edelstein MV, Sukhorukova MV, Ivanchik NV, Skleenova EY, Dekhnich A V, et al. Epidemiology and genetic diversity of colistin nonsusceptible nosocomial Acinetobacter baumannii strains from Russia for 2013-2014. Can J Infect Dis Med Microbiol. 2017;2017:1839190–1839190. doi: 10.1155/2017/1839190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Karah N, Giske CG, Sundsfjord A, Samuelsen Ø. A Diversity of OXA-Carbapenemases and Class 1 Integrons Among Carbapenem-Resistant Acinetobacter baumannii Clinical Isolates from Sweden Belonging to Different International Clonal Lineages. Microb Drug Resist. 2011;17(4):545–549. doi: 10.1089/mdr.2011.0089. [DOI] [PubMed] [Google Scholar]
- 120.Grosso F, Quinteira S, Peixe L. Understanding the dynamics of imipenem-resistant Acinetobacter baumannii lineages within Portugal. Clin Microbiol Infect. 2011;17(8):1275–1279. doi: 10.1111/j.1469-0691.2011.03469.x. [DOI] [PubMed] [Google Scholar]
- 121.Banerjee T, Mishra A, Das A, Sharma S, Barman H, Yadav G. High Prevalence and Endemicity of Multidrug Resistant Acinetobacter spp. in Intensive Care Unit of a Tertiary Care Hospital, Varanasi, India. J Pathog. 2018;2018:9129083–9129083. doi: 10.1155/2018/9129083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Creighton J, Heffernan H, Howard J. Isolation of seven distinct carbapenemase-producing Gram-negative organisms from a single patient. J Antimicrob Chemother. 2017;72(1):317–319. doi: 10.1093/jac/dkw378. [DOI] [PubMed] [Google Scholar]
- 123.Handal R, Qunibi L, Sahouri I, Juhari M, Dawodi R, Marzouqa H, et al. Characterization of Carbapenem-Resistant Acinetobacter baumannii Strains Isolated from Hospitalized Patients in Palestine. Int J Microbiol. 2017;2017:8012104–8012104. doi: 10.1155/2017/8012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Abdulzahra AT, Khalil MAF, Elkhatib WF. First report of colistin resistance among carbapenem-resistant Acinetobacter baumannii isolates recovered from hospitalized patients in Egypt. New Microbes New Infect. 2018;26:53–58. doi: 10.1016/j.nmni.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Quiñones D, Carvajal I, Perez Y, Hart M, Perez J, Garcia S, et al. High prevalence of blaOXA-23in Acinetobacter spp. and detection of blaNDM-1in A.soli in Cuba: Report from National Surveillance Program (2010-2012) New Microbes New Infect. 2015;7:52–56. doi: 10.1016/j.nmni.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rodríguez CH, Nastro M, Fiorilli G, Dabos L, Calvo JL, Fariña ME, et al. Trends in the resistance profiles of Acinetobacter baumannii endemic clones in a university hospital of Argentina. J Chemother. 2016;28(1):25–27. doi: 10.1179/1973947814Y.0000000213. [DOI] [PubMed] [Google Scholar]
- 127.Opazo A, Bello H, Domínguez M, Lima CA, Opazo A, González-Rocha G, et al. First report of blaOXA-23in Acinetobacter baumannii isolates from Chilean hospitals. J Glob Antimicrob Resist. 2015;3(1):54–55. doi: 10.1016/j.jgar.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 128.Nuñez Quezada T, Rodríguez CH, Castro Cañarte G, Nastro M, Balderrama Yarhui N, Dabos L, et al. Outbreak of bla OXA-72-producing Acinetobacter baumannii in South America. J Chemother. 2017;29(5):321–324. doi: 10.1080/1120009X.2016.1158936. [DOI] [PubMed] [Google Scholar]
- 129.Saharman YR, Karuniawati A, Sedono R, Aditianingsih D, Sudarmono P, Goessens WH, et al. Endemic carbapenem-nonsusceptible Acinetobacter baumannii-calcoaceticus complex in intensive care units of the national referral hospital in Jakarta, Indonesia. Antimicrob Resist Infect Control. 2018;7(1):5–5. doi: 10.1186/s13756-017-0296-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cerezales M, Ocampo-Sosa AA, Montes LÁ, Ríos CD, Bustamante Z, Santos J, et al. prevalence of extensively drug-resistant Acinetobacter baumannii at a children hospital in Bolivia. Pediatr Infect Dis J. 2018;37(11):1118–1123. doi: 10.1097/INF.0000000000001962. [DOI] [PubMed] [Google Scholar]
- 131.Chen Y, Yang Y, Liu L, Qiu G, Han X, Tian S, et al. High prevalence and clonal dissemination of OXA-72-producing Acinetobacter baumannii in a Chinese hospital: a cross sectional study. BMC Infect Dis. 2018;18(1):491–491. doi: 10.1186/s12879-018-3359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shirmohammadlou N, Zeighami H, Haghi F, Kashefieh M. Resistance pattern and distribution of carbapenemase and antiseptic resistance genes among multidrug-resistant Acinetobacter baumannii isolated from intensive care unit patients. J Med Microbiol. 2018;67(10):1467–1473. doi: 10.1099/jmm.0.000826. [DOI] [PubMed] [Google Scholar]
- 133.Bado I, Papa-Ezdra R, Delgado-Blas JF, Gaudio M, Gutiérrez C, Cordeiro NF, et al. Molecular characterization of carbapenem-resistant Acinetobacter baumannii in the intensive care unit of Uruguay's University Hospital identifies the first rmtC gene in the species. Microb Drug Resist. 2018;24(7):1012–1019. doi: 10.1089/mdr.2017.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Al-Agamy MH, Khalaf NG, Tawfick MM, Shibl AM, El Kholy A. Molecular characterization of carbapenem-insensitive Acinetobacter baumannii in Egypt. Int J Infect Dis. 2014;22:49–54. doi: 10.1016/j.ijid.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 135.Kazi M, Nikam C, Shetty A, Rodrigues C. Dual‐tubed multiplex‐PCR for molecular characterization of carbapenemases isolated among Acinetobacter spp. and Pseudomonas spp. J Appl Microbiol. 2015;118(5):1096–1102. doi: 10.1111/jam.12770. [DOI] [PubMed] [Google Scholar]
- 136.Hasan B, Perveen K, Olsen B, Zahra R. Emergence of carbapenem-resistant Acinetobacter baumannii in hospitals in Pakistan. J Med Microbiol. 2014;63(1):50–55. doi: 10.1099/jmm.0.063925-0. [DOI] [PubMed] [Google Scholar]
- 137.Irfan S, Turton JF, Mehraj J, Siddiqui SZ, Haider S, Zafar A, et al. Molecular and epidemiological characterisation of clinical isolates of carbapenem-resistant Acinetobacter baumannii from public and private sector intensive care units in Karachi, Pakistan. J Hosp Infect. 2011;78(2):143–148. doi: 10.1016/j.jhin.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 138.Shrestha S, Tada T, Miyoshi-Akiyama T, Ohara H, Shimada K, Satou K, et al. Molecular epidemiology of multidrug-resistant Acinetobacter baumannii isolates in a university hospital in Nepal reveals the emergence of a novel epidemic clonal lineage. Int J Antimicrob Agents. 2015;46(5):526–531. doi: 10.1016/j.ijantimicag.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 139.Jamal S, Al Atrouni A, Rafei R, Dabboussi F, Hamze M, Osman M. Molecular mechanisms of antimicrobial resistance in Acinetobacter baumannii, with a special focus on its epidemiology in Lebanon. J Glob Antimicrob Resist. 2018;15:154–163. doi: 10.1016/j.jgar.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 140.Jung JY, Park MS, Kim SE, Park BH, Son JY, Kim EY, et al. Risk factors for multi-drug resistant Acinetobacter baumannii bacteremia in patients with colonization in the intensive care unit. BMC Infect Dis. 2010;10:228–228. doi: 10.1186/1471-2334-10-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jang TN, Lee SH, Huang CH, Lee CL, Chen WY. Risk factors and impact of nosocomial Acinetobacter baumannii bloodstream infections in the adult intensive care unit: a case-control study. J Hosp Infect. 2009;73(2):143–150. doi: 10.1016/j.jhin.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 142.Ye JJ, Huang CT, Shie SS, Huang PY, Su LH, Chiu CH. Multidrug resistant Acinetobacter baumannii: Risk factors for appearance of imipenem resistant strains on patients formerly with susceptible strains. PLOS ONE. 2010;5(4):e9947. doi: 10.1371/journal.pone.0009947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pagano M, Barin J, Martins AF, Zavascki AP. High Endemic Rates of OXA-23-Producing Carbapenem-Resistant Acinetobacter baumannii Isolates Caused by the Persistence of Major Clones in Hospitals in a Brazilian City 5 Years After an Outbreak. Infect Control Hosp Epidemiol. 2015;36(7):860–862. doi: 10.1017/ice.2015.52. [DOI] [PubMed] [Google Scholar]
- 144.Brito DVD, Brito CS de, Resende DS, Moreira do Ó J, Abdallah VOS, Gontijo PP., Filho Nosocomial infections in a Brazilian neonatal intensive care unit: a 4-year surveillance study. Rev Soc Bras Med Trop. 2010;43(6):633–637. doi: 10.1590/s0037-86822010000600006. [DOI] [PubMed] [Google Scholar]
- 145.Takagi EH, Lincopan N, Cassettari VC, Passadore LF, Mamizuka EM, Martinez MB. Carbapenem-resistant Acinetobacter baumannii outbreak at university hospital. Braz J Microbiol. 2009;40(2):339–341. doi: 10.1590/S1517-838220090002000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dai W, Huang S, Sun S, Cao J, Zhang L. Nosocomial spread of carbapenem-resistant Acinetobacter baumannii (types ST75 and ST137) carrying blaOXA-23-like gene with an upstream ISAba1 in a Chinese hospital. Infect Genet Evol. 2013;14:98–101. doi: 10.1016/j.meegid.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 147.Alyamani EJ, Khiyami MA, Booq RY, Alnafjan BM, Altammami MA, Bahwerth FS. Molecular characterization of extended-spectrum beta-lactamases (ESBLs) produced by clinical isolates of Acinetobacter baumannii in Saudi Arabia. Ann Clin Microbiol Antimicrob. 2015;14(1):38–38. doi: 10.1186/s12941-015-0098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Adams-Haduch JM, Onuoha EO, Bogdanovich T, Tian GB, Marschall J, Urban CM, et al. Molecular epidemiology of carbapenem-nonsusceptible Acinetobacter baumannii in the United States. J Clin Microbiol. 2011;49(11):3849–3854. doi: 10.1128/JCM.00619-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bocanegra-Ibarias P, Pena-López C, Camacho-Ortiz A, Llaca-Díaz J, Silva-Sánchez J, Barrios H, et al. Genetic characterisation of drug resistance and clonal dynamics of Acinetobacter baumannii in a hospital setting in Mexico. Int J Antimicrob Agents. 2015;45(3):309–313. doi: 10.1016/j.ijantimicag.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 150.Lowings M, Ehlers MM, Dreyer AW, Kock MM. High prevalence of oxacillinases in clinical multidrug-resistant Acinetobacter baumannii isolates from the Tshwane region, South Africa-an update. BMC Infect Dis. 2015;15(1):521–521. doi: 10.1186/s12879-015-1246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rynga D, Shariff M, Deb M. Phenotypic and molecular characterization of clinical isolates of Acinetobacter baumannii isolated from Delhi, India. Ann Clin Microbiol Antimicrob. 2015;14(1):40–40. doi: 10.1186/s12941-015-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Anh NT, Nga TV, Tuan HM, Tuan NS, Chau NV, Baker S, et al. Molecular epidemiology and antimicrobial resistance phenotypes of Acinetobacter baumannii isolated from patients in three hospitals in southern Vietnam. J Med Microbiol. 2017;66(1):46–53. doi: 10.1099/jmm.0.000418. [DOI] [PubMed] [Google Scholar]
- 153.Al-Hamad A, Pal T, Leskafi H, Abbas H, Hejles H, Alsubikhy F, et al. Molecular characterization of clinical and environmental carbapenem resistant Acinetobacter baumannii isolates in a hospital of the Eastern Region of Saudi Arabia. J Infect Public Health. 2020;13(4):632–636. doi: 10.1016/j.jiph.2019.08.013. [DOI] [PubMed] [Google Scholar]