ABSTRACT
The development, clinical advancement and licensure of vaccines, and monitoring of vaccine effectiveness could be expedited and simplified by the ability to measure immunological endpoints that can predict a favorable clinical outcome. Antigen-specific and functional antibodies have been described in the context of naturally acquired immunity and vaccination against Shigella, and their presence in serum has been associated with reduced risk of disease in human subjects. The relevance of these antibodies as correlates of protective immunity, their mechanistic contribution to protection (e.g. target antigens, interference with pathogenesis, and participation in microbial clearance), and factors that influence their magnitude and makeup (e.g. host age, health condition, and environment) are important considerations that need to be explored. In addition to facilitating vaccine evaluation, immunological correlates of protection could be useful for identifying groups at risk and advancing immune therapies. Herein we discuss the precedent and value of functional antibodies as immunological endpoints to predict vaccine efficacy and the relevance of functional antibody activity to evaluate protective immunity against shigellosis.
KEYWORDS: Functional antibodies, correlates of protection, threshold of protection, Shigella, protective immunity, trials for developing countries, vaccinology
Shigella disease burden and the need for a vaccine
Diarrheal diseases are the second leading cause of death worldwide, resulting in the loss of about 500,000 children’s lives under the age of 5 per year, primarily in the developing world.1,2 The contribution of Shigella to the global burden of diarrheal morbidity and mortality in this age group has been emphasized by estimates from the Global Enteric Multicenter Study (GEMS),3 the Malnutrition and Enteric Disease (MAL-ED) birth cohort study,4 and the Global Burden of Disease, Injuries, and Risk Factors Study (GBD 2016).5 Frequent and repeated episodes of diarrhea in children living in these resource-poor areas have been associated not only with increased mortality but also with severe and lifelong health impairment, which compromises growth and cognitive ability.6,7 Current prophylaxis to treat acute diarrheal episodes includes oral rehydration and antibiotics. While improved sanitation and access to clean water are certainly effective primary control measures, vaccination might be a more rapid and efficient preventive intervention. The success of this approach is exemplified by the rotavirus vaccine introduction, which resulted in substantial reduction in diarrhea-related hospital visits, admissions8-10 and death9,11-13 in children less than 5 years of age globally. Shigella has also been associated with diarrhea across all adult age groups, with increased incidence in the elderly.5 Shigella and Enterotoxigenic Escherichia coli (ETEC) are the main causes of diarrhea in travelers and military personnel. The development and clinical advancement of a Shigella vaccine are seen as an equitable, cost-effective prevention tool that could be deployed with existing vaccination programs for effective disease control.14
Naturally acquired immunity against shigellosis and the relevance of antibodies
It is known from observational epidemiological and human challenge studies that subjects repeatedly infected with Shigella acquire a natural immunity that prevents or reduces illness following subsequent infection.15-17 In endemic regions, disease incidence diminishes in older children and adults, and pathogen-specific host defenses, lacking in early infancy, progressively increase with age.17-19 This naturally acquired protective immunity tends to be serotype-specific and directed to the O-polysaccharide (OPS).15,16 Early studies involving Israeli soldiers reported associations between LPS-specific serum IgG and reduced illness during Shigella outbreaks.15,16,20 On the basis of this premise, OPS is the main antigen targeted by current vaccine approaches. Serum antibodies against plasmid-encoded antigens have been detected in individuals living in Shigella-endemic regions; these antibodies increase during convalescence and have been implicated in protective immunity.18,19,21 The magnitude of serum antibodies specific for virulence factors such as the invasion plasmid antigen (Ipa) proteins A, B, and C, and VirG at the time of challenge was found to be associated with reduced incidence of disease in US adult volunteers in a controlled infection study.22,23 Similarly, the frequency of IpaB-specific memory B cells in these individuals was linked to lower risk of infection.24 In addition to serum antibodies, antibody-secreting cells (ASC) and antibody in lymphocyte supernatants (ALS) measured in circulating peripheral blood mononuclear cells (PBMC) 7–10 days following exposure to Shigella are used as indicators of robust mucosal immunity.25,26
Vaccine-induced immunity and the relevance of antibodies
There is no approved vaccine to prevent shigellosis. A variety of Shigella vaccine concepts have been proposed and tested mainly for safety and immunogenicity in clinical studies (Reviewed in ref.27). Modeling natural infection, oral Shigella vaccines are among the leading candidates tested in human clinical trials during the past decade. These include the whole cell inactivated S. flexneri 2a Sf2aWC,28 and the live attenuated S. flexneri 2a CVD 1208S,29 S. flexneri 2a SC602,30 and S. sonnei WRSs2 and WRSs3.31 Orally delivered whole cell organisms generally elicit high levels of LPS-specific serum IgG and IgA as well as fecal IgA, and are potent inducers of LPS-specific IgA ASC. Shigella OPS-protein conjugate vaccines have gained interest and support in recent years due to the record of safety and ability to elicit potent systemic immunity. The concept of a Shigella conjugate vaccine was pioneered by John Robbins and colleagues at the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) in the early 1990s. A candidate vaccine produced by this group consisting of OPS from S. sonnei covalently bound to recombinant exoprotein A of Pseudomonas aeruginosa (rEPA) had an efficacy of about 70% in young adults in the Israeli military32 and in 3–4-year-old children in a Phase 3 clinical trial.33 Protection was associated with the presence of high levels of serum IgG against the O-antigen. Unfortunately, the vaccine had no effect in 1–2-year olds, who would be primary targets for immunization. It remains unclear whether the protection seen in adults and in older children derived solely from vaccination or from boosting of pre-existing immunity acquired through natural exposure. A newer Shigella conjugate, Flexyn2a, was engineered based on the same principle, except this time using an elegant and simpler in vivo bioconjugation of the S. flexneri 2a OPS to rEPA. Flexyn2a elicited strong serum antibody and ALS responses in young adult volunteers, and up to 50% protection against severe shigellosis in an experimental human challenge.34,35 Another glycoconjugate vaccine candidate, SF2a-TT15, featuring repeating units of synthetic O-antigen from S. flexneri 2a conjugated to tetanus toxoid, has been shown to be safe and strongly immunogenic in a Phase 1 study.36 An immunogenicity and efficacy study of SF2a-TT15 is ongoing at the University of Maryland in collaboration with Institut Pasteur, University of Tel Aviv, and The Walter Reed Army Institute of Research (WRAIR).
Shigella vaccine candidates that leverage the potential to offer broad protection have also advanced into clinical evaluation. One promising strategy is the Generalized Modules for Membrane Antigen (GMMA), which contain the O-antigen (and can be engineered to express O-antigen from multiple Shigella serotypes) plus outer membrane and periplasmic proteins. The S. sonnei prototype, GSK 1790GAHB, has been shown to be safe and immunogenic in clinical trials in naïve adult populations from France and the United Kingdom37 and in adults living in Kenya, where Shigella is endemic.38 The efficacy of this vaccine is under evaluation in a controlled human challenge study in adult volunteers sponsored by GlaxoSmithKline (NCT035227173). Another example is the macromolecular complex Invaplex, which consists of the native lipopolysaccharide (LPS) and invasion plasmid antigen (Ipa) proteins B and C. The broad spectrum of this vaccine relies on immunity against the Ipa proteins, which are highly conserved among Shigella serotypes. Invaplex has been found to be safe and immunogenic in humans.39 A Phase 1 dose-escalating study of detoxified S. flexneri 2a artificial complex (Invaplex AR-DETOX) administered intramuscularly to adult volunteers is being conducted at WRAIR (NCT03869333).
The inflow of new vaccine concepts and clinical studies, including refined, controlled human infection models (CHIM), and the strategic support of stakeholders have created unprecedented momentum, making this an exciting and hopeful time for a Shigella vaccine to finally materialize.40,41 The upcoming vaccine and challenge studies, in particular, offer an extraordinary opportunity to dissect host responses and mechanisms of protective immunity in the context of different immunization strategies. Specific ways in which antibodies deploy anti-microbial function to prevent Shigella infection as well as the potential of functional antibodies to predict protective immunity against shigellosis are discussed below.
Functional antibodies as immunological correlates of protection
Understanding the mechanisms harnessed by the host immune system to prevent infection and avert pathogenicity is essential to identify correlates of protective immunity.42 Establishing the antimicrobial capacity of vaccine-induced antibodies is important to confirm that these antibodies can operate in such a manner in vivo. Impairment of antibody function (e.g. due to age, immune adaptation, underlying health conditions, or vaccine failure) cannot be discerned by traditional serological methods (such as ELISA) that rely only on antibody binding to an immobilized antigen. In contrast, functional assays appraise not only the magnitude of an antibody response but also the operative anti-microbial activity. A functional outcome may reflect the contribution of multiple antibody specificities as opposed to antibody titers directed to a single, independent antigen, and therefore may be more informative of the breadth of host immunity, and potentially a more robust tool to predict vaccine-induced protection. The production of functional antibodies requires, and therefore indirectly reflects, strong underlying T-cell immunity, which is more difficult to determine and is an impractical primary/secondary clinical trial endpoint.
There are multiple examples for the use of functional antibodies as immune correlates of protection against mucosal bacterial pathogens. Based on evidence that clearance of Streptococcus pneumoniae in the lungs is primarily achieved by opsonophagocytosis,43 opsonophagocytic antibodies have been the primary immunogenicity readout of pneumococcal polysaccharide vaccines.44,45 Bactericidal antibody activity has been associated with protection against meningococcal infection.46,47 Vibriocidal antibodies are indicators of protective immunity against Vibrio cholerae.48,49 These correlates have helped advance vaccine candidates through the regulatory pathway. The assessment of functional antibody responses post-vaccination, when assays are available, is recommended by the World Health Organization (WHO) Expert Committee on Biological Standardization.50 The following sections discuss the relevance of functional antibody to Shigella immunity.
Antibody function relevant to Shigella immunity
Classical studies demonstrated the capacity of serum antibodies from patients with shigellosis to activate complement and promote bactericidal activity.51-53 Immune sera from convalescent individuals were reported to mediate phagocytosis by K-lymphocytes, monocytes, and granulocytes present in circulation.53-55 Bactericidal antibody activity against endemic Shigella strains has been demonstrated in the context of natural infection in adults living in Israel32 and Bangladesh.53,56 Our group reported associations between elevated serum bactericidal antibody (SBA) and opsonophagocytic killing antibody (OPKA) titers at the time of challenge and protection from moderate to severe disease in a challenge study of North American volunteers.22 Shigella SBA activity has been attributed primarily to antibodies specific for the OPS,57 although this may not be the only target of antibody and complement-mediated killing.22 That may also be the case for antibodies mediating opsonophagocytic killing, since both SBA and OPKA tend to follow the same response pattern.22 Adult volunteers immunized with the conjugate vaccine candidates SF2a-TT15 and Flexyn2a had robust SBA titers34,36 and, incidentally, Flexyn2a had >50% efficacy in a subsequent challenge study.35 Detailed analysis of antibody function and clinical outcomes emerging from these and other well-controlled CHIM studies will provide critical insights into the value of functional serological readouts as predictors of vaccine efficacy.
Given that Shigella is an enteric pathogen, protective immune effectors likely operate within the gut. Antibodies, as primary humoral effector molecules, may be produced locally or transported via circulation. We reported the presence of bactericidal and opsonophagocytic killing antibody activity in ALS from human volunteers immunized orally with whole cell inactivated and live attenuated Shigella vaccines.58 ASC are considered robust markers of Shigella-specific B cells (plasmablasts) that originate in and migrate back to the gut,59 and ALS represent the antibodies produced in vitro by transiently circulating ASC.26 It is therefore plausible that antibody-mediated, complement-dependent killing may represent a local (gut mucosal) mechanism of bacterial clearance. Such function would have to be mediated by IgG (or IgM), since IgA does not fix complement. An OPS IgG-mediated local bactericidal and phagocytic killing could explain the protective capacity of the Shigella conjugate vaccine approach. In the early 1990s, John Robbins proposed that IgG could transudate into the intestinal lumen and kill Shigella through complement-mediated lysis at the epithelial cell surface.60,61 We would argue that a combination of vaccine-induced IgG as well as IgM produced shortly after infection (both potent opsonin and complement fixing antibodies) are likely responsible for antibody-mediated protection against Shigella in vivo.
An important and often overlooked study conducted by Carol Tacket in the early 1990s showed that oral administration of bovine colostrum concentrate containing high levels of Shigella LPS-specific immunoglobulin protected adult volunteers against shigellosis, thus providing direct in vivo evidence of IgG functional activity in the gut.62 We have detected LPS-IgG and antibody-mediated complement-dependent Shigella-cidal activity in maternal milk from mothers living in Malawi.63 Together, these observations support a protective role of IgG (and possibly IgM) in mucosal secretions.
What then would be the role for IgA, and is it dispensable in Shigella immunity? A combination of protective effects has been attributed to LPS-specific agglutinating secretory IgA (SIgA), including reduced bacterial attachment to intestinal epithelial cell monolayers, maintenance of tight junction and cell morphology, and inhibition of pro-inflammatory pathways – all in the absence of other immune components.64 Intestinal IgA produced by oral vaccines65 may be an adjunct mode of bacterial immobilization and exclusion that helps preserve barrier integrity.
IgA against Shigella LPS and Ipa proteins have been found in breast milk.66-68 The formation of immune complexes could, likewise, be an effective mechanism of protection in the gut of the breastfeeding infant. The interaction between antibodies and cells in breast milk is an important area that deserves investigation. One study reported that leukocytes isolated from colostrum of healthy mothers and used as effector cells in phagocytic/bactericidal assays were able to kill Shigella in the presence of antibodies.69
Antibodies targeting Shigella antigens other than LPS likely deploy additional effector functions. Antibodies directed to the Ipa proteins would be expected to inhibit TTSS protein translocation70 and prevent invasion of epithelial cells.71 We have shown that combined murine IpaB and IpaD antibodies did not exhibit complement-mediated killing activity, but they inhibited Shigella-induced macrophage toxicity.72 Antibodies against VirG (IcsA) could be important for inhibition of bacterial adhesion73 or cell-to-cell spread.74 Blocking Shigella invasion in this way conceivably averts the release of proinflammatory cytokines and the recruitment of neutrophils, which exacerbates the damage of the intestinal epithelium. The exact operative mechanisms of antibodies against the multiple Shigella antigens (antibody type, interacting cells, and other contributing molecules) and their contribution to protection in vivo remain to be elucidated.
Functional antibodies as immunological correlates of protection against shigellosis and protective thresholds
From the perspective of vaccine development, evaluation and licensure, it would be important and advantageous to identify an immunological endpoint or immune signature that correlates with clinical protection against shigellosis. These immune effectors might be mediators of protection themselves or indirectly reflect other components or determinants of protective immunity.
An immune correlate of protection requires a well-defined cutoff value, or threshold, that could be used to reliably infer vaccine effectiveness, alleviating the need for human challenge or efficacy studies.75 Ideally, a correlate of protection should be established as early as possible in the process of vaccine development. Such a threshold would allow differentiating individuals with adequate immunity from those at risk of contracting disease. It may be an absolute number above which protection is denoted, or relative, meaning that protection increases with higher threshold values.76 As an example, to evaluate group B meningococcal vaccines with an SBA assay that employs baby rabbit complement, a titer ≥1:8 was established as a protective threshold.46 In the case of S. pneumoniae, establishment of an opsonophagocytic threshold titer of ≥1:8 allows the use of non-inferiority trials to assess subsequent vaccines, and was the basis for the licensure of the 13-valent pneumococcal conjugate vaccine (PCV-13).77
Important aspects in the definition of a protective threshold include: differences in populations, age dependency, and vaccine compositions.
Differences in population and pre-existing immunity
Shigella vaccine efficacy traditionally studied in industrialized nations cannot be extrapolated to other geographical settings. Individuals living in endemic regions and repeatedly infected with Shigella acquire natural immunity that includes high levels of antibodies (Figure 1(a)) as well as mucosal and systemic T and B cell-mediated immunity. An initial infection prompts an IgM and modest IgG response, while subsequent infections trigger recall immunity with rapid increases in serum IgG in repeating cycles, as illustrated in Figure 1(a). An SBA response originally mediated by IgM is later supplemented and eventually replaced by an overwhelming IgG-mediated bactericidal activity, as has been shown for V. cholerae.78 In agreement with this model, an increase in the length of exposure to Shigella has been shown to improve protection in field settings.79
Figure 1.
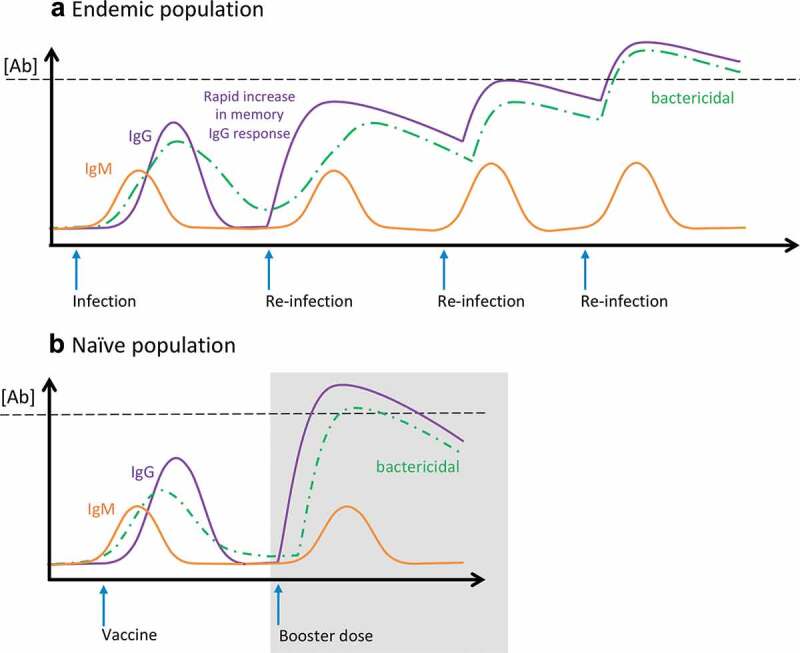
Protective immunity and threshold of protection. Schematic representation of IgM, IgG, and serum bactericidal antibody responses measured following (a) infection and multiple re-infections in an endemic population, and (b) initial vaccination and booster dose administered to a naïve population. Black dashed line represents threshold of protection.
In contrast, populations living in industrialized regions (North America or Europe) are immunologically naïve and highly susceptible to infection (Figure 1(b)).38,80 These individuals typically respond vigorously to vaccine candidates. Ideally, a safe and effective vaccine given to naïve individuals should induce a strong and effective IgG immune response resulting in serum antibody levels above a protective threshold and comparable to, or better than, those found in naturally immune individuals with few required boosters (Figure 1(a,b)). Unfortunately, this was not achieved with previous oral candidates for which immunogenicity and efficacy in an industrialized region could not be reproduced in endemic areas.30,81 The microbiome or other pathogens within the gut microenvironment in these settings might have played a role in diminishing the immune response. The gut microbiome and enteric host factors that may directly affect oral immunization are not expected to influence immune responses induced by the new parenteral OPS conjugate formulations.
Another important consideration in the evaluation of Shigella vaccines, inherent to specific populations, is the blunting of responses by preexisting immunity. This is a complex phenomenon that affects not only responses to Shigella but to other vaccines as well (i.e. measles, pertussis). A variety of possible mechanisms (antibody level, type of antigen administered, B cell receptor interaction, etc.) have been proposed.82 How exactly and why pre-existing immunity precludes further Shigella vaccine responses is not well understood. In recent clinical studies, volunteers have been screened prior to enrollment to exclude those with high levels of serum LPS-IgG, the rationale being that this scenario better represents non-immune vaccine target groups (i.e. young children). Interestingly, immune blunting does not appear to affect in the same way (or to the same extent) individuals from endemic areas who experience multiple cycles of infection that gradually boost immunity (Figure 1(a)). One possibility is the higher stringency of infection compared to vaccination, and the fact that (a more potent) infection may override effectors or responses that prevent vaccine-induced immune activation. For potent vaccines, this may not be an issue. The type of vaccine and route of administration may also influence the outcome. Understanding the influence of pre-existing immunity in immunological priming against Shigella will be critical for the success of any vaccine. The effects of prior immunological exposure on vaccine take (when immune blunting occurs, why it occurs, and who is affected) is therefore a fundamental topic that warrants further investigation.
Differences in age groups
Host susceptibility, immune development, and competence are different for children and adults. Therefore, antibody effector functions and even threshold levels that determine protective immunity in adults may not be the same as those required in young children. Infants and toddlers up to about 2 years of age are more susceptible to Shigella than older children and adults17 and their responses to vaccines are also lower than that of adults.33
Back-tracking to early infancy, Shigella-specific antibodies seem to be efficiently transferred from mothers to infants, as shown by the levels detected in cord blood at birth.83 However, the functionality of these antibodies remains to be investigated. In a study of maternal-infant transfer of functional antibodies against E. coli and Salmonella, Gitlin and colleagues found that unlike the paired maternal sera, most of the cord blood antibodies lacked bactericidal antibody activity – this functionality was contained primarily in the maternal IgM fraction and therefore not transferred through the placenta.84 For newborns who did exhibit serum bactericidal activity, this was found to be IgG-specific and correlated with IgG-mediated functional antibody activity in maternal blood.84 Systemic placentally acquired antibodies wean at about 4 months of age at which time infants rely on passive protection through immune components in maternal milk. Vaccination within this time frame would have the highest impact in preventing disease. The efficacy of this approach would have to be answered by studies in the field.
Because young children lack other potentially enhancing mechanisms such as robust T and B cells, innate immunity, and cross-reactive adaptive immunity, more stringent requirements than those defined for adults may be in order. This has been proposed for seasonal influenza: while a hemagglutination inhibition (HAI) titer of >40 appears sufficient to protect adults against seasonal influenza, young (unprimed) children may require an HAI titer of >330.85,86 Establishing what constitutes protective immunity against shigellosis in young children and identifying thresholds of protection for vaccine target age groups are challenges for the immediate future.
Vaccine composition
Because of the differences in vaccine platforms, antigenic composition, and/or mode of delivery, an immune correlate of protection identified for one vaccine may not be applicable to another. For example, SBA and OPKA may be appropriate to assess OPS-based vaccine efficacy but would not be suitable for protein-based subunits. Mucosal responses induced by oral vaccines (ASC, ALS, fecal antibodies) may not be relevant to conjugate vaccines given parenterally. Different functionality, antibody specificity or even type of immunological readout may be necessary to properly assess some of the new vaccine concepts. A combination of end-points reflecting the contribution of multiple antigens may be necessary to accommodate multivalent (e.g. broadly protective) formulations.
Composite antibody measurements and immune signatures
Instead of a single readout, establishing an immune correlate comprised of an aggregate (or composite) of antibody measurements would be a more robust approach to define protective status. Such composite could include serum antibodies (levels, function, and avidity) and/or mucosal antibodies (ALS levels, function, and avidity). A recent microarray analysis identified the Shigella conserved proteins IpaA, IpaB, and IpaC as an immune signature associated with protection from severe disease.23 Mathematical models87 and machine learning88 tools have the potential to probe complex datasets consisting of multiple parameters across multiple studies to predict measurements that correlate with protective immunity. However, the value of these models is dependent on the breadth and quality of data available. The input from upcoming prospective CHIM studies can break new ground in the field. Systems serology, a new technology that combines the analysis of biophysical features of antibodies and their broad functional capacity using bioinformatic analyses, would be useful to investigate and establish antibody features and novel cell-associated activities relevant for protection against shigellosis. This method was used to interrogate and compare Fc-mediated immune responses from multiple vaccine trials; it allowed successful identification of an “Fc fingerprint” associated with protective immunity against HIV,89 and discrimination of antibody function in latent versus active TB.90
Going forward
What constitutes an effective (protective) response against shigellosis through a “serological lens” remains to be defined. A careful serological analysis of sick versus healthy individuals during case-control and/or household contact studies could yield threshold values to distinguish immune from at-risk individuals. Thresholds of protective immunity can also be established through CHIM. This method, however, traditionally involves a different (non-target) population and an artificial setting (dose, interval between vaccination and challenge, administration of antibiotics post-challenge). Mechanistic analyses are necessary to delineate the contribution of antibodies in promoting bacterial clearance and preventing Shigella infection in the gut mucosa. It will be important to know the antigenic targets and type of antibodies (i.e. IgG subclasses) involved, the intrinsic properties of functional antibodies (e.g. structure, glycan profile), and their interactions with innate immune cells and other host cells. Investigating the capacity of antibodies to interfere with different steps of Shigella pathogenesis and methods that can reliably quantify such antibodies will expand the assay repertoire to facilitate vaccine development and evaluation. Selection, optimization, and harmonization of assays will enable comparative analysis of results derived from multiple studies. The identification and utilization of calibrated reagents (i.e. antigens, standard sera) would be necessary to define and implement a reliable protective threshold or serological cutoff.91 High throughput technologies, including automation, multiplexing,44 and luminescence-based assays92 will facilitate and simplify the implementation of functional assays. A recently reported S. flexneri 2a, S. flexneri 3a, and S. sonnei SBA qualification and demonstration of interlaboratory agreement is a step towards wider adoption of functional assays.93 A consortium sponsored by Bill and Melinda Gates Foundation and coordinated by the National Institute for Biological Standards and Control (NIBSC), with the participation of WRAIR, University of Maryland, University of Tel Aviv, GSK Vaccines Institute for Global Health (GVGH), and Johns Hopkins University is working toward a harmonized LPS IgG assay and the creation of a standard control. Similar efforts would facilitate rigorous and standardized measurement of functional antibodies.
Concluding remarks
There is precedent for the use of functional antibodies as correlates of protection and promise for their use as predictors of protective immunity to Shigella infection. The induction of high levels of antibodies with a defined anti-bacterial effect is indicative of more complex and robust responses that involve T cell activation and B cell expansion in response to infection and vaccination. Functional antibody assays can be performed rigorously in laboratories that conduct low complexity tests and are simple and reliable tools to evaluate vaccine-induced and natural immunity. Upcoming CHIM and field efficacy studies will offer the opportunity to carefully examine markers and thresholds of protection in different settings, including endemic populations. The ultimate goal would be to identify a robust serological correlate of protection in young children, who would benefit the most from a Shigella vaccine.
Acknowledgments
We are grateful to Dr. Ed Oaks for critical reading of this manuscript and Dr. Robert Kaminski for insights and discussions on these topics.
Funding Statement
This work was supported by NIH awards R01AI117734 and R01AI125841 to M.F.P. and a Research Supplement to Promote Diversity in Health-Related Research Program, 3R01AI117734-04S1, to E.N.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.GBD 2017 Causes of Death Collaborators . Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–88. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diarrhoeal diseases- UNICEF DATA . 2018. UNICEF; [accessed 2018 October23]. https://data.unicef.org/topic/child-health/diarrhoeal-disease/.
- 3.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–22. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 4.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, McCormick BJ, McGrath M, Olortegui MP, Samie A, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health. 2015;3(9):e564–e575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khalil IA, Troeger C, Blacker BF, Rao PC, Brown A, Atherly DE, Brewer TG, Engmann CM, Houpt ER, Kang G, et al. Morbidity and mortality due to Shigella and enterotoxigenic Escherichia coli diarrhoea: the Global Burden of Disease Study 1990–2016. Lancet Infect Dis. 2018;18(11):1229–40. doi: 10.1016/S1473-3099(18)30475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Troeger C, Colombara DV, Rao PC, Khalil IA, Brown A, Brewer TG, Guerrant RL, Houpt ER, Kotloff KL, Misra K, et al. Global disability-adjusted life-year estimates of long-term health burden and undernutrition attributable to diarrhoeal diseases in children younger than 5 years. Lancet Glob Health. 2018;6(3):e255–e269. doi: 10.1016/S2214-109X(18)30045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AA.. The impoverished gut – a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol. 2013;10(4):220–29. doi: 10.1038/nrgastro.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah MP, Tate JE, Steiner CA, Parashar UD.. Decline in emergency department visits for acute gastroenteritis among children in 10 US states after implementation of rotavirus vaccination, 2003 to 2013. Pediatr Infect Dis J. 2016;35(7):782–86. doi: 10.1097/INF.0000000000001175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Do Carmo GM, Yen C, Cortes J, Siqueira AA, de Oliveira WK, Cortez-Escalante JJ, Lopman B, Flannery B, de Oliveira LH, Carmo EH, et al. Decline in diarrhea mortality and admissions after routine childhood rotavirus immunization in Brazil: a time-series analysis. PLoS Med. 2011;8(4):e1001024. doi: 10.1371/journal.pmed.1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Palma O, Cruz L, Ramos H, de Baires A, Villatoro N, Pastor D, de Oliveira LH, Kerin T, Bowen M, Gentsch J, et al. Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: case-control study. BMJ. 2010;340:c2825. doi: 10.1136/bmj.c293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, Esparza-Aguilar M, Johnson B, Gomez-Altamirano CM, Parashar U, Patel M. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med. 2010;362(4):299–305. doi: 10.1056/NEJMoa0905211. [DOI] [PubMed] [Google Scholar]
- 12.Bar-Zeev N, King C, Phiri T, Beard J, Mvula H, AC C, Heinsbroek E, Lewycka S, JE T, UD P, et al. Impact of monovalent rotavirus vaccine on diarrhoea-associated post-neonatal infant mortality in rural communities in Malawi: a population-based birth cohort study. Lancet Glob Health. 2018;6(9):e1036–e1044. doi: 10.1016/S2214-109X(18)30314-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, Armah G, Bines JE, Brewer TG, Colombara DV, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 Years. JAMA Pediatr. 2018;172(10):958–65. doi: 10.1001/jamapediatrics.2018.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO Product Development for Vaccines Advisory Committee (PDVAC) meeting – 2018. 26–28 June 2018. [accessed 2019 April29]. https://www.who.int/immunization/research/meetings_workshops/pdvac_june18/en/.
- 15.Cohen D, Block C, Green MS, Lowell G, Ofek I. Immunoglobulin M, A, and G antibody response to lipopolysaccharide O antigen in symptomatic and asymptomatic Shigella infections. J Clin Microbiol. 1989;27:162–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen D, Green MS, Block C, Rouach T, Ofek I. Serum antibodies to lipopolysaccharide and natural immunity to shigellosis in an Israeli military population. J Infect Dis. 1988;157(5):1068–71. doi: 10.1093/infdis/157.5.1068. [DOI] [PubMed] [Google Scholar]
- 17.Raqib R, Qadri F, Sarker P, Mia SM, Sansonnetti PJ, Albert MJ, Andersson J. Delayed and reduced adaptive humoral immune responses in children with shigellosis compared with in adults. Scand J Immunol. 2002;55:414–23. [DOI] [PubMed] [Google Scholar]
- 18.Van de Verg LL, Herrington DA, Boslego J, Lindberg AA, Levine MM. Age-specific prevalence of serum antibodies to the invasion plasmid and lipopolysaccharide antigens of Shigella species in Chilean and North American populations. J Infect Dis. 1992;166(1):158–61. doi: 10.1093/infdis/166.1.158. [DOI] [PubMed] [Google Scholar]
- 19.Oberhelman RA, Kopecko DJ, Salazar-Lindo E, Gotuzzo E, Buysse JM, Venkatesan MM, Yi A, Fernandez-Prada C, Guzman M, Leon-Barua R. Prospective study of systemic and mucosal immune responses in dysenteric patients to specific Shigella invasion plasmid antigens and lipopolysaccharides. Infect Immun. 1991;59:2341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robin G, Cohen D, Orr N, Markus I, Slepon R, Ashkenazi S, Keisari Y. Characterization and quantitative analysis of serum IgG class and subclass response to Shigella sonnei and Shigella flexneri 2a lipopolysaccharide following natural Shigella infection. J Infect Dis. 1997;175(5):1128–33. doi: 10.1086/516452. [DOI] [PubMed] [Google Scholar]
- 21.Oaks EV, Hale TL, Formal SB. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun. 1986;53:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimanovich AA, Buskirk AD, Heine SJ, Blackwelder WC, Wahid R, Kotloff KL, Pasetti MF. Functional and antigen-specific serum antibody levels as correlates of protection against shigellosis in a controlled human challenge study. Clin Vaccine Immunol. 2017;24:2. doi: 10.1128/CVI.00412-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ndungo E, Randall A, Hazen TH, Kania DA, Trappl-Kimmons K, Liang X, Barry EM, Kotloff KL, Chakraborty S, Mani S, et al. A novel Shigella proteome microarray discriminates targets of human antibody reactivity following oral vaccination and experimental challenge. mSphere. 2018;3:4. doi: 10.1128/mSphere.00260-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wahid R, Simon JK, Picking WL, Kotloff KL, Levine MM, Sztein MB. Shigella antigen-specific B memory cells are associated with decreased disease severity in subjects challenged with wild-type Shigella flexneri 2a. Clin Immunol. 2013;148(1):35–43. doi: 10.1016/j.clim.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotloff KL, Nataro JP, Losonsky GA, Wasserman SS, Hale TL, Taylor DN, Sadoff JC, Levine MM. A modified Shigella volunteer challenge model in which the inoculum is administered with bicarbonate buffer: clinical experience and implications for Shigella infectivity. Vaccine. 1995;13:1488–94. [DOI] [PubMed] [Google Scholar]
- 26.Feller AJ, McKenzie R, Taylor DN, Woods CC, Grahek SL, Islam D, Venkatesan MM, Hale TL, Bourgeois AL. Comparative evaluation of the antibody in lymphocyte supernatant (ALS) and enzyme-linked immunospot (ELISPOT) assays for measuring mucosal immune responses to Shigella antigens. Vaccine. 2011;29(47):8487–89. doi: 10.1016/j.vaccine.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mani S, Wierzba T, Walker RI. Status of vaccine research and development for Shigella. Vaccine. 2016;34(26):2887–94. doi: 10.1016/j.vaccine.2016.02.075. [DOI] [PubMed] [Google Scholar]
- 28.Chakraborty S, Harro C, DeNearing B, Bream J, Bauers N, Dally L, Flores J, Van de Verg L, Sack DA, Walker R. Evaluation of the safety, tolerability, and immunogenicity of an oral, inactivated whole-cell Shigella flexneri 2a vaccine in healthy adult subjects. Clin Vaccine Immunol. 2016;23(4):315–25. doi: 10.1128/CVI.00608-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotloff KL, Simon JK, Pasetti MF, Sztein MB, Wooden SL, Livio S, Nataro JP, Blackwelder WC, Barry EM, Picking W, et al. Safety and immunogenicity of CVD 1208S, a live, oral ∆aguaBA ∆sen ∆set Shigella flexneri 2a vaccine grown on animal-free media. HumVaccin. 2007;3(6):268–75. [DOI] [PubMed] [Google Scholar]
- 30.Rahman KM, Arifeen SE, Zaman K, Rahman M, Raqib R, Yunus M, Begum N, Islam MS, Sohel BM, Rahman M, et al. Safety, dose, immunogenicity, and transmissibility of an oral live attenuated Shigella flexneri 2a vaccine candidate (SC602) among healthy adults and school children in Matlab, Bangladesh. Vaccine. 2011;29(6):1347–54. doi: 10.1016/j.vaccine.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 31.Frenck RW Jr., Baqar S, Alexander W, Dickey M, McNeal M, El-Khorazaty J, Baughman H, Hoeper A, Barnoy S, Suvarnapunya AE, et al. A Phase I trial to evaluate the safety and immunogenicity of WRSs2 and WRSs3; two live oral candidate vaccines against Shigella sonnei. Vaccine. 2018;36(32Pt B):4880–89. doi: 10.1016/j.vaccine.2018.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen D, Ashkenazi S, Green MS, Gdalevich M, Robin G, Slepon R, Yavzori M, Orr N, Block C, Ashkenazi I, et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet. 1997;349(9046):155–59. doi: 10.1016/S0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- 33.Passwell JH, Ashkenzi S, Banet-Levi Y, Ramon-Saraf R, Farzam N, Lerner-Geva L, Even-Nir H, Yerushalmi B, Chu C, Shiloach J, et al. Age-related efficacy of Shigella O-specific polysaccharide conjugates in 1–4-year-old Israeli children. Vaccine. 2010;28(10):2231–35. doi: 10.1016/j.vaccine.2009.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riddle MS, Kaminski RW, Di Paolo C, Porter CK, Gutierrez RL, Clarkson KA, Weerts HE, Duplessis C, Castellano A, Alaimo C, et al. Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella flexneri 2a administered to healthy adults: a single-blind, randomized Phase I study. Clin Vaccine Immunol. 2016;23(12):908–17. doi: 10.1128/CVI.00224-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talaat K, Alaimo C, Bourgeois AL, Kaminski RW, Dreyer A, Porter CK, Chakraborty S, Clarkson KA, Brubaker J, Elwood D et al. Flexyn2a, a candidate bioconjugate vaccine against Shigella flexneri 2a induces protective immune response in a controlled human infection model. Paper presented at: Vaccines For Enteric Diseases conference; Albufeira, Portugal; 2017. October 9–11. [Google Scholar]
- 36.Cohen D, Atsmon J, Artaud C, Meron-Sudai S, Gougeon ML, Bialik A, Goren S, Asato V, Ariel-Cohen O, Reizis A et al. Safety and immunogenicity study of SF2a-TT15, a synthetic carbohydrate-based conjugate vaccine against S. flexneri 2a in healthy adult volunteers. Paper presented at 2nd International Vaccines against Shigella and ETEC conference; 2018. June 12–14;Mexico City, Mexico. [Google Scholar]
- 37.Launay O, Lewis DJM, Anemona A, Loulergue P, Leahy J, Scire AS, Maugard A, Marchetti E, Zancan S, Huo Z, et al. Safety profile and immunologic responses of a novel vaccine against Shigella sonnei administered intramuscularly, intradermally and intranasally: results from two parallel randomized phase 1 clinical studies in healthy adult volunteers in Europe. EBio Medicine. 2017;22:164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obiero CW, Ndiaye AGW, Scire AS, Kaunyangi BM, Marchetti E, Gone AM, Schutte LD, Riccucci D, Auerbach J, Saul A, et al. A phase 2a randomized study to evaluate the safety and immunogenicity of the 1790GAHB generalized modules for membrane antigen vaccine against Shigella sonnei administered intramuscularly to adults from a shigellosis-endemic country. Front Immunol. 2017;8:1884. doi: 10.3389/fimmu.2017.01884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tribble D, Kaminski R, Cantrell J, Nelson M, Porter C, Baqar S, Williams C, Arora R, Saunders J, Ananthakrishnan M, et al. Safety and immunogenicity of a Shigella flexneri 2a Invaplex 50 intranasal vaccine in adult volunteers. Vaccine. 2010;28(37):6076–85. doi: 10.1016/j.vaccine.2010.06.086. [DOI] [PubMed] [Google Scholar]
- 40.Porter CK, Lynen A, Riddle MS, Talaat K, Sack D, Gutierrez RL, McKenzie R, DeNearing B, Feijoo B, Kaminski RW, et al. Clinical endpoints in the controlled human challenge model for Shigella: a call for standardization and the development of a disease severity score. PLoS One. 2018;13(3):e0194325. doi: 10.1371/journal.pone.0194325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porter CK, Gutierrez RL, Kotloff KL. Clinical endpoints for efficacy studies. Vaccine. 2019. doi: 10.1016/j.vaccine.2019.03.051. [DOI] [PubMed] [Google Scholar]
- 42.Forthal DN. Functions of antibodies. Microbiol Spectr. 2014;2(4):AID-0019–2014. doi: 10.1128/microbiolspec.AID-0019-2014. [DOI] [PubMed] [Google Scholar]
- 43.Winkelstein JA. The role of complement in the host’s defense against Streptococcus pneumoniae. Rev Infect Dis. 1981;3:289–98. [DOI] [PubMed] [Google Scholar]
- 44.Kim KH, Yu J, Nahm MH. Efficiency of a pneumococcal opsonophagocytic killing assay improved by multiplexing and by coloring colonies. Clin Diagn Lab Immunol. 2003;10(4):616–21. doi: 10.1128/cdli.10.4.616-621.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romero-Steiner S, Frasch CE, Carlone G, Fleck RA, Goldblatt D, Nahm MH. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin Vaccine Immunol. 2006;13(2):165–69. doi: 10.1128/CVI.13.2.165-169.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borrow R, Carlone GM, Rosenstein N, Blake M, Feavers I, Martin D, Zollinger W, Robbins J, Aaberge I, Granoff DM, et al. Neisseria meningitidis group B correlates of protection and assay standardization – international meeting report Emory University, Atlanta, Georgia, United States, 16–17 March 2005. Vaccine. 2006;24(24):5093–107. [DOI] [PubMed] [Google Scholar]
- 47.McIntosh ED, Broker M, Wassil J, Welsch JA, Borrow R. Serum bactericidal antibody assays – the role of complement in infection and immunity. Vaccine. 2015;33(36):4414–21. doi: 10.1016/j.vaccine.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 48.Glass RI, Becker S, Huq MI, Stoll BJ, Khan MU, Merson MH, Lee JV, Black RE. Endemic cholera in rural Bangladesh, 1966–1980. Am J Epidemiol. 1982;116:959–70. doi: 10.1093/oxfordjournals.aje.a113498. [DOI] [PubMed] [Google Scholar]
- 49.Chen WH, Cohen MB, Kirkpatrick BD, Brady RC, Galloway D, Gurwith M, Hall RH, Kessler RA, Lock M, Haney D, et al. Single-dose live oral cholera vaccine CVD 103-HgR protects against human experimental infection with vibrio cholerae O1 El Tor. Clin Infect Dis. 2016;62(11):1329–35. doi: 10.1093/cid/ciw145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.WHO Expert Committee on Biological Standardization, sixty-seventh report. Geneva, Switzerland: World Health Organization; 2017. (WHO technical report series; no 1004) Licence: CC BY-NV-SA 30 IGO. Annex 9- Guidelines on clinical evaluation of vaccines: regulatory expectations. [Google Scholar]
- 51.Roantree RJ, Rantz LA. A study of the relationship of the normal bactericidal activity of human serum to bacterial infection. J Clin Invest. 1960;39(1):72–81. doi: 10.1172/JCI104029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okamura N, Nakaya R, Suzuki K, Kondo S, Hisatsune K, Imagawa Y, Sagara H, Matsubara Y. Differences among Shigella spp. in susceptibility to the bactericidal activity of human serum. J Gen Microbiol. 1988;134(7):2057–65. doi: 10.1099/00221287-134-7-2057. [DOI] [PubMed] [Google Scholar]
- 53.Sayem MA, Ahmad SM, Rekha RS, Sarker P, Agerberth B, Talukder KA, Raqib R. Differential host immune responses to epidemic and endemic strains of Shigella dysenteriae type I. J Health Popul Nutr. 2011;29(5):429–37. doi: 10.3329/jhpn.v29i5.8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lowell GH, MacDermott RP, Summers PL, Reeder AA, Bertovich MJ, Formal SB. Antibody-dependent cell-mediated antibacterial activity: K lymphocytes, monocytes, and granulocytes are effective against Shigella. J Immunol. 1980;125:2778–84. [PubMed] [Google Scholar]
- 55.Reed WP, Albright EL. Serum factors responsible for killing of Shigella. Immunology. 1974;26:205–15. [PMC free article] [PubMed] [Google Scholar]
- 56.Rahman MJ, Sarker P, Roy SK, Ahmad SM, Chisti J, Azim T, Mathan M, Sack D, Andersson J, Raqib R. Effects of zinc supplementation as adjunct therapy on the systemic immune responses in shigellosis. Am J Clin Nutr. 2005;81(2):495–502. doi: 10.1093/ajcn.81.2.495. [DOI] [PubMed] [Google Scholar]
- 57.Lin J, Smith MA, Benjamin WH Jr., Kaminski RW, Wenzel H, Nahm MH. Monoclonal antibodies to shigella lipopolysaccharide are useful for vaccine production. Clin Vaccine Immunol. 2016;23(8):681–88. doi: 10.1128/CVI.00148-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pasetti MF, STS C, SJ H, Mani S, Kotloff K, Harro C, Chakraborty S. Shigella-specific serum bactericidal and opsonophagocytic killing antibodies induced by oral S. flexneri 2a whole-cell killed and live attenuated vaccines. Paper presented at: 2nd International VASE Conference; 2018. June 12–14; Mexico City, Mexico [Google Scholar]
- 59.Toapanta FR, Simon JK, Barry EM, Pasetti MF, Levine MM, Kotloff KL, Sztein MB. Gut-homing conventional plasmablasts and CD27(-) plasmablasts elicited after a short time of exposure to an oral live-attenuated Shigella vaccine candidate in humans. Front Immunol. 2014;5:374. doi: 10.3389/fimmu.2014.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robbins JB, Chu C, Schneerson R. Hypothesis for vaccine development: protective immunity to enteric diseases caused by nontyphoidal salmonellae and shigellae may be conferred by serum IgG antibodies to the O-specific polysaccharide of their lipopolysaccharides. ClinInfect Dis. 1992;15:346–61. [DOI] [PubMed] [Google Scholar]
- 61.Robbins JB, Schneerson R, Szu SC. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171(6):1387–98. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 62.Tacket CO, Binion SB, Bostwick E, Losonsky G, Roy MJ, Edelman R. Efficacy of bovine milk immunoglobulin concentrate in preventing illness after Shigella flexneri challenge. Am J Trop Med Hyg. 1992;47(3):276–83. doi: 10.4269/ajtmh.1992.47.276. [DOI] [PubMed] [Google Scholar]
- 63.Ndungo E, Heine SJ, Franco-Mahecha OL, Shimanovich A, Pasetti MF. Naturally-acquired and vaccine-induced maternal immunity for protection of young infants against shigellosis. Paper presented at: 4th International Neonatal and Maternal Immunization Symposium; 2017. September 10–12; Brussels, Belgium. [Google Scholar]
- 64.Mathias A, Longet S, Corthesy B. Agglutinating secretory IgA preserves intestinal epithelial cell integrity during apical infection by Shigella flexneri. Infect Immun. 2013;81(8):3027–34. doi: 10.1128/IAI.00303-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kotloff KL, Pasetti MF, Barry EM, Nataro JP, Wasserman SS, Sztein MB, Picking WD, Levine MM. Deletion in the Shigella enterotoxin genes further attenuates Shigella flexneri 2a bearing guanine auxotrophy in a phase 1 trial of CVD 1204 and CVD 1208. J Infect Dis. 2004;190(10):1745–54. doi: 10.1086/424680. [DOI] [PubMed] [Google Scholar]
- 66.Cleary TG, Winsor DK, Reich D, Ruiz-Palacios G, Calva JJ. Human milk immunoglobulin A antibodies to Shigella virulence determinants. Infect Immun. 1989;57:1675–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Achi R, Dac Cam P, Forsum U, Karlsson K, Saenz P, Mata L, Lindberg AA. Titres of class-specific antibodies against Shigella and Salmonella lipopolysaccharide antigens in colostrum and breast milk of Costa Rican, Swedish and Vietnamese mothers. J Infect. 1992;25:89–105. [DOI] [PubMed] [Google Scholar]
- 68.Cam PD, Achi R, Lindberg AA, Pal T. Antibodies against invasion plasmid coded antigens of shigellae in human colostrum and milk. Acta Microbiol Hung. 1992;39:263–70. [PubMed] [Google Scholar]
- 69.Morgan DR, DuPont HL, Gonik B, Kohl S. Cytotoxicity of human peripheral blood and colostral leukocytes against Shigella species. Infect Immun. 1984;46:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mounier J, Bahrani FK, Sansonetti PJ. Secretion of Shigella flexneri Ipa invasins on contact with epithelial cells and subsequent entry of the bacterium into cells are growth stage dependent. Infect Immun. 1997;65:774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Menard R, Prevost MC, Gounon P, Sansonetti P, Dehio C. The secreted Ipa complex of Shigella flexneri promotes entry into mammalian cells. Proc Natl Acad Sci USA. 1996;93(3):1254–58. doi: 10.1073/pnas.93.3.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heine SJ, Franco-Mahecha OL, Chen X, Choudhari S, Blackwelder WC, van Roosmalen ML, Leenhouts K, Picking WL, Pasetti MF. Shigella IpaB and IpaD displayed on L. lactis bacterium-like particles induce protective immunity in adult and infant mice. Immunol Cell Biol. 2015;93(7):641–52. doi: 10.1038/icb.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brotcke Zumsteg A, Goosmann C, Brinkmann V, Morona R, Zychlinsky A. IcsA is a Shigella flexneri adhesin regulated by the type III secretion system and required for pathogenesis. Cell Host Microbe. 2014;15(4):435–45. doi: 10.1016/j.chom.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 74.Mounier J, Vasselon T, Hellio R, Lesourd M, Sansonetti PJ. Shigella flexneri enters human colonic Caco-2 epithelial cells through the basolateral pole. Infect Immun. 1992;60:237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.WHO . 2013. Correlates of vaccine-induced protection: methods and implication. WHO/IVB/1301.
- 76.Plotkin SA, Gilbert P. Correlates of Protection. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, editors. Plotkin’s vaccines. 7th ed. Philadelphia (PA): Elsevier; 2017. p. 35–40. [Google Scholar]
- 77.CDC . Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children – advisory committee on immunization practices (ACIP). MMWR. 2010;2010(59):258–61. [PubMed] [Google Scholar]
- 78.Losonsky GA, Yunyongying J, Lim V, Reymann M, Lim YL, Wasserman SS, Levine MM. Factors influencing secondary vibriocidal immune responses: relevance for understanding immunity to cholera. InfectImmun. 1996;64:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cohen D, Green MS, Block C, Slepon R, Lerman Y. Natural immunity to shigellosis in two groups with different previous risks of exposure to Shigella is only partly expressed by serum antibodies to lipopolysaccharide. J Infect Dis. 1992;165(4):785–87. [letter]. doi: 10.1093/infdis/165.4.785. [DOI] [PubMed] [Google Scholar]
- 80.Ekwall E, Cam PD, Trach DD, Taube A, Lindberg AA. Shigella flexneri O-antigen-specific enzyme immunoassay: class specific antibody titres against lipopolysaccharide antigens in healthy Vietnamese and Swedish populations. Serodiagnosis ImmunoTher Infect Dis. 1988;2(1):47–61. doi: 10.1016/0888-0786(88)90036-4. [DOI] [Google Scholar]
- 81.Levine MM, Kotloff KL, Barry EM, Pasetti MF, Sztein MB. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat Rev Microbiol. 2007;5(7):540–53. doi: 10.1038/nrmicro1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Niewiesk S. Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front Immunol. 2014;5:446. doi: 10.3389/fimmu.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thompson CN, Le TP, Anders KL, Nguyen TH, Lu LV, Nguyen VV, Vu TD, Nguyen NM, Tran TH, Ha TT, et al. The transfer and decay of maternal antibody against Shigella sonnei in a longitudinal cohort of Vietnamese infants. Vaccine. 2016;34(6):783–90. doi: 10.1016/j.vaccine.2015.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gitlin D, Rosen FS, Michael JG. Transient 19S gammaglobulin deficiency in the newborn infant, and its significance. Pediatrics. 1963;31:197–208. [PubMed] [Google Scholar]
- 85.Black S, Nicolay U, Vesikari T, Knuf M, Del Giudice G, Della Cioppa G, Tsai T, Clemens R, Rappuoli R. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J. 2011;30(12):1081–85. doi: 10.1097/INF.0b013e3182367662. [DOI] [PubMed] [Google Scholar]
- 86.Ward BJ, Pillet S, Charland N, Trepanier S, Couillard J, Landry N. The establishment of surrogates and correlates of protection: useful tools for the licensure of effective influenza vaccines? Hum Vaccin Immunother. 2018;14(3):647–56. doi: 10.1080/21645515.2017.1413518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Davis CL, Wahid R, Toapanta FR, Simon JK, Sztein MB. A clinically parameterized mathematical model of Shigella immunity to inform vaccine design. PLoS One. 2018;13(1):e0189571. doi: 10.1371/journal.pone.0189571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arevalillo JM, Sztein MB, Kotloff KL, Levine MM, Simon JK. Identification of immune correlates of protection in Shigella infection by application of machine learning. J Biomed Inform. 2017;74:1–9. doi: 10.1016/j.jbi.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chung AW, Kumar MP, Arnold KB, Yu WH, Schoen MK, Dunphy LJ, Suscovich TJ, Frahm N, Linde C, Mahan AE, et al. Dissecting polyclonal vaccine-induced humoral immunity against HIV using systems serology. Cell. 2015;163(4):988–98. doi: 10.1016/j.cell.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lu LL, Chung AW, Rosebrock TR, Ghebremichael M, Yu WH, Grace PS, Schoen MK, Tafesse F, Martin C, Leung V, et al. A functional role for antibodies in tuberculosis. Cell. 2016;167(2):433–443 e414. doi: 10.1016/j.cell.2016.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McArthur MA, Maciel M Jr., Pasetti MF. Human immune responses against Shigella and enterotoxigenic E. coli: current advances and the path forward. Vaccine. 2017;35(49 Pt A):6803–06. doi: 10.1016/j.vaccine.2017.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Necchi F, Saul A, Rondini S. Development of a high-throughput method to evaluate serum bactericidal activity using bacterial ATP measurement as survival readout. PLoS One. 2017;12(2):e0172163. doi: 10.1371/journal.pone.0172163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nahm MH, Yu J, Weerts HP, Wenzel H, Tamilselvi CS, Chandrasekaran L, Pasetti MF, Mani S, Kaminski RW. Development, interlaboratory evaluations, and application of a simple, high-throughput Shigella serum bactericidal assay. mSphere. 2018;3(3). doi: 10.1128/mSphere.00146-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Diarrhoeal diseases- UNICEF DATA . 2018. UNICEF; [accessed 2018 October23]. https://data.unicef.org/topic/child-health/diarrhoeal-disease/.
- WHO Product Development for Vaccines Advisory Committee (PDVAC) meeting – 2018. 26–28 June 2018. [accessed 2019 April29]. https://www.who.int/immunization/research/meetings_workshops/pdvac_june18/en/.


