Abstract
Background and aims
COVID-19 is a multi-system disease, with coagulation abnormalities. D-dimer levels are increased in this disease. We aimed to determine the association of D-dimer levels and mortality and to establish its optimal cut off values in predicting mortality. Association of D-dimer levels with diabetes mellitus has also been established.
Methods
Information on 483 patients with confirmed COVID-19 was retrospectively collected and analyzed. The optimal D-dimer cutoff point and C-statistic of routine tests both on admission and during hospital stay were evaluated by receiver operator characteristic (ROC) curve.
Results
D-dimer elevation (≥0.50 μg/mL) was seen in 80.1% of the hospitalized patients. D-dimer level ≥2.01 μg/mL was a significant predictor of subsequent deaths (P < 0.01; HR, 3.165; 95% CI, 2.013–4.977). High D-dimer values (≥0.50 μg/mL) were observed in 72 of the 75 (96%) cases with a fatal outcome. Median D-dimer value among non-survivors was 6.34 μg/mL and among survivors it was 0.94 μg/mL. A higher proportion of fatal outcomes occurred in patients with underlying disease (89.0%), most prominent of which was diabetes mellitus (66%). The median D-dimer value was found to be significantly high in diabetic patients (1.68 μg/mL).
Conclusions
Among the measured coagulation parameters, D-dimer during hospital stay had the highest C-index to predict in-hospital mortality in COVID-19 patients. D-dimer value ≥ 2.01 μg/mL can effectively predict in-hospital mortality in patients with COVID-19. A significant association of increased D-dimer level has been found with diabetes mellitus and elderly age.
Keywords: Covid-19, Coronoavirus, D-dimer, Mortality, Diabetes
Highlights
-
•
D-dimer ≥2.01 μg/ml FEU (fourfold increase) during hospital stay might be the optimum cutoff to predict mortality.
-
•
Elderly age and diabetes were significantly associated with elevated D-dimer levels, disease severity and mortality.
-
•
Median D-dimer values were calculated for age, gender, survivors/non-survivors, and diabetic/non - diabetics.
1. Introduction
Coronavirus disease (COVID-19) is caused by severe acute respiratory syndrome Coronavirus-2 (SARS-CoV-2) [1]. Although it is well documented that COVID-19 is primarily manifested as a respiratory tract infection, emerging data indicates that it should be regarded as a systemic disease involving multiple systems including cardiovascular, respiratory, gastrointestinal, neurological, hematopoietic, and immune system [2].
SARS-CoV-2 infection induces a profound inflammatory response which triggers the coagulation cascade [3]. Activation of the coagulation cascade in COVID-19 patients is associated with a hypercoagulable state and adverse clinical outcomes including death. Occurrence of dysfunctional coagulation in COVID-19 patients emphasizes the crucial need for a hemostasis-focused laboratory monitoring and therapeutic management [4]. Currently, the best available laboratory diagnostic marker for COVID- 19–associated hemostatic abnormalities (CAHA) is considered to be D-dimer [5]. D-dimer is a biomarker of fibrin formation and degradation that can be measured in the whole blood or in the plasma [6]. Healthy individuals have low levels of circulating D-dimer, whereas elevated levels are found in conditions associated with hypercoagulation and increased fibrinolytic activity.
Elevated levels of D-dimer have also been reported in patients with COVID-19. Elevated D-dimer at the time of admission and markedly increasing D-dimer levels (3to 4fold) over time are associated with high mortality, likely reflecting coagulation activation from infection/sepsis, cytokine storm and impending organ failure [7]. Data on the association between D-dimer levels and mortality in COVID-19 patients has been published and measurement of serial D-dimer has been recommended for COVID-19 patients, however, the optimal cutoff for D-dimer is yet to be well-established [8,9].
In this retrospective study, our primary objective was to determine an optimum cut-off value of D-dimer on admission and during the hospital stay to predict mortality. We also assessed the contribution of Prothrombin Time (PT) and Activated Partial Thromboplastin Time (APTT) to predict mortality independent of D-dimer levels. Association of D-dimer with age, gender, and comorbidities have also been assessed.
The approval for this study was obtained from the Ethics Committee of our institution.
2. Materials & methods
2.1. Study design and participants
Data on the patients with confirmed COVID-19, admitted to our tertiary care hospital, between April 02, 2020, to July 24, 2020, was retrospectively collected through an electronic records system (EMS) and was analyzed. The information included demographic details, laboratory findings, and clinical details including co-morbidities and disease outcomes. Patient’s details were kept confidential.
Patients with a positive result of the nucleic acid test of SARS-CoV-2 by real-time fluorescence RT-PCR as per World Health Organization guidelines were considered as confirmed COVID-19 cases. Criteria for admission to the hospital was moderate to severe disease where the respiratory rate was more than 24/min or SpO2 was less than 93%. COVID-19 confirmed adult patients (18 years or older) who had a definite outcome (discharge or death) were included in the study. The patients who were still admitted to the hospital and the patients discharged against medical advice during this study period were excluded from the study.
2.2. Data collection
D-dimer values within 24 h of admission and the highest values during hospital stay were extracted for all the patients included in the study. The PT and APTT values, on admission, were also extracted.
2.3. Laboratory investigations
Coagulation parameters were analyzed using a Sysmex CS-2400 coagulation analyzer (Sysmex Corporation, Kobe, Japan). D-dimer testing was performed using a latex-enhanced photometric immunoassay (Siemens, Marburg, Germany) and the result was expressed in μg/ml FEU. The laboratory reference interval was 0–0.5 μg/ml FEU. The PT testing was performed using Thromborel S and the APTT testing was performed using Actin FSL, both from Siemens. The laboratory reference interval for PT and APTT was 10–13 s and 20–31 s, respectively.
2.4. Statistical analysis
Continuous and categorical variables were presented as mean ± standard deviation or median (interquartile range [IQR]), as appropriate. Categorical variables were presented as n (%). Event frequencies were compared between the survivor and non-survivor groups wherever necessary with a chi-squared test calculator (P-value). The optimal D-dimer cutoff point and C-statistic of routine tests both on admission and during hospital stay were evaluated by receiver operator characteristic (ROC) curve. Age-adjusted D-dimer cutoff for patients aged over 50 was used to generate the ROC to evaluate the impact of false positives. The outcomes were compared by Kaplan-Meier survival analysis. Hazard ratio (HR) and 95% confidential interval (95% CI) were calculated by log-rank tests. Kaplan-Meier survival analysis was also compared for survivors and non-survivors in patients with co-morbidities. A value of P < 0.05 was accepted as statistically significant. The statistical software package GraphPad Prism 8 statistical software (version 8.4.3, San Diego, CA) was used for analysis.
3. Results
3.1. Baseline characteristics and establishing optimum cutoff value for D-Dimer
The data of 483 patients with COVID-19 were analyzed in the study, according to our inclusion and exclusion criteria. All the COVID-19 patients requiring admission were given prophylactic low molecular weight heparin (LMWH). The median age of the patients was 61 years (IQR, 51–71), ranging from 21 years to 89 years. Of the 483 patients, 59.6% (288) were adults (21–64 years) and 40.3% (195) were elderly (older than 65 years). Majority of the patients were male 69.9% (338), as compared to the female patients 30.1% (145). The basic clinical characteristics of the patients, including age, gender, comorbidities, and D-dimer values are presented in Table 1 . A total of 75 deaths (15.52%) were recorded amongst these admitted patients.
Table 1.
Baseline characteristics of 483 patients with COVID-19.
| Variable | Normal D-dimer (<0.5) | D-dimer >0.5 to 1.5 (mild) | D-dimer >1.5 to 3.0 (moderate) | D-dimer >3.0 (severe) | Total | P-value | |
|---|---|---|---|---|---|---|---|
| n = | 96 | 188 | 66 | 133 | 483 | ||
| Age | Median (IQR) | 54 (48–63) | 63 (51–72) | 62 (54–74) | 63 (55–75) | 61 (51–71) | <0.01 |
| Age (Adult) 21-64 | n (%) | 75 (78.1) | 101 (53.7) | 39 (59.0) | 73 (54.8) | 288 (59.6) | <0.01 |
| Age (Elderly) ≥ 65 | n (%) | 21 (21.8) | 87 (46.2) | 27 (40.9) | 60 (45.1) | 195 (40.3) | <0.01 |
| Gender (Male) | n (%) | 65 (67.7) | 131 (69.6) | 44 (66.6) | 98 (73.6) | 338 (69.9) | <0.01 |
| Gender (Female) | n (%) | 31 (32.2) | 57 (30.31) | 22 (33.3) | 35 (26.3) | 145 (30.0) | 0.22 |
| Mortality | n (%) | 3 [4] | 13 (17.3) | 14 (18.6) | 45 (60) | 75 (15.5) | |
| Underlying condition (comorbidities) | |||||||
| Total | N | 68 | 148 | 60 | 108 | 384 | <0.01 |
| Diabetes | N | 41 | 91 | 47 | 71 | 250 | <0.01 |
| Hypertension | N | 39 | 87 | 42 | 81 | 249 | <0.01 |
| Coronary Artery Disease (CAD) | N | 11 | 26 | 15 | 33 | 85 | 0.04 |
| Chronic Kidney Disease (CKD) | N | 5 | 11 | 11 | 18 | 45 | 0.29 |
| Hypothyroidism | N | 5 | 15 | 6 | 9 | 35 | 0.87 |
| Cancer (any) | N | 1 | 3 | 1 | 3 | 8 | 1.00 |
| Chronic obstructive pulmonary Disease (COPD) | N | 1 | 2 | 1 | 1 | 5 | 1.00 |
| Chronic Liver Disease (CLD) | N | 0 | 2 | 0 | 3 | 5 | 1.00 |
| Pulmonary & Extra Pulmonary Disease | N | 0 | 2 | 0 | 0 | 2 | 1.00 |
| Routine Test on Admission | |||||||
| D-dimer, μg/ml (n = 483) | Median (IQR) | 0.35 (0.22–0.44) | 0.84 (0.65–1.09) | 2.07 (1.69–2.52) | 8.08 (4.86–14.7) | 1.12 (0.56–3.28) | NA |
| D-dimer cases in different groups | (%) | 19.9 | 38.9 | 13.7 | 27.5 | NA | |
| Prothrombin Time (PT) (sec) (n = 252) | Median (IQR) | 11 [11,12] | 12 [[11], [12], [13]] | 12 [12,13] | 13 [[12], [13], [14], [15]] | 12 [[11], [12], [13]] | NA |
| Activated Partial Thromboplastin Time (APTT) (sec) (n = 146) | Median (IQR) | 28 (25.5–32.5) | 30 (28–33) | 33 (25–37) | 31 (26–35) | 31 (26–34) | NA |
The optimum cutoff value for D-dimer on admission for predicting mortality, calculated using the ROC curve was 1.44 μg/ml, with a sensitivity of 60.5%, and a specificity of 74.0% (Table 2 ). The area under the ROC curve (C-index) was 0.683. The optimum cutoff value for D-dimer for predicting mortality, calculated using the highest D-dimer value during hospital stay was 2.01 μg/ml, with a sensitivity of 73.3% and a specificity of 70.0%. The area under the ROC curve was 0.789 (Table 2). For age-adjusted D-dimer, the cutoff value for predicting mortality was 2.1 μg/ml, with a sensitivity of 76.5% and a specificity of 63.5%. The area under the ROC curve (C-index) was 0.766 (Table 2 suggesting no impact of false positives).
Table 2.
Comparison of Sensitivity & Specificity in D-dimer at Admission, during hospital stay & Age adjusted values.
| Optimum D-dimer Values | Admission | On Max D-dimer during hospital stay (n = 483) | Age-adjusted D-dimer cutoff for Age>50 yrs (n = 370) |
|---|---|---|---|
| Cutoff point for D-dimer (μg/ml) | 1.44 | 2.01 | 2.1 |
| Area under curve (95% CI) | 0.683 (0.612–0.754) | 0.789 (0.7345–0.8425) | 0.7661 (0.7066–0.8256) |
| Sensitivity (%) | 60.5 | 73.3 | 76.5 |
| Specificity (%) | 74.0 | 70.0 | 63.5 |
| P-value | <0.01 | <0.01 | <0.01 |
| Likelihood ratio | 2.10 | 2.413 | 2.092 |
| Median D-dimer Value in total Incidence (μg/ml) | 0.82 | 1.14 | 1.54 |
| Median D-dimer Value in Survivors (μg/ml) | 0.76 | 0.945 | 1.14 |
| Median D-dimer Value in Non-Survivors (μg/ml) | 2.02 | 6.34 | 6.415 |
The area under the ROC curve (C-index) of PT was 0.741, while that of APTT was 0.663, indicating PT to be a predictor of mortality in these patients.
Among the measured coagulation parameters, D-dimer during hospital stay had the highest C-index to predict in-hospital mortality in COVID-19 patients.
3.2. High D-dimer level and risk of mortality
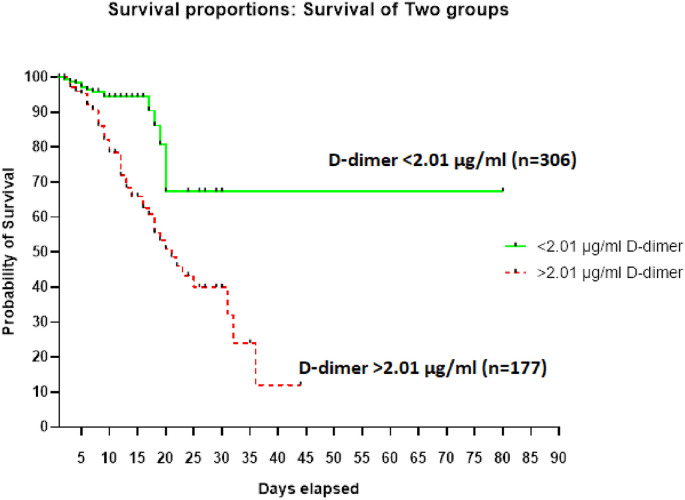
D-dimer elevation (≥0.50 μg/ml) was seen in 80.1% (387/483) of the hospitalized patients (Table 1). The Median D-dimer value among non-survivors was 6.34 μg/ml and it was 0.94 μg/ml among the survivors (Table 2). Kaplan-Meier survival curves for D-dimer levels (Fig. 1 ) showed that D-dimer level ≥2.01 μg/ml was a significant predictor of subsequent deaths (P < 0.01; HR, 3.165; 95% CI, 2.013–4.977) (Fig. 1). High D-dimer values (≥0.50 μg/ml) were observed in 72 of the 75 (96%) cases with a fatal outcome (Table 1). Fifty six of the 75 cases (74.67%) had D-dimer levels of ≥2.01 μg/ml.
Fig. 1.
Kaplan-Meier survival curves for D-dimer levels.
3.3. D-dimer levels and age
The median D-dimer value in elderly was 1.38 μg/ml and in adults it was 0.98 μg/ml (p = 0.05; Table 4). Abnormal D-dimer was observed in 89.2% (174/195) of the hospitalized elderly patients (Table 1). Of the total patients with significantly high D-dimer values, 45.1% (60/133) were elderly (Table 1). Mortality was 20.0% (39/195) in elderly patients and 12.5% (36/288) in adult patients (Table 3 ).
Table 4.
Comparison of Median D-dimer Values in Different groups.
| Median D-dimer Values in | D-dimer (μg/ml) | PValue |
|---|---|---|
| Males | 1.16 | 0.69 |
| Females | 1.10 | |
| Elderly | 1.38 | 0.05 |
| Adults | 0.98 | |
| Patients with Diabetes | 1.68 | <0.01 |
| Patients without Diabetes | 0.8 | |
| Patients with co-morbidities (other than diabetes) | 1.12 | 0.24 |
| Patients without co-morbidities | 0.8 | |
| Non-Survivors | 6.34 | <0.01 |
| Survivors | 0.94 |
Table 3.
Age, Co-morbidities & Gender associated with increased risk of mortality.
| Categories | Findings |
|---|---|
| Median Age of Mortality (Death) | 65 Years |
| Median Age of mortality in males | 65 Years |
| Median Age of mortality in females | 61 Years |
| Mortality in adult patient’s n (%) | 36/288 (12.5) |
| Mortality in elderly patient’s n (%) | 39/195 (20.0) |
| Mortality in adults in total deaths n (%) | 36/75 (48.0) |
| Mortality in elderly in total deaths n (%) | 39/75 (52.0) |
| Mortality of males amongst male’s patients n (%) | 55/338 (16.3) |
| Mortality of females amongst female’s patients n (%) | 20/145 (13.8) |
| Mortality of males in totaldeaths n (%) | 55/75 (73.3) |
| Mortality in females in total deaths n (%) | 20/75 (26.7) |
| Incidence with co-morbiditiesn (%) | 384 (79.5) |
| Mortality with co-morbidities n (%) | 67 (89.3) |
| Mortality without co-morbidities n (%) | 8 (11.8) |
3.4. D-dimer levels and gender
No significant difference in median D-dimer values between genders was noticed. The median D-dimer values were 1.16 μg/ml and 1.10 μg/ml in male and female patients, respectively (p = 0.69; Table 4). Mortality was 16.3% among the male patients (55/338) and 13.8% in the female patients (20/145) (Table 3).
3.5. D-dimer levels and co-morbidities
Most of the infected patients had a comorbidity (79.5%; 384/483) of which the most frequent was diabetes mellitus (65.1%; 250/384). Out of 250 individuals having diabetes, only 41/250 (16.4%) had normal D-dimer whereas 91/250 (36.4%) had mild, 47/250 (18.8%) had moderate and 71/250 (28.4%) had severely elevated D-dimer (Table 1). The median D-dimer values were significantly different between diabetics and non-diabetics (1.68 μg/ml & 0.8 μg/ml respectively, p value <0.01). (Table 4). Difference in median D-dimer value in patients with co-morbidities other than diabetes and patients without any comorbidities was not found to be significant. (1.12 μg/ml & 0.8 μg/ml respectively, p value 0.24) (Table 4). A higher proportion of fatal outcomes occurred in patients with underlying disease (89.0%; 67/75) (Table 3) and 70% (47/67) of these had diabetes. Log rank method in survivor and non-survivors with comorbidities was found to be statistically significant (P < 0.01, HR, 25.01; 95% CI 14.73 to 42.48).
4. Discussion
D-dimer is the fibrin degradation products released upon cleavage of cross-linked fibrin by plasmin [9]. D-dimer is routinely utilized clinically in diagnosing disseminated intravascular coagulation (DIC) and those with low pretest probability for deep vein thrombosis (DVT) and pulmonary embolism (PE) [10]. D-dimer elevation has been reported to be one of the commonest laboratory findings noted in COVID-19 patients requiring hospitalization [11]. Studies have shown that rising D-dimer levels during the course of hospitalization are associated with worst long-term outcomes [12].
The results of this study indicate that D-dimer levels ≥2.01 μg/ml during hospital stay are a predictor of mortality in COVID-19 patients. D-dimer value of ≥2.01 μg/ml had a sensitivity of 73.3% and a specificity of 70.0%, with a C-index of 0.789, achieved with a likelihood ratio of 2.413 (P < 0.0001; HR, 3.165; 95% CI, 2.013–4.977). The cut-off value was similar for age-adjusted D-dimer with a sensitivity of 76.5% and a specificity of 63.5%, suggesting no impact of false positives in patients more than 50 years of age.
In our study, D-dimer levels on admission were not found to be a strong predictor of mortality. D-dimer value of ≥1.44 μg/ml within 24 h of hospital admission had a sensitivity of 60.5% and a specificity of 74.0%, with a C-index of 0.683, achieved with a likelihood ratio of 2.33. The results indicate that D-dimer value during the hospital stay is a better predictor of mortality as compared to D-dimer levels on admission. The time from disease onset to admission may vary amongst patients thereby influencing D-dimer levels on admission. In one study D-dimer levels a few days after admission had a stronger correlation with mortality than those at the admission [13].
In previously published studies, D-dimer value of more than 2 μg/ml on admission has been reported to predict mortality with high sensitivity and specificity. In these studies, the reported all-cause deaths were low and subsequent measurement of D-dimer was not evaluated. Zhang et al. reported an optimum D-dimer the cutoff value of ≥2.0 μg/ml within 24 h of hospital admission to predict in-hospital mortality, with a sensitivity of 92.3% and a specificity of 83.3% and a hazard ratio of 51.5 (95% CI, 12.9–206.7) [11]. In this study there were 13 all-cause deaths during hospitalization out of which 12 were observed among patients with D-dimer levels of ≥2.0 μg/ml. Yao et al. reported D-dimer levels of >2.14 μg/ml on admission as a predictor of mortality, with a sensitivity of 88.2% and a specificity of 71.3%(14). The conclusion of the study was based on 17 reported deaths. Our study included data from 75 fatal outcomes, resulting in a closer approximation of the predictive value of the optimal D-dimer levels.
In our study, the median D-dimer level in non-survivors was significantly higher (6.34 μg/ml) than in survivors (0.94 μg/ml). This corroborates with results from previous studies [14,15,18].
International Society of Thrombosis and Hemostasis interim guidance on recognition and management of coagulopathy recommends PT as the next most important test after D-dimer to be performed in patients with COVID-19 [16]. In our study the areas under the ROC curve of PT and APTT were 0.741 and 0.663 respectively, suggesting PT to be a better predictor of disease mortality. Long et al. had observed a correlation between increased levels of both PT and APTT and mortality in COVID-19 patients [13]. However, in most published studies APTT values have not differed significantly between patients with mild and severe disease [17].
In our study, the elevation of D-dimer and mortality was found to be higher in the elderly (>65yrs). 174 of the 195, i.e., 89.2% of elderly patients had abnormal level D-dimer and 39 of 195, i.e., 20.0% were deceased. This finding is comparable to finding by Long et al. who reported a mortality of 16.5% in the age group of more than 60 yrs [13]. Out of the total mortality recorded, 52.0% (39/75) were in patients more than 65 yrs of age. The median D-dimer value in elderly was 1.38 μg/ml and in adults it was 0.98 μg/ml (p = 0.05).
The incidence of the disease as well as mortality showed a strong association with underlying disease. Comorbidities like diabetes mellitus, hypertension, coronary artery disease, malignancy, COPD, and bronchial asthma were seen in 79.5% (384/483) of the patient. The most frequent comorbidity was diabetes mellitus (65.1%; 250/384). A total of 89.0% (67/75) death events occurred in patients with underlying disease of which 70% patients had diabetes. Sanyaolu et al. have also reported similar findings [19]. There was a significant difference in median D-dimer levels between diabetic and nondiabetic patients. The median D-dimer level in diabetic was found to be 1.68 μg/ml. In a recent publication, Mishra et al. have reported an association of diabetes with increased D-dimer levels in moderately sick COVID 19 patients [20]. Similar to our study, in their study also peak D-dimer levels during the entire course of hospital stay were utilized in statistical analysis. Mukona et al. have emphasized that people with diabetes mellitus are at a greater risk of COVID-19 infection and of having more severe form of disease [21].
We acknowledge some limitations in this study. This was a single-center, retrospective study and thus might have a selection bias. The study findings need to be corroborated with a larger, multicentric study. Considering the heterogeneous etiology of coagulation disturbances, the presence of comorbidities in a significant number of cases with a fatal outcome may be a confounding factor in establishing the association between elevated D-dimer levels and mortality. The dynamic measurement of D-dimer could be more informative in assessing the value of D-dimer as a predictor of mortality. PT and APTT were not measured in all patients and serial evaluation of the two was not performed, limiting the assessment of their role as predictors of the disease outcome. Since there was no follow up, post-discharge clinical status is not available.
5. Conclusion
Coagulopathy is an important complication in patients with COVID-19 and is closely related to the clinical outcome. D-dimer is a reliable and convenient coagulation parameter to predict mortality. A D-dimer value ≥ 2.01 μg/ml can effectively predict in-hospital mortality in patients with COVID-19. D-dimer value on admission is not an effective predictor of in-hospital mortality. A significant association of increased D-dimer level has been found with diabetes and elderly age.
Declaration of competing interest
The authors have no conflict of interest.
Acknowledgement
Ms Malini C, Mr. Madhusudan and Ms. Kavitha from the department of hematology and clinical pathology, Apollo hospital, Chennai for assisting in data collection.
References
- 1.Chaudhry R., Dranitsaris G., Mubashir T., Bartoszko J., Riazi S. A country level analysis measuring the impact of government actions. country preparedness and socioeconomic factors on COVID-19 mortality and related health outcomes. 2020;25:100464. doi: 10.1016/j.eclinm.2020.100464. EClinicalMedicine [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T.N., Politou M. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95(7):834–847. doi: 10.1002/ajh.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agnes D., Lee Y.Y., Connors J.M., Kreuziger L.B., Murphy M., Gernsheimer T. 2020. COVID-19 and Coagulopathy : frequently asked questions; pp. 5–6. [Google Scholar]
- 4.Joly B.S., Siguret V., Veyradier A. Understanding pathophysiology of hemostasis disorders in critically ill patients with COVID-19. Intensive Care Med [Internet] 2020;46(8):1603–1606. doi: 10.1007/s00134-020-06088-1. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thachil J., Cushman M., Srivastava A. A proposal for staging COVID-19 coagulopathy. Res Pract Thromb Haemost. 2020;4(5):731–736. doi: 10.1002/rth2.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weitz J.I., Fredenburgh J.C., Eikelboom J.W. A test in context: D-dimer. J Am Coll Cardiol. 2017;70(19):2411–2420. doi: 10.1016/j.jacc.2017.09.024. [DOI] [PubMed] [Google Scholar]
- 7.Agnes D., Lee Y.Y., Connors J.M., Kreuziger L.B., Murphy M., Gernsheimer T. 2020. COVID-19 and Coagulopathy : frequently asked questions; pp. 5–6. [Google Scholar]
- 8.Sakka M., Connors J.M., Hékimian G., Martin-Toutain I., Crichi B., Colmegna I. Association between D-Dimer levels and mortality in patients with coronavirus disease 2019 (COVID-19): a systematic review and pooled analysis. JMV-Journal Med Vasc [Internet] 2020;45(5):268–274. doi: 10.1016/j.jdmv.2020.05.003. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah S., Shah K., Patel S.B., Patel F.S., Osman M., Velagapudi P. 2020. pp. 295–302. (Elevated D-dimer levels are associated with increased risk of mortality in COVID-19. Cardiol rev.). Publish Ah (6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ay C., Dunkler D., Pirker R., Thaler J., Quehenberger P., Wagner O. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica. 2012;97(8):1158–1164. doi: 10.3324/haematol.2011.054718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemostasis. 2020;18(6):1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long H., Nie L., Xiang X., Li H., Zhang X., Fu X. D-dimer and Prothrombin time are the significant indicators of severe COVID-19 and poor prognosis. BioMed Res Int. 2020:2020. doi: 10.1155/2020/6159720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao Y., Cao J., Wang Q., Shi Q., Liu K., Luo Z. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J Intensive Care. 2020;8(1):1–11. doi: 10.1186/s40560-020-00466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemostasis. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thachil J., Tang N., Gando S., Falanga A., Cattaneo M., Levi M. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemostasis. 2020;18(5):1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiong M., Liang X., Wei Y.D. Changes in blood coagulation in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Br J Haematol. 2020;189(6):1050–1052. doi: 10.1111/bjh.16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou F., Yu T. Evidence of a potential role for D-dimer and Procalcitonin testing used to assess comorbidities of patients hospitalized with COVID-19. 2020;19(March):19–22. [Google Scholar]
- 19.Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2020;2(8):1069–1076. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mishra Y., Pathak B.K., Mohakuda S.S., Tilak T.V.S.V.G.K., Sen S. Relation of D-dimer levels of COVID-19 patients with diabetes mellitus. Diabetes Metab Syndr Clin Res Rev. 2020;14(6):1927–1930. doi: 10.1016/j.dsx.2020.09.035. P H. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mukona D.M., Zvinavashe M. Self- management of diabetes mellitus during the Covid-19 pandemic: recommendations for a resource limited setting. Diabetes Metab Syndr. 2020;14(6):1575–1578. doi: 10.1016/j.dsx.2020.08.022. Epub ahead of print. PMID: 32858475; PMCID: PMC7443206. [DOI] [PMC free article] [PubMed] [Google Scholar]