Dear Editor,
The coronavirus disease 2019 (COVID-19), caused by the SARS-CoV-2 virus, becomes clinically manifest in a broad range from mild symptoms to life-threatening multi-organ failure (MOF). We have read with interest the recent letter by García de Guadiana Romualdo and colleagues1 and the review by Skevaki and colleagues2 examining the significance of biomarkers for risk assessment and prognosis of COVID-19.
A severe course of disease is characterized by a dysregulated immune response, suspected to be initiated by dysregulation of innate immune cells of the granulomonocytic lineage.3 The pro-inflammatory mediator calprotectin (S100A8/A9, MRP 8/14) is reported to be an early signal, mediating the cytokine storm associated with an increased severity of COVID-19.1 , 3 , 4
The expression of calprotectin is predominantly restricted to the intracellular compartment of neutrophil granulocytes, where it presents about half of the total cytosolic protein content. In contrast to routinely used inflammatory biomarkers such as C-reactive protein (CRP) and procalcitonin (PCT), it is released into the bloodstream without need for de novo protein biosynthesis. Thereby, circulating calprotectin possibly has a decisive kinetic advantage in that it might be one of the first responses of an organism to an inflammatory disease.
Previous studies have reported significantly elevated levels of calprotectin in patients with severe COVID-19 and the possible ability of calprotectin to discriminate between mild and severe form of the disease.3, 4, 5 In addition, elevated fecal calprotectin has been shown to associate with thromboembolic events in COVID-19 in the absence of gastrointestinal manifestations.6 However, it is not yet fully elucidated whether changes of calprotectin serum levels occur prior to the progression to severe disease and therefore might be detectable already at an early stage of COVID-19, e.g. in patients in the emergency department (ED), and whether calprotectin is superior compared to traditional biomarkers. Thus, here we evaluated calprotectin levels with regard to prediction of prognosis (subsequent intensive care unit (ICU) admission, MOF, mortality) in ED patients.
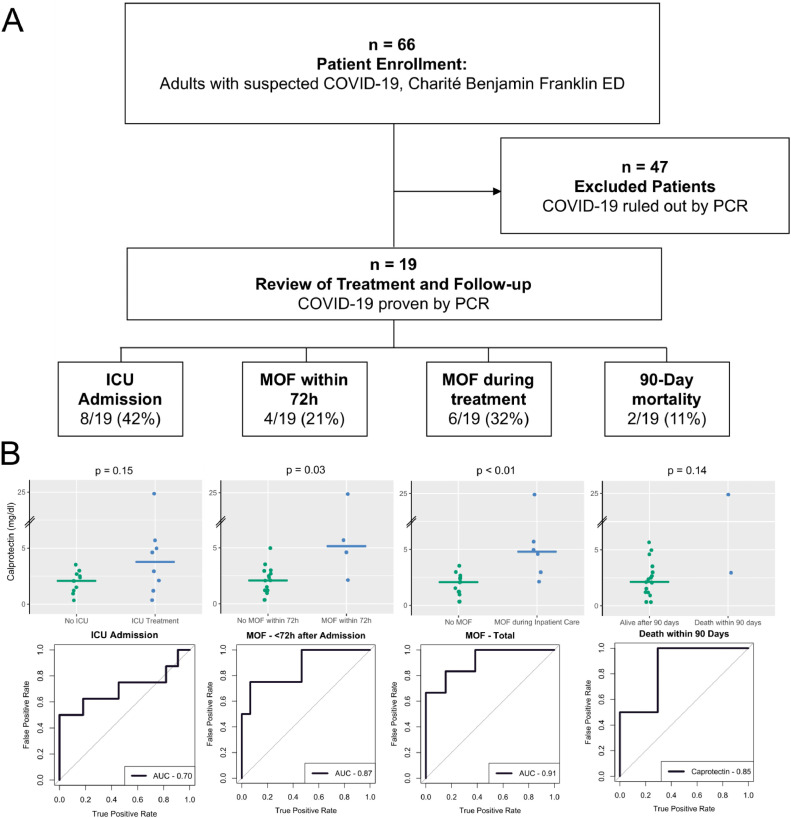
We prospectively enrolled a total of 66 patients presenting to the ED with suspected SARS-CoV-2 infection and isolated serum samples for further investigation. Using PCR testing in pharyngeal swabs, 47 patients were tested negative, and 19 patients were tested positive for SARS-CoV-2 and diagnosed with COVID-19. Main characteristics of SARS-CoV-2 positive patients are presented in Table 1 A. The disease course was evaluated with regard to the clinical endpoints i) MOF, defined as the clinical need for organ replacement of at least two organ systems in regard to the SOFA-Score (Sequential Organ Failure Assessment),7 within either 72 h after admission or ii) during the total hospital stay (total MOF), iii) admission to the ICU, and iv) death, defined as 90-day mortality. These definitions lead to subgroups of n = 8 for ICU admission, n = 4 for MOF within 72 h, n = 6 for total MOF, and n = 2 for 90-day mortality. The study design is presented in Fig. 1 A and the frequency of the described endpoints is depicted in Table 1. In all patients we quantified blood levels and calculated the Area Under the Receiver Operating Characteristic curve (AUROC) and the 95% confidence interval (CI) for calprotectin (measured by turbidimetric method, Gentian AS, Norway) within these subgroups in comparison with the biomarkers routinely used for clinical evaluation of patients admitted to the ED (Table 1B). Routine biomarkers were determined immediately as standard of care and calprotectin was measured in directly isolated serum, which was centrifuged within 30 min after blood withdrawal. With regard to the endpoint MOF incurring within 72 h, calprotectin showed the highest AUROC (0.87) as compared to lactate (0.79), CRP (0.70) and PCT (0.75) (Table 1C). Indeed, patients suffering from MOF within 72 h displayed a two-fold increase in median serum calprotectin at presentation to the ED compared to patients not experiencing MOF within 72 h (5.14 mg/L vs. 2.08 mg/L, p = 0.03, Table 1B). In patients with total MOF i.e. incurring within the total hospital stay, the AUROC of calprotectin was even higher at 0.91 with a comparable difference in median serum values between patients with and without this outcome (4.79 mg/L vs. 2.07 mg/L, p < 0.01, Table 1B). Again, calprotectin displayed the highest AUROC for this endpoint compared to the other biomarkers (Table 1C). With regard to ICU admission, calprotectin was better than CRP and PCT (0.80 vs 0.66 and 0.60 respectively), yet inferior to lactate, although hyperlactatemia being a widely used biomarker for admitting patients to the ICU.8 Thus, the routine in-house strategy presents a potential source of bias in favor for lactate. However, the discriminatory capacity of calprotectin with regard to death was lower compared to lactate, CRP and PCT. Yet, these results should be interpreted with caution based on the low number of patients for this endpoint (90 day mortality: n = 2). Indeed, others have shown a predictive value of calprotectin levels also for in-hospital mortality.1 Values of calprotectin serum levels and AUROCs are visualized within all study groups and endpoints (Figure 2B).
Table 1.
Demographics and Biomarkers by Outcome Groups.
| All | ICU Treatment |
Multi-Organ Failure within 72 h |
Multi-Organ Failure - Total |
90-Day Mortality |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 19) | No(n = 11) | Yes(n = 8) | p | No(n = 15) | Yes(n = 4) | p | No(n = 13) | Yes(n = 6) | P | No(n = 17) | Yes(n = 2) | p | ||||||||
| (A) Demographics | ||||||||||||||||||||
| Age [years] | 67.6 (53.9–72.0) |
67.6 (54.0–70.8) |
65.6 (54.8–76.4) |
0.44 | 67.6 (53.3–71.6) |
67.7 (57.5–78.0) |
0.36 | 57.3 (51.0–70.5) |
74.0 (62.5–77.2) |
0.06 | 67.6 (52.3–71.9) |
68.5 (63.9–73.0) |
0.42 | |||||||
| Female | 11 (58%) | 6 (55%) | 5 (62%) | 1.00 | 9 (60%) | 2 (50%) | 1.00 | 8 (62%) | 3 (50%) | 1.00 | 10 (59%) | 1 (50%) | 1.00 | |||||||
| BMI [kg/m2] | 17; 27.0 (22.3–30.5) | 10; 24.9 (21.9–29.6) |
7; 29.4 (26.4–30.9) |
0.19 | 14; 28.5 (22.6–30.8) |
3; 25.7 (23.9–26.4) |
0.43 | 12; 27.0 (22.1–30.3) |
5; 27.0 (25.7–30.9) |
0.51 | 15; 26.2 (22.2–30.4) |
39.1 (33.0–45.1) |
0.29 | |||||||
| (B) Biomarkers | ||||||||||||||||||||
| Calprotectin [mg/L] | 2.4 (1.4–3.2) |
2.08 (1.36–2.59) |
3.77 (1.90–5.16) |
0.15 | 2.08 (1.205–2.81) |
5.14 (3.98–10.48) |
0.03 | 2.07 (1.20–2.49) |
4.79 (3.36–5.51) |
<0.01 | 2.13 (1.21–2.98) |
13.91 (8.43–19.40) |
0.14 | |||||||
| Lactate [mg/dL] | 15.0 (11.6–21.5) |
13.00 (11.15–15.00) |
21.50 (16.75–31.25) |
0.03 | 13.50 (11.65–18.00) |
32.50 (25.25–44.00) |
0.09 | 13.00 (11.30–15.00) |
26.00 (19.00–33.75) |
0.03 | 13.50 (11.30–18.00) |
28.50 (25.25–31.75) |
0.10 | |||||||
| CRP [mg/L] |
37.7 (22.2–93.4) |
36.60 (23.60–65.90) |
93.40 (22.50–175.70) |
0.27 | 36.60 (22.25–72.20) |
93.40 (61.58–165.25) |
0.26 | 35.90 (18.70–63.00) |
126.80 (85.65–237.70) |
0.02 | 36.60 (18.70–75.60) |
301.50 (285.10–317.90) |
0.01 | |||||||
| PCT [µg/L] |
0.1 (0.1–0.2) |
0.11 (0.07–0.16) |
0.15 (0.07–0.70) |
0.51 | 0.09 (0.065–0.16) |
0.455 (0.18–0.93) |
0.15 | 0.09 (0.06–0.13) |
0.45 (0.12–0.71) |
0.08 | 0.09 (0.06–0.19) |
1.19 (0.96–1.43) |
0.03 | |||||||
| (C) AUROCs by Outcome | ||||||||||||||||||||
| AUROC Calprotectin | 0.70 (0.42–0.99) | 0.87 (0.63–1.00) | 0.91 (0.77–1.00) | 0.85 (0.54–1.00) | ||||||||||||||||
| AUROC Lactate | 0.80 (0.58–1.00) | 0.79 (0.38–1.00) | 0.82 (0.56–1.00) | 0.88 (0.71–1.00) | ||||||||||||||||
| AUROC CRP | 0.66 (0.36–0.96) | 0.70 (0.34–1.00) | 0.83 (0.58–1.00) | 1 | ||||||||||||||||
| AUROC PCT | 0.60 (0.29–0.90) | 0.75 (0.37–1.00) | 0.76 (0.47–1.00) | 1 | ||||||||||||||||
Continuous variables are represented with median and IQR, nominal variables with frequency and column percentage (of valid cases).
For continuous variables, where not all cases have data, the number of valid cases is shown.
P-values are calculated with Mann-Whitney-U test for continuous variables and Fisher's Exact Test for nominal variables.
Area under the Receiver Operating Characteristics (AUROCs) are shown for each biomarker, for each outcome, including 95% confidence intervals. BMI – body mass index; CRP – C-reactive protein; PCT – procalcitonin; AUROC – area under the receiver operating characteristic.
Fig. 1.
(A) Patient enrollment and outcomes and (B) receiver operating characteristics with scatterplots for calprotectin predicting those outcomes. The scatterplots include a broken y axis. The horizontal bar on the scatterplot represents the median calprotectin concentration. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Taken together, our study evaluated the association of calprotectin serum levels at the earliest possible moment, which is when patients are presented to the ED, with COVID-19 disease progression. Our data strongly argue for calprotectin representing a valuable biomarker for risk stratification, in particular with regard to subsequent MOF. Indeed, measurement of calprotectin might add to the biomarker repertoire in the ED since it seems to perform better than traditional markers such as lactate, CRP and PCT. Further, both CRP and PCT may be of low informative value with regard to early patient management in COVID-19 patients evaluated in the ED.
Therefore, we believe that calprotectin represents a novel and useful discriminator in COVID-19 patients admitted to the ED with respect to disease outcome, in particular MOF, with calprotectin measurement in blood samples being easily applicable in routine laboratories.
Declaration of Competing Interest
This study was financially supported by Gentian AS.
References
- 1.Luis Garcia de Guadiana R., Mulero M.D.R., Olivo M.H., Rojas C.R., Arenas V.R., Morales M.G., Abellan A.B., Conesa-Zamora P., Garcia-Garcia J., Hernandez A.C., Morell-Garcia D., Dolores Albaladejo-Oton M., Consuegra-Sanchez L. Circulating levels of GDF-15 and calprotectin for prediction of in-hospital mortality in COVID-19 patients: a case series. J Infect. 2020 doi: 10.1016/j.jinf.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skevaki C., Fragkou P.C., Cheng C., Xie M., Renz H. Laboratory characteristics of patients infected with the novel SARS-CoV-2 virus. J Infect. 2020;81:205–212. doi: 10.1016/j.jinf.2020.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silvin A., Chapuis N., Dunsmore G., Goubet A.G., Dubuisson A., Derosa L., Almire C., Henon C., Kosmider O., Droin N., Rameau P., Catelain C., Alfaro A., Dussiau C., Friedrich C., Sourdeau E., Marin N., Szwebel T.A., Cantin D., Mouthon L., Borderie D., Deloger M., Bredel D., Mouraud S., Drubay D., Andrieu M., Lhonneur A.S., Saada V., Stoclin A., Willekens C., Pommeret F., Griscelli F., Ng L.G., Zhang Z., Bost P., Amit I., Barlesi F., Marabelle A., Pene F., Gachot B., Andre F., Zitvogel L., Ginhoux F., Fontenay M., Solary E. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell. 2020;182:1401–1418. doi: 10.1016/j.cell.2020.08.002. e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi H., Zuo Y., Yalavarthi S., Gockman K., Zuo M., Madison J.A., Blair C.N., Woodard W., Lezak S.P., Lugogo N.L., Woods R.J., Lood C., Knight J.S., Kanthi Y. Neutrophil calprotectin identifies severe pulmonary disease in COVID-19. medRxiv. 2020 doi: 10.1002/JLB.3COVCRA0720-359R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L., Long X., Xu Q., Tan J., Wang G., Cao Y., Wei J., Luo H., Zhu H., Huang L., Meng F., Huang L., Wang N., Zhou X., Zhao L., Chen X., Mao Z., Chen C., Li Z., Sun Z., Zhao J., Wang D., Huang G., Wang W., Zhou J. Elevated serum levels of S100A8/A9 and HMGB1 at hospital admission are correlated with inferior clinical outcomes in COVID-19 patients. Cell Mol Immunol. 2020;17:992–994. doi: 10.1038/s41423-020-0492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giuffre M., Di Bella S., Sambataro G., Zerbato V., Cavallaro M., Occhipinti A.A., Palermo A., Crescenti A., Monica F., Luzzati R., Croce L.S. COVID-19-induced thrombosis in patients without gastrointestinal symptoms and elevated fecal calprotectin: hypothesis regarding mechanism of intestinal damage associated with COVID-19. Trop Med Infect Dis. 2020:5. doi: 10.3390/tropicalmed5030147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.D., Coopersmith C.M., Hotchkiss R.S., Levy M.M., Marshall J.C., Martin G.S., Opal S.M., Rubenfeld G.D., van der Poll T., Vincent J.L., Angus D.C. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seker Y.C., Bozan O., Sam E., Topacoglu H., Kalkan A. The role of the serum lactate level at the first admission to the emergency department in predicting mortality. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.09.088. [DOI] [PubMed] [Google Scholar]