Abstract
Background
At present, the relationships among COVID-19 disease progression, patient prognosis, and immune status are unclear. This single-center retrospective study evaluated the correlation between serum interleukin-6 (IL-6) levels at admission with the severity of COVID-19 pneumonia, as determined by admission to the intensive Care Unit (ICU).
Material/Methods
Patients admitted to The First Affiliated Hospital of Bengbu Medical College in Bengbu City, Anhui Province, China, in January and February 2020 for COVID-19 pneumonia were enrolled in this study. COVID-19 infection was confirmed by the detection of SARS-CoV-2 nucleic acid in throat swab samples using real-time fluorescent reverse transcription PCR. Serum IL-6 concentrations at admission were measured by ELISA. Correlations between serum IL-6 concentrations and ICU admission due to the development of severe COVID-19 pneumonia were evaluated.
Results
This study enrolled 68 patients with novel coronavirus pneumonia. IL-6 concentrations were significantly higher in patients with more severe than less severe COVID-19 pneumonia. Eight of 40 patients with severe COVID-19 pneumonia became critically ill and required ICU admission. IL-6 concentrations were significantly higher in patients with severe COVID-19 pneumonia who were than who were not treated in the ICU. The area under the receiver operating characteristic (ROC) curve (AUC) was 0.816 (P<0.01), indicating that IL-6 was prognostic of disease severity in patients with COVID-19 pneumonia.
Conclusions
Serum IL-6 concentration is closely associated with the severity of COVID-19. Continuous monitoring of IL-6 has clinical value in evaluating patient condition.
MeSH Keywords: COVID-19, Interleukin-6, Pneumonia, Severe Acute Respiratory Syndrome
Background
The novel coronavirus, named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) by the World Health Organization (WHO), was first observed in Wuhan, China, in December 2019 [1]. This virus belongs to the genus Betacoronavirus in the family Coronaviridae in the order Nidovirales. SARS-CoV-2 infection in humans causes immune system dysfunction, including the rapid release of multiple cytokines into body fluids, resulting in acute respiratory distress syndrome (ARDS) and multiple organ failure [2,3].
Coronavirus disease-2019 (COVID-19), which is caused by SARS-CoV-2 infection, has been clinically classified into 4 main categories: mild, common, severe, and critical [4,5]. Many patients with severe COVID-19 experience acute respiratory distress, whereas critically ill patients experience respiratory failure. Although most cases are mild to moderate and can heal spontaneously, some patients develop critical illness, characterized by respiratory dysfunction and/or multiple organ failure. Identifying patients at high risk of critical illness is important, as it can provide these patients with more active treatment and reduce morbidity and mortality rates.
At present, the relationships among COVID-19 disease progression, prognosis, and immune status are unclear, and no effectively predictive biomarkers have been proposed. The progression of novel coronavirus-associated pneumonia to a critical and life-threatening illness is thought to be associated with the cytokine storm, defined as the unregulated and excessive release of pro-inflammatory factors in the body [6,7]. The serum concentrations of interleukin-6 (IL-6), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF) [8–10], and other cytokines have been found to be significantly increased in patients with severe infections of viruses such as SARS-CoV and H1N1 influenza virus [11,12]. These inflammatory factors can recruit and activate immune cells in the lungs. Many immune system cells and tissue fluids accumulate in the lungs of these patients, blocking gas exchange between the alveoli and capillaries and leading to ARDS. Induction of a cytokine storm results in the immune system destroying large numbers of healthy lung cells while killing the virus, thus severely damaging the ventilation function of the lungs. This damage appears on computed tomography (CT) images of the lungs as large white areas, called “white lung” [13,14], and can result in respiratory failure and death.
IL-6 is an important multi-functional pro-inflammatory cytokine [15]. As an acute inflammatory factor, IL-6 mainly stimulates the proliferation and differentiation of cells involved in immune responses and improves their function, as well as participating in inflammatory and fever responses. Several retrospective studies have shown that increased levels of IL-6 are associated with death in patients with severe COVID-19 infection [16,17]. Elevated IL-6 is also associated with disease severity and course, suggesting that this cytokine may be a marker for disease monitoring in patients with severe COVID-19 [18]. Serum concentrations of IL-6 and C-reactive protein (CRP) can effectively assess disease severity, suggesting that IL-6 and CRP may be independent factors predictive of the severity of COVID-19 [19]. The present study evaluated whether serum concentration of IL-6 could predict critical illness in patients with severe COVID-19.
Material and Methods
Patient selection
This study included patients diagnosed with COVID-19 at the First Affiliated Hospital of Bengbu Medical College from January to February 2020. In all patients, infection with SARS-CoV-2 was confirmed by real-time fluorescent reverse transcription PCR detection of virus-specific nucleic acid in sputum and throat swab specimens, using commercially available kits specific for 2019-nCoV (Da An Gene Co., Ltd., Guangzhou, China) and an ABI 7500 thermal cycler.
The clinical severity of COVID-19 was defined according to the Chinese management guidelines for COVID-19 (version 6.0). Patients with general COVID-19 were defined as those with fever and respiratory and other symptoms, with possible pneumonia manifestations on imaging. Patients were categorized as having severe COVID 19 if they had (1) apparent shortness of breath and a respiratory rate ≥30 breaths/min, (2) oxygen saturation at rest <93%, or (3) arterial blood oxygen partial pressure (pO2)/oxygen concentration (FiO2) ≤300 mmHg (1 mmHg=133 Pa). At high altitudes (elevation above 1000 m), pO2/FiO2 was corrected using the formula: pO2/FiO2×[atmospheric pressure (mmHg)/760]. Patients were defined as critically ill if they required admission to the intensive care unit (ICU) for (1) respiratory failure requiring mechanical ventilation, (2) shock, or (3) failure of other organs.
Instruments and reagents
Serum concentrations of IL-6 were determined using IL-6 detection kits and the Cobas E601 electrochemiluminescence (ECL) analyzer (Roche, Basel, Switzerland). All reagents were within their period of validity, with quality control and calibration of these reagents meeting manufacturers’ requirements.
Methods
After a 12-h overnight fast, 2–3 mL of venous blood were drawn in the morning from patients with COVID-19 and healthy individuals and tested within 2 h. Blood samples were centrifuged at 4000 rpm for 5 min, and serum concentrations of IL-6 were measured using the ECL method and IL-6 detection kits. All tests were performed by skilled laboratory authorized personnel in accordance with the manufacturers’ instructions. The normal reference range for IL-6 was 0–7 pg/mL.
Statistical analysis
Normally distributed continuous data were expressed as mean±standard deviation (SD) and compared by t tests, whereas non-normally distributed continuous data were expressed as median (range) and compared by the Wilcoxon rank sum test. Categorical data were expressed as number (percentage) and compared by the chi square test. All statistical analyses were performed using SPSS 21.0 statistical software, with P<0.05 defined as statistically significant.
Results
Baseline characteristics
The study cohort consisted of 68 patients, 37 men and 31 women, ranging in age from 21 to 83 yr, (mean age 48.63±16.05 yr), who had been diagnosed with PCR-confirmed COVID-19. Of these 68 patients, 28 had general COVID-19 and 40 had severe COVID-19. Although there were no significant differences in gender, age, and percentages of patients with diabetes and coronary heart disease between these 2 groups, the percentage of patients with hypertension was significantly higher in patients with severe COVID-19 (P<0.05). In addition, serum IL-6 concentrations were significantly higher in patients with severe than with general COVID-19 (P<0.05; Table 1).
Table 1.
Baseline demographic and clinical characteristics of patients with coronavirus disease-2019 (COVID-19).
| No. (%) Total (N=68) |
Severe group | General group | p Value | |
|---|---|---|---|---|
| Sex | >0.05 | |||
| Female | 31 (45.6%) | 20 | 11 | |
| Male | 37 (54.4%) | 20 | 17 | |
| Age | 59.75±12.67 | 52.94±11.11 | >0.05 | |
| Comorbidities | ||||
| Diabetes | 2 (2.9%) | 1 | 1 | >0.05 |
| Hypertension | 14 (20.6%) | 9 | 5 | <0.05 |
| Cardiovascular disease | 1 (1.5%) | 1 | >0.05 | |
| Interleukin-6 | 45.66±72.35 | 10.63±22.63 | <0.01 |
Clinical characteristics of the progression from severe to critical illness
Of the 40 patients diagnosed with severe COVID-19 pneumonia, 8 developed critical illness after 2 to 7 days (Tables 2, 3). Analysis of IL-6 concentrations at admission and at the time of diagnosis with critical illness in these 8 patients showed that 7 of these patients had elevated IL-6 levels at the time of critical illness, with a maximum 23-fold increase. In contrast, the eighth patient had lower IL-6 levels at diagnosis of critical illness than at admission.
Table 2.
Changes in interleukin-6 (IL-6) levels and time to progression to critical illness in patients with severe coronavirus disease-2019 (COVID-19).
| Critically ill patient (No.) | IL-6 levels at admission | IL-6 levels in critical illness | Time of progression to critical illness |
|---|---|---|---|
| 1 | 218.80 | 919.60 | 4 |
| 2 | 292.16 | 2070.00 | 5 |
| 3 | 42.59 | 745.20 | 4 |
| 4 | 40.63 | 187.00 | 3 |
| 5 | 235.00 | 2742.00 | 6 |
| 6 | 75.17 | 1557.00 | 2 |
| 7 | 28.78 | 672.10 | 7 |
| 8 | 2.55 | 1.50 | 4 |
Table 3.
Baseline demographic and clinical characteristics of patients with severe coronavirus disease-2019 (COVID-19).
| No. (%) Total (N=40) |
Severe group A | General group B | p Value | |
|---|---|---|---|---|
| Sex | >0.05 | |||
| Female | 20 (50%) | 17 | 3 | |
| Male | 20 (50%) | 15 | 5 | |
| Age | 57.81±11.66 | 67.50±14.36 | <0.05 | |
| Comorbidities | ||||
| Diabetes | >0.05 | |||
| Hypertension | 1 (2.5%) | 1 | <0.05 | |
| Cardiovascular disease | ||||
| Interleukin-6 | 27.84±35.58 | 117.94±112.70 | <0.01 |
A comparison of the 8 severely ill patients who progressed to critical illness and the 32 severely ill patients who did not show further progression showed that sex distribution did not differ in these 2 groups (P>0.05), whereas older patients and those with hypertension were more likely to progress to critical illness (P<0.05). In addition, IL-6 concentrations were significantly higher in patients who did than did not progress to critical illness (P<0.01; Figure 1).
Figure 1.
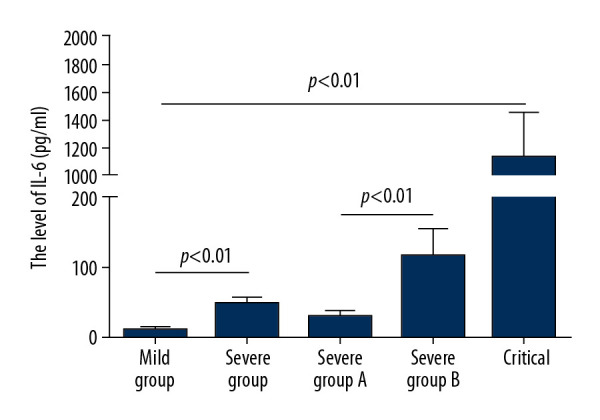
Serum interleukin-6 (IL-6) concentrations in patients with general, severe, severe but not critical, and critical coronavirus disease-2019 (COVID-19).
Analysis by receiver operating characteristic curve
Receiver operating characteristic (ROC) curve analysis was performed to identify the optimal IL-6 concentration predicting COVID-19 intensification. An area under the ROC curve (AUC) of 0.816 was found to predict whether COVID-19 is complicated by severe pneumonia (P<0.01). The optimum critical concentration of IL-6 in patients who progressed to critical illness was 24.3 pg/mL (Figure 2).
Figure 2.
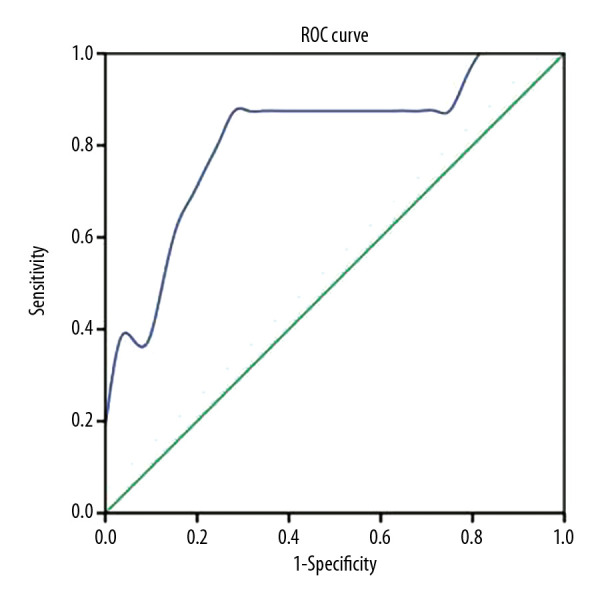
Receiver operating characteristic (ROC) curves of serum interleukin-6 (IL-6) concentrations in the prediction of critical illness in patients with coronavirus disease-2019 (COVID-19).
Discussion
To date, approximately 5 million persons worldwide have been diagnosed with novel coronavirus pneumonia, with a mortality rate over 6%. This high mortality rate has been closely associated with the induced inflammatory storm [20]. Following SARS-CoV-2 infection, the virus can enter immune cells in the lungs by binding to the angiotensin-converting enzyme-2 (ACE-2) receptor. This can result in the activation of lung immune cells, producing large amounts of inflammatory factors, which destroy lung tissue and induce respiratory failure [21,22].
IL-6 is a versatile cytokine with a wide range of functions essential for regulating immune and inflammatory responses and may therefore play a key role in the inflammatory storm [23]. During the acute phase of inflammation, IL-6 mainly stimulates the proliferation, differentiation, and functional improvement of cells involved in immune responses and participates in inflammatory and fever responses. In clinical settings, IL-6 has been used to diagnose infectious diseases, and it is highly significant in the differential diagnosis of bacterial and viral infections. Increased IL-6 is also a biomarker for the severity of hepatitis B virus (HBV) infection [24].
IL-6 may also play a key role in the development and progression of novel coronavirus pneumonia [19,25]. Circulating IL-6 concentrations have been closely associated with the clinical severity of COVID-19. For example, serum IL-6 levels were found to be significantly higher in severely ill patients than in those with mild symptoms [26], suggesting that IL-6 levels are closely associated with the occurrence of severe COVID-19 in adults and could predict the severity of illness in patients with COVID-19. IL-6 levels were also found to be significantly elevated in patients with respiratory insufficiency, suggesting that IL-6 plays an important role in lung injury due to SARS-CoV-2 infection [27]. Severe respiratory distress in patients with highly pathogenic SARS-CoV-2 was found to be caused by IL-6 elevation. Increased levels of IL-6 and CRP were found to be predictive of the need for mechanical ventilation, indicating that measurement of IL-6 can guide the escalation of treatment in patients with COVID-19 – related hyperinflammatory syndrome [28]. Taken together, these findings suggested that monitoring serum concentrations of IL-6 may be crucial for identifying disease progression in patients infected with SARS-CoV-2.
To further understand the relationship between IL-6 and the progression of novel coronavirus pneumonia, we retrospectively analyzed IL-6 levels in serum samples from 68 infected patients. We found that IL-6 levels were significantly higher in patients with severe than with mild COVID-19 symptoms. Of the 40 severely ill patients, 8 subsequently progressed to critically ill status and experienced respiratory failure. The IL-6 levels of these 8 patients were also significantly higher than those of the other 32 severely ill patients, with IL-6 level in 1 patient increasing 23-fold after progression to critical illness. In addition to confirming that IL-6 was closely associated with the severity of infection, the results suggest that IL-6 plays an important role in COVID-19-associated lung injury. During COVID-19 infection, IL-6 may rapidly activate pathogenic T cells, which produce GM-CSF, IL-6, and other factors. GM-CSF further activates CD14+ CD16+ inflammatory monocytes, producing higher quantities of IL-6 and forming a positive feedback loop, resulting in diffuse damage to alveolar and pulmonary capillary endothelial cells. The large accumulation of exudates can block the airways, leading to novel coronavirus pneumonia and ARDS [29]. Because IL-6 may play an important role in the progression of novel coronavirus pneumonia, drugs that target IL-6 receptors may show efficacy in the treatment of those who are severely ill.
IL-6 is closely associated with the degree of infection of SARS-CoV-2. We investigated whether monitoring IL-6 levels in severely ill patients with COVID-19 could predict the development of critical illness. ROC curve analysis indicated that IL-6 was a good predictor of the clinical severity of COVID-19. Serum IL-6 concentrations ≥24.3 pg/mL were associated with a greater likelihood of progression to critical illness status, indicating the need for more active interventions to prevent further deterioration to a life-threatening condition.
Conclusions
This study showed that serum IL-6 concentration was closely associated with the severity of COVID-19, and that a significant increase in IL-6 may indicate that the patient’s condition is worsening and becoming critical. IL-6 levels should be measured immediately after hospital admission of patients with COVID-19 and continuously monitored thereafter. IL-6 evidently has clinical value in evaluating patient condition and predicting its deterioration, indicating the need for active therapeutic measures.
Footnotes
Source of support: This work was supported by grants from the National Natural Science Foundation of China (81702076) and the Bengbu Medical Science and Technology Development Fund (BYKF1795)
References
- 1.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tu YF, Chien CS, Yarmishyn AA, et al. A review of SARS-CoV-2 and the ongoing clinical trials. Int J Mol Sci. 2020;21(7):2657. doi: 10.3390/ijms21072657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coronaviridae Study Group of the International Committee on Taxonomy of Viruss. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–44. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li K, Fang Y, Li W, et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020;30(8):4407–16. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang F, Shi S, Zhu J, et al. Clinical characteristics and outcomes of cancer patients with COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.25972. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Soy M, Keser G, Atagunduz P, et al. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol. 2020;39(7):2085–94. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jose RJ, Manuel A. COVID-19 cytokine storm: The interplay between inflammation and coagulation. Lancet Respir Med. 2020;8(6):e46–47. doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiter RJ, Sharma R, Ma Q, et al. Melatonin inhibits COVID-19-induced cytokine storm by reversing aerobic glycolysis in immune cells: A mechanistic analysis. Med Drug Discov. 2020;6:100044. doi: 10.1016/j.medidd.2020.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao M. Cytokine storm and immunomodulatory therapy in COVID-19: Role of chloroquine and anti-IL-6 monoclonal antibodies. Int J Antimicrob Agents. 2020;55(6):105982. doi: 10.1016/j.ijantimicag.2020.105982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnaldez FI, O’Day SJ, Drake CG, et al. The Society for Immunotherapy of Cancer perspective on regulation of interleukin-6 signaling in COVID-19-related systemic inflammatory response. J Immunother Cancer. 2020;8(1):e000930. doi: 10.1136/jitc-2020-000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lau SKP, Lau CCY, Chan KH, et al. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: Implications for pathogenesis and treatment. J Gen Virol. 2013;94(Pt 12):2679–90. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- 12.Guo X, Zhu Z, Zhang W, et al. Nuclear translocation of HIF-1α induced by influenza A (H1N1) infection is critical to the production of proinflammatory cytokines. Emerg Microbes Infect. 2017;6(5):e39. doi: 10.1038/emi.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yasukawa K, Minami T. Point-of-care lung ultrasound findings in patients with COVID-19 pneumonia. Am J Trop Med and Hyg. 2020;102(6):1198–202. doi: 10.4269/ajtmh.20-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui N, Zou X, Xu L. Preliminary CT findings of coronavirus disease 2019 (COVID-19) Clin Imaging. 2020;65:124–32. doi: 10.1016/j.clinimag.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ulhaq ZS, Soraya GV. Interleukin-6 as a potential biomarker of COVID-19 progression. Med Maladies Infect. 2020;50(4):382–83. doi: 10.1016/j.medmal.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang C, Wu Z, Li JW, et al. Cytokine release syndrome in severe COVID-19: Interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5):105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu T, Zhang J, Yang Y, et al. The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol Med. 2020;12(7):e12421. doi: 10.15252/emmm.202012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. 2020;80(6):607–13. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol. 2020;318(5):H1084–90. doi: 10.1152/ajpheart.00217.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol. 2020;92(7):726–30. doi: 10.1002/jmv.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu Z, Cai T, Fan L, et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int J Infect Dis. 2020;95:332–39. doi: 10.1016/j.ijid.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tangkijvanich P, Vimolket T, Theamboonlers A, et al. Serum interleukin-6 and interferon-gamma levels in patients with hepatitis B-associated chronic liver disease. Asian Pac J Allergy Immunol. 2000;18(2):109–14. [PubMed] [Google Scholar]
- 25.Chen X, Zhao B, Qu Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa449. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao Y, Li T, Han M, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol. 2020;92(7):791–96. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen XY, Yan BX, Man XY. TNFα inhibitor may be effective for severe COVID-19: Learning from toxic epidermal necrolysis. Ther Adv Respir Dis. 2020;14:1753466620926800. doi: 10.1177/1753466620926800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146(1):128–36.e4. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2(−) mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2):216–28. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]


