Abstract
The relationship between body weight and lung function is complex. Using a dyadic multilevel linear modeling approach, treating body mass index (BMI; weight (kg)/height (m)2) and lung function as paired, within-person outcomes, we tested the hypothesis that persons with more rapid increase in BMI exhibit more rapid decline in lung function, as measured by forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and their ratio (FEV1:FVC). Models included random intercepts and slopes and adjusted for sociodemographic and smoking-related factors. A sample of 9,115 adults with paired measurements of BMI and lung function taken at ≥3 visits were selected from a pooled set of 5 US population-based cohort studies (1983–2018; mean age at baseline = 46 years; median follow-up, 19 years). At age 46 years, average annual rates of change in BMI, FEV1, FVC, and FEV1:FVC ratio were 0.22 kg/m2/year, −25.50 mL/year, −21.99 mL/year, and −0.24%/year, respectively. Persons with steeper BMI increases had faster declines in FEV1 (r = −0.16) and FVC (r = −0.26) and slower declines in FEV1:FVC ratio (r = 0.11) (all P values < 0.0001). Results were similar in subgroup analyses. Residual correlations were negative (P < 0.0001), suggesting additional interdependence between BMI and lung function. Results show that greater rates of weight gain are associated with greater rates of lung function loss.
Keywords: body mass index, chronic obstructive lung disease, cohort studies, dyadic models, longitudinal studies, lung function, obesity, spirometry
Abbreviations
- BMI
body mass index
- COPD
chronic obstructive pulmonary disease
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- IQR
interquartile range
- SE
standard error
Obesity and chronic respiratory diseases have increased in prevalence and global public health impact over recent decades (1, 2). According to the Global Burden of Disease Study, high body mass index (BMI) contributed to 4.0 million deaths in 2015, and chronic obstructive pulmonary disease (COPD) and asthma contributed to 3.6 million deaths (1). Understanding interrelationships between the obesity and chronic respiratory disease epidemics is therefore of great public health importance.
Obesity and low lung function are physiologically interdependent: Obesity may restrict ventilatory capacity, reduce exercise capacity, and contribute to breathlessness, while low lung function may reinforce sedentary and obesogenic behavior (3–6). Elevated BMI and low lung function also have shared risk factors, such as aging, maladaptive immune response, obesogenic diet, physical inactivity, and a less diverse microbiome (7). These complex causal networks may engender correlations between within-person changes in BMI and lung function.
Dyadic growth modeling has been applied to understand associations in rates of change between paired individuals (e.g., couples, parent/child) (8, 9). To our knowledge, it has never been applied to paired, within-individual health outcomes such as BMI and lung function. Rather, prior research has relied on nondyadic models that test associations between a given exposure and outcome—whether changes in BMI predict changes in lung function or vice versa (10–32). Dyadic models are better suited for describing the associations between rates of change in BMI and lung function because they provide estimates of the between-person associations of the within-person long-term trends/trajectories in the 2 outcomes and, in addition, the pooled within-person association between the concurrent short-term deviations of the outcomes from their trajectories. Because neither outcome is predicting the other, dyadic models bypass concerns regarding causal ordering (e.g., confounding by prior exposure) (33).
We applied a dyadic growth modeling approach to examine concurrent rates of change in BMI and lung function in a large, US general population-based sample of adults (34). Treating BMI and lung function as a person-level “dyad,” we tested the hypothesis that persons with more rapid rates of gain in BMI would exhibit more rapid rates of lung function loss. We explored whether these associations varied over the life course, across ranges of BMI and lung function at baseline, or according to sociodemographic and clinical factors.
METHODS
NHLBI Pooled Cohorts Study
The National Heart, Lung, and Blood Institute (NHLBI) Pooled Cohorts Study harmonized and pooled data from 9 US epidemiologic cohort studies that conducted lung function assessments over the past 4 decades (34). This report is limited to 5 cohort studies in which 3 or more spirometry assessments were made between 1983 and 2018 (see Web Table 1, available at https://academic.oup.com/aje): the Coronary Artery Risk Development in Young Adults (CARDIA) Study (35); the Cardiovascular Health Study (CHS) (36); the Framingham Heart Study–Offspring Cohort (FHS-O) (37); the Health, Aging and Body Composition (Health ABC) Study (38); and the Multi-Ethnic Study of Atherosclerosis (MESA) (39).
Primary analyses included participants with 3 or more valid spirometry examinations with full data on BMI and covariates. The sample was restricted to participants of non-Hispanic White or African-American race/ethnicity due to low numbers of participants in other racial/ethnic groups (Web Figure 1).
All studies were approved by institutional review boards at participating institutions, and all participants provided written informed consent.
Lung function
Prebronchodilator lung function was measured using water-seal, dry-rolling-seal, or flow-sensing spirometers, following American Thoracic Society guidelines that were current at the time of assessment. To harmonize spirometry data, we applied a standardized quality grading system based upon the 2005 American Thoracic Society/European Respiratory Society guidelines, which define valid examinations as 2 or more acceptable curves reproducible within 150 mL (40). Invalid examinations were excluded (34, 40). This approach was previously found to reduce between-person and within-person variability, outliers, and lung-function trend irregularities.
The lung function outcomes evaluated were forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and their ratio (FEV1:FVC). Standardized z scores, generated using the Global Lung Function Initiative equations (41), were used in sensitivity analyses.
Airflow limitation was defined as an FEV1:FVC ratio less than the lower limit of normal, as defined by the Third National Health and Nutrition Examination Survey reference equations (42). A restrictive pattern was defined as FEV1:FVC ≥ lower limit of normal and FVC < lower limit of normal. Preserved spirometry was defined as the absence of airflow limitation or a restrictive pattern.
Body mass index
Height and weight were measured using standard techniques at each spirometry examination. BMI was defined as weight in kilograms divided by squared height in meters.
BMI was categorized into 4 groups according to the Centers for Disease Control and Prevention definitions (43): obesity (≥30.0), overweight (25.0–29.9), normal weight (18.5–24.9), and underweight (<18.5).
Covariates
Sex, race/ethnicity, educational attainment, birth year, and pack-years of cigarette smoking were self-reported at the baseline examination. Smoking status was self-reported at each spirometry examination; biochemical verification by cotinine was available for 2 cohorts, in which 2%–4% were reclassified as current smokers based on cotinine discordance (44, 45). Baseline clinical chronic respiratory diseases were self-reported COPD, emphysema, chronic bronchitis, and/or asthma; or inhaler use, defined by self-report or medication inventory (34).
Statistical analyses
Data were structured so that there were 2 observations for each person-visit: one observation representing BMI as the outcome and the other representing lung function (FEV1, FVC, or FEV1:FVC) as the outcome. Using mixed linear models (PROC MIXED in SAS, version 9.4; SAS Institute Inc., Cary, North Carolina), a random intercept and random slope for the effect of time were specified separately for each outcome to account for individual differences in within-person rates of change for each outcome. The repeated statement was used to account for unexplained within-person variance in lung function and BMI (residuals), as well as any covariance between concurrent (i.e., same examination) lung function and BMI residuals.
Separate dyadic models were specified for the relationships between rate of change in BMI and rates of change in each of the 3 lung function measures (Figure 1). First, we examined predicted BMI and lung function levels over the life course, together with estimates of the predicted rate of change (slope) at specific ages. Second, we examined the degree of association between individuals’ average rate of change in BMI and their average rates of change in lung function, specifically the correlations (r) and their standard errors. To visualize the bivariate distribution of these 2 rates of change, estimates of each participant’s random effect for rates of change in BMI and lung function were plotted, along with 95% confidence limits. Third, “residual correlations” were examined; this indicates additional, short-term association between individuals’ BMI and lung function, independent of their shared temporal patterns (46).
Figure 1.
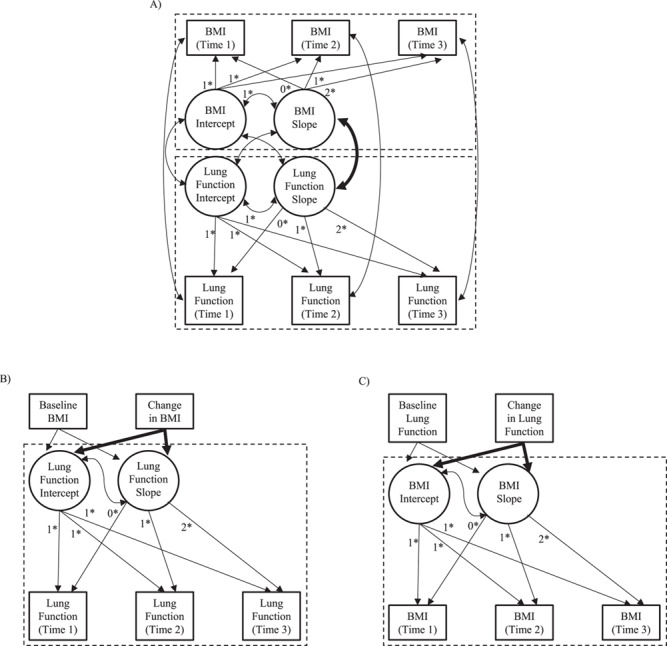
Conceptual distinctions between dyadic and nondyadic growth models for examining associations between weight gain and lung function decline. Rectangles represent observed variables, and circles represent latent variables. Curved arrows represent covariances, and straight arrows represent regression parameters. Fixed parameters are denoted by an asterisk (*). The arrow representing the parameter of interest is bolded in each panel; in dyadic models (A), the focus is on the covariation between simultaneous rates of change in body mass index (BMI; weight (kg)/height (m)2) and lung function. In nondyadic models, the focus is on the estimated (presumed causal) effect of change in BMI on lung function (B) or the estimated effect of change in lung function on BMI (C).
All models treated biological age at measurement as the time scale and adjusted for the following covariates, entered as predictors of both intercept and slope: time-varying age squared, height, height squared, and smoking status; and time-invariant (baseline) sex, educational attainment, birth year, race/ethnicity, study, and pack-years of smoking. Height and height squared were included as covariates because they are major determinants of predicted lung function (42). Sensitivity analyses were performed using weight (kg) and waist circumference (cm) as outcomes, rather than BMI, among participants without baseline clinical chronic respiratory diseases, and including all participants with 2 or more valid spirometry measures.
Stratified analyses were performed to identify differences by baseline BMI and lung function categories, as well as by baseline age group, birth cohort, study cohort, sex, race/ethnicity, and smoking status. Meta-analysis was performed to test for the presence of heterogeneity in slope correlations within each set of stratified analyses using the Q statistic.
For comparison, associations between BMI and lung function were tested using 6 nondyadic models (Figure 1): baseline BMI and rate of change in BMI (i.e., last BMI minus baseline BMI, divided by last age minus baseline age) as predictors of 1) FEV1, 2) FVC, and 3) FEV1:FVC and baseline and rates of change in 4) FEV1, 5) FVC, and 6) FEV1:FVC as predictors of BMI. In these models, the parameter of interest was the impact of change in the predictor on change in the outcome (e.g., the association of change in BMI with the FEV1 slope—the change × age interaction). Estimation of both the effect of BMI on lung function and vice versa violates the assumption that the residuals are independent of the predictors; hence, this was done only for illustrative purposes.
RESULTS
There were 9,115 participants (Table 1) with a median of 4 (interquartile range (IQR), 3–5) observations over a median follow-up period of 19 years (IQR, 10–28; Web Table 2). Fifty-seven percent were female, 73% were non-Hispanic White, and 27% were African-American. At the baseline examination, 49% were never smokers, 31% were former smokers, and 19% were current smokers; among ever smokers, the median number of pack-years of smoking was 9 (IQR, 2–25). At baseline, 4% and 6% reported a prior physician diagnosis of COPD and asthma, respectively.
Table 1.
Baseline Characteristics of Participants in the NHLBI Pooled Cohorts Study, 1983–2018
| Distribution of Participants (n = 9,115) | ||
|---|---|---|
| Characteristic | No. | % |
| Age, yearsa,b | 45.66 (20.36) | |
| Sex | ||
| Female | 5,220 | 57.3 |
| Male | 3,895 | 42.7 |
| Race | ||
| African-American | 2,497 | 27.4 |
| Non-Hispanic White | 6,618 | 72.6 |
| Education | ||
| Grades 1–8 | 178 | 2.0 |
| Grades 9–11 | 635 | 7.0 |
| High school | 2,558 | 28.1 |
| Some college | 2,543 | 27.9 |
| Bachelor’s degree | 1,576 | 17.3 |
| Graduate degree | 1,625 | 17.8 |
| Birth yearc | 1949 (1926–1959) | |
| Height, ma,b | 1.68 (0.09) | |
| Smoking statusb | ||
| Never smoker | 4,499 | 49.4 |
| Former smoker | 2,866 | 31.4 |
| Current smoker | 1,750 | 19.2 |
| Pack-years of smoking (current/former smokers only)b,c | 9.00 (2.48–25.17) | |
| Lung functiona,b | ||
| FEV1, L | 3.01 (0.91) | |
| FVC, L | 3.85 (1.07) | |
| FEV1:FVC ratio | 78.03 (8.29) | |
| Airflow limitationd | 1,021 | 11.2 |
| Restrictive spirometry patterne | 440 | 4.8 |
| Preserved spirometry patternf | 7,654 | 84.0 |
| Percent predicted FEV1a | 95.75 (14.87) | |
| Percent predicted FVCa | 98.51 (13.21) | |
| Body mass indexa,b,g | 25.95 (5.00) | |
| Body mass index categoryb | ||
| Underweight (<18.5) | 206 | 2.3 |
| Normal-weight (18.5–24.9) | 4,194 | 46.0 |
| Overweight (25.0–29.9) | 3,111 | 34.1 |
| Obese (≥30.0) | 1,604 | 17.6 |
| Study | ||
| CARDIA Study | 4,083 | 44.8 |
| CHS | 1,280 | 14.0 |
| FHS-O | 2,237 | 24.5 |
| Health ABC Study | 948 | 10.4 |
| MESA | 567 | 6.2 |
Abbreviations: CARDIA, Coronary Artery Risk Development in Young Adults; CHS, Cardiovascular Health Study; FEV1, forced expiratory volume in 1 second; FHS-O, Framingham Heart Study–Offspring Cohort; FVC, forced vital capacity; Health ABC, Health, Aging and Body Composition; MESA, Multi-Ethnic Study of Atherosclerosis; NHLBI, National Heart, Lung, and Blood Institute.
a Values are presented as mean (standard deviation).
b For time-varying covariates, the baseline observation is shown.
c Values are presented as median (interquartile range).
d Defined as FEV1:FVC ratio less than the lower limit of normal, as defined by the Third National Health and Nutrition Examination Survey reference equations (42).
e Defined as FEV1:FVC ratio ≥ lower limit of normal and FVC < lower limit of normal.
f Defined as the absence of airflow limitation or a restrictive pattern.
g Weight (kg)/height (m)2.
The mean BMI at the baseline examination was 26; 18% of participants were obese, 34% were overweight, 46% were normal-weight, and 2% were underweight (Table 1). Among participants with normal weight at the baseline examination, 34% and 15% transitioned to overweight and obesity, respectively, by the time of their last spirometry visit (Web Table 3).
At baseline, the mean percent predicted FEV1 was 96, the mean percent predicted FVC was 99, the mean FEV1:FVC ratio was 78, and the prevalences of airflow limitation and a restrictive spirometry pattern were 11% and 5%, respectively (Table 1). As of the last spirometry visit, the proportions with incident airflow limitation and restrictive spirometry were 7% and 6%, respectively (Web Table 4).
Changes in BMI and lung function over follow-up
On average, BMI increased over time, although the rate of increase decelerated with age (Figure 2A). The predicted BMI slope was 0.33 kg/m2/year at age 35 years but only 0.03 kg/m2/year at age 65 years. By contrast, lung function decreased over time at an accelerating rate (Figure 2, parts B–D). At 35 years of age, the predicted FEV1 and FVC slopes were −18.98 mL/year and −10.38 mL/year, respectively; at 65 years of age, the predicted FEV1 and FVC slopes were − 36.77 mL/year and − 42.05 mL/year, respectively. Consistent with a relatively greater acceleration in FVC versus FEV1 decline with increasing age, the rate of FEV1:FVC decline decelerated at later ages: −0.29%/year at age 35 years and − 0.15%/year at age 65 years.
Figure 2.
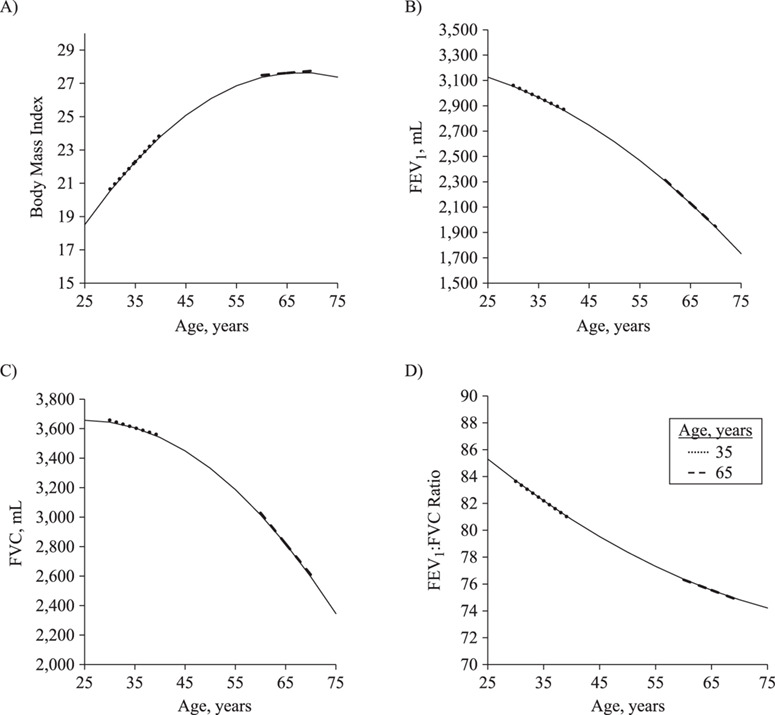
Predicted levels of body mass index (weight (kg)/height (m)2) (age 35 years, B = 0.33; age 65 years, B = 0.03) (A), forced expiratory volume in 1 second (FEV1) (age 35 years, B = −18.98; age 65 years, B = −36.77) (B), forced vital capacity (FVC) (age 35 years, B = −10.38; age 65 years, B = −42.05) (C), and FEV1:FVC ratio (age 35 years, B = −0.29; age 65 years, B = −0.15) (D) between the ages of 25 and 75 years, NHLBI Pooled Cohorts Study, 1983–2018. Dotted and dashed lines show slopes portraying predicted annual rate of change for participants at ages 35 and 65, respectively. Results for body mass index use parameter estimates from the dyadic model with FEV1. NHLBI, National Heart, Lung, and Blood Institute.
Correlations between rates of change in BMI and lung function
Participants with a steeper rate of increase in BMI over time tended to have a steeper rate of decline in FEV1 (r = −0.16 (standard error (SE), 0.02), P < 0.001; Figure 3A). The correlation of within-person slopes was even more negative with respect to FVC (r = −0.26 (SE, 0.02), P < 0.001; Figure 3B). Consistent with these relationships, a steeper BMI gain was associated with a more gradual FEV1:FVC decline (i.e., a less negative slope), as indicated by a positive correlation of within-person slopes (r = 0.11 (SE, 0.02), P < 0.001; Figure 3C). Results obtained when BMI was replaced by weight (FEV1: r = −0.17, P < 0.001; FVC: r = −0.27, P < 0.001; FEV1:FVC: r = 0.11, P < 0.001) (Web Figure 2) or waist circumference (FEV1: r = −0.25, P < 0.001; FVC: r = −0.35, P < 0.001; FEV1:FVC: r = 0.17, P < 0.001) were similar.
Figure 3.
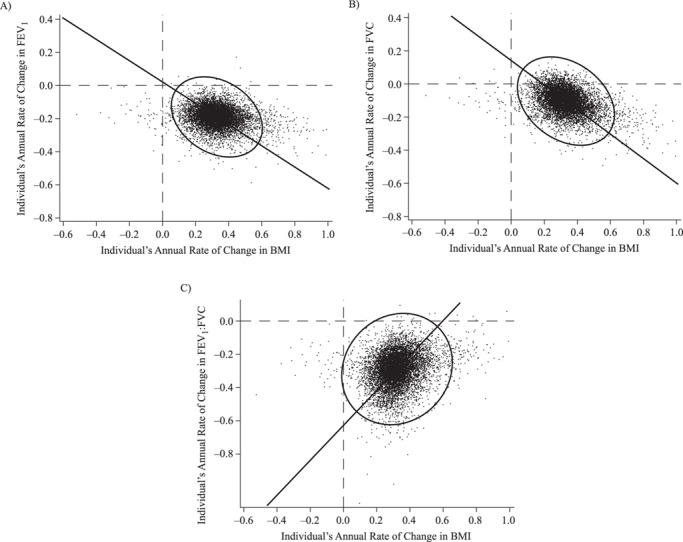
Individuals’ estimated slope coefficients for the associations of body mass index (BMI; weight (kg)/height (m)2) with forced expiratory volume in 1 second (FEV1; r = −0.16) (A), forced vital capacity (FVC; r = −0.26) (B), and FEV1:FVC ratio (r = 0.11) (C), NHLBI Pooled Cohorts Study, 1983–2018. Coefficients for FEV1 and FVC represent lung function change in dL/year. NHLBI, National Heart, Lung, and Blood Institute.
Residual correlations
Correlations between residuals for BMI and those for lung function outcomes were consistently negative (FEV1: r = −0.25 (SE, 0.01), P < 0.001; FVC: r = −0.23 (SE, 0.01), P < 0.001; FEV1:FVC: r = −0.02 (SE, 0.01), P < 0.001) (Web Figure 3). This indicates that, in those examinations where a participant’s BMI was higher (lower) than predicted from her/his covariate-adjusted BMI trajectory, s/he tended to have a lower (higher) lung function than predicted.
Stratified analyses
There was no evidence of heterogeneity in the correlation between individuals’ rates of change in BMI and lung function in analyses stratified by baseline BMI or lung disease (Figure 4). However, there were differences by baseline lung function (Q = 10.46, P = 0.005). Participants with initial airflow limitation exhibited a near-zero correlation between BMI and FEV1 rates of change, even as the inverse correlation with FVC rate of change remained, resulting in the BMI rate of change exhibiting an even greater correlation with FEV1:FVC rate of change (not shown).
Figure 4.
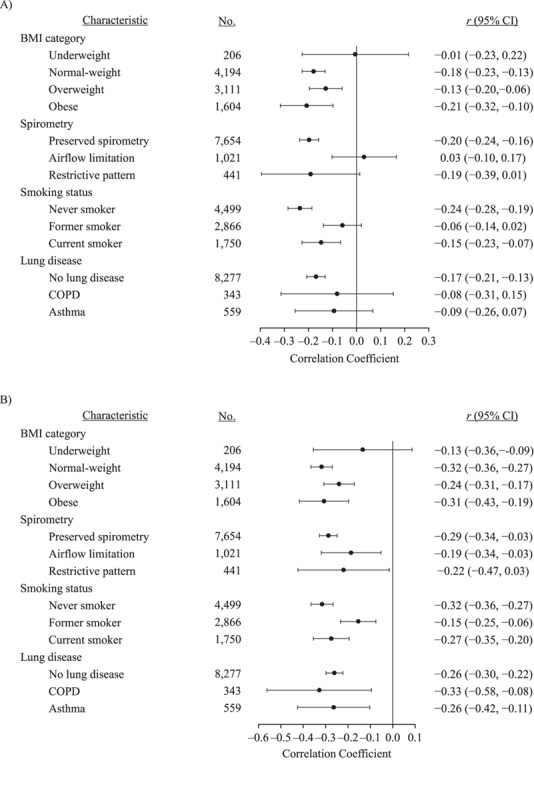
Correlated rates of change between individual slope coefficients for the associations of body mass index (BMI; weight (kg)/height (m)2) with forced expiratory volume in 1 second (FEV1) (A) and forced vital capacity (FVC) (B), NHLBI Pooled Cohorts Study, 1983–2018. There was significant heterogeneity in FEV1 slopes by baseline lung function (Q = 10.46, P = 0.005) and smoking status (Q = 14.49, P < 0.001). There was significant heterogeneity in FVC slopes by smoking status (Q = 9.76, P = 0.008). Bars show the 95% confidence intervals (CIs). COPD, chronic obstructive pulmonary disease; NHLBI, National Heart, Lung, and Blood Institute.
Correlations between rates of change for BMI and lung function also differed by smoking status (FEV1: Q = 14.49, P < 0.001; FVC: Q = 9.76, P = 0.008). The strongest correlations were observed among never smokers and the weakest correlations among former smokers (Figure 4).
Correlations of BMI and FVC rates of change were stronger in males than in females (Q = 4.12, P = 0.042). Correlations of rate of change for BMI with those for FEV1 and FVC were stronger in Black participants (versus White participants) (FEV1: Q = 14.70, P < 0.001; FVC Q = 11.17, P < 0.001), more recent generations (FEV1: Q = 26.88, P < 0.001; FVC: Q = 12.79, P < 0.001), and more contemporary study cohorts (FEV1: Q = 24.71, P < 0.001; FVC: Q = 17.74, P < 0.001) (Web Figure 4).
Reanalysis including participants with 2 or more valid spirometry measures (n = 13,332) somewhat attenuated correlations between rates of change in BMI and lung function (FEV1: r = −0.12; FVC: r = −0.19; FEV1:FVC: r = 0.08 (all P values < 0.0001)).
Comparisons with nondyadic models
Nondyadic models supported conclusions similar to those derived from our dyadic models (Web Table 5), although some results were relatively difficult to interpret or misleading. For example, the estimated effect of change in BMI on FVC intercept was positive, suggesting that persons with a more rapid increase in BMI had a higher FVC; however, the estimated effect of change in BMI on FVC slope was negative, suggesting that a more rapid BMI increase was associated with a more rapid rate of decline in FVC. Contradictory intercept and slope estimates also emerged in the nondyadic models predicting FEV1 and FEV1:FVC from change in BMI.
DISCUSSION
More rapid rates of increase in BMI were associated with greater rates of decline in FEV1 and (especially) FVC in a large, US general population-based sample of adults. These correlations were present across the range of baseline BMI values, consistent with underlying physiological interdependence between weight gain and lung function loss.
To our knowledge, this is the first study to have applied dyadic growth modeling to characterize relationships between weight gain and lung function loss and to compare these with single-outcome growth models. In our data, nondyadic results were inconsistent, complicating their interpretation. Nondyadic models also did not quantify the strength of the association between rates of change in BMI and lung function (i.e., the correlation between the slopes representing their respective rates of change) and were unable to differentiate these long-term associations from correlated residuals. Importantly, if causal relationships are bidirectional, nondyadic models suffer from time-varying confounding by prior exposure (33) and lack of independence of residuals. By contrast, our dyadic growth models identified correlations between rates of change in continuous measures of BMI and lung function that bypassed issues regarding causal ordering, were internally consistent and consistent with our hypotheses (based on physiological and epidemiologic literature), and were straightforward to interpret. Rather than using change scores as a predictor, which is commonly done but inherently introduces error, treating within-person trajectories as latent variables accounts for differential uncertainty in rates of change across participants and increases the precision of estimated correlations between individuals’ rates of change in BMI and lung function (46). Furthermore, our analysis estimated correlations between BMI and lung function residuals, suggesting interdependence between concurrently assessed measures of BMI and lung function that is not accounted for by correlated rates of change or other time-varying covariates.
Our results confirm and extend previously reported associations between increasing weight and decreasing lung function, particularly FVC (24, 26, 28, 47, 48). Our findings are also consistent with cross-sectional data linking the metabolic syndrome to increased prevalence of restrictive lung disease, the hallmark of which is low FVC (49), as well as longitudinal studies showing stronger associations of weight gain with FVC loss than with FEV1 loss (21–31).
Whereas some have argued that the adverse effects of increased BMI on lung function are limited to persons who are already overweight or obese (25, 29), our findings clearly show that the correlation between rate of BMI gain and lung function loss is consistent across categories of baseline BMI. This suggests that alterations in lung mechanics previously associated with massive obesity (50) impact individual lung function trajectories in the context of weight gain across the continuum of weight status. Physiological mechanisms for the observed associations between rate of weight gain and rate of FVC decline may include decreased lung compliance via microatelectasis and/or decreased chest-wall compliance and reduced diaphragmatic excursion due to abdominal fat (4, 51–55). With respect to FEV1 decline, increased airway resistance may be one of the adverse physiological impacts of the shallower breathing favored by decreased lung compliance (11, 56, 57). Furthermore, weight gain is bidirectionally linked with proinflammatory markers (58, 59), which are implicated in airway remodeling and FEV1 decline (60, 61).
Our finding of preserved FEV1 despite weight gain in persons with initial airflow limitation provides some support for the “obesity paradox,” wherein overweight and obesity have been linked with favorable outcomes in COPD, while underweight has been more strongly linked with emphysema (16–20). Physiologically, the “strapping effect” may help to explain our results. Chest wall strapping in experimental paradigms promotes breathing at smaller lung volumes. In persons with airflow obstruction in particular, strapping may raise FEV1 due to increased elastance or increased dilation of small airways (62). Our finding of no association between BMI and FEV1 rates of change in participants with airflow limitation may reflect a lesser decline in FEV1 due to “strapping” by obesity (62–64). That said, our results were nonparadoxical among persons with and without self-reported chronic respiratory diseases. It is also important to consider whether the “paradox” may be explained by smoking. Smoking cessation reduces the rate of lung function decline and promotes weight gain (65). Consistent with this, correlations of rates of BMI gain with decline in FEV1 and FVC were most negative in never smokers and most attenuated in former smokers.
Strengths of this work include the application of a novel, robust analytical method to analyze the association between rates of change in BMI and lung function. We used rigorously quality-controlled, harmonized data from 5 large, US general population-based cohort studies with excellent long-term follow-up.
There were nonetheless a number of limitations. This was a descriptive analysis, so direct clinical applicability is limited. However, results are consistent with prior work showing that an intensive weight-loss program was associated with improved lung function in obese women (13, 66) and that weight loss improves asthma control in obese individuals with severe asthma (67). There is also limited evidence to suggest that inhaler use, which improves lung function, may promote weight loss, although the hypothesized mechanisms for this response relate to metabolic pathways (68).
Restricting the sample to participants with at least 3 observations improved estimation of parameters pertaining to individuals’ rates of change at the expense of potential selection bias. At baseline, participants excluded from the primary analyses were older and had higher BMI and lower lung function. Including participants with 2 or more measures attenuated the associations, but associations persisted.
We did not have measures of abdominal adiposity, which were more strongly associated with lung function than BMI or weight in some prior work (69). Similarly, other lung volume measures, such as total lung capacity and expiratory reserve volume, have been more strongly associated with obesity in some prior studies (12) but were not available in this study. Data on other covariates, such as physical activity, diet, or other environmental exposures, were unavailable in this study, and these variables should be included in future research.
In conclusion, this was to our knowledge the first study to apply dyadic growth modeling to estimate correlations between within-person rates of change in BMI and lung function, showing that persons with more rapid rates of increase in BMI exhibited more rapid rates of decrease in FEV1 and (particularly) FVC. Residual correlations between BMI and lung function suggested negative associations between BMI and lung function not accounted for by the model. Thus, in addition to showing that increased rates of weight gain are associated with increased rates of lung function decline in the general population, this study demonstrated that dyadic growth models are a promising approach for examining associations between interdependent health outcomes.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Division of General Medicine, Columbia University Irving Medical Center, New York, New York (Talea Cornelius, Joseph E. Schwartz, Pallavi Balte, Elizabeth C. Oelsner); Department of Psychiatry and Behavioral Health, Stony Brook School of Medicine, State University of New York at Stony Brook, Stony Brook, New York (Joseph E. Schwartz); Division of Pulmonary, Allergy and Critical Care Medicine, Department of Medicine, University of Alabama at Birmingham, Birmingham, Alabama (Surya P. Bhatt); Division of Nutritional Sciences, Cornell University, Ithaca, New York (Patricia A. Cassano); IMPACCT, University of Technology Sydney, Sydney, New South Wales, Australia (David Currow); Wolfson Palliative Care Research Centre, University of Hull, Hull, United Kingdom (David Currow and Miriam Johnson); Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, Minnesota (David R. Jacobs, Jr.); Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois (Ravi Kalhan); Department of Biostatistics, School of Public Health, University of Washington, Seattle, Washington (Richard Kronmal); Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, North Carolina (Laura Loehr); Department of Medicine, School of Medicine, Boston University, Boston, Massachusetts (George T. O’Connor); Department of Medicine, McGill University Health Centre, Montreal, Quebec, Canada (Benjamin Smith); Tougaloo College, Tougaloo, Mississippi (Wendy B. White); and Department of Critical Care Medicine, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania (Sachin Yende).
This work was supported as follows by grants from the US National Institutes of Health (NIH) and the US Environmental Protection Agency (EPA). National Heart, Lung, and Blood Institute (NHLBI) Pooled Cohorts Study: The NHLBI Pooled Cohorts Study was supported by NIH/NHLBI grants R21-HL121457, R21-HL-129924, and K23-HL-130627. Coronary Artery Risk Development in Young Adults (CARDIA) Study: CARDIA was conducted and supported by the NHLBI in collaboration with the University of Alabama at Birmingham (grants HHSN268201800005I and HHSN268201800007I), Northwestern University (grant HHSN268201800003I), the University of Minnesota (grant HHSN268201800006I), and the Kaiser Foundation Research Institute (grant HHSN268201800004I). Cardiovascular Health Study (CHS): CHS research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, and N01HC85086 and grants U01HL080295 and U01HL130114 from the NHLBI, with an additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by the National Institute on Aging (NIA) (grant R01AG023629). Framingham Heart Study–Offspring Cohort (FHS-O): The FHS-O was supported by the Framingham Heart Study (NHLBI, NIH, and Boston University School of Medicine) and by NHLBI contracts N01-HC-25195 and HHSN268201500001I. Health, Aging and Body Composition (Health ABC) Study: Research in the HABC Study was supported by NIA contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; NIA grant R01-AG028050; and National Institute of Nursing Research grant R01-NR012459. The research was supported in part by the intramural research program at the NIA. Multi-Ethnic Study of Atherosclerosis (MESA): MESA was supported by NIH/NHLBI grants R01-HL-077612, R01-HL-093081, R01-HL-130605, RC1-HL-100543, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169.
This manuscript has been reviewed by the CARDIA investigators for scientific content. A full list of CHS principal investigators and institutions can be found at CHS-NHLBI.org. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This publication was developed under a STAR research assistance agreement (no. RD831697 (MESA Air)) awarded by the EPA. It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors, and the EPA does not endorse any products or commercial services mentioned in this publication.
S.P.B. has received research grants from the NIH and ProterixBio, Inc. (Billerica, Massachusetts) and consulting fees from Sunovion Pharmaceuticals, Inc. (Marlborough, Massachusetts) and has served on the advisory boards of Sunovion and GlaxoSmithKline plc (London, United Kingdom). R.K. reports receiving grants from the NHLBI, Boehringer Ingelheim GmbH (Ingelheim am Rhein, Germany), PneumRx (Mountain View, California), Spiration Inc. (Redmond, Washington), AstraZeneca LP (London, United Kingdom), and GlaxoSmithKline and personal/consulting fees from Boehringer Ingelheim, AstraZeneca, CVS CareMark (Woonsocket, Rhode Island), Aptus Health (Reading, Massachusetts), GlaxoSmithKline, and Boston Scientific Corporation (Marlborough, Massachusetts). G.T.O. reports receiving grants from Janssen Pharmaceuticals, Inc. (Titusville, New Jersey) and personal/consulting fees from AstraZeneca. D.C. is a paid consultant and receives payment for intellectual property with Mayne Pharma (Mayne Pharma International Pty. Ltd., Salisbury, South Australia, Australia). The other authors report no competing interests relevant to the present work.
REFERENCES
- 1. GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD 2016 Causes of Death Collaborators Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jakes RW, Day NE, Patel B, et al. Physical inactivity is associated with lower forced expiratory volume in 1 second: European Prospective Investigation Into Cancer-Norfolk prospective population study. Am J Epidemiol. 2002;156(2):139–147. [DOI] [PubMed] [Google Scholar]
- 4. Koenig SM. Pulmonary complications of obesity. Am J Med Sci. 2001;321(4):249–279. [DOI] [PubMed] [Google Scholar]
- 5. Currow DC, Dal Grande E, Sidhu C, et al. The independent association of overweight and obesity with breathlessness in adults: a cross-sectional, population-based study. Eur Respir J. 2017;50(3):1700558. [DOI] [PubMed] [Google Scholar]
- 6. Vermeulen F, Garcia G, Ninane V, et al. Activity limitation and exertional dyspnea in adult asthmatic patients: what do we know? Respir Med. 2016;117:122–130. [DOI] [PubMed] [Google Scholar]
- 7. Peters U, Suratt BT, Bates JH, et al. Beyond BMI: obesity and lung disease. Chest. 2017;153(3):702–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cornelius T, Gettens K, Gorin AA. Dyadic dynamics in a randomized weight loss intervention. Ann Behav Med. 2016;50(4):506–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kashy DA, Donnellan MB, Burt SA, et al. Growth curve models for indistinguishable dyads using multilevel modeling and structural equation modeling: the case of adolescent twins’ conflict with their mothers. Dev Psychol. 2008;44(2):316–329. [DOI] [PubMed] [Google Scholar]
- 10. Must A, Spadano J, Coakley EH, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–1529. [DOI] [PubMed] [Google Scholar]
- 11. Rubinstein I, Zamel N, DuBarry L, et al. Airflow limitation in morbidly obese, nonsmoking men. Ann Intern Med. 1990;112(11):828–832. [DOI] [PubMed] [Google Scholar]
- 12. Jones RL, Nzekwu MMU. The effects of body mass index on lung volumes. Chest. 2006;130(3):827–833. [DOI] [PubMed] [Google Scholar]
- 13. Aaron SD, Fergusson D, Dent R, et al. Effect of weight reduction on respiratory function and airway reactivity in obese women. Chest. 2004;125(6):2046–2052. [DOI] [PubMed] [Google Scholar]
- 14. Hakala K, Stenius-Aarniala B, Sovijärvi A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest. 2000;118(5):1315–1321. [DOI] [PubMed] [Google Scholar]
- 15. Tantisira K, Weiss S. Complex interactions in complex traits: obesity and asthma. Thorax. 2001;56(suppl 2):ii64–ii73. [PMC free article] [PubMed] [Google Scholar]
- 16. Hanson C, Rutten EP, Wouters EF, et al. Influence of diet and obesity on COPD development and outcomes. Int J Chron Obstruct Pulmon Dis. 2014;9:723–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eriksson B, Backman H, Bossios A, et al. Only severe COPD is associated with being underweight: results from a population survey. ERJ Open Res. 2016;2(3):00051–02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Poulain M, Doucet M, Major GC, et al. The effect of obesity on chronic respiratory diseases: pathophysiology and therapeutic strategies. Can Med Assoc J. 2006;174(9):1293–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zammit C, Liddicoat H, Moonsie I, et al. Obesity and respiratory diseases. Int J Gen Med. 2010;3:335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilson DO, Rogers RM, Wright EC, et al. Body weight in chronic obstructive pulmonary disease. The National Institutes of Health Intermittent Positive-Pressure Breathing Trial. Am Rev Respir Dis. 1989;139(6):1435–1438. [DOI] [PubMed] [Google Scholar]
- 21. Wang ML, McCabe L, Petsonk EL, et al. Weight gain and longitudinal changes in lung function in steel workers. Chest. 1997;111(6):1526–1532. [DOI] [PubMed] [Google Scholar]
- 22. Chinn DJ, Cotes JE, Reed JW. Longitudinal effects of change in body mass on measurements of ventilatory capacity. Thorax. 1996;51(7):699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Y, Horne SL, Dosman JA. Body weight and weight gain related to pulmonary function decline in adults: a six year follow up study. Thorax. 1993;48(4):375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wise RA, Enright PL, Connett JE, et al. Effect of weight gain on pulmonary function after smoking cessation in the Lung Health Study. Am J Respir Crit Care Med. 1998;157(3):866–872. [DOI] [PubMed] [Google Scholar]
- 25. Carey IM, Cook DG, Strachan DP. The effects of adiposity and weight change on forced expiratory volume decline in a longitudinal study of adults. Int J Obes Relat Metab Disord. 1999;23(9):979–985. [DOI] [PubMed] [Google Scholar]
- 26. Bottai M, Pistelli F, Di Pede F, et al. Longitudinal changes of body mass index, spirometry and diffusion in a general population. Eur Respir J. 2002;20(3):665–673. [DOI] [PubMed] [Google Scholar]
- 27. Oostrom SH, Engelfriet PM, Verschuren WM, et al. Aging-related trajectories of lung function in the general population—the Doetinchem Cohort Study. PloS One. 2018;13(5):e0197250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moualla M, Qualls C, Arynchyn A, et al. Rapid decline in lung function is temporally associated with greater metabolically active adiposity in a longitudinal study of healthy adults. Thorax. 2017;72(12):1113–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thyagarajan B, Jacobs DR Jr, Apostol GG, et al. Longitudinal association of body mass index with lung function: the CARDIA Study. Respir Res. 2008;9(1):Article 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abramson MJ, Kaushik S, Benke GP, et al. Symptoms and lung function decline in a middle-aged cohort of males and females in Australia. Int J Chron Obstruct Pulmon Dis. 2016;11:1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rossi A, Fantin F, Di Francesco V, et al. Body composition and pulmonary function in the elderly: a 7-year longitudinal study. Int J Obes (Lond). 2008;32(9):1423–1430. [DOI] [PubMed] [Google Scholar]
- 32. Wu TD, Ejike CO, Wise RA, et al. Investigation of the obesity paradox in chronic obstructive pulmonary disease, according to smoking status, in the United States. Am J Epidemiol. 2019;188(11):1977–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mansournia MA, Etminan M, Danaei G, et al. Handling time varying confounding in observational research. BMJ. 2017;359:j4587. [DOI] [PubMed] [Google Scholar]
- 34. Oelsner EC, Balte PP, Cassano PA, et al. Harmonization of respiratory data from 9 US population-based cohorts: the NHLBI Pooled Cohorts Study. Am J Epidemiol. 2018;187(11):2265–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105–1116. [DOI] [PubMed] [Google Scholar]
- 36. Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1(3):263–276. [DOI] [PubMed] [Google Scholar]
- 37. Kannel WB, Feinleib M, McNamara PM, et al. An investigation of coronary heart disease in families: the Framingham Offspring Study. Am J Epidemiol. 1979;110(3):281–290. [DOI] [PubMed] [Google Scholar]
- 38. Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol (1985). 2001;90(6):2157–2165. [DOI] [PubMed] [Google Scholar]
- 39. Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. [DOI] [PubMed] [Google Scholar]
- 40. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. [DOI] [PubMed] [Google Scholar]
- 41. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the Global Lung Function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. [DOI] [PubMed] [Google Scholar]
- 43. Centers for Disease Control and Prevention Healthy weight. About adult BMI. https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html. Updated 2017 Accessed January 1, 2019.
- 44. Rodriguez J, Jiang R, Johnson WC, et al. The association of pipe and cigar use with cotinine levels, lung function, and airflow obstruction: a cross-sectional study. Ann Intern Med. 2010;152(4):201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wagenknecht LE, Burke GL, Perkins LL, et al. Misclassification of smoking status in the CARDIA Study: a comparison of self-report with serum cotinine levels. Am J Public Health. 1992;82(1):33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kenny DA, Kashy DA, Cook WL. Dyadic Analysis. New York, NY: Guilford Press; 2006. [Google Scholar]
- 47. Lazarus R, Gore CJ, Booth M, et al. Effects of body composition and fat distribution on ventilatory function in adults. Am J Clin Nutr. 1998;68(1):35–41. [DOI] [PubMed] [Google Scholar]
- 48. Wannamethee SG, Shaper AG, Whincup PH. Body fat distribution, body composition, and respiratory function in elderly men. Am J Clin Nutr. 2005;82(5):996–1003. [DOI] [PubMed] [Google Scholar]
- 49. Ford ES, Cunningham TJ, Mercado CI. Lung function and metabolic syndrome: findings of National Health and Nutrition Examination Survey 2007–2010. J Diabetes. 2014;6(6):603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ray CS, Sue DY, Bray G, et al. Effects of obesity on respiratory function. Am Rev Respir Dis. 1983;128(3):501–506. [DOI] [PubMed] [Google Scholar]
- 51. Lourenco RV. Diaphragm activity in obesity. J Clin Invest. 1969;48(9):1609–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Naimark A, Cherniack RM. Compliance of the respiratory system and its components in health and obesity. J Appl Physiol. 1960;15:377–382. [DOI] [PubMed] [Google Scholar]
- 53. Hedenstierna G, Santesson J. Breathing mechanics, dead space and gas exchange in the extremely obese, breathing spontaneously and during anaesthesia with intermittent positive pressure ventilation. Acta Anaesthesiol Scand. 1976;20(3):248–254. [DOI] [PubMed] [Google Scholar]
- 54. Sharp JT, Henry JP, Sweany SK, et al. The total work of breathing in normal and obese men. J Clin Invest. 1964;43:728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pelosi P, Croci M, Ravagnan I, et al. Total respiratory system, lung, and chest wall mechanics in sedated-paralyzed postoperative morbidly obese patients. Chest. 1996;109(1):144–151. [DOI] [PubMed] [Google Scholar]
- 56. Zerah F, Harf A, Perlemuter L, et al. Effects of obesity on respiratory resistance. Chest. 1993;103(5):1470–1476. [DOI] [PubMed] [Google Scholar]
- 57. Watson RA, Pride NB. Postural changes in lung volumes and respiratory resistance in subjects with obesity. J Appl Physiol (1985). 2005;98(2):512–517. [DOI] [PubMed] [Google Scholar]
- 58. Cottam DR, Mattar SG, Barinas-Mitchell E, et al. The chronic inflammatory hypothesis for the morbidity associated with morbid obesity: implications and effects of weight loss. Obes Surg. 2004;14(5):589–600. [DOI] [PubMed] [Google Scholar]
- 59. Duncan BB, Schmidt MI, Chambless LE, et al. Fibrinogen, other putative markers of inflammation, and weight gain in middle-aged adults—the ARIC Study. Obes Res. 2000;8(4):279–286. [DOI] [PubMed] [Google Scholar]
- 60. Donaldson GC, Seemungal TA, Patel IS, et al. Airway and systemic inflammation and decline in lung function in patients with COPD. Chest. 2005;128(4):1995–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bousquet J, Jeffery PK, Busse WW, et al. Asthma: from bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med. 2000;161(5):1720–1745. [DOI] [PubMed] [Google Scholar]
- 62. Taher H, Bauer C, Abston E, et al. Chest wall strapping increases expiratory airflow and detectable airway segments in computer tomographic scans of normal and obstructed lungs. J Appl Physiol (1985). 2018;124(5):1186–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Abston E, Comellas A, Reed RM, et al. Higher BMI is associated with higher expiratory airflow normalised for lung volume (FEF25–75:FVC) in COPD. BMJ Open Respir Res. 2017;4(1):e000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Eberlein M, Schmidt GA, Brower RG. Chest wall strapping. An old physiology experiment with new relevance to small airways diseases. Ann Am Thorac Soc. 2014;11(8):1258–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. O’Hara P, Connett JE, Lee WW, et al. Early and late weight gain following smoking cessation in the Lung Health Study. Am J Epidemiol. 1998;148(9):821–830. [DOI] [PubMed] [Google Scholar]
- 66. Stenius-Aarniala B, Poussa T, Kvarnström J, et al. Immediate and long term effects of weight reduction in obese people with asthma: randomised controlled study. BMJ. 2000;320(7238):827–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dias-Júnior SA, Reis M, Carvalho-Pinto RM, et al. Effects of weight loss on asthma control in obese patients with severe asthma. Eur Respir J. 2014;43(5):1368–1377. [DOI] [PubMed] [Google Scholar]
- 68. Liu AG, Arceneaux KP 3rd, Chu JT, et al. The effect of caffeine and albuterol on body composition and metabolic rate. Obesity (Silver Spring). 2015;23(9):1830–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ochs-Balcom HM, Grant BJ, Muti P, et al. Pulmonary function and abdominal adiposity in the general population. Chest. 2006;129(4):853–862. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


