Dear Editor
Several groups1, 2, 3, 4 have reported that saliva specimens perform as well or better than nasopharyngeal swabs (NPS) in hospital, emergency care and mass screening settings when testing for SARS-CoV-2 with reverse transcriptase real time PCR (RT-qPCR). In contrast, others found saliva less sensitive than NPS in community5 or mildly infected outpatient settings6. To better understand saliva's performance in the SARS-CoV-2 RT-qPCR assay, we collected paired NPS and saliva from self-reported mild symptomatic or asymptomatic individuals at two community testing sites in Tucson, Arizona between late July and early September 2020. The study was reviewed and approved by the Advarra Institutional Research Board.
Self-collection of saliva was performed using either the SDNA-1000 Saliva Collection device (Spectrum Solutions LLC, USA) or a sterile dry 50 ml conical vial followed by NPS collection within 10 min by medical staff. All samples were processed within 12 h of collection using the Beckman RNAdvance Viral XP Reagent kit and the CDC 2019 nCoV Real-Time RT-PCR Diagnostic Panel with One Step PrimerScriptTM III RT-PCR Kit (Takara Bio Inc. Japan). Using this platform, the viral yield of saliva was comparable to that of NPS using contrived samples and serial dilution of positive saliva samples.
A total of 943 pairs of samples were collected and tested, of which 108 pairs had positive results (11.5%). This included n = 54 samples in which only NPS samples were positive (“NPS-only”), n = 8 in which only saliva was positive (”saliva-only”) and n = 46 in which both NPS and saliva were positive (“double positive”).
The overall positive agreement of saliva to NPS (saliva sensitivity) was 46% (95% CI: 36.6%–55.7%). The average saliva cycle threshold (Ct) value was 26.8 ± 5.9 (N1, same for all below) which was significantly higher than 23.2 ± 8.5 (paired t-test, p < 10−4) of NPS, consistent with several earlier reports4, 5, 6. Of 843 NPS negatives, 8 saliva specimens were positive. The saliva specificity relative to NPS was 99.1% (n = 843, 95% CI: 98.1% to 99.5%) with an average Ct of 34.4 ± 3.4.
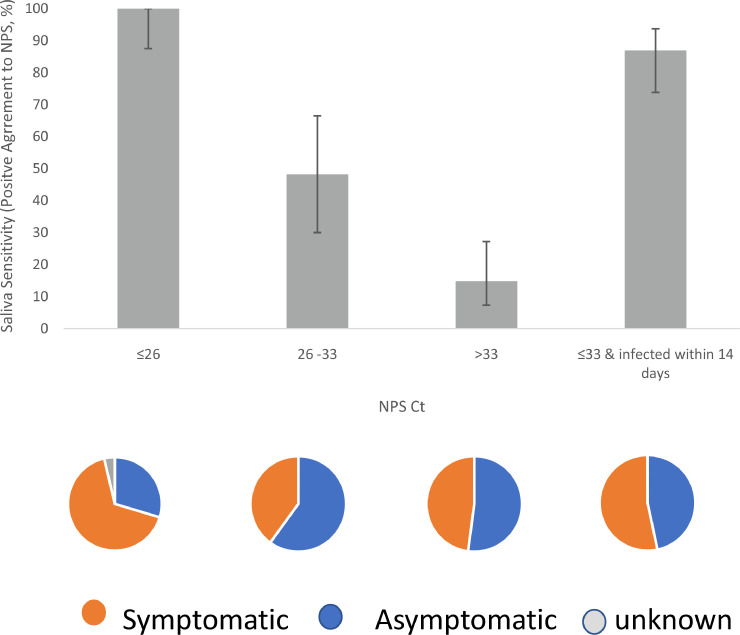
Saliva sensitivity varied inversely with NPS Ct. When NPS Ct was lower than 26, saliva was positive in all NPS positive samples (n = 27, sensitivity 100%, 95% CI: 87.5%–100%). When NPS Ct was between 26 and 33, only 48.0% of the positive NPS samples had paired positive saliva (n = 25, 95% CI: 30.0%–66.5%). When NPS Ct was greater than 33, the saliva sensitivity further decreased to 14.6% (n = 48, 95% CI: 7.3%–27.2%) (Fig. 1 ). Although the lowest NPS Ct groups had a significantly higher number of symptomatic individuals (p = 0.04, Fig. 1 pie chart), the overall saliva sensitivity is not related to the symptoms (Table 1 ).
Fig. 1.
Bar graph: The relationship between saliva sensitivity (or positive agreement to NPS) and the NPS Ct range. Saliva sensitivity from left to right: 100% (n = 27), 48.0% (n = 25), and 14.6% (n = 48), corresponding to the NPS Ct range of 26 and lower, between 26 and 33, and higher than 33. After excluding individuals infected longer than 14 days, saliva sensitivity was 86.7% when NPS Ct was 33 and lower (n = 45) (the right most bar). Error bar represents the 95% confidence interval. Pie chart: The composition of symptomatic (orange) and asymptomatic (blue) individuals in the corresponding NPS Ct range. Percentage of symptomatic individuals from left to right: 66.7%, 40.0%, 47.9%, and 53.3%. The lowest NPS Ct group (the left most pie) had a significant number of symptomatic individuals (p = 0.04).
Table 1.
Demographic distribution of double positive, NPS-only positive, and saliva-only positive individuals
| NPS+Saliva+ | NPS+Saliva- | NPS-Saliva+ | p-value* | |
|---|---|---|---|---|
| Symptom | ||||
| No | 18 | 30 | 5 | 0.226 |
| Yes | 27 | 24 | 3 | |
| Age | ||||
| 18–29 | 28 | 15 | 3 | 0.003 |
| 30–49 | 10 | 25 | 1 | |
| 50 or above | 8 | 14 | 4 | |
| Sex | ||||
| Male | 22 | 16 | 2 | 0.201 |
| Femal | 22 | 34 | 4 | |
| Race | ||||
| NH White | 15 | 17 | 3 | 0.378 |
| Hispanic | 30 | 31 | 4 | |
| other or unknown | 1 | 6 | 1 | |
| Saliva device | ||||
| SDNA1000 | 27 | 40 | 6 | 0.235 |
| Conical | 19 | 14 | 2 | |
| Collection center | ||||
| Site one: n = 637 | 39 | 50 | 3 | 0.001 |
| Site two: n = 335 | 6 | 3 | 5 |
Fisher exact test via Monte carlo simulation
Among NPS-only positive individuals, a total of 22 had onset of symptoms 14 days earlier or SARS-COV-2 positivity longer than 14 days whereas there was only one such case among double positives. After excluding these individuals, the saliva sensitivity reached 86.7% (n = 45, 95% CI: 73.8%–93.7%) for NPS Ct of 33 and lower (Fig. 1). Our results corroborate early reports that saliva positivity declines more rapidly than that of NPS after two weeks of infection2 , 6.
We examined the effects of other factors on saliva sensitivity, including age, gender, race, saliva collection device, and collection site (Table 1). The double positive group had a significant number of individuals younger than age 30 (p=0.003). Gender, race, and collection device had no significant impact on saliva sensitivity. Of note, the two specimen collection sites had significantly different testing result profiles. Site 1 had the majority of positive cases (positivity rate of NPS and saliva combined was 14.4%), whereas Site 2 had a much lower positivity rate of 4.5%. Saliva appeared to be more sensitive than NPS at Site 2. Site 2 had 4 NPS-only positives and 5 saliva-only positives, whereas Site 1 had 50 NPS-only positives and 3 saliva-only positives. Although the total number of positive cases at Site 2 was small (Table 1), the difference is significant (p = 0.001). Moreover, of 3 NPS-only positives at Site 2, one was inconclusive on saliva testing and two were known positive for more than 14 days at the time of this study. The two sites generated two different results with saliva more sensitive at one site, and less at another.
The differences cannot be readily attributed to procedural variations. The two collection sites had the same rotating medical staff, followed the same collection and transportation protocol, and collected the paired specimens on the same days. The samples from two sites were randomly batched together for the lab analysis. The population at Site 2 was more suburban and socially distanced with an average age of 47.8 years, compared to 34.4 years at Site 1. Since the performance of laboratory tests can vary as a function of the prevalence of the disease, the disparate results profiles across sites could be related to the differences in the SARS-CoV-2 positivity at the two testing sites. It is also possible that the observed differences were due to demographic differences or a chance occurrence.
Nearly half (47%) of all NPS positives in our cohort had Ct higher than 33. Some had prolonged presence of the virus and other had unknown date of initial infection, likely a true picture of many communities. Most of those people were tested negative by saliva. Previous studies have shown there is much lower likelihood of isolating live SARS-CoV-2 virus from test samples when Ct > 33–357 , 8. Detecting viral RNA does not equate with infectious virus being present and transmissible. Further work is needed to establish the relationship between RT-qPCR Ct values in saliva and viral infectivity9, particularly in populations with a high prevalence rate10.
Because its collection is non-invasive and does not require trained medical staff, saliva is a desirable specimen for COVID-19 screening and diagnostics. Our results indicate that RT-qPCR testing of saliva in a community-based population can effectively identify infected individuals with the high viral loads in a timely fashion, which is important for identifying those who may have the greatest potential to spread the virus.
Declaration of Competing Interest
None.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
We would like to thank Dr. Theresa Cullen and Pima County Health Department for their support in this study. We are also grateful to Dr. Carlos M. Perez-Velez (Pima County Health Department and University of Arizona College of Medicine) for discussing the testing issues and critically reviewing this manuscript, and Mr. Zhongxu Zhu for helping with Fisher exact test using R-software.
Reference
- 1.Azzi L, Carcano G, Gianfagna F, Grossi P, Gasperina D D, Genoni A. Saliva is a reliable tool to detect SARS-CoV-2. J Infect. 2020:e45–e50. doi: 10.1016/j.jinf.2020.04.005. doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwasaki S, Fujisawa S, Nakakubo S, Kamada K, Yamashita Y, Fukumoto T. Comparison of SARS-CoV-2 detection in nasopharyngeal swab and saliva. J Infect. 2020:e145–e147. doi: 10.1016/j.jinf.2020.05.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyllie A, Fournier J, Casanovas-Massana A, Campbell M, Tokuyama M, Vijayakumar P. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N Engl J Med. 2020:1283–1286. doi: 10.1056/NEJMc2016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yokota I, Shane P Y, Okada K, Unoki Y, Yang Y, Inao T. Mass screening of asymptomatic persons for SARS-CoV-2 using saliva. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1388. ciaa 1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skolimowska K, Rayment M, Jones R, Madona P, Moore L S P, Randell P. Non-invasive saliva specimens for the diagnosis of COVID-19: caution in mild outpatient cohorts with low prevalence. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.07.015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker D, Sandoval E, Amin A, De Hoff P, Diets A, Leonetti N. Saliva is less sensitive than nasopharyngeal swabs for COVID-19 detection in the community setting. medRxiv preprint. 2020 doi: 10.1101/2020.05.11.20092338. [DOI] [Google Scholar]
- 7.Bullard J, Dust K, Funk D, Strong J E, Alexander D, Garnett L. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa638. ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singanayagam A, Patel M, Charlett A, Bernal J L, Saliba V, Ellis J. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill. 2020 doi: 10.2807/1560-7917.ES.2020.25.32.2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krupp K, Madhivanan P, Perez-Velez C M. Should qualitative RT-PCR be used to determine release from isolation of COVID-19 patients? J Infect. 2020:459–461. doi: 10.1016/j.jinf.2020.06.030. doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woloshin S, Patel N, Kesselheim A. False negative tests for SARS-CoV-2 infection —challenges and implications. N Engl J Med. 2020:e38. doi: 10.1056/NEJMp2015897. [DOI] [PubMed] [Google Scholar]