Abstract
Coronavirus disease 2019 (COVID-19) may present as acute abdomen, although the pathophysiology remains obscure. We report the case of a 45-year-old-man with severe COVID-19 pneumonia with associated pulmonary embolism who presented with acute abdomen. He underwent emergency laparotomy and resection of an ischaemic area of the jejunum. Postoperatively, he had septic shock, acute respiratory distress syndrome and acute kidney injury necessitating continuous renal replacement therapy. We administered antibiotics and therapeutic anticoagulation along with two sessions of haemoadsorption by CytoSorb filter, in conjunction with continuous renal replacement therapy. The patient survived. Bowel ischaemia due to thromboembolic disease should be promptly treated. Extracorporeal blood purification may be useful in managing sepsis in severe COVID-19.
Keywords: Acute abdomen, COVID-19, extracorporeal blood purification therapies, sepsis, thromboembolic disease
Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease, coronavirus disease 2019 (COVID-19), pandemic emerged in China in 2019 and quickly spread worldwide [1]. A minority of patients can develop life-threatening disease, which is characterized by acute respiratory distress syndrome (ARDS), sepsis, multisystem organ failure (MSOF), cytokine storm, thromboembolic disease and rarely extrapulmonary manifestations [[2], [3], [4], [5]]. Amongst the extrapulmonary manifestations, severe gastrointestinal disorders such as surgical abdomen-like presentations were described [[6], [7], [8], [9], [10], [11], [12]]. The diagnostic and therapeutic challenges related to an acute abdomen presentation in COVID-19 were evident since the beginning of the pandemic [13]. However, scarce data exist regarding the underlying pathophysiology of acute abdomen in COVID-19.
We report a case of a patient with severe COVID-19 and thromboembolic disease who developed sepsis due to bowel perforation. The putative role of extracorporeal blood purification therapies in the management of COVID-19 related hyperinflammation and perioperative sepsis is also discussed.
The study was approved by the institutional review board of King Saud Medical City, Riyadh, Kingdom of Saudi Arabia (H-01-R-053, IORG0010374, serial no. H1RI-23-20-03), and written informed consent was obtained from the patient for publication of this case report and accompanying images.
Case report
During the COVID-19 outbreak in Saudi Arabia, a previously healthy 45-year-old Asian man was admitted to our emergency department with recent (6 days) onset of fever (temperature 39°C), cough, dyspnoea, diarrhoea, vomiting and abdominal pain. The patient's height and weight were 1.68 m and 75 kg respectively, body mass index was calculated as 24.1 kg/m2. Physical examination revealed bilateral crackles on lung bases and severe pain located in the epigastrium along with rigidity and guarding of the abdominal wall. The saturation of peripheral oxygen (SpO2) was 82% on room air; heart rate was 109 beats per minute, and blood pressure was 89/47 mm Hg.
The patient was promptly intubated, mechanically ventilated and resuscitated by the administration of low-dose noradrenaline and crystalloid fluids. The patient was found to be positive for SARS-CoV-2 infection via real-time PCR (RT-PCR) performed on nasopharyngeal swabs using the QuantiNova Probe RT-PCR kit (Qiagen, Germantown, MD, USA) in a LightCycler 480 RT-PCR system (Roche, Basel, Switzerland) [14,15]. Electrocardiogram, cardiac enzymes and echocardiograph results were normal. Baseline laboratory findings showed leukocytosis (20 × 103 white blood cells; reference range, 4–10 × 10³) with lymphocytopenia (0.47 × 103/L; reference range, 1.1–3.2 × 10⁹/L) and increased C-reactive protein (99 mg/L; reference range, 0–7 mg/L), lactate dehydrogenase (997 U/L; reference range, 100–190 U/L), lactate (6 mmol/L; reference range, 1.0 to 2.5 mmol/L) and d-dimers (4.1 μg/mL; reference range, 0 to 0.5 μg/mL).
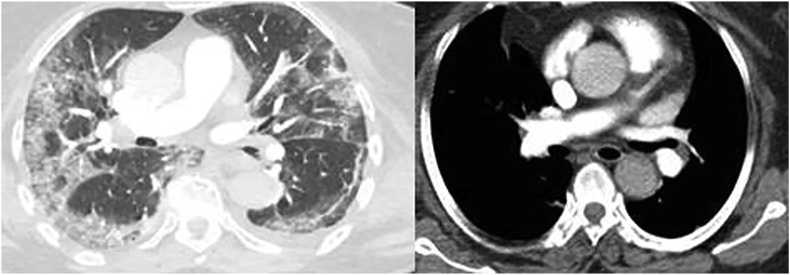
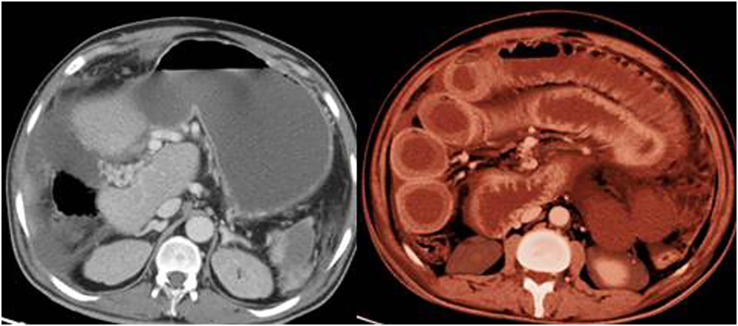
At admission, his blood urea nitrogen was 6.1 mmol/L (reference range, 2.5–6.4 mmol/L) and his creatinine was 119 μmol/L (reference range, 71–115 μmol/L). The rest of his blood count, coagulation profile and biochemistry report were within normal limits. Chest computed tomographic (CT) scans revealed bilateral peripheral ground-glass opacities with associated infiltrates and pulmonary embolism (Fig. 1). Emergency abdominal CT scans depicted thickened bowel wall and portal vein thrombosis but no clear picture of intestinal perforation (Fig. 2). Duplex ultrasonography of the lower limbs excluded deep vein thrombosis. However, the patient's clinical status did not improve. He therefore underwent emergency exploratory laparotomy. Intraoperatively, a contained small intestinal perforation with associated peritonitis was found; resection of an ischaemic area of the jejunum was thus performed, without any complications. Histopathologic examination via haematoxylin and eosin staining of a resected small intestine specimen revealed epithelial necrosis with haemorrhagic infarction changes and neutrophilic inflammation with fibrin deposition within the lamina propria.
Fig. 1.
Contrast chest computed tomographic scans revealing bilateral peripheral ground-glass opacities (left) and thrombus formation in right pulmonary artery (right) in patient with coronavirus disease 2019 (COVID-19).
Fig. 2.
Emergency contrast abdominal computed tomographic scans depicting portal vein thrombosis (left) and thickened bowel wall (right) in patient with coronavirus disease 2019 (COVID-19).
Postoperatively, the patient was admitted to our level III COVID-19–designated intensive care unit (ICU). At ICU admission, we administered empiric therapy with ribavirin (400 mg tablet every 12 hours), meropenem (0.5 g intravenously every 12 hours) and vancomycin (15 mg/kg intravenously loading dose) that were both adjusted for the patient's renal function, as well as therapeutic anticoagulation with enoxaparin 75 mg subcutaneously once daily (dosing adjusted to his body weight and renal function), ARDS-net ventilation (positive end-expiratory pressure of 9 cm H2O), administration of hydrocortisone and vasopressors and supportive ICU care [16]. The patient developed acute kidney injury (AKI) according to the RIFLE (risk, injury, failure) criteria [17]. His blood urea nitrogen was 7.9 mmol/L (reference range, 2.5–6.4 mmol/L) and creatinine was 309 μmol/L (reference range, 71–115 μmol/L).
We speculated that the development of AKI was partially due to severe COVID-19 and perioperative septic shock, as notion supported by the results of the CT scans with contrast that were performed at admission. The patient developed mild metabolic acidosis and hyperkalaemia while remaining anuric and in a state of shock necessitating the administration of vasopressors (day 1 after ICU admission). We promptly applied continuous renal replacement therapy (CRRT) as per Kidney Disease Improving Global Outcomes (KDIGO) 2019 guidelines [18]. Two sessions of CRRT were administered for two consecutive days (24 hours a day). In addition, we performed extracorporeal blood purification therapy by means of the CytoSorb filter (CytoSorbents Europe GmbH, Berlin, Germany) connected to the CRRT device in order to treat persistent sepsis and to mitigate the ensuing MSOF [[19], [20], [21], [22]]. The CytoSorb filter was used according to the manufacturer's guidelines [23]. Briefly, the filter was connected after haemofiltering via a closed-loop circuit to the CRRT pump (Prismaflex, Baxter Deutschland, Unterschleißheim, Germany) with an optimal ultrafiltration rate of 250 to 400 mL/min. The filter was changed after 24 hours of use. Fortunately, the patient experienced no severe coagulopathy, and transfusion was deemed necessary only once with two packs of red blood cells while he was receiving CRRT (day 1). After two sessions of CRRT with CytoSorb, we were able to initiate diuresis and wean the patient off vasopressors (day 3). The patient's lactate level and all inflammatory biomarkers were normalized, and CRRT was discontinued 3 days after ICU admission. No bleeding episodes were observed, although he received full anticoagulation therapy due to venous thromboembolism. His oxygenation progressively improved; the PaO2/FiO2 ratio exceeded 300 on day 6.
The patient was extubated on day 7 after ICU admission. His renal function was normalized approximately 2 weeks after ICU admission. We administered broad-spectrum antibiotics for a total of 2 weeks, and therapeutic anticoagulation was provided with low-molecular-weight heparin for a month. His RT-PCR for COVID-19 was negative on day 19. The workup for autoimmune disorders included lupus anticoagulant, antiphospholipid antibodies (anticardiolipin, anti–β2-glycoprotein I antibodies), antineutrophil cytoplasmic antibodies and thrombophilia screening (i.e. levels of proteins C and S, homocysteine, factor V Leiden); no abnormalities were found. All follow-up blood, urine and sputum cultures for common bacteria were negative approximately 24 days after ICU admission. The patient was discharged in a good clinical condition 34 days after ICU admission. Oral rivaroxaban was prescribed for another 2 months [24,25]. The patient is being followed by a multidisciplinary physician's group including our outreach team.
Discussion
An increased prevalence of thromboembolic disease in critically ill patients with COVID-19 affecting their morbidity and mortality has been previously reported [26,27]. The administration of prophylactic anticoagulation in mechanically ventilated patients with severe COVID-19 is mandatory [[28], [29], [30], [31], [32]]. The administration of enhanced prophylactic anticoagulation and/or therapeutic anticoagulation in critically ill COVID-19 patients should outweigh the risks of bleeding complications [32]. Our COVID-19 patient with venous thromboembolic disease had increased levels of d-dimer but no risk factors for developing thromboembolism (Padua prediction score < 4). His refractory ARDS could be attributed to a dual-hit pathology of severe lung parenchymal inflammation and the presence of pulmonary embolism due to COVID-19 [33], as well as progressive development of abdominal sepsis.
In this case report, COVID-19 presented as septic shock and acute abdomen due to contained small bowel perforation on the grounds of thromboembolic disease. Scarce data exist about the presentation of COVID-19 as an acute abdomen; in most reported cases, no definitive underlying pathology is documented [[6], [7], [8],[10], [11], [12]]. In other studies, the underlying mechanism is not attributed to bowel pathology but rather to extraintestinal causes such as pancreatitis or cholecystitis [9,34]. However, bowel ischaemia due to arterial thromboembolism was recently described in patients with severe COVID-19 [35,36]. Hence, the presentation of COVID-19 as an acute abdomen cannot be underestimated and should be promptly diagnosed irrespective of the workup challenges [[10], [11], [12]].
In our patient, although imaging studies were inconclusive, the clinical picture indicated a surgical emergency, which was then confirmed intraoperatively. Prompt surgical intervention was a vital management step even though the application of strict isolation measures for COVID-19 patients intraoperatively is challenging [13]. Postoperatively, the patient developed AKI necessitating CRRT due to sepsis and ensuing MSOF. Extracorporeal haemoadsorption by means of a CytoSorb filter was used to complement CRRT. This combination therapy aimed to restore the patient's homeostasis and counteract sepsis. Extracorporeal blood purification therapies using a variety of cartridges for patients with sepsis have been previously reported, with variable results [[20], [21], [22]]. In our case, after two sessions of CRRT with the CytoSorb filter, inflammatory biomarkers and lactate levels were normalized. Moreover, renal function was restored and oxygenation gradually improved. Prompt surgical intervention and the administered empiric treatment, including antibiotics, anticoagulation therapy and ICU supportive care, could have also contributed to the patient's clinical recovery. However, given the severity of his clinical picture, we speculate that the prompt application of CRRT with haemoadsorption might have helped. Presumably the early application of haemoadsorption mitigated a full-blown picture of COVID-19–related hyperinflammation and/or the development of treatment-refractory septic shock.
The patient had an uneventful recovery without any bleeding episodes despite receipt of full anticoagulation therapy and was finally discharged from hospital. However, the patient's long-term rehabilitation challenges and his pulmonary function after COVID-19 pneumonia with associated pulmonary embolism remain to be further elucidated. Our patient had typical features of COVID-19 along with severe gastrointestinal manifestations. Also, he had thromboembolic disease and a laboratory profile characterized by increased inflammatory biomarkers and d-dimers. Hypercoagulability, vascular dysfunction and cytokine storm may be the underlying mechanisms enabling the development of thromboembolic disease in severe COVID-19.
No clear evidence of diffuse intravascular coagulation was observed in this case, which presumably reflects to versatile underlying pathophysiology in the development of thromboinflammation in COVID-19 [[3], [4], [5],[26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]]. SARS-CoV-2 can bind to the angiotensin-converting enzyme 2 receptor, and by gaining direct cell entry may promote endotheliitis [4,27,33,[37], [38], [39]], which can be further exacerbated by dysregulated responses of the renin–angiotensin–aldosterone and immune systems [4,[37], [38], [39]]. The detrimental effects of thromboembolic disease on survival in severe COVID-19 have been previously documented by clinical and histopathology studies [[26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36],[39], [40], [41], [42], [43]]. Also, after hospital discharge, some COVID-19 patients may require additional prophylaxis for venous thromboembolism. Our critically ill patient with severe thromboembolic disease was mechanically ventilated and hospitalized for a prolonged time; hence rivaroxaban was prescribed at hospital discharge as outpatient venous thromboembolism prophylaxis [24,25,43]. Moreover, we should consider the fact that the natural course of SARS-CoV-2 viraemia and the host's natural immunity remain obscure; reinfections and/or recurrently positive RT-PCR results have been reported [[44], [45], [46], [47], [48]].
Conclusion
Despite the inherent limitations of reports of a single case which prevent its generalizability, we documented that COVID-19 can present with severe gastrointestinal manifestations, which could be further linked to underlying thromboinflammation. Further study of such cases may enable us to tailor cost-effective diagnostic and therapeutic strategies for the management of COVID-19 patients who present with an acute abdomen. Apart from extraintestinal causes, bowel ischaemia due to venous and/or arterial thromboembolic disease should be promptly diagnosed and treated. The application of CRRT and extracorporeal blood purification techniques could be a useful tool in the management of perioperative sepsis, AKI and cytokine storm, which are all poor prognostic factors in critically ill patients with COVID-19. Future larger prospective studies are needed to confirm or refute the present findings.
Conflict of interest
None declared.
Acknowledgements
We acknowledge all healthcare workers involved in the diagnosis and treatment of COVID-19 patients in Riyadh, Saudi Arabia.
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J., HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faqihi F., Alharthy A., Alodat M., Kutsogiannis D.J., Brindley P.G., Karakitsos D. Therapeutic plasma exchange in adult critically ill patients with life-threatening SARS-CoV-2 disease: a pilot study. J Crit Care. 2020 doi: 10.1016/j.jcrc.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sellevoll H.B., Saeed U., Young V.S., Sandbæk G., Gundersen K., Mala T. Acute abdomen as an early symptom of COVID-19. Tidsskr Nor Laegeforen. 2020 doi: 10.4045/tidsskr.20.0262. [DOI] [PubMed] [Google Scholar]
- 7.Blanco-Colino R., Vilallonga R., Martín R., Petrola C., Armengol M. Suspected acute abdomen as an extrapulmonary manifestation of COVID-19 infection. Cir Esp. 2020;98:295–296. doi: 10.1016/j.ciresp.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seeliger B., Philouze G., Cherkaoui Z., Felli E., Mutter D., Pessaux P. Acute abdomen in patients with SARS-CoV-2 infection or co-infection. Langenbecks Arch Surg. 2020 doi: 10.1007/s00423-020-01948-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazrouei S.S.A., Saeed G.A., Al Helali A.A. COVID-19–associated acute pancreatitis: a rare cause of acute abdomen. Radiol Case Rep. 2020;15:1601–1603. doi: 10.1016/j.radcr.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed A.O.E., Badawi M., Ahmed K., Mohamed M.F.H. Case report: COVID-19 masquerading as an acute surgical abdomen. Am J Trop Med Hyg. 2020 doi: 10.4269/ajtmh.20-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdalhadi A., Alkhatib M., Mismar A.Y., Awouda W., Albarqouni L. Can COVID-19 present like appendicitis? ID Cases. 2020;21 doi: 10.1016/j.idcr.2020.e00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed A.O.E., Mohamed S.F., Saleh A.O., Al-Shokri S.D., Ahmed K., Mohamed M.F.H. Acute abdomen–like-presentation associated with SARS-CoV-2 infection. ID Cases. 2020;21 doi: 10.1016/j.idcr.2020.e00895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai M., Wang G., Zhang L., Gao J., Xia Z., Zhang P. Performing abdominal surgery during the COVID-19 epidemic in Wuhan, China: a single-centred, retrospective, observational study. Br J Surg. 2020;107:e183–e185. doi: 10.1002/bjs.11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO) Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. Interim guidance 2020. https://www.who.int/publications-detail/laboratory-testing-for-2019-novel-coronavirus-in-suspectedhuman-cases-20200117 Available at:
- 15.Chan J.F., Yip C.C., To K.K., Tang T.H., Wong S.C., Leung K.H. J Clin Microbiol. 2020 Apr 23;58(5):e00310–e00320. doi: 10.1128/JCM.00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saudi Ministry of Health Coronavirus disease 19 (COVID-19) guidelines, revised version 1.7. 25 May 2020. https://covid19.moh.gov.sa Available at:
- 17.Ostermann M., Chang R.W. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007;35:1837–1843. doi: 10.1097/01.CCM.0000277041.13090.0A. [DOI] [PubMed] [Google Scholar]
- 18.Wang A.Y., Akizawa T., Bavanandan S., Hamano T., Liew A., Lu K.C. 2017 Kidney Disease: Improving Global Outcomes (KDIGO) Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) guideline update implementation: asia summit conference report. Kidney Int Rep. 2019;4:1523–1537. doi: 10.1016/j.ekir.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katagiri D., Ishikane M., Ogawa T. Continuous renal replacement therapy for a patient with severe COVID-19. Blood Purif. 2020 doi: 10.1159/000508062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawchar F., Laszlo I., Oveges N., Trasy D., Ondrik Z., Molnar Z. Extracorporeal cytokine adsorption in septic shock: a proof of concept randomized, controlled pilot study. J Crit Care. 2019;49:172–178. doi: 10.1016/j.jcrc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Dellinger R.P., Bagshaw S.M., Antonelli M., Foster D.M., Klein D.J., Marshall J.C. Effect of targeted polymyxin B hemoperfusion on 28-day mortality in patients with septic shock and elevated endotoxin level: the EUPHRATES randomized clinical trial. JAMA. 2018;320:1455–1463. doi: 10.1001/jama.2018.14618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piccinni P., Dan M., Barbacini S., Carraro R., Lieta E., Marafon S. Early isovolaemic haemofiltration in oliguric patients with septic shock. Intensive Care Med. 2006;32:80–86. doi: 10.1007/s00134-005-2815-x. [DOI] [PubMed] [Google Scholar]
- 23.CytoSorbents US FDA grants CytoSorb® emergency use authorization for use in patients with COVID-19 infection. https://cytosorbents.com/us-fda-authorize-cytosorb-for-use-COVID-19 [press release]. 13 April 2020. Available at:
- 24.Barnes G.D., Burnett A., Allen A., Blumenstein M., Clark N.P., Cuker A. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombol. 2020;50:72–81. doi: 10.1007/s11239-020-02138-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weitz J.I., Rascob G.E., Spyropoulos A., Spiro T.E., De Sanctis Y., Xu J. Thromboprophylaxis with rivaroxaban in acutely ill medical patients with renal impairment: insights from the MAGELLAN and MARINER trials. Thromb Haemost. 2020;120:515–524. doi: 10.1055/s-0039-1701009. [DOI] [PubMed] [Google Scholar]
- 26.Cui S., Chen S., Li X., Liu S., Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alharthy A., Faqihi F., Abuhamdah M., Noor A.F., Nasim N., Balhamar A. A prospective, longitudinal evaluation of point-of-care lung ultrasound in critically ill patients with severe COVID-19 pneumonia. J Ultrasound Med. 2020 doi: 10.1002/jum.15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fraissé M., Logre E., Pajot O., Mentec H., Plantefève G., Contou D. Thrombotic and hemorrhagic events in critically ill COVID-19 patients: a French monocenter retrospective study. Crit Care. 2020;24:245. doi: 10.1186/s13054-020-03025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paranjpe I., Fuster V., Lala A., Russak A.J., Glicksberg B.S., Levin M.A. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76:123–124. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alharthy A., Faqihi F., Memish Z.A., Karakitsos D. Lung injury in COVID-19—an emerging hypothesis. ACS Chem Neurosci. 2020;11:2156–2158. doi: 10.1021/acschemneuro.0c00422. [DOI] [PubMed] [Google Scholar]
- 34.Ying M., Lu B., Pan J., Lu G., Zhou S., Wang D. From the COVID-19 Investigating and Research Team COVID-19 with acute cholecystitis: a case report. BMC Infect Dis. 2020;20:437. doi: 10.1186/s12879-020-05164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung S., Quiwa J.C., Pillai A., Onwu C., Tharayil Z.J., Gupta R. Superior mesenteric artery thrombosis and acute intestinal ischemia as a consequence of COVID-19 infection. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.925753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Besutti G., Bonacini R., Iotti V., Marini G., Riva N., Dolci G. Abdominal visceral infarction in 3 patients with COVID-19. Emerg Infect Dis. 2020;26:1926–1928. doi: 10.3201/eid2608.201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181:894–904. doi: 10.1016/j.cell.2020.03.045. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helms J., Tacquard C., Severac F., Leonard-Lorant I., Ohana M., Delabranche X. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Menter T., Haslbauer J.D., Nienhold R., Savic S., Hopfer H., Deigendesch N. Histopathology. 2020 Aug;77(2):198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deshpande C. Thromboembolic findings in COVID-19 autopsies: pulmonary thrombosis or embolism? Ann Intern Med. 2020;173:394–395. doi: 10.7326/M20-3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fox S.E., Akmatbekov A., Harbert J.L., Fox S.E., Akmatbekov A., Harbert J.L. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alharthy A., Faqihi F., Papanikolaou J., Balhamar A., Blaivas M., Memish Z.A. Thrombolysis in severe COVID-19 pneumonia with massive pulmonary embolism. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lan L., Xu D., Ye G., Xia C., Wang S., Li Y. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020;323:1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng K.I., Wang X.B., Jin X.H., Liu W.Y., Gao F., Chen Y.P. A case series of recurrent viral RNA positivity in recovered COVID-19 Chinese patients. Version 2. J Gen Intern Med. 2020;35:2205–2206. doi: 10.1007/s11606-020-05822-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu R., Jiang Z., Gao H., Huang D., Jiang D., Chen F. Recurrent positive reverse transcriptase–polymerase chain reaction results for coronavirus disease 2019 in patients discharged from a hospital in China. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.10475. [DOI] [PMC free article] [PubMed] [Google Scholar]