Abstract
Low physical activity (PA) among older adults increases the risk of cardiovascular disease (CVD) and mortality through metabolic disorders such as type 2 diabetes. We aimed to elucidate the extent to which diabetes mediates the effect of nonoccupational PA levels on CVD and mortality among older Mexican Americans. This study included 1,676 adults from the Sacramento Area Latino Study on Aging (1998–2007). We employed Cox proportional hazards regression models to investigate associations of PA level with all-cause mortality, fatal CVD, and nonfatal CVD events. Utilizing causal mediation analysis within a counterfactual framework, we decomposed the total effect of PA into natural indirect and direct effects. Over a median of 8 years of follow-up, low PA (<25th percentile) was associated with increased risks of all-cause mortality (hazard ratio (HR) = 1.36, 95% confidence interval (CI): 1.06, 1.75), fatal CVD (HR = 2.05, 95% CI: 1.42, 2.97), and nonfatal CVD events (HR = 1.67, 95% CI: 1.18, 2.37) in comparison with high PA (>75th percentile). Diabetes mediated 11.0%, 7.4%, and 5.2% of the total effect of PA on all-cause mortality, fatal CVD, and nonfatal CVD events, respectively. Our findings indicate that public health interventions targeting diabetes prevention and management would be a worthwhile strategy for preventing CVD and mortality among older Mexican Americans with insufficient PA levels.
Keywords: cardiovascular disease, diabetes, mediation analysis, Mexican Americans, mortality, physical activity
Abbreviations
- CI
confidence interval
- CVD
cardiovascular disease
- HR
hazard ratio
- MET
metabolic equivalent of task
- PA
physical activity
- SALSA
Sacramento Area Latino Study on Aging
Physical inactivity is widely recognized as an important public health problem that increases risk of type 2 diabetes, cardiovascular disease (CVD), and mortality (1–5). Previous studies suggest that type 2 diabetes may be a mediator on the causal pathway from physical inactivity to these long-term adverse outcomes (6, 7). The prevalence of physical inactivity is higher among older adults (42%) and among Hispanics (40%) compared with younger adults (22%–33%) and non-Hispanic Whites (26%) (8). Moreover, a prior study using 3 national surveys showed that older Hispanics reported lower levels of physical activity (PA) than older non-Hispanic Whites (9), underscoring the importance of investigating the health burden of physical inactivity in this minority population.
According to the 2020 Diabetes Statistics Report, 1 in 10 people in the United States has diabetes, and the prevalence rises to 21.4% for those aged ≥65 years (10). While prescribing exercise is important for management of type 2 diabetes and subsequent long-term adverse outcomes (11), initiating and maintaining active lifestyle interventions in older adults is challenging due to multiple barriers, such as comorbidity, fatigue, pain, poor perceived health, and misconceptions about benefits of PA (12). In addition, in a recent study from the National Health and Nutrition Examination Survey (2016–2017), investigators reported that type 2 diabetes prevalence in Mexican Americans is nearly double that in non-Hispanic Whites (10, 13). Long-term health outcomes from diabetes may also differ by race/ethnicity—for example, prior studies found that diabetes showed weaker associations with CVD but stronger associations with mortality among Hispanics compared with non-Hispanic Whites (14, 15). Therefore, understanding the causal pathway from PA to CVD and mortality through diabetes is crucial in order to prevent such long-term adverse outcomes in older Mexican Americans and reduce health disparities by race/ethnicity.
In the present study, using causal mediation analysis (16, 17), we aimed to investigate whether associations between nonoccupational PA and CVD (including stroke) or all-cause mortality are mediated by type 2 diabetes among older Mexican Americans. To address a potential role that sex hormones may have in modifying effects of PA on the cardiometabolic system (18, 19), we also investigated the mediation effects by sex. The distinction between direct and indirect effects provides valuable information about whether public health interventions targeting diabetes prevention and management would be beneficial for mitigating the overall risk of long-term adverse health outcomes among older Mexican Americans who are physically inactive.
METHODS
Study design and patients
We included participants with PA information from the Sacramento Area Latino Study on Aging (SALSA), a cohort study of community-dwelling older Mexican Americans in the Sacramento area of California. Eligibility criteria included 1) being 60 years of age or older at the time of enrollment in 1998–1999, 2) residing in a 6-county area in the Sacramento Valley region (Sacramento, Yolo, Sutter, Solano, San Joaquin, and Placer counties), and 3) self-identifying as Latino, Mexican, Central American, or Mexican American. Participants were contacted in 3 stages: 1) by mail, 2) by telephone, and 3) by door-to-door neighborhood enumeration. Participants who referred themselves were screened for eligibility, including residing in a targeted census tract and having a household on the sampling list. The overall response rate among those contacted was 85%. A total of 1,789 participants were initially enrolled, and they were followed with interviews and examinations in their homes every 12–15 months for up to 7 study visits through the end of 2007. Among the 1,789 participants, 1,676 had PA information at baseline. More details about sampling and study procedures have been provided elsewhere (20). All procedures described here were approved by the institutional review boards of the universities involved (University of California, San Francisco; University of California, Los Angeles; University of California, Davis; University of Michigan; and University of North Carolina).
Measurement of variables
Physical activity
At baseline, participants were asked to report the average number of hours per week they spent in 18 different types of non-work-related activities that are common among older adults. Metabolic equivalents of task (METs) were assigned to each activity based on the Compendium of Physical Activities (21). This value was multiplied by the reported amount of time (hours/week) spent performing the activity (MET-hours/week). Cumulative PA measures were calculated to represent moderate- to vigorous-intensity PA levels by summing MET-hours/week values for 9 activities that require a 3-fold or greater increase over the metabolic rate achieved by sitting quietly (≥3 METs) (22): taking walks; walking around the neighborhood; dancing; hunting, camping, or boating; swimming or engaging in workouts; golfing or other moderate exercise; gardening or yard work; house repairs; and heavy housework (22, 23). Participants were then categorized into the following 3 groups according to their PA levels: low PA (<25th percentile; <20 MET-hours/week), medium PA (25th–75th percentiles; 20–97 MET-hours/week), and high PA (>75th percentile; >97 MET-hours/week).
Diabetes
We classified diabetes as fasting glucose level ≥7.0 mmol/L, use of antidiabetic medication, or self-report of a physician’s diagnosis at baseline, as in previous studies (10, 24). Fasting glucose level was measured with the Cobas Mira Chemistry Analyzer (Roche Diagnostics Corporation, Indianapolis, Indiana). Medication use was assessed by means of a medicine cabinet inventory of prescription medicines.
Other covariates
At the baseline interview, participants reported their age, sex (male, female), educational level (0, 1–8, 9–12, or ≥13 years of education), country of birth (United States, other), marital status (single, married), tobacco smoking status (current, former, or never smoker), alcohol intake (frequent (daily) drinker, moderate (weekly) drinker, occasional (monthly) drinker, or rarely/never drinking), activities of daily living status, instrumental activities of daily living status, current employment status (yes, no), and type of lifetime occupation (nonmanual, manual, other). According to previous literature (25, 26), limitation in activities of daily living was defined on the basis of whether or not the participant report having difficulty with ≥1 activities. Similarly, limitation in instrumental activities of daily living was defined on the basis of whether or not the participant reported having difficulty with ≥3 activities (25, 26). Acculturation was assessed using the Geriatric Acculturation Ratings Scale for Mexican Americans, a modified version of the Acculturation Ratings Scale for Mexican Americans-II for use in older Latinos that consisted of 19 items assessing English and Spanish language and media use, childhood and current friendships, contact with Latin America, and dietary practices (27). Systolic and diastolic blood pressure measurements were made with an automatic digital blood pressure monitor while the participant was seated, and 2 measurements taken within a 10-minute interval were averaged. Hypertension was based on measured systolic blood pressure (≥140 mm Hg), diastolic blood pressure (≥90 mm Hg), self-report of a physician’s diagnosis, and/or use of antihypertensive medication (28). Low-density lipoprotein cholesterol level was measured from a morning fasting serum sample using LDL Direct Liquid Select Cholesterol Reagent (catalog number 7120; Equal Diagnostics, Inc., Exton, Pennsylvania). Prescription statin use was also self-reported. Body mass index (weight (kg)/height (m)2) was calculated on the basis of the participant’s measured height (tape measure) and weight (Tanita scale). Waist circumference was measured with a tape at the level of maximum indentation over the abdomen, following a standard protocol.
Ascertainment of outcomes
The primary outcome was all-cause mortality, with secondary outcomes being fatal and nonfatal CVD events (including stroke). Mortality data were ascertained through May 2010, using online obituary surveillance, review of the Social Security Death Index and the National Death Index, review of vital statistics data files from California, and interviews with family members. If participants were not identified as deceased, they were assumed to be alive and censored at the date of the last contact. Fatal CVD events were defined as death for which any of the following International Classification of Diseases, Tenth Revision (ICD-10) codes were mentioned anywhere on the death certificate: codes I20–I25 (ischemic heart disease), code I50 (heart failure), and code I63 or I64 (stroke). Nonfatal CVD events were ascertained by self-report at each visit and by phone call; that is, participants were asked whether a physician had diagnosed any of the following: myocardial infarction, angina, catheterization or coronary artery bypass grafting, stroke, heart failure, or atrial fibrillation. For analyses of nonfatal CVD events, 612 persons with a self-reported history of CVD at baseline were excluded to estimate effects of PA on primary CVD events.
Statistical analyses
Crude and multivariable Cox proportional hazards regression models were employed for estimating effects of categorical PA exposure (low, medium, high) on the risks of all-cause mortality, fatal CVD events, and nonfatal CVD events in separate models while adjusting for potential confounders. Missing data in each variable were replaced using multiple-imputation algorithms which included all of the above-mentioned covariates in the model (29). We first adjusted for age, sex, educational level, country of birth, and marital status (model 1). We then further adjusted for score on the Geriatric Acculturation Ratings Scale for Mexican Americans, smoking status, alcohol intake, activities of daily living, instrumental activities of daily living, current employment status, and type of lifetime occupation, in addition to the variables in model 1 (model 2). We also performed competing-risk analysis with the method proposed by Fine and Gray (30), considering the competing risks for fatal and nonfatal CVD events. In this competing-risk analysis, we estimated the subdistribution hazard by constructing risk sets that included both persons without any event and those who had competing events such as cancer-related mortality (30, 31).
In mediation analyses, we aimed to quantify the degree to which diabetes mediates the associations between PA and long-term outcomes, including all-cause mortality, fatal CVD, and nonfatal CVD events, adjusting for the potential confounders included in model 2 (see Web Figure 1, available at https://academic.oup.com/aje). We employed a marginal structural approach based on the counterfactual framework to estimate the natural direct and indirect effects (32, 33). The natural direct effect is the effect of PA on long-term outcomes via pathways that do not involve diabetes while diabetes status is allowed to vary according to determinants of diabetes, except PA. The natural indirect effect represents the effect of PA on long-term outcomes due to the effect that PA has on diabetes; that is, we estimate the hazard ratios for the counterfactual outcomes given a physically “active” status if diabetes status changed to what it would be given a physically “inactive” status. Robust 95% confidence intervals were estimated by repeating the analysis on 10,000 bootstrapped samples. The mediated proportion was computed as the log of the natural indirect effect divided by the log of the total effect. We included cross-product terms for exposure and mediator in the model, but there was no indication of an interaction. More detailed discussion and coding tutorials using R software are provided elsewhere (16).
Because previous studies have suggested that there is a difference in the effect of PA levels on long-term adverse outcomes by sex (3, 34, 35), we also conducted stratum-specific analyses to estimate the causal mediation effects of diabetes on the pathway between PA and long-term adverse outcomes according to sex. We also calculated P values for multiplicative interaction terms between PA level and sex for the total effects on long-term adverse outcomes.
We performed several sensitivity analyses. First, we additionally adjusted for other metabolic factors such as body mass index, waist circumference, hypertension, prescription statin use, and low-density lipoprotein cholesterol levels, which we did not include in the main model because we assumed that they are more likely to be mediators than confounders in the pathway from physical inactivity to CVD and mortality. Second, we reanalyzed the data using ≤8.3 MET-hours/week (i.e., 500 MET-minutes/week) as the cutoff for a low PA level based on the recommendation of the Physical Activity Guidelines for Americans (22, 36). Finally, we fitted Aalen’s additive hazard models, which allowed us to estimate hazard differences without the assumption of proportionality (17). Using this model, we can estimate the actual number of additional events that provide insight into the potential public health interventions. Effect estimates presented here may be considered statistically significant if the 95% confidence interval does not include the null value.
Statistical analyses were conducted using Stata, version 15 (StataCorp LLC, College Station, Texas) and R, version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria). Sample syntax for each analysis is shown in the Web Appendix.
RESULTS
The mean age of participants was 70.3 years, and 41.7% were male (Table 1). PA levels were generally lower in participants with lower educational levels and those who rarely/never consumed alcohol but were higher among those in nonmanual occupations. The low-PA group exhibited the highest prevalences of diabetes, hypertension, obesity, prescription statin use, and history of CVD events. We found similar characteristics by PA level when we excluded participants with a history of CVD at baseline (Web Table 1).
Table 1.
Baseline Clinical Characteristics of Participants According to Physical Activity Level, Sacramento Area Latino Study on Aging, 1998–2007
| Physical Activity Level, MET-hours/week | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Total (n  1,676) 1,676)
|
Low (20) (n  419) 419)
|
Medium (20–97) (n  838) 838)
|
High (>97) (n  419) 419)
|
|||||
| Variable | No. | % | No. | % | No. | % | No. | % |
| Male sex | 699 | 41.7 | 127 | 30.3 | 356 | 42.5 | 216 | 51.6 |
| Age, yearsa | 70.3 (6.8) | 71.4 (7.6) | 70.1 (6.6) | 69.8 (5.9) | ||||
| US birth | 823 | 49.1 | 201 | 48.0 | 411 | 49.1 | 211 | 50.4 |
| Education, years | ||||||||
| 0 | 216 | 12.9 | 62 | 14.8 | 107 | 12.8 | 47 | 11.2 |
| 1–8 | 794 | 47.4 | 219 | 52.3 | 392 | 46.8 | 183 | 43.7 |
| 9–12 | 385 | 23.0 | 91 | 21.7 | 193 | 23.0 | 101 | 24.1 |
| ≥13 | 281 | 16.8 | 47 | 11.2 | 146 | 17.4 | 88 | 21.0 |
| Married | 979 | 58.4 | 223 | 53.2 | 492 | 58.7 | 264 | 63.0 |
| Acculturation scorea,b | 22.0 (13.0) | 20.7 (12.7) | 21.9 (13.1) | 23.4 (12.9) | ||||
| Tobacco smoking (all types) | ||||||||
| Current smoker | 187 | 11.2 | 48 | 11.5 | 92 | 11.0 | 47 | 11.2 |
| Former smoker | 713 | 42.5 | 168 | 40.1 | 367 | 43.8 | 178 | 42.5 |
| Never smoker | 776 | 46.3 | 203 | 48.4 | 379 | 45.2 | 194 | 46.3 |
| Alcohol consumption | ||||||||
| Frequent (daily) | 147 | 8.8 | 27 | 6.4 | 73 | 8.7 | 47 | 11.2 |
| Moderate (weekly) | 181 | 10.8 | 25 | 6.0 | 89 | 10.6 | 67 | 16.0 |
| Occasional (monthly) | 153 | 9.1 | 24 | 5.7 | 86 | 10.3 | 43 | 10.3 |
| Yearly/rarely/never | 1,195 | 71.3 | 343 | 81.9 | 590 | 70.4 | 262 | 62.5 |
| ADL difficultyc | 200 | 11.9 | 112 | 26.7 | 66 | 7.9 | 22 | 5.3 |
| IADL difficultyd | 788 | 47.0 | 266 | 63.5 | 383 | 45.7 | 139 | 33.2 |
| Lifetime occupation | ||||||||
| Nonmanual | 362 | 21.6 | 68 | 16.2 | 194 | 23.2 | 100 | 23.9 |
| Manual | 1,000 | 59.7 | 241 | 57.5 | 496 | 59.2 | 263 | 62.8 |
| Other | 314 | 18.7 | 110 | 26.3 | 148 | 17.7 | 56 | 13.4 |
| Currently employed | 285 | 17.0 | 70 | 16.7 | 152 | 18.1 | 63 | 15.0 |
| Statin use | 141 | 8.4 | 41 | 9.8 | 68 | 8.1 | 32 | 7.6 |
| LDL cholesterol level, mmol/La | 3.2 (0.9) | 3.0 (0.9) | 3.2 (0.9) | 3.2 (0.9) | ||||
| Diabetes | 542 | 32.3 | 166 | 39.6 | 256 | 30.6 | 120 | 28.6 |
| Hypertension | 1,136 | 67.8 | 309 | 73.8 | 547 | 65.3 | 280 | 66.8 |
| Body mass indexe | ||||||||
| <25 | 324 | 19.3 | 83 | 19.8 | 158 | 18.9 | 83 | 19.8 |
| 25–29 | 628 | 37.5 | 135 | 32.2 | 326 | 38.9 | 167 | 39.9 |
| ≥30 | 724 | 43.2 | 201 | 48.0 | 354 | 42.2 | 169 | 40.3 |
| Waist circumference, cma | 97.0 (13.3) | 98.9 (13.8) | 96.4 (13.4) | 96.2 (12.5) | ||||
| Cardiovascular disease | 612 | 36.5 | 187 | 44.6 | 300 | 35.8 | 125 | 29.8 |
Abbreviations: ADL, activities of daily living; IADL, instrumental activities of daily living; LDL, low-density lipoprotein; MET, metabolic equivalent of task.
a Values are expressed as mean (standard deviation).
b Acculturation was estimated using the Geriatric Acculturation Ratings Scale for Mexican Americans. Possible scores range from 0 to 56, and a lower score means the respondent is less acculturated (i.e., more Mexican-oriented).
c For estimation of ADL level, participants were asked whether they had any difficulty engaging in the following: walking, bathing, brushing hair and teeth, eating, putting clothes on, and moving from the bed to a chair. ADL difficulty was based on whether or not the participant reported having difficulty with ≥1 activities.
d For estimation of IADL level, participants were asked whether they had any difficulty engaging in the following: moving heavy objects, kneeling, lifting weights over 10 pounds (4.5 kg), extending arms above shoulders, getting up from kneeling, standing up from a chair, climbing stairs, writing, walking 0.25 mile (0.4 km), walking 10 steps, using a telephone, managing money, cooking, performing housework, and shopping. IADL difficulty was based on whether or not the participant reported having difficulty with ≥3 activities.
e Weight (kg)/height (m)2.
The median duration of follow-up for all-cause mortality was 7.7 years (interquartile range, 4.7–8.4), during which 579 deaths were identified. A total of 263 fatal CVD events and 369 nonfatal CVD events were identified. All-cause mortality was higher in the low-PA group than in the high-PA group (hazard ratio (HR) = 1.36, 95% confidence interval (CI): 1.06, 1.75) after model 2 adjustments (Table 2). The low-PA group also experienced higher risks of both fatal CVD (HR = 2.05, 95% CI: 1.42, 2.97) and nonfatal CVD events (HR = 1.67, 95% CI: 1.18, 2.37) than the high-PA group. The medium-PA group showed higher risks of nonfatal CVD compared with the high-PA group (HR = 1.38, 95% CI: 1.03, 1.85). These results were not altered in the competing-risks survival regression model (Table 2, Web Table 2).
Table 2.
Incidence of All-Cause Mortality and Fatal and Nonfatal Cardiovascular Disease Events by Physical Activity Level, Sacramento Area Latino Study on Aging, 1998–2007
| Unadjusted Results | Model 1 a | Model 2 b | ||||||
|---|---|---|---|---|---|---|---|---|
| Outcome and Physical Activity Level, MET-hours/week | No. of Incident Cases | Total No. of Participants | HR | 95% CI | HR | 95% CI | HR | 95% CI |
| All-cause mortality | ||||||||
| High (>97) | 115 | 419 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Medium (20–97) | 262 | 838 | 1.21 | 0.97, 1.51 | 1.25 | 1.00, 1.56 | 1.13 | 0.90, 1.41 |
| Low (<20) | 202 | 419 | 1.90 | 1.50, 2.39 | 1.85 | 1.46, 2.34 | 1.36 | 1.06, 1.75 |
| P for trendc | <0.001 | <0.001 | 0.03 | |||||
| Fatal CVD event | ||||||||
| High (>97) | 46 | 419 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Medium (20–97) | 111 | 838 | 1.25 | 0.89, 1.77 | 1.25 | 0.89, 1.77 | 1.07 | 0.75, 1.52 |
| Low (<20) | 106 | 419 | 2.88 | 2.03, 4.06 | 2.75 | 1.93, 3.91 | 2.05 | 1.42, 2.97 |
| P for trend | <0.001 | <0.001 | 0.001 | |||||
| Nonfatal CVD event | ||||||||
| High (>97) | 84 | 294 | 1.00 | Referent | 1.00 | Referent | 1.00 | Referent |
| Medium (20–97) | 192 | 538 | 1.52 | 1.15, 2.02 | 1.51 | 1.13, 2.00 | 1.38 | 1.03, 1.85 |
| Low (<20) | 93 | 232 | 1.97 | 1.42, 2.74 | 1.89 | 1.35, 2.64 | 1.67 | 1.18, 2.37 |
| P for trend | <0.001 | 0.001 | 0.004 | |||||
Abbreviations: CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; MET, metabolic equivalent of task.
a Adjusted for age, sex, educational level, country of birth, and marital status.
b Adjusted for acculturation, smoking, alcohol intake, activities of daily living, instrumental activities of daily living, current employment status, and type of occupation, in addition to the variables in model 1.
c The median MET value was assigned for each physical activity category (low, 7 MET-hours/week; medium, 48.5 MET-hours/week; high, 147.5 MET-hours/week).
We estimated that diabetes mediated 11.0% of the effect of PA (low vs. high) on all-cause mortality (total effect: HR = 1.36 (95% CI: 1.02, 1.81); indirect effect: HR = 1.04 (95% CI: 1.00, 1.09)) (Table 3). For fatal CVD events and nonfatal CVD events, we estimated that diabetes mediated 7.4% (total effect: HR = 2.05 (95% CI: 1.40, 3.09); indirect effect: HR = 1.05 (95% CI: 1.00, 1.14)) and 5.2% (total effect: HR = 1.67 (95% CI: 1.18, 2.45); indirect effect: HR = 1.03 (95% CI: 0.96, 1.10)) of the effect of PA, respectively. The mediation effects of diabetes on the association between PA (medium vs. high) and these outcomes were small, and the 95% confidence intervals included the null value. The results were qualitatively consistent when we additionally adjusted for metabolic factors (Web Table 3) and when we used the recommended PA levels as the cutoff point for defining low PA (Web Table 4).
Table 3.
Direct and Indirect (Through Diabetes) Effects of Physical Activity Level on the Incidence of All-Cause Mortality and Fatal and Nonfatal Cardiovascular Disease Events (Hazard Ratio Scale), Sacramento Area Latino Study on Aging, 1998–2007a
| Total Effect | Direct Effect | Indirect Effect | |||||
|---|---|---|---|---|---|---|---|
| Outcome and Physical Activity Level, MET-hours/week | HR | 95% CI | HR | 95% CI | HR | 95% CI | % Mediated b |
| All-cause mortality | |||||||
| Medium (20–97) vs. high (>97) | 1.13 | 0.89, 1.42 | 1.13 | 0.87, 1.42 | 1.00 | 0.97, 1.05 | 2.6 |
| Low (<20) vs. high (>97) | 1.36 | 1.02, 1.81 | 1.32 | 0.99, 1.77 | 1.04 | 1.00, 1.09 | 11.0 |
| Fatal CVD events | |||||||
| Medium (20–97) vs. high (>97) | 1.07 | 0.75, 1.58 | 1.06 | 0.75, 1.56 | 1.00 | 0.95, 1.07 | 6.9 |
| Low (<20) vs. high (>97) | 2.05 | 1.40, 3.09 | 1.94 | 1.34, 2.96 | 1.05 | 1.00, 1.14 | 7.4 |
| Nonfatal CVD events | |||||||
| Medium (20–97) vs. high (>97) | 1.38 | 1.02, 1.91 | 1.38 | 1.03, 1.92 | 1.00 | 0.97, 1.03 | 0.0 |
| Low (<20) vs. high (>97) | 1.67 | 1.18, 2.45 | 1.63 | 1.17, 2.41 | 1.03 | 0.96, 1.10 | 5.2 |
Abbreviations: CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio.
a Results were adjusted for age, sex, educational level, country of birth, marital status, acculturation, smoking, alcohol intake, activities of daily living, instrumental activities of daily living, current employment status, and type of occupation. We performed 10,000 iterations for bootstrapping to estimate 95% CIs.
b The percent mediated was calculated as log(indirect effect)/log(total effect) and therefore depended on both the total effect and the indirect effect.
In stratum-specific analyses, we estimated that diabetes mediated 55.8% of the effect of PA (low vs. high) on all-cause mortality for males, but there was no evidence of mediation among females (Figure 1, Web Table 5). For fatal and nonfatal CVD events separately, we estimated that diabetes mediated 22.9% and 22.1% of the effect for males but not females.
Figure 1.
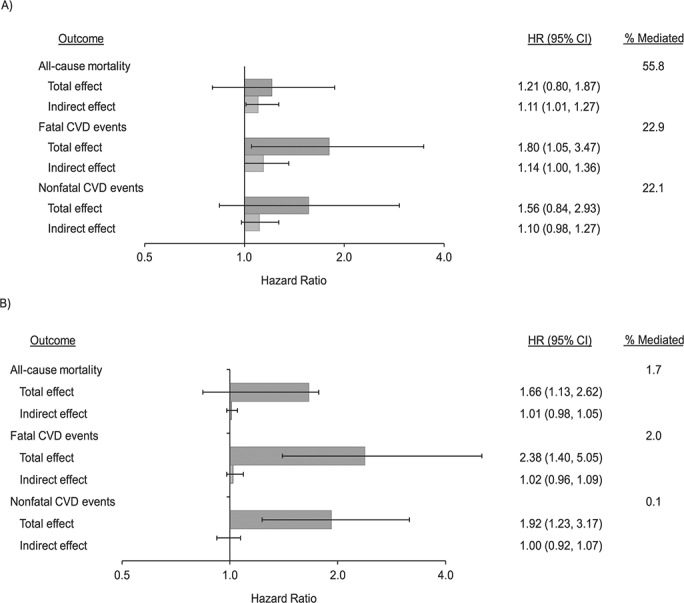
Decomposition of the effect of physical activity level (low vs. high) on the incidence of all-cause mortality, fatal cardiovascular disease (CVD), and nonfatal CVD events via diabetes, by sex, Sacramento Area Latino Study on Aging, 1998–2007. A) men; B) women. Hazard ratios (HRs) were adjusted for age, sex, educational level, country of birth, marital status, acculturation, smoking, alcohol intake, activities of daily living, instrumental activities of daily living, current employment status, and type of occupation. We performed 10,000 iterations for bootstrapping to estimate 95% confidence intervals (CIs) for the total effect and the indirect effect (through diabetes). The x-axis is shown on the log scale. The percent mediated was calculated as log(indirect effect)/log(total effect).
In Aalen’s additive hazard model, we estimated that diabetes mediated 7.0% of the effect of PA on all-cause mortality among participants with low (vs. high) PA (total effect: 4,184 additional cases per 100,000 person-years; indirect effect: 292 additional cases per 100,000 person-years)) (Web Table 6). For fatal and nonfatal CVD events among those with low (vs. high) PA, we estimated that diabetes mediated 4.3% (total effect: 2,744 additional cases per 100,000 person-years; indirect effect: 118 additional cases per 100,000 person-years) and 2.2% (direct effect: 2,929 additional cases per 100,000 person-years; indirect effect: 63 additional cases per 100,000 person-years) of the effect, respectively.
DISCUSSION
In this population-based study of older Mexican Americans, diabetes mediated around 5%–10% of the association of physical inactivity with all-cause mortality, fatal CVD events, and nonfatal CVD events. Mediation effects of diabetes on these outcomes were much more prominent among males than among females.
To the best of our knowledge, this is the first study to have quantified the extent to which diabetes mediates the association of PA with all-cause mortality and CVD events in older Mexican Americans. Given the high prevalence of type 2 diabetes in this population and the challenges of prescribing exercise in older adults (12, 13), our findings underscore the importance of public health interventions for the prevention of type 2 diabetes and its long-term sequelae among physically inactive older Mexican Americans. While it is well known that PA influences risk of type 2 diabetes and CVD, including randomized controlled trials in older adults and meta-analyses (6, 7, 37–40), the extent to which type 2 diabetes mediates the association between PA and CVD has not yet been explored sufficiently. Previous studies suggesting that type 2 diabetes may mediate the association between PA and long-term adverse outcomes have approached this question by evaluating the change in the estimate with and without adjusting for the potential mediators (6, 7). However, such an approach does not always validly assess mediation, nor does it quantify this effect (17, 41). Here, we used more appropriate methods—that is, proportional and additive hazard models based on the counterfactual framework (16, 32).
The underlying mechanisms through which diabetes may mediate the association between PA and long-term adverse outcomes include improved energy balance, reduction of adiposity, and reduction of inflammation through high PA (42, 43). High PA also affects myosin phenotypic characteristics, increases mitochondrial activity and volume, and increases glucose transporter type 4 protein expression, which may reduce risk of type 2 diabetes through improved insulin sensitivity and subsequently reduce risk of CVD and mortality (44–46).
Consistent with prior studies (3, 34, 35), the estimated total effect of low PA on long-term outcomes was larger in females than in males, but the term for interaction between PA and sex was not statistically significant. In contrast, the proportion of the estimated effect of low PA on long-term adverse outcomes mediated by diabetes was larger in males than in females. In the San Antonio Heart Study, a cohort study of Mexican Americans aged 25–64 years, Monterrosa et al. (5) reported an association between low PA and type 2 diabetes incidence in males only. Biologically, higher PA is associated with lower levels of testosterone and estradiol in postmenopausal women but with higher testosterone levels in men (18, 19). The Mexican Americans in SALSA are older (ages 60–93 years) than participants in the previous studies, and PA might affect pathways involved in long-term adverse outcomes other than type 2 diabetes (e.g., endogenous levels of sex hormones); these might have larger contributions to the overall effect of PA on adverse health outcomes in females than in males. The observed sex discrepancy might also be associated with the PA health paradox—that is, high leisure-time PA decreases risks of CVD outcomes but high occupational PA increases this risk due to sustained inflammatory responses and 24-hour elevated heart rate (47). Because men are more likely to have manual occupations with higher PA demands than females, the benefits of engaging in nonoccupational PA for long-term adverse outcomes (especially the direct pathway that does not go through diabetes) might have been diluted by higher occupational PA levels even after controlling for current employment status and type of occupation in men. Lastly, previous studies have found sex differences in type 2 diabetes due to obesity (42, 48, 49). In general, females have a stronger obesity-related risk of diabetes due to abdominal adiposity than males, and the impact of physical inactivity on this type of obesity is greater (48, 50, 51). Thus, future studies are needed to prospectively estimate mediating effects of both obesity and type 2 diabetes from PA to long-term adverse outcomes to elucidate mechanisms underlying sex differences (52).
The population-based longitudinal design of this study was a major strength, and it enabled us to analyze the incidence of long-term adverse outcomes over 10 years in an understudied older ethnic minority population. However, our study also had several limitations. First, while there is some evidence showing that low PA increases type 2 diabetes incidence (34, 49) and we assumed that self-reported PA reflected participants’ long-term PA levels, we still have to consider the possibility of reverse causation (i.e., a diagnosis of diabetes might have affected PA levels). Persons diagnosed with diabetes would be expected to be encouraged by their health-care providers to increase their PA levels; thus, a reversal of temporality would be expected to induce a bias toward the null. Conversely, some persons diagnosed with diabetes a very long time ago may have become physically inactive due to complications of diabetes. Second, there was potential misclassification of the exposure, mediator, and outcomes. PA levels were estimated at baseline on the basis of self-reports, and no information was collected regarding trends in PA levels over the follow-up period. We classified the participants as having diabetes based on self-report, medication use, and fasting glucose level, as in previous studies (10, 24), but we lacked information on hemoglobin A1c level, oral glucose tolerance testing, and the presence of diabetes-related antibodies (e.g., glutamic acid decarboxylase antibodies) at baseline. Relying on self-reporting of nonfatal CVD events could have introduced potential outcome misclassification. Moreover, mortality surveillance might have been less accurate after active follow-up ended in 2008. Even though misclassification was probably nondifferential with respect to exposure, the bias this may generate is not always bias toward the null in mediation analysis (17). Third, individuals had to survive to at least 60 years of age to participate in this study. Our estimates might have been affected by the nonenrollment of persons with disabilities and mortality among people with diabetes before age 60 years. Fourth, given that SALSA participants were residents of the Sacramento area, our findings may not be generalizable to older Mexican Americans elsewhere. Further multiregional studies with longitudinal measures of PA and diabetes are needed to overcome these limitations and to help better establish temporality.
Our model was based on the assumption that there are no other unmeasured confounders and no mediator-outcome confounders affected by exposure (17). However, for example, metabolic factors (e.g., obesity, hypertension, and dyslipidemia) might be affected by PA and also affect both diabetes status and long-term health outcomes. Therefore, we may overestimate the indirect effect if we do not control for metabolic factors, and on the other hand, we may underestimate the direct effect when we control for metabolic factors (i.e., adjust for factors that are intermediate in the pathway and not operating directly through diabetes). Moreover, if there is unmeasured confounding between metabolic factors and outcomes, controlling for such metabolic factors could induce collider-stratification bias (53). However, we found qualitatively consistent results when we adjusted for metabolic factors, indicating that these potential biases did not change our main findings substantially. As we mentioned above, more advanced mediation analysis with multiple mediators would be helpful to fully address this issue in the future but would require larger samples (52).
In conclusion, the present study suggested that diabetes mediates the estimated effects of physical inactivity on long-term adverse outcomes among older Mexican Americans, particularly men. Given the rapidly growing older adult population with a high prevalence of diabetes and the challenges of prescribing exercise in older adults, our findings suggest that public health interventions targeting diabetes prevention and management (e.g., active diabetes screening programs focusing on physically inactive older adults) would be worthwhile strategies for preventing long-term adverse outcomes in older Mexican Americans.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Fielding School of Public Health, University of California, Los Angeles, Los Angeles, California (Kosuke Inoue, Elizabeth R. Mayeda, Kimberly C. Paul, I-Fan Shih, Qi Yan, Yu Yu, and Beate R. Ritz); Department of Epidemiology and Biostatistics, School of Medicine, University of California, San Francisco, San Francisco, California (Mary Haan); and Department of Neurology, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California (Beate R. Ritz).
K.I. was supported by the Burroughs Wellcome Fund Interschool Training Program in Chronic Diseases and a fellowship in epidemiology at the University of California, Los Angeles. E.R.M. was supported by National Institute on Aging grant R00AG053410. K.P. was supported by a Burroughs Wellcome Fund Population and Laboratory Based Sciences Award and by the National Institute of Environmental Health Sciences (Ruth L. Kirschstein National Research Service F32 Award ES028087). The Sacramento Area Latino Study on Aging was supported by the National Institute of Environmental Health Sciences (grant R01 ES023451), the National Institute on Aging (grants AG012975 and AG033751), and the National Institute of Diabetes and Digestive and Kidney Diseases (grant DK060753).
We thank the study participants for their contributions.
The funders played no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, and approval of the manuscript; or the decision to submit the manuscript for publication.
Conflict of interest: none declared.
REFERENCES
- 1. GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lobelo F, Rohm Young D, Sallis R, et al. Routine assessment and promotion of physical activity in healthcare settings: a scientific statement from the American Heart Association. Circulation. 2018;137(18):e495–e522. [DOI] [PubMed] [Google Scholar]
- 3. Arem H, Moore SC, Patel A, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med. 2015;175(6):959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burchfiel CM, Sharp DS, Curb JD, et al. Physical activity and incidence of diabetes: the Honolulu Heart Program. Am J Epidemiol. 1995;141(4):360–368. [DOI] [PubMed] [Google Scholar]
- 5. Monterrosa AE, Haffner SM, Stern MP, et al. Sex difference in lifestyle factors predictive of diabetes in Mexican-Americans. Diabetes Care. 1995;18(4):448–456. [DOI] [PubMed] [Google Scholar]
- 6. Reddigan JI, Ardern CI, Riddell MC, et al. Relation of physical activity to cardiovascular disease mortality and the influence of cardiometabolic risk factors. Am J Cardiol. 2011;108(10):1426–1431. [DOI] [PubMed] [Google Scholar]
- 7. Hamer M, Stamatakis E. Physical activity and risk of cardiovascular disease events: inflammatory and metabolic mechanisms. Med Sci Sports Exerc. 2009;41(6):1206–1211. [DOI] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention Status Report for Step It Up! The Surgeon General’s Call to Action to Promote Walking and Walkable Communities. Atlanta, GA: Centers for Disease Control and Prevention; 2017. https://www.cdc.gov/physicalactivity/walking/call-to-action/index.htm. Accessed April 8, 2020. [Google Scholar]
- 9. Keadle SK, McKinnon R, Graubard BI, et al. Prevalence and trends in physical activity among older adults in the United States: a comparison across three national surveys. Prev Med. 2016;89:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Centers for Disease Control and Prevention National Diabetes Statistics Report, 2020 . Atlanta, GA: Centers for Disease Control and Prevention; 2020. https://www.cdc.gov/diabetes/data/statistics/statistics-report.html. Accessed April 8, 2020. [Google Scholar]
- 11. American Diabetes Association 5. Lifestyle management: Standards of Medical Care in Diabetes—2019. Diabetes Care. 2019;42(suppl 1):S46–S60. [DOI] [PubMed] [Google Scholar]
- 12. Brawley LR, Rejeski WJ, King AC. Promoting physical activity for older adults: the challenges for changing behavior. Am J Prev Med. 2003;25(3 suppl 2):172–183. [DOI] [PubMed] [Google Scholar]
- 13. Xu G, Liu B, Sun Y, et al. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ. 2018;362:k1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Golden SH, Brown A, Cauley JA, et al. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors—an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2012;97(9):E1579–E1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep. 2013;13(6):814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nordahl H, Lange T, Osler M, et al. Education and cause-specific mortality: the mediating role of differential exposure and vulnerability to behavioral risk factors. Epidemiology. 2014;25(3):389–396. [DOI] [PubMed] [Google Scholar]
- 17. VanderWeele TJ. Mediation analysis: a practitioner’s guide. Annu Rev Public Health. 2016;37:17–32. [DOI] [PubMed] [Google Scholar]
- 18. Bertone-Johnson ER, Tworoger SS, Hankinson SE. Recreational physical activity and steroid hormone levels in postmenopausal women. Am J Epidemiol. 2009;170(9):1095–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shiels MS, Rohrmann S, Menke A, et al. Association of cigarette smoking, alcohol consumption, and physical activity with sex steroid hormone levels in US men. Cancer Causes Control. 2009;20(6):877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haan MN, Mungas DM, Gonzalez HM, et al. Prevalence of dementia in older Latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003;51(2):169–177. [DOI] [PubMed] [Google Scholar]
- 21. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of Physical Activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 suppl):S498–S504. [DOI] [PubMed] [Google Scholar]
- 22. Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320(19):2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shih I-F, Haan MN, Paul KC, et al. The roles of physical activity and inflammation in mortality, cognition, and depressive symptoms among older Mexican Americans. Am J Epidemiol. 2019;188(11):1944–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mayeda ER, Haan MN, Kanaya AM, et al. Type 2 diabetes and 10-year risk of dementia and cognitive impairment among older Mexican Americans. Diabetes Care. 2013;36(9):2600–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu JH, Haan MN, Liang J, et al. Diabetes as a predictor of change in functional status among older Mexican Americans: a population-based cohort study. Diabetes Care. 2003;26(2):314–319. [DOI] [PubMed] [Google Scholar]
- 26. Aiello AE, Haan MN, Pierce CM, et al. Persistent infection, inflammation, and functional impairment in older Latinos. J Gerontol A Biol Sci Med Sci. 2008;63(6):610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. González HM, Haan MN, Hinton L. Acculturation and the prevalence of depression in older Mexican Americans: baseline results of the Sacramento Area Latino Study on Aging. J Am Geriatr Soc. 2001;49(7):948–953. [DOI] [PubMed] [Google Scholar]
- 28. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–520. [DOI] [PubMed] [Google Scholar]
- 29. Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10(4):585–598. [DOI] [PubMed] [Google Scholar]
- 30. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 31. Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pearl J. The causal mediation formula—a guide to the assessment of pathways and mechanisms. Prev Sci. 2012;13(4):426–436. [DOI] [PubMed] [Google Scholar]
- 33. Richiardi L, Bellocco R, Zugna D. Mediation analysis in epidemiology: methods, interpretation and bias. Int J Epidemiol. 2013;42(5):1511–1519. [DOI] [PubMed] [Google Scholar]
- 34. Jeon CY, Lokken RP, Hu FB, et al. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care. 2007;30(3):744–752. [DOI] [PubMed] [Google Scholar]
- 35. Elhakeem A, Cooper R, Whincup P, et al. Physical activity, sedentary time, and cardiovascular disease biomarkers at age 60 to 64 years. J Am Heart Assoc. 2018;7(16):e007459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. World Health Organization Physical activity and older adults. https://www.who.int/dietphysicalactivity/factsheet_olderadults/en/. Accessed November 27, 2019.
- 37. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Franco OH, Laet C, Peeters A, et al. Effects of physical activity on life expectancy with cardiovascular disease. Arch Intern Med. 2005;165(20):2355–2360. [DOI] [PubMed] [Google Scholar]
- 39. Berlin JA, Colditz GA. A meta-analysis of physical activity in the prevention of coronary heart disease. Am J Epidemiol. 1990;132(4):612–628. [DOI] [PubMed] [Google Scholar]
- 40. Koolhaas CM, Dhana K, Golubic R, et al. Physical activity types and coronary heart disease risk in middle-aged and elderly persons: the Rotterdam Study. Am J Epidemiol. 2016;183(8):729–738. [DOI] [PubMed] [Google Scholar]
- 41. VanderWeele TJ, Robinson WR. On the causal interpretation of race in regressions adjusting for confounding and mediating variables. Epidemiology. 2014;25(4):473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mozaffarian D, Hao T, Rimm EB, et al. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rothenbacher D, Hoffmeister A, Brenner H, et al. Physical activity, coronary heart disease, and inflammatory response. Arch Intern Med. 2003;163(10):1200–1205. [DOI] [PubMed] [Google Scholar]
- 44. Borghouts LB, Keizer HA. Exercise and insulin sensitivity: a review. Int J Sports Med. 2000;21(1):1–12. [DOI] [PubMed] [Google Scholar]
- 45. Short KR, Vittone JL, Bigelow ML, et al. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52(8):1888–1896. [DOI] [PubMed] [Google Scholar]
- 46. Röckl KSC, Witczak CA, Goodyear LJ. Signaling mechanisms in skeletal muscle: acute responses and chronic adaptations to exercise. IUBMB Life. 2008;60(3):145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Holtermann A, Krause N, Beek AJ, et al. The physical activity paradox: six reasons why occupational physical activity (OPA) does not confer the cardiovascular health benefits that leisure time physical activity does. Br J Sports Med. 2018;52(3):149–150. [DOI] [PubMed] [Google Scholar]
- 48. Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 2016;37(3):278–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Aune D, Norat T, Leitzmann M, et al. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol. 2015;30(7):529–542. [DOI] [PubMed] [Google Scholar]
- 50. Tobias M. Global control of diabetes: information for action. Lancet. 2011;378(9785):3–4. [DOI] [PubMed] [Google Scholar]
- 51. Logue J, Walker JJ, Colhoun HM, et al. Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia. 2011;54(12):3003–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Steen J, Loeys T, Moerkerke B, et al. Flexible mediation analysis with multiple mediators. Am J Epidemiol. 2017;186(2):184–193. [DOI] [PubMed] [Google Scholar]
- 53. Greenland S. Quantifying biases in causal models: classical confounding vs collider-stratification bias. Epidemiology. 2003;14(3):300–306. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


