Abstract
In recent years the global market for monoclonal antibodies (mAbs) became a multi-billion-dollar business. This success is mainly driven by treatments in the oncology and autoimmune space. Instead, development of effective mAbs against infectious diseases has been lagging behind. For years the high production cost and limited efficacy have blocked broader application of mAbs in the infectious disease space, which instead has been dominated for almost a century by effective and cheap antibiotics and vaccines. Only very few mAbs against RSV, anthrax, Clostridium difficile or rabies have reached the market. This is about to change. The development of urgently needed and highly effective mAbs as preventive and therapeutic treatments against a variety of pathogens is gaining traction. Vast advances in mAb isolation, engineering and production have entirely shifted the cost-efficacy balance. MAbs against devastating diseases like Ebola, HIV and other complex pathogens are now within reach. This trend is further accelerated by ongoing or imminent health crises like COVID-19 and antimicrobial resistance (AMR), where antibodies could be the last resort. In this review we will retrace the history of antibodies from the times of serum therapy to modern mAbs and lay out how the current run for effective treatments against COVID-19 will lead to a quantum leap in scientific, technological and health care system innovation around mAb treatments for infectious diseases.
Keywords: Monoclonal antibody, serum therapy, mAb, Innovation, COVID-19
1. Introduction
Antibodies play a fundamental role in the humoral immunity to infection. They bind to soluble toxins, blocking their function, and to antigens on the surface of pathogens, which neutralizes their capacity to infect human cells or tag them for destruction. Tagged pathogens are eliminated through complement activation, antibody-dependent cellular cytotoxicity (ADCC) or antibody-dependent cellular phagocytosis (ADCP) by immune cells [1,2]. All antibodies are composed of an antigen-binding fragment (Fab) that confers specificity to its target, and a crystallizable fragment (Fc) that drives the biological function. Changes in both Fab and Fc regions affect the specificity, durability, and the outcome of the antibody-dependent response [3]. The simple biological design of antibodies coupled with an extensive versatility and an array of potent effector functions makes them of prime interest to be used as biological agents to treat disease.
The discovery of how to isolate and clone antibodies gave rise to the generation of humanized and human monoclonal antibodies (mAbs), and enabled their use as therapies in the fields of oncology and autoimmunity [4]. Despite their successes in these field, the use of mAbs to treat infectious diseases remained limited, until recently. Complex pathogens trained by evolution to avoid destruction by antibodies are notoriously difficult to tackle [[5], [6], [7], [8]]. Coupled with other hurdles in clinical development and implementation, progression of mAbs has been challenging: first generation mouse or mouse-derived mAbs have had low efficacy and elicited anti-antibody immunity. To reach efficacious concentrations, patients required hospitalization and antibody infusion. High production costs translated into preventively high treatment costs. While many of these factors were acceptable for settings like oncology, they were unsurmountable in the infectious disease space. Only few molecules have reached the market.
This is about to change, since considerable progress in isolation of rare, potent and fully human mAbs together with enhanced molecular understanding of antibody functionality are paving the way towards novel mAb-based therapies against several difficult-to-treat pathogens. A multitude of antibodies are in pre-clinical and clinical evaluation, and preventive or therapeutic mAb treatments against devastating diseases like Ebola, HIV and other complex pathogens advancing in their development. Moreover, the COVID-19 pandemic is also fueling new interest in this field, giving a strong impulse to antibody-based therapy research. An unprecedented quest for effective treatments is ongoing. Multiple vaccine technologies, monoclonal antibody formats and even serum therapy are evaluated in parallel. We can expect a wealth of pre-clinical and clinical data, to inform systems biology, hypothesis testing and tremendous knowledge gain to spark future innovation.
In this review, we discuss how antibodies are used to treat infections, what are the current limitations, and how innovative therapy approaches could shape the future of the antibody-based treatments.
2. Active & passive immunizations – two sides of a coin for effective infectious disease management
2.1. Serum therapies
Antibody-derived therapies have been in use for more than a century. It all started with the observations of von Behring and Kitasato, describing how antibodies could neutralize toxins and confer protection [9]. These preparations, derived from the serum of immunized animals or convalescent human donors (“serum therapies”) were introduced in the 20th century to treat bacterial toxins, in particular diphtheria and tetanus, and viruses such as cytomegalovirus (CMV), hepatitis, herpes simplex, measles, rabies, respiratory syncytial virus (RSV), smallpox and varicella zoster [10].
Passive serum therapy results in an immediate effect and it is highly advantageous to treat microorganisms due to the polyclonality of the response targeting different antigens. However, there are also disadvantages: the short-lived protection, the limited standardization and supply and, particularly, the safety concerns, as it often led to hypersensitivity reactions [11]. While hyperimmune sera from animals is still used today to treat diphtheria, the advent of antibiotics marked a decline in the use of serum therapy for bacterial infections, which it is today limited to hyperimmunoglobulin-based products for the treatment of a handful of pathogens and for rabies post-exposure prophylaxis [12]. Nonetheless, passive immune therapy was one of the first treatments used for COVID-19. This is yet a different kind of application that is not based on a standardized product, since plasma samples are not pooled and used individually, as we will describe later in this review.
2.2. Monoclonal antibodies
The advent of the hybridoma technology, with immortalization of single B cell clones, allowed culture of antibody-producing cells and the generation of large quantities of identical immunoglobulins (monoclonal antibodies, mAbs), greatly increasing the capacity to design precision therapies. The use of mAbs with defined target epitope and reproducible efficacy has important advantages over serum therapies [11]. Indeed, mAbs are well defined biological products with high specificity, as they are directed to a key antigen of the pathogen with minimal off-target effects. They can have effector functions modulating different biological effects depending on the pathogen. Moreover, they can be scaled up for mass production by in vitro culture and have the possibility to be engineered to refine their characteristics even further. Avoiding the origin from human or animal serum/plasma also reduces the risk of contamination from undetected pathogens or other factors. Finally, mAbs targeting different epitopes can be combined to achieve synergistic or additive effects, also in combination with other types of therapies [13]. Combination can be achieved also by engineering two or more mAbs into multi-specific antibody formats, as described later.
The success story of mAbs for infectious agents is reflected by the multiple molecules that are already approved or in late-stage clinical development (summarized in Table 1 ). Currently approved molecules are: Ibalizumab (Trogarzo) to treat drug-resistant HIV-1, raxibacumab (ABthrax) and obiltoxaximab (Anthim) for prophylaxis and treatment of anthrax, bezlotoxumab (Zinplava) for prevention of Clostridium difficile infection recurrence, palivizumab (Synagis®) for prevention and treatment of RSV [[13], [14], [15]], and R-Mab (Rabishield) and RabiMabs (Twinrab) for rabies post-exposure prophylaxis [16]. Effective mAb therapies against Ebola are close to licensure: ansuvimab (mAb114) and the mAb cocktail REGN-EB3 are under marketing approval by the FDA. In addition, ansuvimab is also considered to be used during the current Ebola outbreak in Congo.
Table 1.
Approved and most advanced mAbs in clinical development targeting infectious disease.
| Target | mAb (brand name) | Company | Format | Technology | Indication& | Stage |
|---|---|---|---|---|---|---|
| Respiratory syncytial virus (RSV) Fusion glycoprotein | Palivizumab (Synagis) | MedImmune / AbbVie Inc. | Humanized IgG1 | Hybridoma | Prevention of RSV infection | US Approval 1998 |
| Bacillus anthracis Protective Antigen (PA) | Raxibacumab (Abthrax) | GlaxoSmithKline / Human Genome Sciences (HGSI) | Human IgG1 | Transgenic mice | Anthrax infection | US Approval 2012 |
| Bacillus anthracis Protective Antigen (PA) | Obiltoxaximab (Anthim) | Elusys Therapeutics | Chimeric IgG1 | Hybridoma | Prevention of inhalational anthrax | US Approval 2016 |
| Clostridium difficile enterotoxin B | Bezlotoxumab (Zinplava) | Merck | Human IgG1 | Transgenic mice | Prevention of Clostridium difficile infection recurrence | US Approval 2016 |
| Rabies Virus glycoprotein G | SII Rmab (Rabishield) | Serum Institute of India / MassBiologics | Human IgG1 | Transgenic mice | Rabies Post-exposure prophylaxis | India Approval 2016 |
| CD4 | Ibalizumab (Trogazo) | Taimed Biologics / Theratechnologies | Humanized IgG4 | Hybridoma | Drug-resistant HIV-1 | US Approval 2018 |
| Rabies Virus glycoprotein G | RabiMabs, M777−16-3 / MAb 62−71-3 Mab5 (Twinrab) | Zydus Cadila | Mixture of murine IgG1 (M777−16-3) and IgG2b (62−71-3) | Hybridoma | Rabies post-exposure prophylaxis | India Approval 2019 |
| Zaire Ebola virus (EBOV) glycoprotein | Ansuvimab / mAb114 | Ridgeback Biotherpeutics / Vir Biotechnology / Humabs Biomed | Human IgG1 | Human donor | Ebola virus infection | Under FDA approval (2020) |
| Zaire Ebola virus (EBOV) glycoprotein | REGN-EB3 | Regeneron Pharmaceuticals | Mixture of 3 human IgG1 (REGN3470, 3471, and 3479) | Transgenic mice | Ebola virus infection | Under FDA approval (2020) |
| Staphylococcus aureus Hla | Tosatoxumab / AR-301 (Salvecin) | Aridis Pharmaceuticals / Kenta Biotech | Human IgG1 | Human donor | Treatment of ventilator- and hospital-associated pneumonia | Ph III (NCT03816956) |
| SARS-CoV-2 Spike protein | REGN10933 and REGN10987 combination therapy | Regeneron Pharmaceuticals | Human IgG1 | Transgenic mice | COVID-19 | Ph III (NCT04426695) |
| SARS-CoV-2 Spike protein | Ly-CoV555 | Eli Lilly | Human IgG1 | Human donor | COVID-19 | Ph III (NCT04497987) |
| CCR-5 | Leronlimab / PRO 140 / PA-14 | CytoDyn / Progenics Pharmaceuticals | Humanized IgG4 | Hybridoma | HIV-1 infection | Ph III (NCT03902522) |
| CD4 | UB-421 / dB4 / dB4C7 | United BioPharma | Humanized IgG1 | Hybridoma | HIV-1 infection | Ph III (NCT03149211) |
| Rabies virus glycoprotein | NM-57 / SO-57 / SOJB | North China Pharmaceutical | Rabies Post-exposure prophylaxis | Ph III | ||
| Respiratory syncytial virus (RSV) Fusion glycoprotein | Nirsevimab / MEDI-8897 | Sanofi & AstraZeneca / MedImmune | Human IgG1, half-life extended | Human donor | Prevention of RSV infection | Ph II-III (NCT03959488) |
| Heptatitis B virus HBsAg | Lenvervimab / GC-1102 | GC Pharma | Humanized IgG1 | Phage display library from HBV vaccinees | Prevention and treatment of hepatitis-B virus (HBV) | Ph II-III (NCT03801798) |
| SARS-CoV-2 Spike protein | VIR-7831 (GSK4182136) | Vir Biotechnology / GlaxoSmithKline | Half-life extended human mAb | Human donor | COVID-19 | Ph II-III (NCT04545060) |
| Ebola virus (EBOV) glycoprotein | Larcaviximab / ZMapp | Mapp Biopharmaceutical | Chimeric IgG1 | Hybridoma | Ebola infection | Ph II-III (NCT03719586) |
| HIV-1 Envelope protein CD4 binding site (CD4bs) | VRC-HIVMAB060−00-AB / VCR01 | NIAID | Human IgG1 | Human donor | Prevention of HIV-1 infection | Ph II (NCT02716675) |
2.3. Active immunization
Active immunization (vaccination) is a highly complementary approach to achieve protection from infectious diseases. Preventive vaccination with the aim of inducing both potent antibody response and durable immunity usually has to be administered well advanced of a potential pathogen encounter, but confers much longer protection and immune memory. Vaccines stimulate an immune response toward key pathogen antigenic targets without exposing the individual to the risk of the disease. The advantage of vaccination is that it is not limited to the generation of antibodies, as it also recruits effector cells from both the innate and the adaptive branches of the immune system. Nonetheless, pathogen-specific antibodies are a key component of vaccination, and levels of antibodies can associate with protection from the disease [17,18]. Neutralizing antibodies are correlates of protection for polio, measles, rabies, diphtheria and tetanus vaccines. Other correlates, dependent on effector functions of the antibodies (complement fixation and killing, antibody-dependent cell cytotoxicity (ADCC), opsonophagocytosis) are used to measure the efficacy of chickenpox, pneumococcal, meningococcal and rubella vaccines. Moreover, binding antibody levels are also correlates of protection– likely through a fraction of the binding antibodies contributing to neutralization or opsonophagocytosis – for hepatitis A, hepatitis B, Hemophilus influenzae B, and Lyme disease.
Over the last century huge progress has been made in vaccinology leading to a vast number of different vectors and production technologies, each with individual benefits and limitations. Most of them have been used by different research groups or companies to produce COVID-19 vaccines in the run to quickly find an effective vaccine.
2.4. The virtuous cycle of co-development of active (vaccines) and passive (mAbs) immunization treatments
There is tremendous benefit in co-development of vaccines and mAbs [19]. MAbs from convalescent patients help to identify and improve vaccine antigens [7]. Leveraging the human immune system by vaccinating subjects with potent vaccine formulations containing optimized antigens and strong adjuvants will drive more focused, potent and highly functional antibodies. Co-development of vaccines and mAbs will drive a virtuous cycle of mutual improvement. First generation vaccines and mAbs will hopefully contain or solve the COVID-19 crisis. Continuous molecule improvement will enhance success and drive innovation.
3. How to overcome bottlenecks in the development of mAbs against infectious diseases
The benefits of active and passive immunization are highly complementary. Passive transfer of antibodies is immediately effective after injection, acting directly against their target. Passive transfer can be used in “emergency setting” to provide immediate protection, and in immunocompromised or in extreme ages individuals (newborn and elderly) who are at risk of infection and often mount a suboptimal immune response. In these cases, mAbs provide an alternative or complementary way to vaccination to protect. Recently, it had been described that mAbs can also have an effect in activating T cell response in HIV-infected patients [[20]]. Moreover, manufacturing methods are efficient and applicable to multiple antibodies without the need to change the production facility, with costs that are rapidly declining in the latest years.
So why are there so few antibody-based therapies for infectious diseases today?
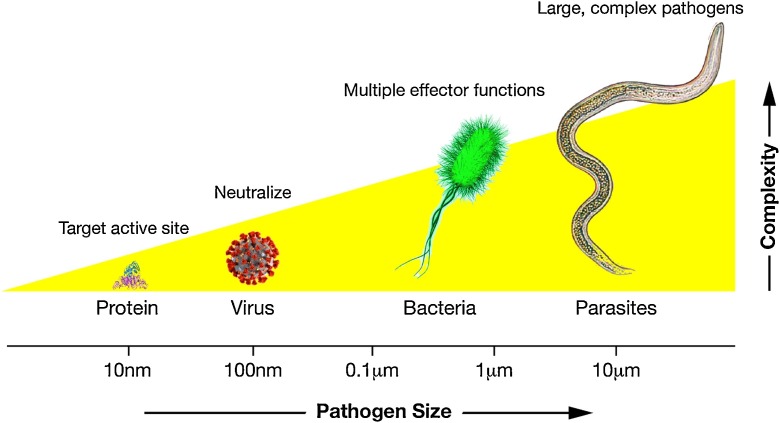
Initial roadblocks have been high cost and low efficacy of first generation mAb therapies. The nature of the pathogen plays an important role: antibodies target only a specific antigen. This makes it difficult to target pathogens of increased size and complexity (Fig. 1 ). Moreover, co-evolution of pathogens with host drives immune evasion and redundant virulence mechanisms, which makes it even more challenging to target more complex pathogens with just a single mAb.
Fig. 1.
Pathogens complexity increases with size. Soluble toxins can be blocked by inhibiting the active site, and a single mAb can also neutralize a virus conferring protection. However, larger bacteria or more complex parasites require engagement of multiple effector functions to be destroyed, thus increasing the difficulty to be treated by single monoclonal antibodies.
The few broadly conserved epitopes across a variety of strains from a given pathogen are frequently hidden and less accessible to classical antibodies. Tissue homing to the site of infection is another important roadblock. Achieving relevant mAb concentrations in the respiratory tract, the gut or other mucosal sites is more difficult than treating systemic infections. We should also consider that the immunoglobulin isotype used for the majority of commercially available human mAbs is IgG1 with a limited access to mucosal tissues. A broader range of molecules, including other IgG subclasses and Ig isotypes to access relevant infection sites and with enhanced effector functions, will be key for the success in this field.
Aside from mechanistic limitations of the biology of antibodies, a recent report1 from Wellcome and IAVI recognized that accessibility of patients to this treatment is one of the main bottlenecks in the use of mAbs. Initial roadblocks have been high cost and low efficacy of first generation of mAb therapies. Today improved technologies and reduced production costs are delivering more potent mAbs at a more accessible cost. Nevertheless availability, including registration and inclusion on national medicine lists and reimbursement through public health systems, and affordability, with lack of lower-priced biosimilar mAbs as a critical problem for low-income countries, are still considered the two biggest barriers impeding global access.
Regulatory approval pathways are also not well optimized for emerging threats, an area of rapid emergence of effective mAb treatments. Unpredictable outbreaks may impede the conduct of controlled clinical trials, requiring the use of other supportive evidence such as human challenge trials and acceptance of assessment based mainly on animal trials and safety evaluation in healthy volunteers [21].
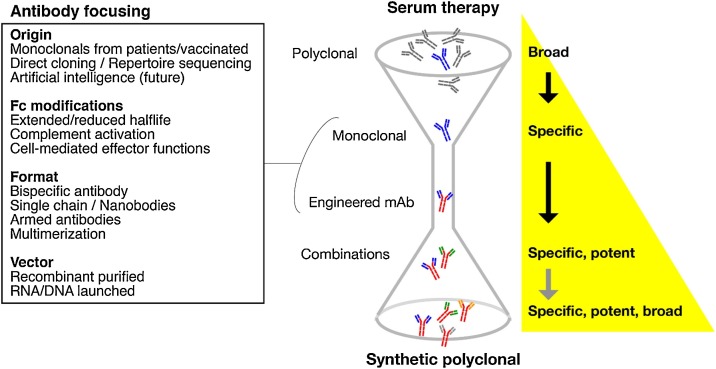
Fig. 2 lays out how innovation in mAb isolation and engineering is helping to develop effective antibody treatments against infectious diseases.
Fig. 2.
Efficacy of antibody-based therapy can be focused using novel technological approaches. Serum therapy presents the broadest activity, due to the polyclonality of the antibodies (obtained either from animals or convalescent patients). Monoclonal antibodies (mAbs) are specific for a single epitope, and can be engineered to increase their potency, efficacy, and half-life. Combination of mAbs as cocktails or, in the future, as synthetically designed polyclonals could enhance the broadness of the treatment, avoiding the side effects and the low specificity of conventional serum therapy.
4. Recent advancement in antibody-based treatments of infectious diseases
Recently, mAb-based therapies have greatly accelerated, bringing new innovative products to development (Table 1). Novel engineering technologies (e.g. half-life extension, bi-specific or even more complex formats, Fc modifications) have brought to higher functionality and greater efficacy, thereby removing important bottlenecks for the development of pathogen-specific mAbs. A review of the clinicaltrials.gov platform on September 2020 shows 75 active clinical trials using monoclonal antibodies to treat or prevent infections. Mostly target viral pathogens: mainly HIV-1 and SARS-CoV-2, but also influenza A, RSV, herpes virus, Chikungunya virus, Zika virus, BK virus, human papilloma virus, Epstein-Barr virus, and Hepatitis B; while mAbs for non-viral pathogens under investigation target Plasmodium falciparum, Pseudomonas aeruginosa, Clostridium difficile and Staphylococcus aureus. The number of mAb trials is rapidly increasing, denoting a renewed interest to develop mAbs to treat and prevent disease. For comparison, in 2016 the clinical development of mAbs to treat infection was limited to 38 active trials (12). The number of mAbs is even higher in preclinical evaluation, both in vitro and in animal models. COVID-19 gave an enormous impulse to the development of mAbs as complementary approach to vaccination, a change that will likely reflects in the near future on other pathogens of major health concerns.
4.1. RSV
Human respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infections in the very young. The RSV fusion protein (F) is essential for virus entry because it mediates viral and host membrane fusion. Antibodies that target F protein can prevent viral entry and reduce disease caused by RSV. This line of research brought in 1998 to the licensure of Palivizumab, the first clinically approved monoclonal antibody against an infectious disease. Palivizumab is a humanized mAb targeting a neutralizing epitope present both in the Post-fusion (PostF) and Pre-fusion (PreF) form of the antigen and preventing RSV disease in high risk infants [14]. However, the observation that the vast majority of potent neutralizing mAbs isolated from convalescent patients recognize exclusively the PreF led to the development of PreF-specific mAbs as possible best new molecules and of PreF antigen as the best target for the development of an RSV vaccine [22]. Nirsevimab (MEDI8897) is a “second generation” more potent PreF specific mAb obtained by introducing five amino acid substitutions in the CDRs of the D25 progenitor that improved the charge complementarity between the heavy-chain CDRs and the RSV F2 subunit and by extending the half-life through YTE amino acid substitutions in the Fc domain. Compared to Palivizumab, MEDI8897 showed superiority both in-vitro and in animal infection models in neutralizing a broad range of RSV strains [23]. A Ph2b study recently showed that nirsevimab is efficacious in reducing medically attended RSV-confirmed lower respiratory tract infections in pre-term infants [24]. A currently on-going Ph3 clinical study (NCT03979313) will assess if nirsevimab can be given to healthy late- and full-term infants, to offer preventive protection for all neonates. This would be the first time that an optimized mAb would be given to such a broad target neonatal population, highlighting the potential of mAbs to protect from infectious diseases as complementary treatment in very early stages of life, when the immune system is not yet properly formed and ready to respond to vaccination.
4.2. Influenza virus
Influenza A and B are major public health threats, for their capacity to rapidly evade the immune system and to cause pandemics [25]. Influenza’s antigenic drift require annual selection of most prominent seasonal viral strains for vaccine development, and often results in low efficacy treatments. Moreover, current antiviral treatments are suboptimal with emergence of drug-resistant viruses [26].
Monoclonal antibodies have the potential to reduce viral escape by binding conserved epitopes and neutralizing multiple influenza strains. Monoclonals with the greatest breadth consistently target the stem region of the influenza virus receptor hemagglutinin (HA) [27]. Such broadly neutralizing antibodies (bnAbs) can be isolated from human B cells in the peripheral blood of subjects either vaccinated or previously infected by the virus. Several bnAbs have been evaluated in clinical studies, few of which have reached phase 2 [28]. Alleviation of symptoms from uncomplicated influenza infection was demonstrated for VIS410 [29], MHAA4549A [30], and MEDI8852 (in combination with the antiviral drug oseltamivir) [31]. However, the clinical results of testing therapeutically anti-stem mAbs both in an outpatient setting and in hospitalized patients has shown no or very poor efficacy, likely due to the limited therapeutic window of intervention for antivirals. Importantly, MHAA4549A did not meet the primary end point of reducing the time to normalization of respiratory function compared to placebo plus oseltamivir in hospitalized patients, and showed no significant improvement of other secondary endpoints such as time to ICU or hospital discharge and 30-day mortality [32]. In this context, a different approach might be needed, such as the one pursued in a phase 2 study for VIR-2482 mAb (NCT04033406), which was to develop a half-life extended mAb for preventing, rather than treating, Influenza A virus related illness.
4.3. Human immunodeficiency virus (HIV)
The discovery of broadly neutralizing antibodies (bnAbs) from the serum of individuals following viral infections – HIV, influenza virus, EBOV and Lassa virus – has contributed to fuel the mAb research, and HIV has served as a prototype virus for many studies in this field [33]. Rational design of viral envelope (Env) stabilized probes, allowed isolation of bnAbs targeting conserved areas of vulnerability of Env [34]. However, elicitation of anti-HIV bnAbs in healthy subjects by vaccination was proven challenging, due to the many unusual features of these antibodies, including extensive amino acid mutations and very long and unusual HCDR3s. For this reason, active vaccination strategies aim at priming B cells encoding bnAb precursors, using sequential immunogens that will guide the antibody affinity maturation towards the acquisition of such unusual features [35]. To date, many advances have been made in developing precursor-targeting immunogens of different bnAbs classes, and some of them are moving to clinical stage development [[36], [37], [38], [39], [40], [41], [42]].
Aside from active immunization, injection of bnAbs remains an attractive therapeutic option for HIV. Their neutralization activity of free virus particles and the contribution to cell-mediated killing of HIV infected cells could integrate daily antiretroviral regimens, increasing compliance. Therefore, parallel to a HIV vaccine development, both preclinical and clinical bnAbs research is rapidly advancing. Passive transfer experiments in non-human primates demonstrated the ability of bnAbs to protect against SHIV challenge [43]. Moreover, administration of bnAbs to chronically infected animals resulted in rapid decline of viral RNA in peripheral blood, gastrointestinal mucosa and lymph nodes [44]. Following the encouraging results of preclinical studies, bnAbs have entered clinical trials for the prevention and treatment of HIV-1 infection. Antibodies against the CD4 binding site of HIV Env, VRC01, 3BNC117, VRC01-LS and VRC07−523LS, as well as antibodies targeting the glycan-rich V3 loop epitope, 10–1074 and PGT121, are being tested in humans [45]. The most advanced bnAb, the CD4-binding VRC01, is also being evaluated in Antibody Mediated Prevention (AMP) studies (NCT02716675, NCT02568215), to assessing its protective effect in individuals at high risk of HIV infection [46]. Despite their potential as valid treatment option to protect from or treat HIV infection, passive immunization with bnAbs faces critical challenges: suppression of viremia appears to be transient, with the virus readily escaping due to its high mutation rate, and the virus reservoir waiting quiescently in the host cells appear to be difficult to target. For this reason, a cocktail of modified, half-life extended bnAbs that targets different epitopes will certainly be required for HIV treatment, to avoid breakthroughs of resistant viral variants [45,47].
4.4. Emerging infectious diseases (EID)
Lately, mAb approaches had an enormous impact in the context of emerging infectious disease (EID) outbreaks, in which the process of vaccine development for new pathogens may be difficult and prolonged [48]. In this area, a notable example is the rapid development of protective antibodies against Ebola virus (EBOV). At the time of this first Ebola outbreak, which resulted in over 28,000 cases and 11,000 deaths,2 there were no approved vaccines or therapeutics. Passive antibody treatment was readily recognized as an innovative counter measure as potential solution for the Ebola crisis [49], and mAbs were included in the WHO list of investigational therapies.3 Yet, this trial provided the first clear example that mAbs could be efficacious medicines in a therapeutic setting (versus prophylactic or post-exposure prophylactic settings for prior approved anti-infective mAbs). ZMapp, a mixture of three chimeric monoclonal antibodies directed against EBOV glycoprotein to inhibit virus particle cell entry, was developed and resulted 100 % effective in reverting disease in non-human primates [50]. However, assessment in a randomized, controlled trial (PREVAIL II) reported that, although a 40 % lower risk of death was calculated for those who received ZMapp, the difference was not statistically significant and could not justify superiority to the optimized standard of care alone [51]. More recently, a mixture of three antibodies (REGN EB3) [52], and a single monoclonal (mAb144) [53], outperformed ZMapp in the PALM randomized, controlled clinical trial [54], and are now seeking regulatory approval.
Advancement of single B cell isolation technologies allowed the identification of potent antibody targeting other EID pathogens. Antibodies targeting Dengue and Zika viruses have not reached clinical stage yet but demonstrated potential to be developed into effective therapeutics [[55], [56], [57], [58], [59]]. Recently, TY014, a fully human anti-yellow fever E protein antibody, induced complete abrogation of viremia in a proof of concept study in YF17D vaccinated subjects [60]. This is an important evidence of the therapeutic efficacy of anti-infective mAb.
4.5. COVID-19
Lessons from previously described fields of investigation funneled into the isolation of potent anti-SARS-CoV-2 antibodies. In the span of few months, over 20 scientific manuscripts appeared on PubMed describing antibody-based treatments for COVID-19, revamping the field of antibody discovery. Not only, the development of mAbs and their testing in clinical trials happened at an unprecedented speed. During the pandemic, mAbs were discovered, produced and advanced to proof-of-concept clinical trials in just 6 months, halving the traditional timelines, thanks to a universal convergence of technologies and strategies [61].
At the time of writing, approximately 29.7 million people were infected and over 937,000 died of COVID-19, inducing global action by both public and private sectors to address the pandemic4 . A significant fraction of COVID-19 patients was hospitalized for severe respiratory symptoms and required treatment in intensive care units [[62], [63], [64]]. Currently, there are no preventative vaccines to contain SARS-CoV-2 infection. Several groups are developing potent antibody-based treatments. The rationale lies in antibody-mediated neutralization of viral entry to host cells [65]. One notable approach is the use of hyperimmune serum, isolated from convalescent patients5 . Passive immunization was proven effective for other coronaviruses, suggesting its employment as a rapid treatment option for SARS-CoV-2 [66]. A perspective non-controlled study on 10 severe COVID patients reported improved clinical outcome and certain absorption of lung lesions within 7 days from transfusion of plasma from recovered donors, suggesting the beneficial effect of the therapy [67]. In another study on 6 critically ill patients, convalescent plasma treatment had an effect on viral shedding but not on mortality rate [68]. Larger controlled studies evaluating the benefits and harms of convalescent plasma might be necessary to assess the efficacy and safety of this treatment, addressing also the concern of possible enhanced disease with the use of polyclonal hyperimmune globulin therapy [69,70]
Potent neutralizing mAbs have been isolated from COVID-19-experienced donors. Several studies reported that antibodies specific to SARS-CoV-2 proteins can be found in the majority of COVID-19 patients within days after symptoms onset [[71], [72], [73]]. Wang and colleagues firstly reported two mAbs blocking the interaction of the viral spike protein with its human receptor ACE2 [74]. Shortly after, several other potent neutralizing mAbs were isolated from convalescent patients [[75], [76], [77], [78]]. A mAb was also isolated from a phage library obtained by immunizing mice with a recombinant SARS-CoV-2 spike receptor binding domain (RBD) [79]. Due to cross-reactivity with SARS, investigators have also characterized an antibody originated from SARS survivor able to neutralize SARS-CoV-2 [80]. Passive transfer of neutralizing mAbs was demonstrated to protect from disease in small animal model [81]. Initial results on human patients were also promising. Ly-CoV555 proof of concept data from an interim analysis of a phase 2 clinical trial showed a reduced rate of hospitalization for treated patients compared to placebo,6 and the antibody cocktail REGN−COV2 rapidly reduced viral load and associated symptoms in non-hospitalized COVID-19 patients7
The current use of mAbs for COVID-19 is limited to bedside administration in controlled clinical trials 8 . However, other applications are envisioned. In particular, a cocktail of multiple mAbs could avoid viral escape mutations and obtain a superior synergistic effect [82,83]. While the scientific community works on the development of a plethora of different vaccination approaches and drugs, mAbs are most likely the first de novo COVID-19 treatment to be developed [84,85].
4.6. Imminent and future Healthcare crises like Antimicrobial resistance (AMR)
Future outbreaks and pandemics will occur. Another crisis on the rise is antimicrobial resistance, with a variety of different pathogens posing imminent multiple threat. Monoclonal antibodies represent an important therapeutic tool to fight antimicrobial resistance (AMR) [21,86]. Antibodies are highly selective, targeting surface antigens or toxins and can complement their action with antimicrobials without the risk to be affected by existing resistance mechanisms. They have minimal off-target effect, reducing the unwanted elimination of commensal bacteria thus preserving the gut microflora, which is increasingly recognized as fundamental contributor to human health [87]. Moreover, they can contribute to limit the use of antibiotics, which could result in less selective pressure for the emergence of resistant strains. Limitations in the use of mAbs to treat bacteria lie in the escape mechanisms of these pathogens, including the production of a capsule or biofilm that hide the bacterial surface from being targeted by antibodies, the variability of epitopes located on exopolysaccharides (i.e. different serotypes exist), the inhibition of immune cell recruitment and the secretion of proteases [86]. Indeed, failures have been encountered in fighting Staphylococcus aureus infection when a single antigen was targeted [88]. Nonetheless, next-generation antibodies or combinations might surpass the limitations of the previously failed studies. Suvratoxumab (MEDI4893) and AR-301, targeting S. aureus alpha toxin, are currently being evaluated in patients suffering from hospital-acquired pneumonia. Suvratoxumab was shown to have 31.9 % relative risk reduction of S. aureus pneumonia compared to placebo, but the results were not statistically significant [89]. A similar approach in the same target population was pursued with a cocktail of two mAbs, ASN100, that cross-neutralized alpha toxin and 5 additional leukocidins [90]. However, the clinical development of ASN100 was halted due to failed effectiveness in high-risk, mechanically ventilated patients with S. aureus pneumonia (NCT02940626).
5. mAb engineering and novel formats will pave the way towards successful treatments for infectious diseases
Antibodies have proven their high value in antitumor therapy, becoming first choice treatments for several cancers. Discovery in the area of tumor therapy is expanding to innovative approaches that increase the efficacy of antibody treatments, including alternative formats like bifunctional antibodies and combination therapies [91]. From these approaches, lessons can be learned and applied to treat infections.
This knowledge, together with recent learnings from systems serology on key features for antibody functionality and the advent of non-conventional antibody-like formats currently explored in pre-clinical models, could pave the way towards successful mAb therapies for complex bacteria, fungi or even parasites.
5.1. Bi-specific antibodies
Bi-specific antibodies (bsAbs), displaying Fabs binding two distinct targets, have been investigated for the past five decades. Despite the promise of expanded therapeutic applications, bsAbs-based treatments encountered numerous roadblocks. A notable example is catumaxomab, targeting T lymphocyte antigen CD3 and epithelial cell adhesion molecule EpCA. After being approved for malignant ascites, it was withdrawn from the market for serious adverse events due to unspecific T cell activation in the liver [92]. Active bsAbs research also involves infectious disease agents [93]. Bispecific antibodies are considered to be equivalent to antibody cocktails, as was observed when neutralizing pertussis toxin in mouse model [94]. An example of use of bsAbs is MGD014, an antibody targeting CD3 and HIV-1 Env, currently in Phase 1 clinical trial (NCT03570918). This strategy enables recruitment and activation of T cells to infected cells and subsequent T-cell mediated lysis [95]. Other exploratory bsAbs we used to treat hepatitis B virus, cytomegalovirus, Ebola virus and Pseudomonas aeruginosa infections. Both HBV and CMV approaches used anti-CD3 bsAbs to redirect T cells to the infection site and promote virus clearance [96,97]. A different approach was used to target Ebola virus (EBOV) infection: in a mouse challenge model, extracellular EBOV GP-specificity was exploited to direct bsAbs into the endosome during viral uptake where the second arm of the antibody could block the binding site of the EBOV NPC1 intracellular receptor, conferring broad protection [98]. This “trojan horse” approach was also used to enhance killing against P. aeruginosa in mice, employing a bsAb specific for the surface polysaccharide Psl and the bacterial component PcrV [99,100]. Although the bispecific format could lead to potent therapeutic options, current limitation lies in the correct design and compatibility of the mechanistic effects of the two arms. In case of Staphylococcus aureus, bsAb targeting clumping factor A (CflA) and alpha toxin were less protective in vivo than the binary cocktail, likely due to interference of the anti-ClfA bsAb arm on the alpha toxin neutralization [101,102]. To further expand the capacity of multi-specific antibodies, a tri-specific format could be employed. This approach was used to display specificities of three HIV bnAb antibodies, which conferred protection in non-human primates against infection with a mixture of SHIVs in contrast to single bnAbs [103].
5.2. Armed antibodies
In the recent years, several antibodies armed with cytotoxic drugs or radionuclides have been developed to enhance therapeutic potential or immune response against cancer [104]. The rationale of armed antibodies is the selective delivery of the small molecule to the target cells, with increased efficiency of internalization and drug release.
An antibody-antibiotic conjugate was reported for the treatment of Staphylococcus aureus infection [105]. Authors tethered a rifamycin derivative to a mAb targeting the cell wall teichoic acid, inducing superior infection control compared to standard vancomycin treatment. This approach could allow selective targeting of non-replicating bacteria reservoirs, opening to new potential treatment options. Antibodies were also used to efficiently deliver viral antigens in a targeted manner. Anti-CD19-CD22 antibodies combined to HLA II-restricted Epstein-Barr virus (EBV) epitopes induced in-vitro superior T cell-mediated killing of multiple Burkitt's lymphoma cell lines compared to EBV peptides alone [106]. Armed antibodies could provide a valuable opportunity to re-design molecules that previously failed in preclinical or clinical stages, due to lack of selectivity for desired targets [107].
5.3. Nanobodies
Camelid antibodies can be composed of two chains like the other mammals, including humans, but also of a single heavy chain variant. The variable portion of these antibodies VHH, named nanobody, has been used for a range of applications [108]. Like other described classes of antibodies, nanobodies find their primary application in oncology and inflammation, but they are also exploited in diagnostics [109]. Among several candidates in development, Caplacizumab is the only nanobody approved for human use, for the treatment of thrombotic thrombocytopenic purpura [110,111]. Nanobodies have been developed also against infectious agents, as dengue virus, hepatitis C virus, poliovirus, norovirus, HIV, rabies virus and rotavirus [112]. The nanobody ALX0171 was developed against RSV as an inhalation product. In animal model, ALX0171 nanobody showed therapeutic effects [113], and results from a clinical trial in hospitalized infants suffering from RSV lower respiratory tract infection are anticipated (NCT02979431). Recently, SARS-CoV-2 specific nanobodies were isolated from llamas, either immunized with prefusion-stabilized coronavirus spikes [114], or by in vitro phage display panning of camelid repertoire [115].
Nanobodies have several advantages over conventional antibodies: smaller size, thermal and chemical stability, adaptability to multiple formats, high solubility, higher in vivo tissue penetration and targeting, lower susceptibility to steric hindrances, retaining comparable antigenic affinity and specificity of conventional antibodies [112]. Nanobodies also have disadvantages: poor half-life and risk of immunogenicity, due to their camelid origin, are the two main drawbacks of these molecules.
5.4. Fc modifications
The Fc portion of the antibody drives a myriad of effector functions necessary to eliminate pathogens and regulate its half-life in the blood stream. Understanding effector functions is important for passive immunization but also for active vaccination, for which it has been recognized that vaccine-specific titers alone do not always predict efficacy [17]. An example of the importance of the Fc-dependent function comes from anthrax studies, where Fc class mutations that increase FcyR binding significantly improve the in vivo protection of anti-anthrax antibodies in humanized mice [116]. Alteration of Fc domain was used to enhance NK-mediated antibody-mediated cytotoxicity (ADCC) [117]. EBOV-specific antibodies targeting the GP chalice bowl and the fusion loop have been demonstrated to require FcyR engagement for optimal in vivo antiviral activity [118]. On the contrary, in the case of Dengue fever, antibody-dependent enhancement of disease is a critical concern for disease management and vaccination. Therefore, therapeutic antibodies may be engineered to reduce the immune effector functions responsible of antibody-dependent disease enhancement (ADE) [119]. Among others, the LALA double mutation (Leu234Ala, Leu235Ala) in the IgG1 Fc region reduced binding to FcyR and did not enhance infection while maintaining neutralization capacity in vitro and in vivo [120].
Because most of the licensed antibodies and related development efforts rely on the use of IgG-class antibodies, glycosylation has been extensively exploited to regulate FcR binding and half-life of the antibody. Exploratory studies are accumulating evidence of the role of glycosylation in infectious disease [121]. A notable example is the removal of fucose on IgG antibodies, which results in enhanced affinity for FcyRIIIa and cytotoxic effect [122,123]. Aside from cell-mediated effects, extensive work has been conducted to manipulate the sequence and glycosylation to alter complement activation [124], including point mutations impacting antibody hexamerization and enhanced complement deposition [125], or isotype hybridization [126].
Half-life is also critical for mAb based therapies, as it impacts serum persistence and activity. Half-life extension is important in case of HIV, where long-acting bnAbs may provide an alternative to routine antiretroviral therapy. In a Phase 1 study involving VRC01, a CD4-binding bnAb, incorporation of Fc mutations (M428 L and N434S) made the in vivo half-life 4-fold greater than unmodified VRC01 [127]. Another example is nirsevimab (MEDI8897), a highly potent human antibody optimized from antibody D25, which targets the pre-fusion conformation of the RSV F protein. In animal models, it is 9 times more potent than palivizumab at reducing pulmonary viral loads and with a threefold increased half-life thanks to three amino acid substitutions in the Fc region [128]. In preterm infants, half-life of nirsevimab was 62.5–72.9 days [129]; and a single intramuscular injection induced 70 % protection from RSV-associated lower respiratory tract infection over the whole RSV season [24].
Aside from Fc domain sequence and glycosylation alteration, isotype switch can be used to increased efficacy and homing. In particular, IgA is a well-studied isotype for mucosal immunity [130]. Examples of protective effect of IgA mAb against tuberculosis or influenza in animals were previously published [131,132]. More recently, a SARS-CoV-2-reactive IgA was isolated, with potent neutralizing activity [133].
5.5. DNA-RNA encoded antibodies
Synthetically designed nucleotide-encoded monoclonal antibodies are a novel delivery method for antibody therapy. The advantages are the simpler design and cheaper production, very much like nucleotide-based subunit vaccines, but also the potential to achieve higher titers and for prolonged periods of time.
DNA-encoded antibodies (DMAbs) - delivered through plasmid DNA injection and electroporation - have been used in preclinical models for the treatment of cancer [[134], [135], [136]] and cardiovascular disease [137], but also for the prophylaxis of infection. Mice received a single inoculation of anti-influenza A DMAb and survived lethal virus challenge, a similar protection achieved by conventional antibody delivery [138]. Furthermore, protection from viral challenge was also achieved by single intramuscular injection of an anti-chikungunya virus DMAb, which conferred more rapidly serum titers than DNA vaccine [139].
In recent years, also mRNA-based in vivo mAb expression has entered the field [140]. The first mAb to be launched by a nucleoside-modified mRNA formulated with lipid nanoparticle (LNP) was VRC01, an antibody against HIV, which outperformed the purified recombinant VRC01 mAb injection, with higher and more durable serum levels [141]. The feasibility of mRNA-launched antibodies was further demonstrated in preclinical models of rabies, botulism and lymphoma [142]. Interestingly, mRNA was used to achieve site-specific delivery of antibodies: naked mRNA encoding for Palivizumab, an anti-RSV antibody, was delivered via intratracheal aerosol, resulting in local production and reduced RSV infection post-challenge [143]. An anti-chikungunya highly potent neutralizing mAb isolated from human B cells of a survivor has induced protection from viral infection in mice and macaques when administered intravenously as mRNA encapsulated in lipid nanoparticles. The preclinical results achieved in this study paved the way for the start of a clinical trials of mRNA-based passive immunotherapy for human chikungunya infection currently on-going [144].
6. Conclusions
In the COVID-19 era, we are observing a quantum leap in the number of polyclonal and monoclonal antibodies under development, as well as vaccines – made with different technologies. We can expect a wealth of pre-clinical and clinical data, to inform systems biology and hypothesis testing. The scientific knowledge gain established around a single pathogen will be unprecedented. Comparison of efficacy data from passive and active immunization will help to deconvolute the complex data sets.
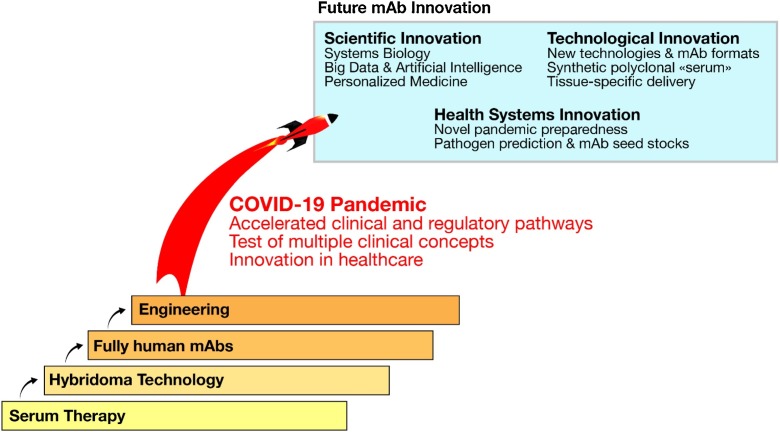
Future health crises will benefit from this leap in science, technology and pandemic preparedness (Fig. 3 ). The important role of mAbs will be further fueled by the notion that they can be rapidly isolated, tested and produced on large scale, that they are safe and can theoretically be used in any patient.
Fig. 3.
Fig. 2: Quantum leap driven by recent technology advancements and COVID19 crisis. Single leaps towards more efficacious treatments have been done when new technologies – hybridoma, humanization, Fc engineering – were introduced. The COVID-19 pandemics induced a considerable step forward, bringing accelerated development pathways. In the future, innovation in the mAb space could bring new ways to approach the use of antibodies.
MAb therapy in animal models of SARS-CoV-2 infection demonstrated the potential benefit of mAb-based therapies, and results of clinical trials assessing their efficacy in humans are eagerly expected. In this period of rapid scientific advancement, innovative technologies were also considered to design anti-SARS-CoV-2 antibodies. Examples are single-chain antibody constructs and Fc-engineered mAbs to avoid Antibody-Dependent Enhancement (ADE). RNA platform technology, used for one of the most advanced COVID-19 vaccines in clinical development, could soon become a tool to provide unparalleled simplicity of vector design and antibody production, lowering costs [145]. Moreover, the use of antibody cocktails could guide the development of preparations with greater efficacy.
Advancements of discovery methods could further improve identification of “best in class” mAbs and their development. A recent report described the use of computational-based analysis to identify additional members of a class of influenza-specific monoclonals [146]. This approach could lead to a faster development of mAbs with characteristics of interest and, using reverse vaccinology 2.0 [7], to discovery of antigens for future, better vaccines.
It is important to consider, however, that accessibility is still a major roadblock for mAb-based preventive and treatment options. Despite the rapid advancement of platform technologies and the decrease of mAb production costs, low income countries might be the last ones to get access to this type of cure.
Tradename statements
Trogarzo is a tradename of TaiMed Biologics; ABthrax is a tradename of GlaxoSmithKline; Anthim is a tradename by Elusys Therapeutics; ZINPLAVA is a trademark of Merck Sharp & Dohme Corp; Synagis is a tradename of Arexis AB; Rabishield is a tradename of Serum Institute of India; Twinrab is a tradename of Zydus Cadila; Salvecin is a tradename of Aridis Pharmaceuticals.
Authors contribution
SP, OF, and AKS wrote the manuscript and contributed to the ideas and concepts it contains. All authors reviewed and approved the manuscript.
Funding
This work was sponsored by GlaxoSmithKline Biologicals SA.
Declaration of Competing Interest
SP, OF, and AS are permanent employees of the GSK group of companies.
Acknowledgments
The authors wish to thank Giorgio Corsi for the artwork, Catherine Mallia for the editorial assistance and Rino Rappuoli and Sanjay Phogat for reading our manuscript and providing valuable feedback.
Footnotes
Expanding access to monoclonal antibody-based products: A global call to action. https://www.iavi.org/news-resources/expanding-access-to-monoclonal-antibody-based-products-a-global-call-to-action (accessed September 18th, 2020)
WHO, Ebola virus disease, https://www.who.int/health-topics/ebola/#tab=tab_1 (accessed on September 18th, 2020)
WHO, Categorization and prioritization of drugs for consideration for testing or use in patients infected with Ebola, https://www.who.int/medicines/ebola-treatment/2015_0703TablesofEbolaDrugs.pdf?ua=1 (accessed on September 18th, 2020)
WHO, Coronavirus disease (COVID-19), https://covid19.who.int/ (accessed on September 18th, 2020)
FDA Coordinates National Effort to Develop Blood-Related Therapies for COVID-19, https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-coordinates-national-effort-develop-blood-related-therapies-covid-19 (accessed on September 18th, 2020)
Lilly announces proof of concept data for neutralizing antibody LY-CoV555 in the COVID-19 outpatient setting, https://investor.lilly.com/news-releases/news-release-details/lilly-announces-proof-concept-data-neutralizing-antibody-ly
Regeneron's REGN-COV2 Antibody Cocktail Reduced Viral Levels and Improved Symptoms in Non-Hospitalized COVID-19 Patients, https://investor.regeneron.com/news-releases/news-release-details/regenerons-regn-cov2-antibody-cocktail-reduced-viral-levels-and (accessed on October 7th, 2020)
Clinical trials of monoclonal antibodies to prevent COVID-19, https://www.nih.gov/news-events/news-releases/clinical-trials-monoclonal-antibodies-prevent-covid-19-now-enrolling. (accessed on September 18th, 2020)
References
- 1.Bonilla F.A., Oettgen H.C. Adaptive immunity. J. Allergy Clin. Immunol. 2010;125:S33–40. doi: 10.1016/j.jaci.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 2.Marshall J.S., Warrington R., Watson W., Kim H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018;14:49. doi: 10.1186/s13223-018-0278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu L.L., Suscovich T.J., Fortune S.M., Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat. Rev. Immunol. 2018;18:46–61. doi: 10.1038/nri.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hafeez U., Gan H.K., Scott A.M. Monoclonal antibodies as immunomodulatory therapy against cancer and autoimmune diseases. Curr. Opin. Pharmacol. 2018;41:114–121. doi: 10.1016/j.coph.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Tan J., Piccoli L., Lanzavecchia A. The antibody response to plasmodium falciparum: cues for vaccine design and the discovery of receptor-based antibodies. Annu. Rev. Immunol. 2019;37:225–246. doi: 10.1146/annurev-immunol-042617-053301. [DOI] [PubMed] [Google Scholar]
- 6.Crowe J.E., Jr. Principles of broad and potent antiviral human antibodies: insights for vaccine design. Cell Host Microbe. 2017;22:193–206. doi: 10.1016/j.chom.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rappuoli R. Reverse vaccinology 2.0: human immunology instructs vaccine antigen design. J. Exp. Med. 2016;213:469–481. doi: 10.1084/jem.20151960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlsson Hedestam G.B. The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat. Rev. Microbiol. 2008;6:143–155. doi: 10.1038/nrmicro1819. [DOI] [PubMed] [Google Scholar]
- 9.von Behring E., Kitasato S. [The mechanism of diphtheria immunity and tetanus immunity in animals. 1890] Mol. Immunol. 1991;28(1317):1319–1320. [PubMed] [Google Scholar]
- 10.Graham B.S., Ambrosino D.M. History of passive antibody administration for prevention and treatment of infectious diseases. Curr. Opin. HIV AIDS. 2015;10:129–134. doi: 10.1097/COH.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casadevall A., Dadachova E., Pirofski L.A. Passive antibody therapy for infectious diseases. Nat. Rev. Microbiol. 2004;2:695–703. doi: 10.1038/nrmicro974. [DOI] [PubMed] [Google Scholar]
- 12.Sparrow E., Friede M., Sheikh M., Torvaldsen S. Therapeutic antibodies for infectious diseases. Bull. World Health Organ. 2017;95:235–237. doi: 10.2471/BLT.16.178061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu R.M. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020;27:1. doi: 10.1186/s12929-019-0592-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson S. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J. Infect. Dis. 1997;176:1215–1224. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 15.The IMpact-RSV Study Group Palivizumab, a Humanized Respiratory Syncytial Virus Monoclonal Antibody, Reduces Hospitalization From Respiratory Syncytial Virus Infection in High-risk Infants. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 16.Sparrow E. Recent advances in the development of monoclonal antibodies for rabies post exposure prophylaxis: a review of the current status of the clinical development pipeline. Vaccine. 2019;37(Suppl 1):A132–A139. doi: 10.1016/j.vaccine.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Pulendran B., Ahmed R. Immunological mechanisms of vaccination. Nat. Immunol. 2011;12:509–517. doi: 10.1038/ni.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plotkin S.A. Correlates of protection induced by vaccination. Clin. Vaccine Immunol. 2010;17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andreano E., Seubert A., Rappuoli R. Human monoclonal antibodies for discovery, therapy, and vaccine acceleration. Curr. Opin. Immunol. 2019;59:130–134. doi: 10.1016/j.coi.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Niessl J. Combination anti-HIV-1 antibody therapy is associated with increased virus-specific T cell immunity. Nat. Med. 2020;26:222–227. doi: 10.1038/s41591-019-0747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pelfrene E., Mura M., Cavaleiro Sanches A., Cavaleri M. Monoclonal antibodies as anti-infective products: a promising future? Clin. Microbiol. Infect. 2019;25:60–64. doi: 10.1016/j.cmi.2018.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McLellan J.S. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science. 2013;342:592–598. doi: 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin M.P. Safety, tolerability, and pharmacokinetics of medi8897, the respiratory syncytial virus prefusion f-targeting monoclonal antibody with an extended half-life, in healthy adults. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.01714-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffin M.P. Single-Dose Nirsevimab for Prevention of RSV in Preterm Infants. N. Engl. J. Med. 2020;383:415–425. doi: 10.1056/NEJMoa1913556. [DOI] [PubMed] [Google Scholar]
- 25.Krammer F. Influenza. Nat. Rev. Dis. Primers. 2018;4:3. doi: 10.1038/s41572-018-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beigel J., Bray M. Current and future antiviral therapy of severe seasonal and avian influenza. Antiviral Res. 2008;78:91–102. doi: 10.1016/j.antiviral.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laursen N.S., Wilson I.A. Broadly neutralizing antibodies against influenza viruses. Antiviral Res. 2013;98:476–483. doi: 10.1016/j.antiviral.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sedeyn K., Saelens X. New antibody-based prevention and treatment options for influenza. Antiviral Res. 2019;170 doi: 10.1016/j.antiviral.2019.104562. [DOI] [PubMed] [Google Scholar]
- 29.Hershberger E. Safety and efficacy of monoclonal antibody VIS410 in adults with uncomplicated influenza A infection: Results from a randomized, double-blind, phase-2, placebo-controlled study. EBioMedicine. 2019;40:574–582. doi: 10.1016/j.ebiom.2018.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beigel J.H. Advances in respiratory virus therapeutics - a meeting report from the 6th isirv antiviral group conference. Antiviral Res. 2019;167:45–67. doi: 10.1016/j.antiviral.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ali S.O. Evaluation of MEDI8852, an anti-influenza a monoclonal antibody, in treating acute uncomplicated influenza. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.00694-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim J.J. A phase 2 randomized, double-blind, placebo-controlled trial of MHAA4549A, a monoclonal antibody, plus oseltamivir in patients hospitalized with severe influenza a virus infection. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.00352-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker L.M., Burton D.R. Passive immunotherapy of viral infections:’ super-antibodies’ enter the fray. Nat. Rev. Immunol. 2018;18:297–308. doi: 10.1038/nri.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burton D.R., Hangartner L. Broadly neutralizing antibodies to HIV and their role in vaccine design. Annu. Rev. Immunol. 2016;34:635–659. doi: 10.1146/annurev-immunol-041015-055515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andrabi R., Bhiman J.N., Burton D.R. Strategies for a multi-stage neutralizing antibody-based HIV vaccine. Curr. Opin. Immunol. 2018;53:143–151. doi: 10.1016/j.coi.2018.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jardine J. Rational HIV immunogen design to target specific germline B cell receptors. Science. 2013;340:711–716. doi: 10.1126/science.1234150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jardine J.G. HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science. 2016;351:1458–1463. doi: 10.1126/science.aad9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGuire A.T. Specifically modified Env immunogens activate B-cell precursors of broadly neutralizing HIV-1 antibodies in transgenic mice. Nat. Commun. 2016;7:10618. doi: 10.1038/ncomms10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian M. Induction of HIV neutralizing antibody lineages in mice with diverse precursor repertoires. Cell. 2016;166:1471–1484. doi: 10.1016/j.cell.2016.07.029. e1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Medina-Ramirez M. Design and crystal structure of a native-like HIV-1 envelope trimer that engages multiple broadly neutralizing antibody precursors in vivo. J. Exp. Med. 2017;214:2573–2590. doi: 10.1084/jem.20161160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steichen J.M. A generalized HIV vaccine design strategy for priming of broadly neutralizing antibody responses. Science. 2019;366 doi: 10.1126/science.aax4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saunders K.O. Targeted selection of HIV-specific antibody mutations by engineering B cell maturation. Science. 2019;366 doi: 10.1126/science.aay7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pegu A. A meta-analysis of passive immunization studies shows that serum-neutralizing antibody titer associates with protection against SHIV challenge. Cell Host Microbe. 2019;26:336–346. doi: 10.1016/j.chom.2019.08.014. e333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barouch D.H. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013;503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y., Cao W., Sun M., Li T. Broadly neutralizing antibodies for HIV-1: efficacies, challenges and opportunities. Emerg. Microbes Infect. 2020;9:194–206. doi: 10.1080/22221751.2020.1713707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gilbert P.B. Basis and statistical design of the passive HIV-1 antibody mediated prevention (AMP) test-of-Concept efficacy trials. Stat. Commun. Infect. Dis. 2017;9 doi: 10.1515/scid-2016-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stephenson K.E., Wagh K., Korber B., Barouch D.H. Vaccines and broadly neutralizing antibodies for HIV-1 prevention. Annu. Rev. Immunol. 2020;38:673–703. doi: 10.1146/annurev-immunol-080219-023629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marston H.D., Paules C.I., Fauci A.S. Monoclonal antibodies for emerging infectious diseases - borrowing from history. N. Engl. J. Med. 2018;378:1469–1472. doi: 10.1056/NEJMp1802256. [DOI] [PubMed] [Google Scholar]
- 49.Casadevall A., Pirofski L.A. The Ebola epidemic crystallizes the potential of passive antibody therapy for infectious diseases. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qiu X. Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp. Nature. 2014;514:47–53. doi: 10.1038/nature13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Group P.I.W. A randomized, controlled trial of ZMapp for ebola virus infection. N. Engl. J. Med. 2016;375:1448–1456. doi: 10.1056/NEJMoa1604330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sivapalasingam S. Safety, pharmacokinetics, and immunogenicity of a co-formulated cocktail of three human monoclonal antibodies targeting Ebola virus glycoprotein in healthy adults: a randomised, first-in-human phase 1 study. Lancet Infect. Dis. 2018;18:884–893. doi: 10.1016/S1473-3099(18)30397-9. [DOI] [PubMed] [Google Scholar]
- 53.Gaudinski M.R. Safety, tolerability, pharmacokinetics, and immunogenicity of the therapeutic monoclonal antibody mAb114 targeting Ebola virus glycoprotein (VRC 608): an open-label phase 1 study. Lancet. 2019;393:889–898. doi: 10.1016/S0140-6736(19)30036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mulangu S. A randomized, controlled trial of ebola virus disease therapeutics. N. Engl. J. Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teoh E.P. The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3003888. [DOI] [PubMed] [Google Scholar]
- 56.Fibriansah G. A potent anti-dengue human antibody preferentially recognizes the conformation of E protein monomers assembled on the virus surface. EMBO Mol. Med. 2014;6:358–371. doi: 10.1002/emmm.201303404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fibriansah G. DENGUE VIRUS. Cryo-EM structure of an antibody that neutralizes dengue virus type 2 by locking E protein dimers. Science. 2015;349:88–91. doi: 10.1126/science.aaa8651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robinson L.N. Structure-Guided Design of an Anti-dengue Antibody Directed to a Non-immunodominant Epitope. Cell. 2015;162:493–504. doi: 10.1016/j.cell.2015.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Q. Molecular determinants of human neutralizing antibodies isolated from a patient infected with Zika virus. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aai8336. [DOI] [PubMed] [Google Scholar]
- 60.Low J.G. Phase 1 trial of a therapeutic anti-yellow fever virus human antibody. N. Engl. J. Med. 2020;383:452–459. doi: 10.1056/NEJMoa2000226. [DOI] [PubMed] [Google Scholar]
- 61.Kelley B. Developing therapeutic monoclonal antibodies at pandemic pace. Nat. Biotechnol. 2020;38:540–545. doi: 10.1038/s41587-020-0512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang X. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou F. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Phua J. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir. Med. 2020;8:506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiang S., Hillyer C., Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses: (trends in immunology 41, 355-359; 2020) Trends Immunol. 2020 doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Casadevall A., Pirofski L.A. The convalescent sera option for containing COVID-19. J. Clin. Invest. 2020;130:1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duan K. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zeng Q.L. Effect of Convalescent Plasma Therapy on Viral Shedding and Survival in Patients With Coronavirus Disease 2019. J. Infect. Dis. 2020;222:38–43. doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Alwis R., Chen S., Gan E.S., Ooi E.E. Impact of immune enhancement on Covid-19 polyclonal hyperimmune globulin therapy and vaccine development. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abraham J. Passive antibody therapy in COVID-19. Nat. Rev. Immunol. 2020;20:401–403. doi: 10.1038/s41577-020-0365-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Long Q.X. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020 doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 72.Siracusano G., Pastori C., Lopalco L. 2020. Humoral Immune Responses in COVID-19 Patients: A Window on the State of the Art. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wu F. 2020. Neutralizing Antibody Responses to SARS-CoV-2 in a COVID-19 Recovered Patient Cohort and Their Implications; pp. 2003–2030. 20047365 (2020) [Google Scholar]
- 74.Wang C. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 2020;11:2251. doi: 10.1038/s41467-020-16256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu Y. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science. 2020 doi: 10.1126/science.abc2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brouwer P.J.M. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science. 2020;369:643–650. doi: 10.1126/science.abc5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wec A.Z. Broad neutralization of SARS-related viruses by human monoclonal antibodies. Science. 2020;369:731–736. doi: 10.1126/science.abc7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu L. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020 doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 79.Lv Z. Structural basis for neutralization of SARS-CoV-2 and SARS-CoV by a potent therapeutic antibody. Science. 2020 doi: 10.1126/science.abc5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pinto D. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020 doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- 81.Rogers T.F. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020 doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hansen J. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020 doi: 10.1126/science.abd0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baum A. Antibody cocktail to SARS-CoV-2 spike protein prevents rapid mutational escape seen with individual antibodies. Science. 2020 doi: 10.1126/science.abd0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cohen J. The race is on for antibodies that stop the new coronavirus. Science. 2020;368:564–565. doi: 10.1126/science.368.6491.564. [DOI] [PubMed] [Google Scholar]
- 85.Ledford H. Antibody therapies could be a bridge to a coronavirus vaccine - but will the world benefit? Nature. 2020;584:333–334. doi: 10.1038/d41586-020-02360-y. [DOI] [PubMed] [Google Scholar]
- 86.Bebbington C., Yarranton G. Antibodies for the treatment of bacterial infections: current experience and future prospects. Curr. Opin. Biotechnol. 2008;19:613–619. doi: 10.1016/j.copbio.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 87.Lynch S.V., Pedersen O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016;375:2369–2379. doi: 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 88.Sause W.E. Antibody-based biologics and their promise to combat Staphylococcus aureus infections. Trends Pharmacol. Sci. 2016;37:231–241. doi: 10.1016/j.tips.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.M. G. Sanchez et al., B14. Late breaking clinical trials. pp. A7358-A7358.
- 90.Magyarics Z. Randomized, double-blind, placebo-controlled, single-ascending-dose study of the penetration of a monoclonal antibody combination (asn100) targeting staphylococcus aureus cytotoxins in the lung epithelial lining fluid of healthy volunteers. Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.00350-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Corraliza-Gorjon I. New strategies using antibody combinations to increase Cancer treatment effectiveness. Front. Immunol. 2017;8:1804. doi: 10.3389/fimmu.2017.01804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Borlak J. Immune-mediated liver injury of the cancer therapeutic antibody catumaxomab targeting EpCAM, CD3 and Fcgamma receptors. Oncotarget. 2016;7:28059–28074. doi: 10.18632/oncotarget.8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Labrijn A.F., Janmaat M.L., Reichert J.M., Parren P. Bispecific antibodies: a mechanistic review of the pipeline. Nat. Rev. Drug Discov. 2019;18:585–608. doi: 10.1038/s41573-019-0028-1. [DOI] [PubMed] [Google Scholar]
- 94.Wagner E.K., Wang X., Bui A., Maynard J.A. Synergistic neutralization of pertussis toxin by a bispecific antibody in vitro and in vivo. Clin. Vaccine Immunol. 2016;23:851–862. doi: 10.1128/CVI.00371-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sung J.A. Dual-Affinity Re-Targeting proteins direct T cell-mediated cytolysis of latently HIV-infected cells. J. Clin. Invest. 2015;125:4077–4090. doi: 10.1172/JCI82314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kruse R.L. In Situ Liver Expression of HBsAg/CD3-Bispecific Antibodies for HBV Immunotherapy. Mol. Ther. Methods Clin. Dev. 2017;7:32–41. doi: 10.1016/j.omtm.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meng W. Targeting human-cytomegalovirus-Infected cells by redirecting t cells using an Anti-CD3/Anti-Glycoprotein B bispecific antibody. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.01719-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wec A.Z. A "Trojan horse" bispecific-antibody strategy for broad protection against ebolaviruses. Science. 2016;354:350–354. doi: 10.1126/science.aag3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.DiGiandomenico A. A multifunctional bispecific antibody protects against Pseudomonas aeruginosa. Sci. Transl. Med. 2014;6 doi: 10.1126/scitranslmed.3009655. [DOI] [PubMed] [Google Scholar]
- 100.Thanabalasuriar A. Bispecific antibody targets multiple Pseudomonas aeruginosa evasion mechanisms in the lung vasculature. J. Clin. Invest. 2017;127:2249–2261. doi: 10.1172/JCI89652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tkaczyk C. Targeting Alpha Toxin and ClfA with a Multimechanistic Monoclonal-Antibody-Based Approach for Prophylaxis of Serious Staphylococcus aureus Disease. mBio. 2016;7 doi: 10.1128/mBio.00528-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tkaczyk C. Multimechanistic monoclonal antibodies (mabs) targeting staphylococcus aureus alpha-toxin and clumping factor a: activity and efficacy comparisons of a mab combination and an engineered bispecific antibody approach. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.00629-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu L. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science. 2017;358:85–90. doi: 10.1126/science.aan8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thomas A., Teicher B.A., Hassan R. Antibody-drug conjugates for cancer therapy. Lancet Oncol. 2016;17:e254–e262. doi: 10.1016/S1470-2045(16)30030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lehar S.M. Novel antibody-antibiotic conjugate eliminates intracellular S. Aureus. Nature. 2015;527:323–328. doi: 10.1038/nature16057. [DOI] [PubMed] [Google Scholar]
- 106.Yu X. Antigen-armed antibodies targeting B lymphoma cells effectively activate antigen-specific CD4+ T cells. Blood. 2015;125:1601–1610. doi: 10.1182/blood-2014-07-591412. [DOI] [PubMed] [Google Scholar]
- 107.Liu R., Wang R.E., Wang F. Antibody-drug conjugates for non-oncological indications. Expert Opin. Biol. Ther. 2016;16:591–593. doi: 10.1517/14712598.2016.1161753. [DOI] [PubMed] [Google Scholar]
- 108.Jovcevska I., Muyldermans S. The Therapeutic Potential of Nanobodies. BioDrugs. 2020;34:11–26. doi: 10.1007/s40259-019-00392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chanier T., Chames P., Engineering Nanobody. Toward Next Generation Immunotherapies and Immunoimaging of Cancer. Antibodies Basel (Basel) 2019;8 doi: 10.3390/antib8010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peyvandi F. Caplacizumab reduces the frequency of major thromboembolic events, exacerbations and death in patients with acquired thrombotic thrombocytopenic purpura. J. Thromb. Haemost. 2017;15:1448–1452. doi: 10.1111/jth.13716. [DOI] [PubMed] [Google Scholar]
- 111.Morrison C. Nanobody approval gives domain antibodies a boost. Nat. Rev. Drug Discov. 2019;18:485–487. doi: 10.1038/d41573-019-00104-w. [DOI] [PubMed] [Google Scholar]
- 112.Konwarh R. Nanobodies: Prospects of Expanding the Gamut of Neutralizing Antibodies Against the Novel Coronavirus, SARS-CoV-2. Front. Immunol. 2020;11:1531. doi: 10.3389/fimmu.2020.01531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Larios Mora A. Delivery of ALX-0171 by inhalation greatly reduces respiratory syncytial virus disease in newborn lambs. MAbs. 2018;10:778–795. doi: 10.1080/19420862.2018.1470727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wrapp D. Structural Basis for Potent Neutralization of Betacoronaviruses by Single-Domain Camelid Antibodies. Cell. 2020;181:1436–1441. doi: 10.1016/j.cell.2020.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huo J. Neutralizing nanobodies bind SARS-CoV-2 spike RBD and block interaction with ACE2. Nat. Struct. Mol. Biol. 2020 doi: 10.1038/s41594-020-0469-6. [DOI] [PubMed] [Google Scholar]
- 116.Bournazos S. Human IgG Fc domain engineering enhances antitoxin neutralizing antibody activity. J. Clin. Invest. 2014;124:725–729. doi: 10.1172/JCI72676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jefferis R. Isotype and glycoform selection for antibody therapeutics. Arch. Biochem. Biophys. 2012;526:159–166. doi: 10.1016/j.abb.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 118.Bournazos S. Differential requirements for FcgammaR engagement by protective antibodies against Ebola virus. Proc. Natl. Acad. Sci. U. S. A. 2019;116:20054–20062. doi: 10.1073/pnas.1911842116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Khandia R. Modulation of dengue/zika virus pathogenicity by antibody-dependent enhancement and strategies to protect against enhancement in zika virus infection. Front. Immunol. 2018;9:597. doi: 10.3389/fimmu.2018.00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ramadhany R. Antibody with an engineered Fc region as a therapeutic agent against dengue virus infection. Antiviral Res. 2015;124:61–68. doi: 10.1016/j.antiviral.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 121.Irvine E.B., Alter G. Understanding the role of antibody glycosylation through the lens of severe viral and bacterial diseases. Glycobiology. 2020;30:241–253. doi: 10.1093/glycob/cwaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zeitlin L. Enhanced potency of a fucose-free monoclonal antibody being developed as an Ebola virus immunoprotectant. Proc Natl Acad Sci U S A. 2011;108:20690–20694. doi: 10.1073/pnas.1108360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li T. Modulating IgG effector function by Fc glycan engineering. Proc. Natl. Acad. Sci. U. S. A. 2017;114:3485–3490. doi: 10.1073/pnas.1702173114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Saunders K.O. Conceptual approaches to modulating antibody effector functions and circulation half-life. Front. Immunol. 2019;10:1296. doi: 10.3389/fimmu.2019.01296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gulati S. Complement alone drives efficacy of a chimeric antigonococcal monoclonal antibody. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Natsume A., Niwa R., Satoh M. Improving effector functions of antibodies for cancer treatment: enhancing ADCC and CDC. Drug Des. Devel. Ther. 2009;3:7–16. [PMC free article] [PubMed] [Google Scholar]
- 127.Gaudinski M.R. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a Phase 1 open-label clinical trial in healthy adults. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhu Q. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aaj1928. [DOI] [PubMed] [Google Scholar]
- 129.Domachowske J.B. Safety, tolerability and pharmacokinetics of medi8897, an extended half-life single-dose respiratory syncytial virus prefusion f-targeting monoclonal antibody administered as a single dose to healthy preterm infants. Pediatr. Infect. Dis. J. 2018;37:886–892. doi: 10.1097/INF.0000000000001916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Corthesy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front. Immunol. 2013;4:185. doi: 10.3389/fimmu.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Seibert C.W. Recombinant IgA is sufficient to prevent influenza virus transmission in guinea pigs. J. Virol. 2013;87:7793–7804. doi: 10.1128/JVI.00979-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Balu S. A novel human IgA monoclonal antibody protects against tuberculosis. J. Immunol. 2011;186:3113–3119. doi: 10.4049/jimmunol.1003189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ejemel M. A cross-reactive human IgA monoclonal antibody blocks SARS-CoV-2 spike-ACE2 interaction. Nat. Commun. 2020;11:4198. doi: 10.1038/s41467-020-18058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Duperret E.K. Synthetic DNA-Encoded Monoclonal Antibody Delivery of Anti-CTLA-4 Antibodies Induces Tumor Shrinkage In Vivo. Cancer Res. 2018;78:6363–6370. doi: 10.1158/0008-5472.CAN-18-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hollevoet K., De Smidt E., Geukens N., Declerck P. Prolonged in vivo expression and anti-tumor response of DNA-based anti-HER2 antibodies. Oncotarget. 2018;9:13623–13636. doi: 10.18632/oncotarget.24426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Perales-Puchalt A., Duperret E.K., Muthumani K., Weiner D.B. Simplifying checkpoint inhibitor delivery through in vivo generation of synthetic DNA-encoded monoclonal antibodies (DMAbs) Oncotarget. 2019;10:13–16. doi: 10.18632/oncotarget.26535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Khoshnejad M. Development of novel DNA-Encoded PCSK9 monoclonal antibodies as lipid-lowering therapeutics. Mol. Ther. 2019;27:188–199. doi: 10.1016/j.ymthe.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Elliott S.T.C. DMAb inoculation of synthetic cross reactive antibodies protects against lethal influenza A and B infections. Npj Vaccines. 2017;2:18. doi: 10.1038/s41541-017-0020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Muthumani K. Rapid and long-term immunity elicited by dna-encoded antibody prophylaxis and dna vaccination against chikungunya virus. J. Infect. Dis. 2016;214:369–378. doi: 10.1093/infdis/jiw111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Van Hoecke L., Roose K. How mRNA therapeutics are entering the monoclonal antibody field. J. Transl. Med. 2019;17:54. doi: 10.1186/s12967-019-1804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pardi N. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nat. Commun. 2017;8:14630. doi: 10.1038/ncomms14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thran M. mRNA mediates passive vaccination against infectious agents, toxins, and tumors. EMBO Mol. Med. 2017;9:1434–1447. doi: 10.15252/emmm.201707678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tiwari P.M. Engineered mRNA-expressed antibodies prevent respiratory syncytial virus infection. Nat. Commun. 2018;9:3999. doi: 10.1038/s41467-018-06508-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kose N. A lipid-encapsulated mRNA encoding a potently neutralizing human monoclonal antibody protects against chikungunya infection. Sci. Immunol. 2019;4 doi: 10.1126/sciimmunol.aaw6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.J. Cohen, Designer antibodies could battle COVID-19 before vaccines arrive, https://www.sciencemag.org/news/2020/08/designer-antibodies-could-battle-covid-19-vaccines-arrive. doi:10.1126/science.abe1740.
- 146.Finn J.A. Identification of structurally related antibodies in antibody sequence databases using rosetta-derived position-specific scoring. Structure. 1993;(2020) doi: 10.1016/j.str.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]