Abstract
Objective
The aim of this study was to evaluate the efficacy of anakinra in patients who were admitted to hospital for severe COVID-19 pneumonia requiring oxygen therapy.
Methods
A prospective, open-label, interventional study in adults hospitalized with severe COVID-19 pneumonia was conducted. Patients in the interventional arm received subcutaneous anakinra (100 mg twice daily for 3 days, followed by 100 mg daily for 7 days) in addition to standard treatment. Main outcomes were the need for mechanical ventilation and in-hospital death. Secondary outcomes included successful weaning from supplemental oxygen and change in inflammatory biomarkers. Outcomes were compared with those of historical controls who had received standard treatment and supportive care.
Results
A total of 69 patients were included: 45 treated with anakinra and 24 historical controls. A need for mechanical ventilation occurred in 14 (31%) of the anakinra-treated group and 18 (75%) of the historical cohort (p < 0.001). In-hospital death occurred in 13 (29%) of the anakinra-treated group and 11 (46%) of the historical cohort (p = 0.082). Successful weaning from supplemental oxygen to ambient air was attained in 25 (63%) of the anakinra-treated group compared with 6 (27%) of the historical cohort (p = 0.008). Patients who received anakinra showed a significant reduction in inflammatory biomarkers.
Conclusion
In patients with severe COVID-19 pneumonia and high oxygen requirement, anakinra could represent an effective treatment option and may confer clinical benefit.
Trial registration number
ISRCTN74727214.
Keywords: COVID-19, COVID-19 pneumonia, Hyperinflammation, Mechanical ventilation, Anakinra
Introduction
The coronavirus disease 19 (COVID-19) pandemic, instigated by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), had caused more than 36 million cases and over 1 million deaths worldwide as of October 9, 2020 (World Health Organization, 2020). While the majority of symptomatic COVID-19 cases are mild, approximately 5% develop severe illness with abrupt clinical deterioration and rapid progression to acute hypoxic respiratory failure, often requiring advanced respiratory support (Wu and McGoogan, 2020). This worsening typically coincides with declining viral titres, and usually occurs 7–10 days after onset of symptoms (Joynt and Wu, 2020). During this phase patients frequently exhibit extreme increases in proinflammatory cytokines, especially interleukin-1 (IL-1), interleukin-6 (IL-6), and interleukin-10 (IL-10), eventually leading to hyperinflammation (Yang et al., 2020, Petrilli et al., 2020). IL-1 is an extremely potent proinflammatory cytokine, which has been linked to SARS-CoV-2-induced lung injury (Liao et al., 2020) and implicated in the pathogenesis of the inflammatory phase of COVID-19 (Bordoni et al., 2020), and may serve as a therapeutic target for COVID-19 pneumonia (Ong et al., 2020).
It is becoming increasingly recognized that there are two distinct, albeit overlapping, pathological and phenotypic subsets of COVID-19 pneumonia, namely viral pneumonitis and virus-triggered overreacting immune response (Siddiqi and Mehra, 2020). The latter phenotype is the most severe form and is typified by rapid progression to acute respiratory failure, often requiring invasive ventilatory support (Yang et al., 2020). Patients with severe COVID-19 pneumonia on invasive ventilatory support have excessive mortality (Karagiannidis et al., 2020). This excess death is mostly attributed to extreme hyperinflammation, resulting in severe acute lung injury (Tay et al., 2020).
One of the key challenges posed by the COVID-19 pandemic is the paucity of proven effective therapies to date (ASHP, 2020). Although attempting to inhibit the virus’s replication may be a viable option in the early stages of the disease, this approach appears to be less effective in the later stages, when the inflammatory phenotype dominates the clinical picture (Polak et al., 2020). This supports the view that properly timed anti-inflammatory therapeutic strategies could improve patients’ clinical outcomes and prognosis (Mehta et al., 2020). One plausible approach to impede this virally induced hyperinflammation in patients with severe COVID-19 pneumonia is through interleukin-1 (IL-1) antagonism (Van De Veerdonk and Netea, 2020).
Anakinra is a bioengineered form of the naturally occurring IL-1 receptor antagonist (IL-1ra), which blocks the biological activity of the proinflammatory cytokines IL-1α and IL-1β (Van De Veerdonk and Netea, 2020) and is approved for the treatment of a wide variety of diseases (Cavalli and Dinarello, 2018). The anakinra safety profile (Kullenberg et al., 2016), combined with the utility of IL-1 antagonism in conditions that share many immunological and clinical features with the hyperinflammatory phase of COVID-19 (Cardone et al., 2020), warranted assessing anakinra as a potential therapeutic agent in severe COVID-19 pneumonia with associated hyperinflammation.
Currently, there is no known published prospective clinical trial evidence supporting the efficacy of anakinra for the treatment of COVID-19 pneumonia. However, limited data on the use of anakinra in patients with COVID-19 pneumonia exist (Huet et al., 2020, Cavalli et al., 2020a). We aimed to evaluate the efficacy of anakinra in patients who were admitted to hospital for severe COVID-19 pneumonia and required oxygen therapy. We hypothesized that anakinra might be beneficial in this patient population.
Methods
Study design and patients
The OMA-COVID-19 study was a single-centre prospective interventional trial evaluating the effectiveness of anakinra in hospitalized patients with severe COVID-19 pneumonia, and who required oxygen therapy, against retrospectively derived historical controls.
Patients were included in the interventional arm if they were aged >18 years and were admitted to Sultan Qaboos University Hospital (SQUH), a 490-bed referral teaching hospital in Muscat, Oman, with severe COVID-19-pneumonia, defined as bilateral lung infiltrates on a chest X-ray, a positive SARS-CoV-2 RT-PCR assay, and any of the following: (1) respiratory rate >30 breaths/min and peripheral capillary oxygen saturation (SpO2) of <90% on room air; (2) SpO2 ≤93% on oxygen ≥ 6 L/min; or (3) acute respiratory distress syndrome (ARDS). Exclusion criteria included: refusal of the patient to participate; patients already on invasive mechanical ventilation; those with a known allergy to anakinra; pregnant or breastfeeding women; patients with active or untreated tuberculosis, apparent risk for gastrointestinal perforation (abdominal surgery, active inflammatory bowel disease, or active endoscopy-proven peptic ulcer disease); those with active cancer, active bacterial or fungal infection, chronic liver disease, an absolute neutrophil count of <1.5 × 109/L, or platelet count of <50 × 109/L; and patients who had been enrolled concurrently in other clinical trials. The historical control group was identified retrospectively from the SQUH COVID-19 register, which commenced on April 1, 2020, and incuded all hospitalized patients with COVID-19 disease. Patients who did not receive anakinra and fulfilled the same inclusion and exclusion criteria as those of the interventional arm were included as historical controls.
The OMA-COVID-19 study protocol was approved by the institutional medical research and ethics committee (Ref. No. SQU-EC/169/2020, MREC #2136) and registered under international standard randomized controlled trial number ISRCTN74727214. All patients were given both oral and written information by their physicians about anakinra and the study protocol, and could either accept or refuse enrolment. Obtaining patients’ written consent was mandatory for enrolment, and their choice was documented in the institutional electronic medical record. The patients’ agreement to the use of their data for research was also obtained.
Procedures
Patients in the interventional arm received subcutaneous anakinra (Swedish Orphan Biovitrum, Stockholm, Sweden) at a dose of 100 mg twice daily for 3 days, followed by 100 mg daily for a maximum of 7 days, in addition to the standard treatment at SQUH and supportive care. The dose of anakinra was adjusted to renal function and reduced to one daily injection of 100 mg for the first 3 days, followed by an injection every other day for the next 7 days, in patients under dialysis or with a glomerular filtration rate of less than 30 mL/min. Historical controls received standard treatment and supportive care. Standard treatment at SQUH at the time included parenteral β-lactam antibiotics (intravenous ceftriaxone 2 g per day or intravenous piperacillin–tazobactam 4.5 g three times per day) for 7 days, a macrolide antibiotic (intravenous azithromycin 500 mg per day for 3 days), and thromboembolic prophylaxis for all hospitalized patients, unless contraindicated. Supportive care comprised supplemental oxygen therapy and ventilatory support. Some patients in the interventional arm received a maximum of three doses of intravenous dexamethasone (6 mg per day) before enrollment. Many historical controls received a 5-day course of intravenous methylprednisolone (40 mg twice per day for 5 days).
The data were recorded in an electronic database. Data were collected confidentially and coded according to the institutional regulations regarding patient information and confidential treatment of all data. The study design and data collection were approved by the institutional medical research ethics committee.
Demographic and clinical characteristics, including age, sex, ethnicity, hypertension, diabetes, chronic cardiac/pulmonary/renal/hepatic/neurological diseases, and CXR severity index (Brixia score) were recorded. Clinical manifestations, including fever, respiratory rate, oxygen saturation, and oxygen therapy requirement, were recorded at baseline and day 14, or on discharge from hospital. Similarly, biomarkers of inflammation, including IL-6, CRP, serum ferritin, and D-dimer, and other laboratory parameters, including neutrophil/lymphocyte/platelet count, lactate dehydrogenase (LDH), alanine and aspartate aminotransferase, and creatinine, were also recorded at baseline and day 14, or on discharge from hospital. Plain chest X-rays were performed on admission and on day 14 or discharge, whichever was earlier. The chest radiologist independently reviewed and reported CXR Brixia scores for radiological grading of the severity of COVID-19 pneumonia.
Outcomes
The main outcomes were the need for invasive mechanical ventilation (IMV) and in-hospital death. Secondary outcomes included differences in mean oxygen therapy requirements between day 0 and day 4, day 14, or discharge, and changes in inflammatory biomarkers of disease severity (d-dimer, absolute lymphocyte count, serum ferritin, LDH, CRP, and IL-6 levels) between day 0 and day 14 or discharge from the hospital.
In the interventional arm, day 0 corresponded to the date of initiation of anakinra. In the historical controls, day 0 corresponded to the first day during which each patient fulfilled the inclusion criteria defined for the interventional arm. Safety outcomes were occurrence — within 14 days — of invasive bloodstream infection (BSI), thrombocytopenia (platelet count <50 × 109/L), neutropenia (absolute neutrophil count <1.5 × 109/L), increase in liver aminotransferase enzymes (defined as > five times the upper limit of normal), and injection site reactions.
Statistical analysis
Descriptive statistics were used to describe the data. For categorical variables, frequencies and percentages were reported. Differences between groups (anakinra versus the historical cohort) were analyzed using Pearson’s χ2 tests (or Fisher’s exact tests for expected cells <5). For continuous variables, mean and standard deviation were used to present the data, while analysis was performed using Student’s t-tests. For non-normally distributed continuous variables, median and interquartile range (IQR) were used to summarize the data, and analysis was performed using Wilcoxon-Mann-Whitney tests. Outcomes were adjusted for the significant differences at baseline via multivariate logistic regression, using the simultaneous method. An a-priori two-tailed level of significance was set at <0.05. Statistical analyses were conducted using STATA version 16.1 (STATA Corporation, College Station, TX, USA).
Power analysis
Grasselli et al. (2020) reported that more than 80% of patients with severe COVID-19 pneumonia required IMV. We hypothesized that, to reduce the need for IMV by 50% between the anakinra arm and the historical cohort (80% to 30%), 46 patients were needed (23 on each arm) with power of 90% at the 5% alpha (significance) level. Because of missing data and losses to follow-up, an additional 23 patients were added for a total of 69 subjects (study sample).
Results
From June 15 to July 25, 2020, 45 consecutive patients were enrolled in the interventional arm. From April 1 to June 14, 2020, 24 consecutive patients with the same inclusion and exclusion criteria as the interventional arm were retrospectively identified from the hospital COVID-19 register, and used as historical controls. Baseline demographic data, clinical characteristics, CXR Brixia severity index, and laboratory biomarkers of inflammation for patients in the two groups are compared in Table 1 .
Table 1.
Demographic and clinical characteristics, Brixia severity index, and laboratory biomarkers at baseline.
| Characteristic, mean (SD) unless specified otherwise | Anakinra-treated (n = 45) | Historical controls (n = 24) | p-Value |
|---|---|---|---|
| Age, years | 49.8 ± 16.0 | 51.7 ± 14.8 | 0.630 |
| Age category, n (%) | |||
| <50 years | 23 (51%) | 11 (46%) | |
| 50–69 years | 16 (36%) | 11 (46%) | 0.720 |
| ≥70 years | 6 (13%) | 2 (8%) | |
| Male gender, n (%) | 35 (78%) | 17 (71%) | 0.524 |
| Ethnicity, n (%) | |||
| Arab (Omani) | 34 (76%) | 12 (50%) | |
| Asian | 10 (22%) | 12 (50%) | 0.029 |
| Other | 1 (2%) | 0 | |
| Comorbidities, n (%) | |||
| 0–1 | 33 (73%) | 14 (58%) | 0.203 |
| ≥2 | 12 (27%) | 10 (42%) | |
| Diabetes mellitus | 16 (36%) | 12 (50%) | 0.245 |
| Hypertension | 17 (38%) | 11 (46%) | 0.516 |
| CXR Brixia score of ≥8 | 39/41 (95%) | 19/22 (86%) | 0.333 |
| Duration of symptoms before inclusion, mean (IQR), days | 10 (7–12) | 7 (4–10) | 0.009 |
| SpO2 at presentation, % | 82 ± 8% | 81 ± 12% | 0.449 |
| RR at presentation, breaths/min | 30 ± 8 | 32 ± 7 | 0.173 |
| Fever at enrolment (temperature ≥38 °C) | 15 (33%) | 7 (29%) | 0.724 |
| Clinical inclusion parameters | |||
| ARDS, n (%), mean PaO2/FiO2 | 12 (27%), 123 | 4 (17%), 112 | 0.390 |
| Pneumonia plus SpO2 of ≤93% under oxygen at ≥6 L/min, n (%) | 27 (60%) | 18 (75%) | 0.213 |
| Pneumonia plus SpO2 of <90% on room air plus RR of >30 breaths/min | 6 (13%) | 2 (8%) | 0.704 |
| Oxygen therapy at inclusion | |||
| On NIV, n (%), mean FiO2 | 12 (27%), 0.9 | 4 (17%), 1 | 0.390 |
| On FM/NRM n (%), mean O2, L/min | 33 (73%), 12 | 20 (83%), 15 | 0.390 |
| Concomitant treatments, n (%) | |||
| β-lactam antibiotics | 44 (98%) | 24 (100%) | 1.000 |
| Macrolide antibiotics | 38 (84%) | 22 (92%) | 0.480 |
| Oseltamivir | 21 (47%) | 11 (46%) | 1.000 |
| Enoxaparin (40 mg) OD | 45 (100%) | 24 (100%) | n/a |
| Corticosteroids | 25 (56%) | 16 (67%) | 0.371 |
| Dexamethasone 6 mg IV OD | 24 | 3 | |
| <3 doses | 17 (38%) | 2 (8%) | 1.000 |
| ≥3 doses | 7/45 (16%) | 1 (4%) | |
| Methylprednisolone 40 mg IV BD | 1 (2%) | 13 (54%) | <0.001 |
| <4 doses | 0 (0%) | 0 (0%) | n/a |
| ≥4 doses | 1 (2%) | 13 (54%) | <0.001 |
| IFN + KAL + RIBAV | 1 (2%) | 3 (13%) | 0.118 |
| Hydroxychloroquine | 0 (0%) | 5 (21%) | 0.004 |
| Tocilizumab | 0 (0%) | 1 (4%) | 0.348 |
| Inflammatory biomarkers at inclusion, median (IQR) | |||
| IL-6, pg/mL (normal: 0–7) | 55 (18–98) | 98 (91–255) | 0.103 |
| Ferritin, μg/L (normal: 13–150) | 1088 (650–1622) | 1460 (990–2385) | 0.051 |
| CRP, mg/L (normal: 0–5) | 150 (82–178) | 139 (89–245) | 0.604 |
| LDH, U/L (normal: 135–214) | 455 (384–578) | 582 (445–696) | 0.107 |
| d-dimer, mg/L FEU (normal: 0.2–0.7) | 0.7 (0.5–1.4) | 1.0 (0.7–1.8) | 0.105 |
| ALC, ×109/L (normal: 1.2–3.8) | 1.6 (1.2–2.4) | 0.8 (0.6–1.0) | <0.001 |
| ANC, ×109/L (normal: 1–4.8) | 5.8 (4.0–7.8) | 6.2 (3.7–7.9) | 0.947 |
| Biomarker thresholds of severity at inclusion, n (%) | |||
| IL-6 > 60 pg/mL | 20/42 (48%) | 4/5 (80%) | 0.348 |
| Ferritin > 1500 μg/L | 11/43 (26%) | 8/19 (42%) | 0.193 |
| CRP > 150 mg/L | 22/44 (50%) | 11/24 (46%) | 0.743 |
| LDH > 300 U/L | 33/37 (89%) | 16/17 (94%) | 1.000 |
| d-dimer > 1 mg/L FEU | 11/43 (26%) | 12/24 (50%) | 0.044 |
| ALC ≤ 0.8 × 109/L | 17/41 (41%) | 14/24 (58%) | 0.189 |
| Patients with biomarker threshold for hyperinflammation, n (%) | 33/45 (73%) | 17/24 (71%) | 0.825 |
| Other laboratory parameters, median (IQR) | |||
| PLT count, ×109/L (normal: 150–450) | 227 (205–297) | 227 (177–277) | 0.168 |
| ALT, IU/L (normal: 0–33) | 44 (27–73) | 29 (20–50) | 0.069 |
| AST, IU/L (normal: 0–32) | 51 (33–71) | 52 (32–66) | 0.959 |
| Creatinine, mmol/L (normal: 48–84) | 78 (64–96) | 75 (59–103) | 0.997 |
Abbreviations: SD: standard deviation; IQR: interquartile range; RR: respiratory rate; ARDS: acute respiratory distress syndrome; PaO2/FiO2: ratio of arterial oxygen partial pressure to fractional inspired oxygen; SpO2: oxygen saturation; NIV: non-invasive ventilation; FM: face mask; NRM: non-rebreather mask; OD: once daily; n/a: not applicable; IV: intravenously; BD: twice daily; IFN: interferon; KAL: kaletra (lopinavir-ritonavir); RIBAV: ribavirin; IL-6: interleukin-6; CRP: C-reactive protein; LDH: lactate dehydrogenase; ALC: absolute lymphocyte count; ANC: absolute neutrophil count; PLT: platelets; ALT: alanine aminotransferase; AST: aspartate aminotransferase.
Significant differences between the two groups were a lower Asian ethnicity (22% vs 50%; p = 0.029), a longer duration of symptoms before inclusion (10 days vs 7 days; p = 0.009), a lower proportion of patients treated with hydroxychloroquine (0% vs 21%; p = 0.004), a lower proportion of patients treated with ≥4 doses of 40 mg of intravenous methylprednisolone (2% vs 54%; p < 0.001), a higher lymphocyte count (1.6 × 109/L vs 0.8 × 109/L; p < 0.001), and a lower proportion of patients with d-dimer >1 mg/L FEU (26% vs 50%; p = 0.044) in the anakinra-treated than in the historical control group, respectively. Clinical inclusion criteria, requirements for oxygen therapy, and disease severity as determined by the Brixia severity score and biomarker-defined severity thresholds were balanced between the anakinra-treated and historical groups (Table 1). A total of 33/45 (73%) of the patients in the interventional arm and 17/24 (71%) of the historical patients met the laboratory definition for hyperinflammation (CRP > 150 mg/L, or IL-6 > 60 pg/mL, or ferritin >1500 μg/L).
The need for IMV occurred in 14 (31%) of 45 patients in the anakinra-treated group compared with 18 (75%) of 24 patients in the historical group (p < 0.001) (Table 2 ). The treatment effect of anakinra on the need for IMV persisted and remained significant even after multivariate adjustment of the significant variables at baseline (adjusted odds ratio (aOR) 0.27; 95% confidence interval (CI) 0.07–0.97; p = 0.046) and when stratified by the presence (36% vs 76%; p = 0.016) or absence (17% vs 71%; p = 0.045) of hyperinflammation (Table 2). This treatment effect on the need for IMV was likewise demonstrated at day 4 (20% vs 58%; p = 0.033) and day 14 (23% vs 50%; p = 0.046) in the anakinra-treated compared with the historical group, respectively.
Table 2.
Clinical outcomes
| Outcome, n (%) unless specified otherwise | Anakinra-treated (n = 45) | Historical controls (n = 24) | p-Value |
|---|---|---|---|
| Need for IMV | 14 (31%) | 18 (75%) | <0.001 |
| Need for IMV, stratified by hyperinflammatory statea | |||
| With hyperinflammation | 12/33 (36%) | 13/17 (76%) | 0.016 |
| Without hyperinflammation | 2/12 (17%) | 5/7 (71%) | 0.045 |
| In-hospital death | 13 (29%) | 11 (46%) | 0.159 |
| In-hospital death, stratified by hyperinflammatory state | |||
| With hyperinflammation | 11/33 (33%) | 7/17 (41%) | 0.757 |
| Without hyperinflammation | 2/12 (17%) | 4/7 (57%) | 0.129 |
| In-hospital death, stratified by ethnicity | |||
| Arab (Omani) | 8/34 (24%) | 8/12 (67%) | 0.013 |
| Asian | 5/10 (50%) | 3/12 (25%) | 0.378 |
| Progression to NIV | 10 (30%) | 7 (35%) | 0.723 |
| Progression to NIV, stratified by hyperinflammatory state | |||
| With hyperinflammation | 8/33 (24%) | 4/17 (24%) | 1.000 |
| Without hyperinflammation | 2/12 (17%) | 3/7 (43%) | 0.305 |
| Progression from NIV to IMV | 13 (59%) | 9 (82%) | 0.258 |
| Progression from NIV to IMV, stratified by hyperinflammatory state | |||
| With hyperinflammation | 11/33 (33%) | 7/17 (41%) | 0.638 |
| Without hyperinflammation | 2/12 (17%) | 2/7 (29%) | 0.603 |
| Weaned off NIV | 6 (27%) | 1 (9%) | 0.408 |
| Weaned off IMV | 7 (50%) | 7 (39%) | 0.530 |
| Oxygen requirement (day 4) | |||
| On ambient air | 2 (4%) | 1 (4%) | 1.000 |
| On oxygen by NC/FM/NRM | 25 (56%) | 5 (21%) | 0.006 |
| On NIV | 9 (20%) | 4 (17%) | 1.000 |
| On IMV | 9 (20%) | 14 (58%) | 0.033 |
| Oxygen requirement (day 14 or day of discharge) | |||
| On ambient air | 25 (63%) | 6 (27%) | 0.008 |
| On oxygen by NC/FM/NRM | 6 (15%) | 4 (18%) | 0.733 |
| On NIV | 0 (0%) | 1 (4%) | 0.375 |
| On IMV | 9 (23%) | 11 (50%) | 0.046 |
| Time to defervescence, days (median) | 1 | 2 | 0.019 |
| Inflammatory biomarkers (day 14 or day of discharge), median (IQR) | |||
| IL-6, pg/mL (0–7) | 6.6 (2.8–6.6) | 124 (94–271) | <0.001 |
| Ferritin, m/L (13–150) | 945 (601–1637) | 1139 (763–1980) | 0.254 |
| CRP, mg/L (0–5) | 9 (4–59) | 94 (23–182) | 0.001 |
| LDH, U/L (135–214) | 278 (210–387) | 485 (321–794) | 0.011 |
| d-dimer, mg/L FEU (0.2–0.7) | 0.9 (0.5–2.6) | 3.7 (2.1–11.2) | 0.001 |
| ALC, ×109/L (1.2–3.8) | 1.6 (1.2–2.4) | 1.4 (0.9–1.6) | 0.109 |
| Safety outcomes | |||
| Bloodstream infectionb | 5 (11%) | 4/22 (18%) | 0.461 |
| Neutropenia (ANC < 1.5 × 109/L) | 3 (7%) | 0/22 (0%) | 0.545 |
| Thrombocytopenia (PLT < 50 × 109/L) | 2 (5%) | 0/22 (0%) | 1.000 |
| ALT (>5 times rise) | 6 (14%) | 2/22 (9%) | 0.704 |
| AST (>5 times rise) | 0/41 (0%) | 1/22 (5%) | 0.349 |
| Injection site reaction | 0/45 (0%) | n/a | n/a |
Abbreviations: IMV: invasive mechanical ventilation; NIV: non-invasive ventilation; FM: face mask: NRM, non-rebreather mask; IQR: interquartile range; IL-6: interleukin-6; CRP: C-reactive protein; LDH: lactate dehydrogenase; ALC: absolute lymphocyte count; ANC: absolute neutrophil count; PLT: platelets. ALT: alanine aminotransferase; AST: aspartate aminotransferase.
Hyperinflammation was defined as C-reactive protein >150 mg/L, or interleukin-6 > 60 pg/mL, or ferritin > 1500 μg/L.
Bloodstream infection: four of five isolates in the anakinra-treated group and all four isolates in the historical controls were Staphylococcus epidermidis. The remaining patient in the anakinra-treated group had Brevibacterium sp.
In-hospital death occurred in 13 (29%) of 45 patients in the interventional group compared with 11 (46%) of 24 patients in the historical cohort (p = 0.082). This mortality difference did not reach statistical significance.
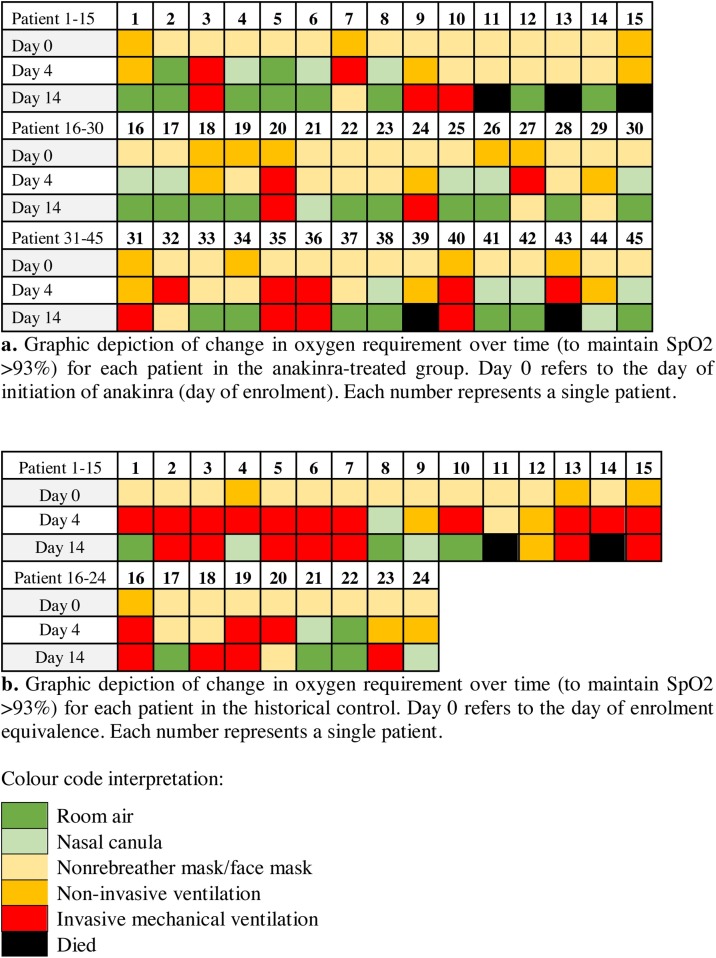
Among the 40 patients in the interventional group who were either alive on day 14 or discharged, 25 (63%) were off oxygen supplementation (on ambient air), compared with only six (27%) in the historical group (p = 0.008) (Figure 1 ).
Figure 1.
Change in oxygen requirement over time. (a) Graphic depiction of change in oxygen requirement over time (to maintain SpO2 >93%) for each patient in the anakinra-treated group. Day 0 refers to the day of initiation of anakinra (day of enrolment). Each number represents a single patient. (b) Graphic depiction of change in oxygen requirement over time (to maintain SpO2 >93%) for each patient in the historical control group. Day 0 refers to the day of enrolment equivalence. Each number represents a single patient.
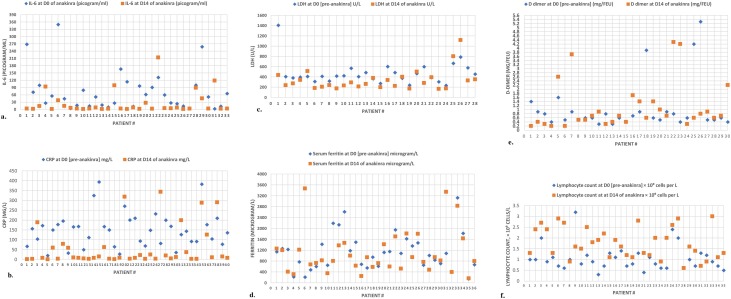
The effect of anakinra on normalization/near normalization of the inflammatory biomarkers at day 14 or discharge was significant for IL-6 (6.6 pg/mL vs 124 pg/mL; p < 0.001), CRP (9 mg/L vs 94 mg/L; p = 0.001), d-dimer (0.9 mg/L vs 3.7 mg/L FEU; p = 0.001), and LDH (278 U/L vs 485 U/L; p = 0.011), respectively (Table 2 and Figure 2 ).
Figure 2.
The effect of anakinra on inflammatory biomarkers. (a) Changes in IL-6 concentrations between D0 (day 0) and D14 (day 14) among patients in the anakinra group (comparison data available for 33 patients). (b) Changes in CRP concentrations between D0 (day 0) and D14 (day 14) among patients in the anakinra group (comparison data available for 40 patients). (c) Changes in LDH concentrations between D0 (day 0) and D14 (day 14) among patients in the anakinra group (comparison data available for 28 patients).
A total of 11% of anakinra-treated patients and 18% of historical controls developed bloodstream infection (p = 0.461). All infections in both groups were caused by Staphylococcus epidermidis, except one in the interventional group where Brevibacterium sp. was isolated. Increases in alanine aminotransferase levels (>5 times the upper limit) were observed in 14% and 9% of anakinra-treated and historical controls, respectively (p = 0.704). Neutropenia and thrombocytopenia occurred in 7% and 5% of anakinra-treated and historical patients, respectively. No injection site reactions were observed.
Discussion
In this prospective, open-label, interventional trial (OMA-COVID-19) of hospitalized patients with severe COVID-19 pneumonia requiring oxygen therapy, anakinra significantly reduced the need for invasive mechanical ventilation, improved respiratory function, and reduced COVID-19-associated hyperinflammation.
Thus far, no specific treatment has been conclusively shown to reduce the need for IMV in patients with COVID-19 pneumonia requiring oxygen therapy. Similarly, no published clinical trial evidence on the efficacy of anakinra in this patient population has been reported. Nonetheless, there are several encouraging reports from case series, and retrospective and prospective cohorts, on the use of anakinra in patients with COVID-19 pneumonia (Huet et al., 2020, Cavalli et al., 2020a, Navarro-Millán et al., 2020, Aouba et al., 2020, Pontali et al., 2020).
Our study demonstrated that anakinra, as a therapeutic intervention for severely ill patients with COVID-19 pneumonia, has the potential to avert the need for IMV. We found that 69% of anakinra-treated patients responded favourably and did not need IMV. This is in contrast with the historical patients, of whom only 25% achieved this outcome (p < 0.001), suggesting that targeting IL-1 via the use of anakinra may be an effective approach to avoid IMV in these patients. Interestingly, this treatment effect of anakinra was noted in patients with and without demonstrable hyperinflammation. This unexpected, though interesting, observation may be explained by shortcomings in the biomarker thresholds used in this and other studies (Manson et al., 2020) to define hyperinflammation. We believe that these biomarker thresholds signify advanced inflammation and may under-recognize a subset of patients (labelled as without hyperinflammation) with early inflammation, who may still benefit from the anti-inflammatory effect of anakinra.
The therapeutic effect of anakinra on deferring the need for IMV was not seen in the subset of patients who required non-invasive ventilation (59% vs 82%; p = 0.258). This latter observation suggests that there may be a therapeutic window — before advanced respiratory failure — where anakinra might be most beneficial (Langer-Gould et al., 2020). It may also suggest that low-dose subcutaneous anakinra may not be adequate for controlling inflammation in patients with advanced respiratory failure, a finding consistent with a previous report (Cavalli et al., 2020a).
While the search for effective therapeutics for severe COVID-19 pneumonia is underway, oxygen therapy is currently the main means of supporting these patients. All patients in this study were severely ill and required oxygen therapy on enrolment (Table 1). Successful weaning from supplemental oxygen to ambient air at day 14 was attained in 63% of patients in the interventional group compared with 27% of patients in the historical control group. This treatment effect was significant (p = 0.008) and could translate to a shorter time to recovery and to hospital discharge, thus easing hospital pandemic surge capacity.
Several studies have indicated that a subset of patients with severe COVID-19 pneumonia experience a hyperinflammatory state, inferred by a concurrent rise in biomarkers of inflammation above defined thresholds (Manson et al., 2020). Applying these thresholds, we found that 73% of anakinra-treated patients and 71% of historical patients had biomarker-defined hyperinflammation on inclusion, denoting advanced stages, poor prognosis, and possibly biological plausibility for anakinra. The anti-inflammatory effect of anakinra was examined in our study. Patients who received anakinra showed significant reductions in IL-6, CRP, LDH, and d-dimer levels compared with historical controls (Table 2 and Figure 2).
Although, anakinra has been reported to reduce COVID-19 mortality in small observational studies (ASHP, 2020), our study showed no significant difference in in-hospital mortality between the anakinra-treated and historical groups (29% vs 46%; p = 0.159). We believe that the inability of our study to demonstrate mortality benefit may be explained by the late initiation of anakinra, a large proportion of patients in the advanced hyperinflammatory state at inclusion (73%), and possibly an underpowered design, rather than a true lack of treatment effect. Whether earlier administration of anakinra will decrease in-hospital mortality is unknown. Despite the lack of demonstrable, statistically significant, between-group mortality difference, the treatment effect of anakinra may still be clinically relevant. Intriguingly, a significant difference in mortality, favouring anakinra, was observed for Arab ethnicity (24% vs 67%; p = 0.013). This ethnicity difference in treatment outcomes, disadvantaging Asian ethnicity, is consistent with reported worse clinical outcomes from COVID-19 in this population (ICNARC, 2020, Pan et al., 2020). Although cause of death was not systematically examined in our study, seven patients in the anakinra group (54%) and nine patients in the historical group (82%) died from ARDS. Additionally, two patients (one from each group) were presumed to have died from systemic thromboembolism.
A distinctive feature of the OMA-COVID-19 study was the use of Brixia score, a CXR severity scoring system designed exclusively for assessment of COVID-19 pneumonia (Borghesi and Maroldi, 2020a). In a previous study, a Brixia score of 8 or more was found to be predictive for risk of in-hospital death (Borghesi et al., 2020b). In our study, 39/41 (95%) of anakinra-treated patients and 19/22 (86%) of historical controls had CXR Brixia score of ≥8 at inclusion, suggesting that the majority of patients in both groups had severe disease and were at high risk of death.
When there was no contraindication, a combination of β-lactam and macrolide antibiotics was prescribed at our institution as standard of care for hospitalized patients with severe COVID-19 pneumonia. A total of 98% and 100% of patients in the intervention and historical groups, respectively, received β-lactam antibiotics, while 84% and 92% received azithromycin. Nearly half the patients in both cohorts were prescribed oseltamivir (47% and 46%, respectively) at the discretion of the treating physician. All patients in both groups were treated with prophylactic-dose, low-molecular-weight heparin for thromboprophylaxis. More patients in the historical control group (54% vs 2%; p < 0.0.001) received long therapeutic courses of steroids (methyl prednisone), which might have created bias, obscuring detection of a significant mortality benefit from anakinra. It is important to mention that corticosteroids are the only therapeutic intervention thus far to conclusively prove a mortality benefit for patients with COVID-19 pneumonia (Lamontagne et al., 2020).
Of the historical controls, 21% received hydroxychloroquine in combination with azithromycin, compared to none in the intervention group. This combination has been shown in a recent systematic review and meta-analysis to significantly increase mortality (Fiolet et al., 2021), and may have introduced bias favouring anakinra.
Treatment with anakinra was safe and well tolerated, and its discontinuation did not result in respiratory deterioration. We observed similar rates of bacteremia (11% vs 18%) and increases in alanine aminotransferase (14% vs 9%) in anakinra-treated and historical controls, respectively. All bacteremia events were self-limited, and none required antimicrobial therapy. The similar occurrence of transient changes in liver enzymes in both groups suggests that hyperinflammation may have been the cause (Cavalli and Dagna, 2020b). Neutropenia and thrombocytopenia occurred in 7% and 5% of anakinra-treated patients, respectively, versus none observed among the historical controls.
We acknowledge several limitations of this study. First, the small sample size was a potential hindrance to definitive conclusions. Second, the lack of a randomized design could have introduced bias. Third, the intervention group was compared with a historical control group over a 10-week period, possibly introducing a non-contemporaneous bias. Fourth, the historical controls differed from the anakinra-treated patients in several potentially confounding variables.
Despite the aforementioned limitations, OMA-COVID-19 had several strengths. Unlike previous reports, this study was one of only a few clinical trials, and the first to be published. OMA-COVID-19 enrolled a heterogeneous population with severe COVID-19 pneumonia, but with variable degrees of inflammation and different requirements for oxygen therapy, permitting assessment of the treatment effect in various clinical phenotypes. Our study suggests that anakinra could represent an effective treatment option for patients with severe COVID-19 pneumonia, and may confer benefit by averting the need for invasive mechanical ventilation and shortening the need for oxygen therapy in a substantial number of patients.
Conclusion
In patients with severe COVID-19 pneumonia and high oxygen requirement, anakinra is a safe and effective therapeutic approach for deferring mechanical ventilation, shortening the need for supplemental oxygen, and controlling SARS-CoV-2-triggered inflammation. Our study has provided further evidence of the utility of anakinra in this patient population. Further adequately powered, randomized clinical trials are warranted to corroborate our findings.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding source
This study was sponsored by the medical research centre of Sultan Qaboos University, Oman. OQ (a non-pharmaceutical national company in Oman) funded the cost of anakinra.
Ethical approval
This study was approved by the medical research and ethics committee of the College of Medicine and Health Sciences at Sultan Qaboos University, Oman (Ref. No. SQU-EC/169/2020, MREC #2136). Trial registration number: ISRCTN74727214.
Authors’ contributions
AB conceptualized and designed the study, interpreted the data, and drafted and revised the manuscript. IZ designed the study, analyzed the data, and critically revised the manuscript. MB, AK, SM, HB, JA, and IB conceptualized and designed the study, and revised the manuscript. MK interpreted the laboratory data, conceptualized the study, and revised the manuscript. SB interpreted the data on Brixia scores and revised the manuscript. AA, AL, BR, KB, KZ, NE, BD, BJ, FR, FY, GK, IS, JL, MG, MA, NA, SR, SK, WA, ZN, and ZB recruited patients for the study, provided clinical care for study patients, contributed to the acquisition of the data, and revised the manuscript. OB collected and summarized all data related to this study, analyzed the data for historical controls, and critically revised the manuscript.
Acknowledgements
We would like to thank the medical research centre of Sultan Qaboos University, Oman and the Oman Qaboos company for supporting this research.
References
- Aouba A., Baldolli A., Geffray L., Verdon R., Bergot E., Martin-Silva N., et al. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann Rheum Dis. 2020;79:1381. doi: 10.1136/annrheumdis-2020-217706. [DOI] [PubMed] [Google Scholar]
- ASHP . 2020. American Society of Health-System Pharmacists (ASHP) COVID-19 resources (2020). Assessment of evidence for COVID-19-related treatments. Available at: https://www.ashp.org/-/media/assets/pharmacy-practice/resource-centers/Coronavirus/docs/ASHP-COVID-19-Evidence-Table.ashx. [Accessed 12 October 2020] [Google Scholar]
- Bordoni V., Sacchi A., Cimini E., Notari S., Grassi G., Tartaglia E., et al. An inflammatory profile correlates with decreased frequency of cytotoxic cells in coronavirus disease 2019. Clin Infect Dis. 2020;71(November (16)):2272–2275. doi: 10.1093/cid/ciaa577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghesi A., Maroldi R. COVID-19 outbreak in Italy: experimental chest X-ray scoring system for quantifying and monitoring disease progression. Radiol Med. 2020;125(5):509–513. doi: 10.1007/s11547-020-01200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghesi A., Zigliani A., Golemi S., Carapella N., Maculotti P., Farina D., et al. Chest X-ray severity index as a predictor of in-hospital mortality in coronavirus disease 2019: a study of 302 patients from Italy. Int J Infect Dis. 2020;96:291–293. doi: 10.1016/j.ijid.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone M., Yano M., Rosenberg A.S., Puig M. Lessons learned to date on COVID-19 hyperinflammatory syndrome: considerations for interventions to mitigate SARS-CoV-2 viral infection and detrimental hyperinflammation. Front Immunol. 2020;11:1131. doi: 10.3389/fimmu.2020.01131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli G., Dinarello C.A. Anakinra therapy for non-cancer inflammatory diseases. Front Pharmacol. 2018;9:1157. doi: 10.3389/fphar.2018.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D., et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2(6):e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli G., Dagna L. Anakinra for COVID-19: how to interpret elevations in serum liver enzymes. Arthritis Rheumatol. 2020;(September) doi: 10.1002/art.41525. (ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiolet T., Guihur A., Rebeaud M.E., Mulot M., Peiffer-Smadja N., Mahamat-Saleh Y. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27(January (1)):19–27. doi: 10.1016/j.cmi.2020.08.022. S1198-743X (20)30505-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323:1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- Huet T., Beaussier H., Voisin O., Jouveshomme S., Dauriat G., Lazareth I., et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393–e400. doi: 10.1016/S2665-9913(20)30164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICNARC . 2020. Intensive Care National Audit and Research Centre report on COVID-19 in critical care. England, Wales, and Northern Ireland. 9 October 2020. ICNARC COVID-19 study case mix program database. Available at: file:///C:/Users/infec_000/Downloads/ICNARC_COVID-19_Report_2020-10-09.pdf.pdf. [Accessed 12 October 2020]. [Google Scholar]
- Joynt G.M., Wu W.K. Understanding COVID-19: what does viral RNA load really mean? Lancet Infect Dis. 2020;20(6):635–636. doi: 10.1016/S1473-3099(20)30237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannidis C., Mostert C., Hentschker C., Voshaar T., Malzahn J., Schillinger G., et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020;8(9):853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullenberg T., Löfqvist M., Leinonen M., Goldbach-Mansky R., Olivecrona H. Long-term safety profile of anakinra in patients with severe cryopyrin-associated periodic syndromes. Rheumatology (Oxford) 2016;55(8):1499–1506. doi: 10.1093/rheumatology/kew208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamontagne F., Agoritsas T., MacDonald H., Leo Y.S., DIaz J., Agarwal A., et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- Langer-Gould A., Smith J.B., Gonzales E.G., Castillo R.D., Figueroa J.G., Ramanathan A., et al. Early identification of COVID-19 cytokine storm and treatment with anakinra or tocilizumab. Int J Infect Dis. 2020;99:291–297. doi: 10.1016/j.ijid.2020.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26(6):842–844. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- Manson J.J., Crooks C., Naja M., Ledlie A., Goulden B., Liddle T., et al. COVID-19-associated hyperinflammation and escalation of patient care: a retrospective longitudinal cohort study. Lancet Rheumatol. 2020;2(10):e594–e602. doi: 10.1016/S2665-9913(20)30275-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Millán I., Sattui S., Lakhanpal A., Zisa D., Siegel C., Crow M. Use of anakinra to prevent mechanical ventilation in severe COVID-19: a case series. Arthritis Rheumatol. 2020;72(December (12)):1990–1997. doi: 10.1002/art.41422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong E.Z., Chan Y.F.Z., Leong W.Y., Lee N.M.Y., Kalimuddin S., Haja Mohideen S.M., et al. A dynamic immune response shapes COVID-19 progression. Cell Host Microbe. 2020;27(6) doi: 10.1016/j.chom.2020.03.021. 879–882.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D., Sze S., Minhas J.S., Bangash M.N., Pareek N., Divall P., et al. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. EClinicalMedicine. 2020;23 doi: 10.1016/j.eclinm.2020.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak S.B., Van Gool I.C., Cohen D., von der Thüsen J.H., van Paassen J. A systematic review of pathological findings in COVID-19: a pathophysiological timeline and possible mechanisms of disease progression. Mod Pathol. 2020:1–11. doi: 10.1038/s41379-020-0603-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontali E., Volpi S., Antonucci G., Castellaneta M., Buzzi D., Tricerri F., et al. Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease. J Allergy Clin Immunol. 2020;146(1):213–215. doi: 10.1016/j.jaci.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi H.K., Mehra M.R. COVID-19 illness in native and immunosuppressed states: a clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39(5):405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van De Veerdonk F.L., Netea M.G. Blocking IL-1 to prevent respiratory failure in COVID-19. Crit Care. 2020;24(1):445. doi: 10.1186/s13054-020-03166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update. 5 October 2020. Available at: www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. [Accessed 11 October 2020] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Yang L., Liu S., Liu J., Zhang Z., Wan X., Huang B., et al. COVID-19: immunopathogenesis and immunotherapeutics. Signal Transduct Target Ther. 2020;5(1):128. doi: 10.1038/s41392-020-00243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]