Abstract
Introduction
Taking folic acid containing supplements prior to and during early pregnancy reduces the risk of neural tube defects. Neural tube defects occur prior to 28 days postconception, after which, there is no proven benefit of continuing to take folic acid. However, many women continue to take folic acid containing supplements throughout the pregnancy. At higher intakes, folic acid is not converted to its active form and accumulates in circulation as unmetabolised folic acid (UMFA). Recently, concerns have been raised about possible links between late gestation folic acid supplementation and childhood allergy, metabolic disease and autism spectrum disorders. We aim to determine if removing folic acid from prenatal micronutrient supplements after 12 weeks gestation reduces circulating levels of maternal UMFA at 36 weeks gestation.
Methods and analysis
This is a parallel-design, double-blinded randomised controlled trial. Women ≥12 and <16 weeks’ gestation with a singleton pregnancy and able to give informed consent are eligible to participate. Women (n=100; 50 per group) will be randomised to receive either a micronutrient supplement containing 0.8 mg of folic acid or a micronutrient supplement without folic acid daily from enrolment until delivery. The primary outcome is plasma UMFA concentration at 36 weeks gestation. Secondary outcomes include red blood cell folate and total plasma folate concentration. We will assess whether there is a difference in mean UMFA levels at 36 weeks gestation between groups using linear regression with adjustment for baseline UMFA levels and gestational age at trial entry. The treatment effect will be described as a mean difference with 95% CI.
Ethics and dissemination
Ethical approval has been granted from the Women’s and Children’s Health Network Research Ethics Committee (HREC/19/WCHN/018). The results of this trial will be presented at scientific conferences and published in peer-reviewed journals.
Trial registration number
ACTRN12619001511123.
Keywords: nutrition & dietetics, obstetrics, public health
Strengths and limitations of this study.
We will determine if discontinuing folic acid supplementation after 12 weeks of gestation results in lower levels of unmetabolised folic acid.
Unmetabolised folic acid is a biomarker of excess folic, and has been associated with a number of adverse pregnanacy outocomes.
This study is not powered to determine the effect of continuing folic acid supplements after the first trimester on clinical outcomes.
The study findings will be generalisable to countries which like Australia have mandatory folic acid fortification.
This research will inform the need for larger trials to determine if folic acid beyond the first trimester leads to adverse maternal and infant health outcomes.
Introduction
Evidence from randomised controlled trials1 2 and a large public health intervention3 showed that taking folic acid containing supplements prior to and during early pregnancy reduces the incidence of neural tube defects (NTD). Based on these findings, public health agencies around the world issued recommendations advising women to take folic acid supplements prior to conception and during early pregnancy.4 For example, in Australia, the government recommends that women trying to become pregnant take a folic acid supplement of 0.5 mg/day 12 weeks prior to conceiving and for the first 12 weeks of pregnancy.5
The neural tube closes in the first month of pregnancy, beyond this time there is no proven benefit of taking folic acid.6 However, many women continue to take folic acid as part of a prenatal vitamin and mineral supplement throughout pregnancy.7 In Australia, for example, a randomised controlled trial of pregnant women showed that more than 80% of women were taking a prenatal supplement containing folic acid at some time during their pregnancy,8 with the market leading supplement containing 0.8 mg of folic acid. Furthermore, almost 80 countries, including Australia, have mandated the addition of folic acid to food staples, typically wheat flour, to reduce NTDs in unplanned pregnancies.9 As such, the combination of food fortification along with prenatal supplement use may expose women and their fetus to excessive amounts of folic acid.
There is emerging evidence that higher intakes of folic acid in pregnancy may have negative health effects on the offspring including autism spectrum disorders10–12 and insulin resistance.13 An increased risk of childhood allergic disease is chief among these concerns with several studies reporting an inverse association with folic acid14–24 However, results are inconsistent and some studies report no relationship between folic acid intake and allergy outcomes in offspring25–27 or a reduction in risk of allergic disease.28 These studies vary greatly in regard to the timing and measurement of exposure and only one study differentiated between maternal total plasma folate and maternal plasma unmetabolised folic acid (UMFA).29
Folic acid is the synthetic form of the vitamin folate that is used in supplements and fortified foods because of its high bioavailability and stability compared with naturally occurring folate in food.30 Once consumed, folic acid must be converted into an active form, 5-methyltetrahydrofolate.31 At higher intakes folic acid is not converted to its active form and accumulates in plasma as UMFA.32 Circulating UMFA has been proposed as a biomarker of excess folic acid intake.33 Without proven benefit and with the suggestion of harm, the amount of folic acid in prenatal supplements may need to be reduced after the first trimester. We aim to determine if removing folic acid from prenatal multivitamin supplements after the first trimester (12 weeks gestation) reduces the accumulation of maternal UMFA measured at 36 weeks gestation.
Hypotheses
Removing folic acid from prenatal supplements after 12 weeks of gestation will limit the accumulation of UMFA in maternal plasma at 36 weeks of gestation.
Methods and analysis
Trial design
A multicentre two-arm parallel design, double-blinded randomised controlled trial.
Participating centres
The sponsoring institution and Trial Coordinating Centre is the South Australian Health and Medical Research Insititute (SAHMRI) based at the Women’s and Children’s Hospital (WCH). We will also seek approval to conduct the trial at Flinders Medical Centre (FMC), Adelaide, South Australia.
Study population
Participants are pregnant women with a singleton pregnancy enrolled between ≥12 and <16 weeks gestation. Enrolment commenced on 18 December 2019 and recruitment is ongoing. Data collection will continue through to May 2021.
Eligibility criteria
Inclusion criteria
To be eligible for participation women must be:
Carrying a singleton pregnancy ≥12 and <16 weeks gestation.
Currently taking a folic acid containing supplement and planning to continue this throughout pregnancy.
Able to give informed consent.
Exclusion criteria
Women will be ineligible for trial participation if they meet any the following criteria:
Carrying a fetus with a confirmed or suspected fetal abnormality.
Unwilling to cease current folic acid containing supplement/s.
Past history of an NTD affected pregnancy.
Currently taking medication known to interfere with folate metabolism (eg, methotrexate, sulphasalazine, anticonvulsants, antimalarials or barbiturates).
Known haemolytic anaemia or haemoglobinopathy.
Known to carry the TT variant of the methylene tetrahydrofolate reductase gene (MTHFR C77T) polymorphism.
Intolerance or allergy to prenatal vitamin and mineral supplements.
Study treatments
Participating women will be randomised to receive either a micronutrient supplement in tablet form, containing 0.8 mg folic acid (the dose in the most commonly used supplement in Australia or an identical micronutrient supplement containing no folic acid. The composition of micronutrients within the intervention and control supplements are formulated to approximate leading brands of prenatal micronutrient supplements available in Australia (table 1). Intervention and control supplements are identical in size, shape, colour and packaging and only differ in the removal of folic acid from the intervention supplement. Women will be asked to consume one supplement per day from enrolment (≥12 and <16 weeks of gestation) until delivery.
Table 1.
Ingredients of supplements for intervention and control groups
| Ingredients | Intervention | Control | Unit |
| Folic acid | 0 | 0.8 | mg |
| Calcium | 250 | 250 | mg |
| Iron | 27 | 27 | mg |
| Thiamine | 1.4 | 1.4 | mg |
| Riboflavin | 1.4 | 1.4 | mg |
| Niacinamide | 18 | 18 | mg |
| Vitamin B6 | 1.9 | 1.9 | mg |
| Vitamin B12 | 2.6 | 2.6 | μg |
| Pantothenic acid | 6 | 6 | mg |
| Biotin | 30 | 30 | mg |
| Vitamin C | 85 | 85 | mg |
| Vitamin E | 13.5 | 13.5 | IU |
| Magnesium | 50 | 50 | mg |
| Zinc | 7.5 | 7.5 | mg |
| Manganese | 2.0 | 2.0 | mg |
| Iodine | 0.22 | 0.22 | mg |
| Copper | 1 | 1 | mg |
| Selenium | 30 | 30 | μg |
| Vitamin D3 | 10 | 10 | μg |
| b-carotene | 2500 | 2500 | IU |
Manufacture of study supplements
Intevention and control supplements are manufactured in a licensed facility in accordance with the Code of Good Manufacturing Practice (GMP) for Medicinal Products and have been donated to the trial by Factors Group of Companies, Coquitlam, British Columbia, Canada. The supplements are packaged and labelled in accordance with GMP including an individual product identifier, batch number, expiry date and the statement ‘for clinical trial use only’. The pharmacist or the investigator’s designee maintains accurate records of the dispensing of study product. Unused study supplements will be destroyed in compliance with applicable regulations.
Monitoring adherence to study treatment
Research personnel will maintain regular contact with participating women to monitor and encourage supplement adherence and study compliance and answer any questions as they arise. At each contact, women will be asked if they have missed any supplements in the last week and if so, how many have been missed. Women will be asked to return unused supplements at the final study visit (36 weeks’ gestation) and the proportion of supplements returned will serve as the primary measure of compliance. A woman will be classified as compliant if she takes greater than 80% of her study supplements. At this visit women will be issued with enough supplements to last until the delivery of their baby.
Outcome measures
The primary outcome is maternal plasma UMFA concentration at 36 weeks gestation.
Secondary outcomes
Maternal plasma total and red blood cell folate levels at 36 weeks’ gestation.
Gestational age at birth, birth weight, birth length, birth head circumference.
Safety outcomes
Neonatal complications requiring admission to the neonatal unit.
Pregnancy complications requiring hospital admission.
Serious adverse events defined as: maternal or fetal (>20 weeks) deaths, fetal loss (<20 weeks), maternal or neonatal admissions to intensive care and major congenital anomalies.
Participant timeline
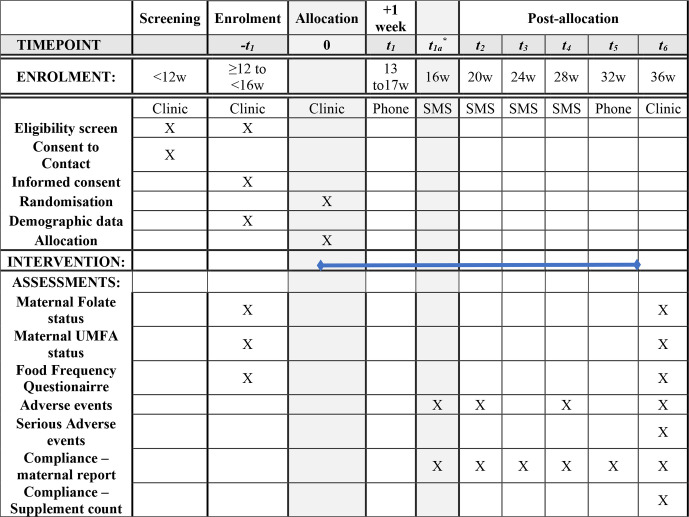
Women will be randomised and asked to cease their current prenatal supplements immediately and for the duration of the study. At enrolment, following informed consent and prior to commencement of the study treatment, research personnel will collect baseline clinical and demographic data including: contact details, self-reported ethnicity, gravida, parity, age, supplement and prescription drug use, weight, height, highest level of education, occupation and smoking status. Maternal dietary intakes of folate and other one-carbon nutrients during early and late pregnancy will be collected with the use of an 80-item semiquantitative Food-Frequency Questionnaire—Dietary Questionnaire for Epidemiological Studies (V.3.2).34 A 10 mL venous blood sample will be collected by venepuncture to assess UMFA, folate status and full blood count. The time the woman last ate and drank as well as the time her last supplement was taken will be recorded. Research personnel will contact the participant 1 week following the enrolment visit and then monthly to ensure adherence and record adverse events (figure 1). At 36 weeks’ gestation participating women will attend a clinic appointment for collection of venous blood sample for UMFA and folate analysis and full blood count. Participants will be asked to return unused supplements which will be counted as a measure of compliance. The Food-Frequency Questionnaire will be repeated and women will be given enough supplements to last the remainder of their pregnancy. Following delivery, research personnel will extract details of pregnancy, labour and birth from the woman and her baby’s medical records. Blood samples will be analysed for UMFA according to established methods.35 Plasma folate (nmol/L) and erythrocyte folate (nmol/L) concentrations will be determined using the folate microbiological assay harmonised by the Centers for Disease Control and Prevention.36
Figure 1.
Folic acid trial schedule. *This timepoint will be completed only for women enrolled <14 weeks gestation. SMS, short message service; UMFA, unmetabolised folic acid.
Sample size
A sample size of 90 women (45 per group) will provide >90% power to detect a standardised difference in mean UMFA concentration at 36 weeks gestation between groups of 0.60 (two-tailed alpha =0.05, correlation between UMFA concentrations at baseline and 36 weeks of gestation=0.60).29 Calculations were performed based on a standardised mean difference (mean difference divided by SD of outcome at 36 weeks gestation) due to considerable variability in the literature in the reported SD for UMFA concentration in pregnancy.16 29 A standardised mean difference of 0.60 represents a medium effect size and would demonstrate biologically excessive folic acid consumption. To allow for 10% lost to follow-up, we will randomise 50 women per group.
Recruitment
Pregnant women will be recruited through a combination of flyers, posters, a digital media campaign and through in-person recruitment at antenatal clinics. Women who meet eligibility criteria and agree to participate are invited to attend an enrolment appointment at our research clinics at the WCH or FMC, Adelaide between 12 and 16 weeks gestation.
Randomisation procedures
Participants will be randomised using a secure web-based randomisation service. Allocation will follow a computer-generated randomisation schedule using balanced variable block sizes, prepared by an independent statistician who is not involved with trial participants or data analysis. A unique four-digit study identification number and a coloured coded study pack are assigned to each participant. Stratification will be by gestational age at trial entry 12 to ≤14 weeks or >14 to 16 weeks gestation.
Blinding
The independent unblinded statistician (not involved in any other way in the trial) allocated two colours to the intervention group and two colours to the control group. Supplements were subsequently packaged and labelled with a colour by two unblinded staff members who have no other involvement in the trial. Research personnel, participants and their family, care providers, outcome assessors and data analysts remain blinded to colour allocation and therefore randomisation group.
The intervention and control supplements are identical in size, shape, colour, packaging and labelling and uniquely identified by the coloured product identification label (yellow, pink, blue or green) only. The randomisation code for an individual participant may be unblinded by the independent statistician in the event of an emergency.
Data collection and trial management
Data are collected by trained research personnel and entered directly into an electronic case report form with password protection and defined user-level access Research Electronic Data Capture (REDCap). A record of all women approached, screened for eligibility and consented will be recorded.37 Once consented and randomised, REDCap has been designed to automatically calculate study milestones for each participant. This information is readily available for clinical trial staff to enable scheduling of appointments and sample collection. Summary reports including screening data, enrolment, appointment attendance, sample collection, serious adverse events and study completion are generated from REDCap and reviewed at monthly trial steering committee meetings. Electronic data are stored on secure servers at South Australia Health and Medical Research Institute and released only to persons authorised to receive those data.
Data and safety monitoring
We do not anticipate any serious adverse events related to participation in this trial. Regardless, an independent (blinded) clinician will review all serious adverse events and determine whether there is any likelihood that involvement in the trial could have contributed to the event. Determinations of causality will be made from medical records retrieved for this purpose. All serious adverse events will be captured and reported to the Human Research Ethics Committee.
Statistical analysis
Statistical analyses will be performed on an intention-to-treat basis according to a pre-specified statistical analysis plan. For the primary outcome, we will assess whether there is a difference in mean UMFA levels at 36 weeks gestation between groups using linear regression, with adjustment for baseline UMFA and the stratification variable gestational age at trial entry (12 to ≤14 weeks or >14 weeks). The treatment effect will be described as a mean difference with 95% CI. Secondary outcomes will be analysed using linear and logistic regression models for continuous and binary outcomes, respectively, again with adjustment for gestational age at trial entry. Safety outcomes will be compared between groups using Fisher exact tests. In all analyses, a two-sided p<0.05 will be taken to indicate statistical significance.
Ethics and dissemination
Human Research Ethics Approval
This protocol, the informed consent and participant information document and all participant communication have been approved by the Women’s and Children’s Health Network Research Ethics Committee (HREC) (HREC/19/WCHN/018) and Governance (SSA/19/WCHN/080). Governance approval has also been obtained from FMC. Any subsequent modifications will be reviewed and approved by the HREC and governance of each study site. The study will be conducted in compliance with the current approved version of the protocol. Any change to the protocol document or informed consent form that affects the scientific intent, study design, patient safety or may affect a participant’s willingness to continue participation in the study will be considered a major amendment. All such amendments will be submitted to the HREC for approval. Any other changes to the protocol (such as administrative changes to dates and study personnel) will be considered minor amendments and will be notified to the HREC as appropriate.
Confidentiality
Participant confidentiality is strictly held in trust by the participating investigators, research staff and their agents. This confidentiality is extended to cover testing of biological samples in addition to the clinical information relating to participants. Regulatory authorities may inspect all documents and records required to be maintained by the Investigator, including but not limited to, medical records for the women and/or infants in this study subject to individuals having obtained approval/clearance through State/National Governments and HREC as required by local laws. Clinical information will not be released without written permission of the parent, except as necessary for monitoring by HREC or regulatory agencies.
Patient and public involvement
The study was supported by a consumer advisory group which provided input to the protocol. A Consumer representative from our SAHMRI Women and Kids Consumer Advisory Group partnered with us for the design of the study, informational material to support the intervention, and the burden of the intervention from the participant’s perspective. We will meet with the consumer representative for this trial and the full Consumer Advisory group on a regular basis for the duration of the study. At the end of the study, the consumer advisory group will be given the opportunity to comment on the findings and contribute to the dissemination plan.
Dissemination plan
Study findings will be submitted for peer-reviewed publication and for presentation at appropriate local and international conferences. In addition, study findings will be disseminated to participants through a one-page lay summary. Results will be made available to the wider community through social media avenues and the SAHMRI website.
Supplementary Material
Footnotes
Twitter: @kpb2901
Contributors: KPB, TG, MM, DP and MS conceived the trial and proposed the trial design; TS advised on sample size calculations, trial design and analysis; SW and TG designed the prenatal supplement and had it manufactured; DS advised on analytical methodology; DS, TG and KPB drafted the protocol, all authors contributed to refinement of the study and approved the final manuscript.
Funding: This study is sponsored by the South Australian Health and Medical Research Institute (Adelaide, Australia). This study is supported by grants in aid from the Women’s and Children’s Hosptial Foundation (Best_WCHFG_2020). An Ella McKnight Scholarship from the Royal Australian and New Zealand College of Obstetricians and Gynaecologists supports MS. KPB is supported by a Women’s and Children’s Hospital Foundation, MS McLeod Postdoctoral Research Fellowship. DS is supported by the Australian Government Research Training Program Scholarship from The University of Adelaide. The study product is donated by Factors Group of Companies, Coquitlam, British Columbia, Canada.
Disclaimer: The funder/s have no role in the study design; collection, management, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication and have no authority over any of these activities.
Competing interests: MM reports that she has a financial relationship outside the submitted work with Trajan Nutrition as a member of the board. SW is a consultant for the Factors Group of Companies. DS, TG, DP, TS MS and KPB have nothing to disclose.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Wald N, Sneddon J. Prevention of neural tube defects: results of the medical research council vitamin study. MRC vitamin study research group. Lancet 1991;338:131. [PubMed] [Google Scholar]
- 2.Czeizel AE, Dudás I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med 1992;327:1832–5. 10.1056/NEJM199212243272602 [DOI] [PubMed] [Google Scholar]
- 3.Crider KS, Qi YP, Devine O, et al. . Modeling the impact of folic acid fortification and supplementation on red blood cell folate concentrations and predicted neural tube defect risk in the United States: have we reached optimal prevention? Am J Clin Nutr 2018;107:1027–34. 10.1093/ajcn/nqy065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Who recommendations on antenatal care for a positive pregnancy experience. Geneva, Switzerland: World Health Organization, 2016: 152. [PubMed] [Google Scholar]
- 5.RANZCOG Antenatal care during pregnancy, 2020. Available: https://ranzcog.edu.au/womens-health/patient-information-resources/antenatal-care-during-pregnancy
- 6.Sadler TW. Embryology of neural tube development. Am J Med Genet C Semin Med Genet 2005;135C:2–8. 10.1002/ajmg.c.30049 [DOI] [PubMed] [Google Scholar]
- 7.McNulty B, McNulty H, Marshall B, et al. . Impact of continuing folic acid after the first trimester of pregnancy: findings of a randomized trial of folic acid supplementation in the second and third trimesters. Am J Clin Nutr 2013;98:92–8. 10.3945/ajcn.112.057489 [DOI] [PubMed] [Google Scholar]
- 8.Makrides M, Best K, Yelland L, et al. . A randomized trial of prenatal n-3 fatty acid supplementation and preterm delivery. N Engl J Med 2019;381:1035–45. 10.1056/NEJMoa1816832 [DOI] [PubMed] [Google Scholar]
- 9.Australian Institute of Health and Welfare Monitoring the health impacts of mandatory folic acid and iodine fortification 2016, 2016. Available: https://www.aihw.gov.au/reports/food-nutrition/monitoring-health-impacts-of-mandatory-folic-acid/contents/table-of-contents
- 10.Levine SZ, Kodesh A, Viktorin A, et al. . Association of maternal use of folic acid and multivitamin supplements in the periods before and during pregnancy with the risk of autism spectrum disorder in offspring. JAMA Psychiatry 2018;75:176–84. 10.1001/jamapsychiatry.2017.4050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghavan R, Riley AW, Volk H, et al. . Maternal multivitamin intake, plasma folate and vitamin B12 levels and autism spectrum disorder risk in offspring. Paediatr Perinat Epidemiol 2018;32:100–11. 10.1111/ppe.12414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeSoto M, Hitlan R. Synthetic folic acid supplementation during pregnancy may increase the risk of developing autism. J Pediatr Biochem 2016;2:251–61. [Google Scholar]
- 13.Yajnik CS, Deshpande SS, Jackson AA, et al. . Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune maternal nutrition study. Diabetologia 2008;51:29–38. 10.1007/s00125-007-0793-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McStay C, Prescott S, Bower C, et al. . Maternal folic acid supplementation during pregnancy and childhood allergic disease outcomes: a question of timing? Nutrients 2017;9:123 10.3390/nu9020123 [DOI] [Google Scholar]
- 15.Whitrow MJ, Moore VM, Rumbold AR, et al. . Effect of supplemental folic acid in pregnancy on childhood asthma: a prospective birth cohort study. Am J Epidemiol 2009;170:1486–93. 10.1093/aje/kwp315 [DOI] [PubMed] [Google Scholar]
- 16.McGowan EC, Hong X, Selhub J, et al. . Association between folate metabolites and the development of food allergy in children. J Allergy Clin Immunol Pract 2020;8:132–40. 10.1016/j.jaip.2019.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bekkers MBM, Elstgeest LEM, Scholtens S, et al. . Maternal use of folic acid supplements during pregnancy, and childhood respiratory health and atopy. Eur Respir J 2012;39:1468–74. 10.1183/09031936.00094511 [DOI] [PubMed] [Google Scholar]
- 18.Kiefte-de Jong JC, Timmermans S, Jaddoe VWV, et al. . High circulating folate and vitamin B-12 concentrations in women during pregnancy are associated with increased prevalence of atopic dermatitis in their offspring. J Nutr 2012;142:731–8. 10.3945/jn.111.154948 [DOI] [PubMed] [Google Scholar]
- 19.Dunstan JA, West C, McCarthy S, et al. . The relationship between maternal folate status in pregnancy, cord blood folate levels, and allergic outcomes in early childhood. Allergy 2012;67:50–7. 10.1111/j.1398-9995.2011.02714.x [DOI] [PubMed] [Google Scholar]
- 20.Zetstra-van der Woude PA, De Walle HEK, Hoek A, der WPAZ, Walle H, et al. . Maternal high-dose folic acid during pregnancy and asthma medication in the offspring. Pharmacoepidemiol Drug Saf 2014;23:1059–65. 10.1002/pds.3652 [DOI] [PubMed] [Google Scholar]
- 21.Veeranki SP, Gebretsadik T, Mitchel EF, et al. . Maternal folic acid supplementation during pregnancy and early childhood asthma. Epidemiology 2015;26:934–41. 10.1097/EDE.0000000000000380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang T, Gu Y, Wei X, et al. . Periconceptional folic acid supplementation and vitamin B12 status in a cohort of Chinese early pregnancy women with the risk of adverse pregnancy outcomes. J Clin Biochem Nutr 2017;60:136–42. 10.3164/jcbn.16-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parr CL, Magnus MC, Karlstad Øystein, et al. . Maternal folate intake during pregnancy and childhood asthma in a population-based cohort. Am J Respir Crit Care Med 2017;195:221–8. 10.1164/rccm.201604-0788OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Håberg SE, London SJ, Stigum H, et al. . Folic acid supplements in pregnancy and early childhood respiratory health. Arch Dis Child 2009;94:180–4. 10.1136/adc.2008.142448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Granell R, Heron J, Lewis S, et al. . The association between mother and child MTHFR C677T polymorphisms, dietary folate intake and childhood atopy in a population-based, longitudinal birth cohort. Clin Exp Allergy 2008;38:320–8. 10.1111/j.1365-2222.2007.02902.x [DOI] [PubMed] [Google Scholar]
- 26.Martinussen MP, Bracken MB, Triche EW, et al. . Folic acid supplementation in early pregnancy and the risk of preeclampsia, small for gestational age offspring and preterm delivery. Eur J Obstet Gynecol Reprod Biol 2015;195:94–9. 10.1016/j.ejogrb.2015.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magdelijns FJH, Mommers M, Penders J, et al. . Folic acid use in pregnancy and the development of atopy, asthma, and lung function in childhood. Pediatrics 2011;128:e135–44. 10.1542/peds.2010-1690 [DOI] [PubMed] [Google Scholar]
- 28.Fortes C, Mastroeni S, Mannooranparampil TJ, et al. . Pre-natal folic acid and iron supplementation and atopic dermatitis in the first 6 years of life. Arch Dermatol Res 2019;311:361–7. 10.1007/s00403-019-01911-2 [DOI] [PubMed] [Google Scholar]
- 29.Pentieva K, Selhub J, Paul L, et al. . Evidence from a randomized trial that exposure to supplemental folic acid at recommended levels during pregnancy does not lead to increased Unmetabolized folic acid concentrations in maternal or cord blood. J Nutr 2016;146:494–500. 10.3945/jn.115.223644 [DOI] [PubMed] [Google Scholar]
- 30.Bailey LB, Gregory JF. Folate metabolism and requirements. J Nutr 1999;129:779–82. 10.1093/jn/129.4.779 [DOI] [PubMed] [Google Scholar]
- 31.Blakley RL. IUPAC-IUB joint Commission on Biochemical Nomenclature (JCBN). Nomenclature and symbols for folic acid and related compounds. Recommendations 1986. Eur J Biochem 1987;168:251–3. 10.1111/j.1432-1033.1987.tb13413.x [DOI] [PubMed] [Google Scholar]
- 32.Pfeiffer CM, Sternberg MR, Fazili Z, et al. . Unmetabolized folic acid is detected in nearly all serum samples from US children, adolescents, and adults. J Nutr 2015;145:520–31. 10.3945/jn.114.201210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plumptre L, Masih SP, Ly A, et al. . High concentrations of folate and unmetabolized folic acid in a cohort of pregnant Canadian women and umbilical cord blood. Am J Clin Nutr 2015;102:848–57. 10.3945/ajcn.115.110783 [DOI] [PubMed] [Google Scholar]
- 34.Hodge A, Patterson AJ, Brown WJ, et al. . The anti cancer council of Victoria FFQ: relative validity of nutrient intakes compared with weighed food records in young to middle-aged women in a study of iron supplementation. Aust N Z J Public Health 2000;24:576–83. 10.1111/j.1467-842X.2000.tb00520.x [DOI] [PubMed] [Google Scholar]
- 35.Zayed A, Bustami R, Alabsi W, et al. . Development and validation of a rapid high-performance liquid chromatography⁻tandem mass spectrometric method for determination of folic acid in human plasma. Pharmaceuticals 2018;11. 10.3390/ph11020052. [Epub ahead of print: 27 05 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang M, Sternberg MR, Pfeiffer CM. Harmonizing the calibrator and microorganism used in the folate microbiological assay increases the comparability of serum and whole-blood folate results in a CDC Round-Robin study. J Nutr 2018;148:807–17. 10.1093/jn/nxy030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulz KF, Altman DG, Moher D, et al. . Consort 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010;8:18. 10.1186/1741-7015-8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.