Abstract
Objective
The objective of this study was to determine the rate of perioperative SARS-CoV-2 infection among gynecologic cancer patients undergoing major surgery.
Methods
The database of the Turkish Ministry of Health was searched in order to identify all consecutive gynecologic cancer patients undergoing major surgery between March 11, 2020 and April 30, 2020 for this retrospective, nationwide, cohort study. The inclusion criteria were strictly founded on a final histopathological diagnosis of a malignant gynecologic tumor. COVID-19 cases were diagnosed by reverse transcriptase- polymerase chain reaction testing for SARS-CoV-2. The rate of perioperative SARS-CoV-2 infection and the 30-day mortality rate of COVID-19 patients were investigated.
Results
During the study period, 688 women with gynecologic cancer undergoing major surgery were identified nationwide. The median age of the patients was 59 years. Most of the surgeries were open (634/688, 92.2%). There were 410 (59.6%) women with endometrial cancer, 195 (28.3%) with ovarian cancer, 66 (9.6%) with cervical cancer, 14 (2.0%) with vulvar cancer and 3 (0.4%) with uterine sarcoma. The rate of SARS-CoV-2 infections confirmed within 7 days before or 30 days after surgery was 46/688 (6.7%). All but one woman was diagnosed postoperatively (45/46, 97.8%). The rates of intensive care unit admission and invasive mechanical ventilation were 4/46 (8.7%) and 2/46 (4.3%), respectively. The 30-day mortality rate was 0%.
Conclusion
In the COVID-19 era, gynecologic cancer surgery may be performed with an acceptable rate of perioperative SARS-CoV-2 infection if the staff and the patients strictly adhere to the established infection control measures.
Keywords: Coronavirus infection, Gynecologic neoplasms, Period, Perioperative, Severe acute respiratory syndrome, Surgery
Highlights
-
•
This is the first nationwide study describing the feasibility of major gynecologic cancer surgery during COVID-19 pandemic.
-
•
COVID-19 will be one of the most important concerns of health care systems for a long time.
-
•
Gynecologic cancer surgery may be performed with an acceptable rate of perioperative SARS-CoV-2 infection.
-
•
Improved surgical outcomes can be achieved by strictly adhering to restricted infectious triage protocols.
-
•
As the pandemic persists, the risk of disease progression associated with treatment delay will become a more critical issue.
1. Introduction
Coronavirus disease-2019 (COVID-19), caused by the “severe acute respiratory syndrome coronavirus-2” (SARS-CoV-2), has become a major threat for humanity, turning out to be a pandemic. As SARS-CoV-2 is a novel virus, the human population has got neither prior immunity nor a vaccine to fight against this contagious disease [1]. The elderly are the most disadvantageous group, as well as those with known comorbidities [2].
Patients with cancer who encountered SARS-CoV-2 have been reported to have a high risk of developing severe events such as intensive care unit (ICU) admission, invasive ventilation, or death when compared to the patients without cancer [3]. Besides, among cancer patients, a recent history of surgery or chemotherapy had a higher risk of severe events than those who did not receive treatment [3]. Similarly, a history of malignancy has been reported as the fourth most common risk factor of reaching adverse endpoints such as ICU admission, invasive ventilation, or death [4]. According to a recent study from the United States, the authors reported a case-fatality rate of 25% for patients with solid tumors and COVID-19 [5]. Additionally, malignant disease and major surgery have been recently reported to significantly increase the risk of 30-day mortality in patients undergoing surgery with perioperative SARS-CoV-2 infection [6]. However, these earlier data are mainly based on limited series with several confounding factors, and the data about cancer surgery and COVID-19 is still developing. Besides, the COVID-19 pandemic may worsen the outcome of patients with cancer indirectly by causing a significant delay in the diagnosis of cancer, resulting in an increase in the proportion of patients in advanced stage disease [7]. A recent systematic review suggests that prioritization and triage criteria should be determined for each country and institution in order to maintain patient safety and balance the risk between disease progression and viral exposure. [8]
Based on this information, addressing patients with cancer as a vulnerable population during the COVID-19 pandemic [3,4,6], several surgical societies [[8], [9], [10], [11], [12]] published guidelines in order to prioritize and triage the surgical procedures; most of them suggested withholding or postponing gynecologic cancer surgery. However, the American College of Surgeons (ACS) classified most gynecologic cancer cases as semi-urgent [13]; emphasizing significant delay could result in significant patient harm.
It has become difficult to provide timely care for gynecologic cancer patients in the COVID-19 era. The main issue seems to avoid loss of opportunity without placing the gynecologic cancer patients at an increased risk of SARS-CoV-2 infection [14]. However, Wainstein et al. [15] have stated that cancer surgery, in general, should not be delayed for most patients. It has also been suggested that definitive surgery may be done for women with gynecologic cancer if resources permit [16]. Although it is obvious that women with gynecologic cancer are at high risk for contracting SARS-CoV-2, it should be kept in mind that they are simultaneously at high risk for experiencing poor outcomes with a delay in cancer care [17]. Alternative approaches are recommended to be contemplated only in overstretched hospitals with restricted access to operating rooms [14].
The rate of developing SARS-CoV-2 infection was 6.1% among women undergoing gynecologic cancer surgery in a small case series from China [18]. The authors have suggested that women with gynecologic cancer are susceptible for COVID-19 and rapid deterioration [18]. Another small Chinese study [19] identified the lack of strict guidelines for the management of gynecologic cancer in an endemic region. It is obvious that the data to address the impact of SARS-CoV-2 infection on gynecologic cancer surgery are very limited in the literature. Additionally, the rate of perioperative SARS-CoV-2 infection among women undergoing gynecologic cancer surgery has not been delineated yet. To our knowledge, there are no solid data about the outcomes of operated gynecologic cancer patients who had contracted the novel coronavirus. The primary objective of this retrospective, nationwide, cohort study was to determine the rate of perioperative SARS-CoV-2 infection among gynecologic cancer patients undergoing major surgery. Our secondary aim was to determine the outcome of women those contracted perioperative SARS-CoV-2 and to address the risk factors for developing perioperative COVID-19 in this specific population.
2. Materials and methods
The database of the Turkish Ministry of Health was searched in order to identify all consecutive gynecologic cancer patients undergoing surgery between March 11, 2020 and April 30, 2020 for this retrospective, nationwide, cohort study. The inclusion criteria were strictly founded on a pathologic diagnosis of a malignant gynecologic tumor based on the final histopathological report. The study was approved by the Institutional Review Board of Ankara City Hospital (Date: April 27, 2020, Number: 004). Each woman included in the current study provided an informed consent at admission for her medical information to be used for research purposes.
The Bupa schedule of surgical procedures [20] was used for classifying the grade of surgery as minor (procedures described as minor and intermediate in the Bupa schedule) or major (procedures described as major and complex major in the Bupa schedule). Women who had undergone minor procedures and those with incomplete medical information were excluded. We also excluded women with a final pathologic diagnosis of endometrial intraepithelial neoplasia (EIN), atypical endometrial hyperplasia, and borderline ovarian tumor (BOT).
As COVID-19 testing was not widely available during the early and peak phases of the pandemic in Turkey, routine preoperative screening for SARS-CoV-2 infection was not mandatory during the study period. However, a reverse transcriptase-polymerase chain reaction (RT-PCR) testing was performed for preoperative women with suspicious symptoms, or in the case of an ascertained suspicious contact with a verified COVID-19 patient; as a necessity of national filiation (contact tracing) activities. For postoperative patients, fever lasting more than 3 days or fever accompanied by respiratory symptoms were the established indications for COVID-19 testing. COVID-19 cases were diagnosed by RT-PCR testing for SARS-CoV-2. For women discharged from the hospital, COVID-19 testing was performed for suspicious symptoms or a suspicious contact with a verified COVID-19 patient.
The demographic and clinical characteristics of the patients were abstracted from the electronic medical records of the Turkish Ministry of Health database. Comorbidities such as chronic obstructive lung disease (yes/no), diabetes mellitus (yes/no), ischemic heart disease (yes/no), chronic renal disease (yes/no), and chronic liver disease (yes/no) were noted. Women with ≥2, ≥3, and ≥4 comorbidities were classified into three separate groups for statistical analyses. The date of surgery, type of surgery (open or minimally invasive), type of gynecologic cancer diagnosed (endometrial, ovarian, cervical, vulvar, vaginal, uterine sarcoma, others), the surgical procedure applied (major or complex major), execution of lymphadenectomy (either retroperitoneal or inguinofemoral) (yes/no), anesthesia used (regional or general), and length of hospital stay (days) were recorded. The timing of COVID-19 diagnosis was recorded as preoperative or postoperative. For women with a postoperative COVID-19 diagnosis, the interval (days) between the date of surgery and COVID-19 diagnosis was recorded as the “time to SARS-CoV-2 infection.” Women with a postoperative positive test were also assessed whether they were diagnosed with COVID-19 within 14 days after hospital discharge or not.
The primary outcome measure for gynecologic cancer patients undergoing major surgery was the rate of SARS-CoV-2 infection confirmed within 7 days before or 30 days after surgery. The primary outcomes for women with COVID-19 were need for hospitalization, admission to ICU, the number of days in the ICU (for women who have recovered), utilization of invasive mechanical ventilation, and 30-day mortality (the day of surgery defined as day 0).
Statistical Package for Social Sciences (SPSS) version 23.0 (IBM Corp., Armonk, NY, USA) was used for performing all statistical analyses. Continuous variables were expressed in terms of medians and ranges, whereas binary variables were reported in terms of counts and percentages. Simple logistic regression analysis was performed in order to determine the correlation of patient characteristics with perioperative SARS-CoV-2 infection. Variables with a p value < 0.05 in univariate analysis were included in the multiple logistic regression analysis. The impact of each factor on perioperative SARS-CoV-2 infection was evaluated. A p-value < 0.05 was considered to indicate statistical significance.
3. Results
During the study period, 1166 women with gynecologic cancer undergoing surgery were identified nationwide. Of these women, 363 underwent minor surgery and were excluded. Among 803 women undergoing major surgery, there were 39 women with BOTs, 28 with EIN, and 45 with atypical endometrial hyperplasia. Those patients were excluded as well as three women with incomplete medical information. Therefore, 688 patients were included in the final analysis (Fig. 1 ). The demographic and clinical characteristics of gynecologic cancer patients undergoing major surgery in the COVID-19 era are shown in Table 1 .
Fig. 1.
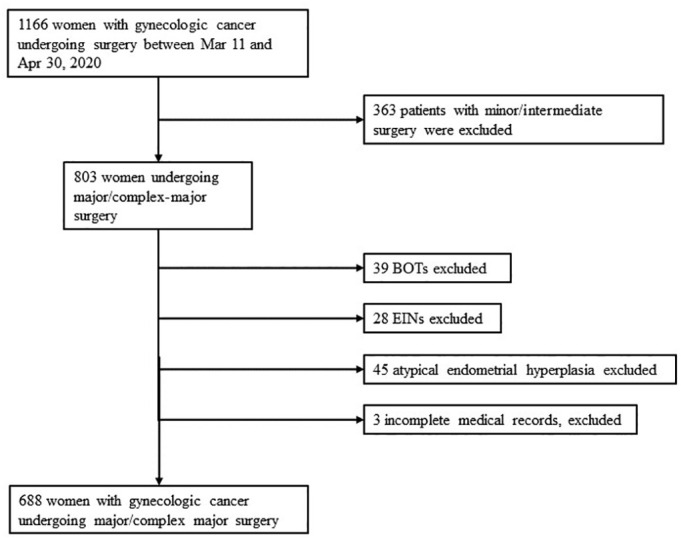
Flowchart of the study.
Table 1.
Clinical and demographic characteristics of the patients.
| Characteristic (n = 688) | n (%) |
|---|---|
| Age, median (range) | 59 (15–88) |
| <65 years | 470 (68.3%) |
| >65 years | 218 (31.7%) |
| Co-morbidity⁎ | |
| Yes | 476 (69.2%) |
| No | 212 (30.8%) |
| Co-morbidity types | |
| Hypertension | 427 (62.1%) |
| Diabetes Mellitus† | 261 (387.9%) |
| Chronic pulmonary disease‡ | 200 (29.1%) |
| Coronary heart disease | 87 (12.6%) |
| Chronic renal disease | 14 (2.0%) |
| Chronic liver disease | 1 (0.1%) |
| Severity of co-morbidities | |
| Presence of ≥2 co-morbidities | 325 (47.2%) |
| Presence of ≥3 co-morbidities | 128 (18.6%) |
| Presence of ≥4 co-morbidities | 26 (3.8%) |
| Type of surgeries | |
| Major surgery | 92 (13.4%) |
| Complex-major surgery | 596 (86.6%) |
| Type of anesthesia | |
| General anesthesia | 665 (96.7%) |
| Regional anesthesia | 23 (13.3%) |
| Surgical approach | |
| Open surgery | 634 (92.2%) |
| MIS | 50 (7.3%) |
| Conversion from MIS to open | 4 (0.6%) |
| Gynecologic cancer types | |
| Endometrial cancer | 410 (59.6%) |
| Ovarian cancer | 195 (28.3%) |
| Cervical cancer | 66 (9.6%) |
| Vulvar cancer | 14 (2.0%) |
| Uterine sarcoma | 3 (0.4%) |
| Lymphadenectomy | |
| Performed | 557 (81.0%) |
| Not performed | 131 (19.0%) |
Abbreviations: MIS, minimally invasive surgery.
Presence of at least one co-morbidity.
Need for insulin or oral anti diabetic medication,
Including chronic obstructive pulmonary disease, chronic asthma, chronic bronchitis, other chronic obstructive and restrictive pulmonary diseases.
The median age of the patients was 59 years (range: 15–88 years). With regard to comorbidities, there were 261 (37.1%) women with diabetes mellitus, 427 (62.1%) with hypertension, 200 (29.1%) with chronic obstructive lung disease, 87 (12.6%) with ischemic heart disease, 14 (2.0%) with chronic renal disease, and one (0.1%) with chronic liver disease. There were 325 (47.2%) women with at least two comorbidities.
Ninety-two (13.4%) women underwent major surgery, whereas 596 (86.6%) underwent complex major surgery. Most of the surgeries were open (634/688, 92.2%). There were 50 (7.3%) minimally invasive surgeries and four (0.6%) conversions from laparoscopy to laparotomy. Twenty-three (3.3%) women received regional anesthesia while general anesthesia was performed for 665 (96.7%) patients. Most of the patients (557/688, 81.0%) underwent lymph node dissection. There were 410 (59.6%) women with endometrial cancer, 195 (28.3%) with ovarian cancer, 66 (9.6%) with cervical cancer, 14 (2.0%) with vulvar cancer and 3 (0.4%) with uterine sarcoma.
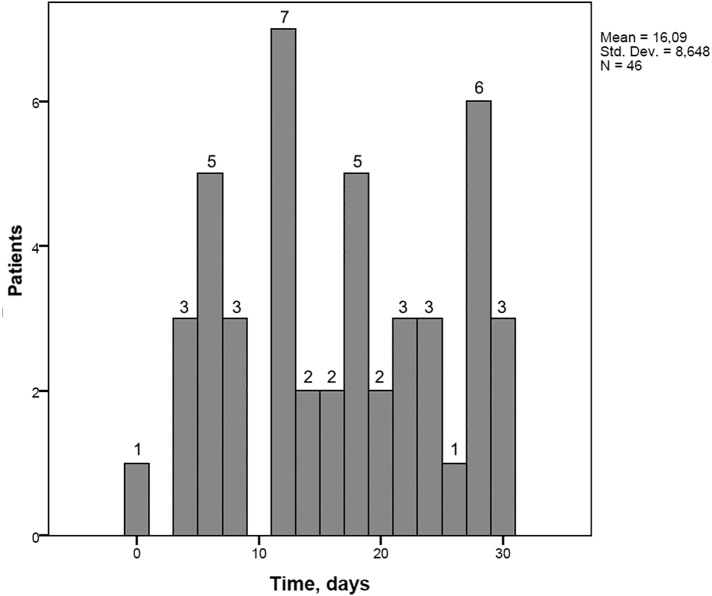
The number of SARS-CoV-2 infections confirmed within 7 days before or 30 days after surgery was 46 (6.7%). All but one woman was diagnosed postoperatively (45/46, 97.8%). Four (8.7%) women were diagnosed to have SARS-CoV-2 infection during hospitalization. Of those, one (25.0%) woman was diagnosed on the operation day (but preoperatively), whereas the remaining three (75.0%) were diagnosed in the postoperative setting; on postoperative day 3, 4, and 5; respectively. Forty-two (91.3%) women had COVID-19 after they had been discharged from the hospital. The median time to SARS-COV-2 infection was 8.0 days (range, 2–28) for women who were diagnosed after hospital discharge. Of those, 10 (23.8%) were diagnosed >14 days later after discharge, whereas 32 (76.2%) were diagnosed to have SARS-CoV-2 infection within 14 days after hospital discharge. For 45 women with a COVID-19 diagnosis in the postoperative period, the median time to detection of SARS-CoV-2 infection was 16.5 days (range, 3–30). The frequency of COVID-19 diagnosis with regard to the day of surgery among women undergoing major gynecologic cancer surgery in the COVID-19 era is shown in Fig. 2 .
Fig. 2.
Frequency of COVID-19 diagnosis regarding the day of surgery among women undergoing major gynecologic cancer surgery in the COVID-19 era.
Excluding those who were diagnosed with COVID-19 during hospitalization (n = 4), eight (19.1%) women needed hospitalization because of COVID-19 whereas 34 (80.9%) were treated at home self-isolated. The median length of hospital stay was 6.5 days (range 2–21 days) for women contracting SARS-CoV-2 perioperatively. There were four (33.3%) ICU admissions among 12 women treated at hospital. The median length of ICU stay was 3.0 days (range, 1–11 days). Two (2/12, 16.6%) needed invasive mechanical ventilation. Of those in need of invasive mechanic ventilation, one of them stayed in ICU for 5 days, whereas the other stayed in the ICU for 11 days. The 30-day mortality was 0% among women who had got perioperative SARS-CoV-2 infection. Among all women with SARS-CoV-2 infection, the rates of ICU admission and invasive mechanical ventilation were 4/46 (8.7%) and 2/46 (4.3%), respectively. The demographic and clinical characteristics of women gynecologic cancer patients undergoing major surgery with perioperative SARS-CoV-2 infection are demonstrated in Table 2 .
Table 2.
Demographic and clinical characteristics of women undergoing gynecologic cancer surgery with perioperative SARS-CoV-2 infection.
| n (%) | |
|---|---|
| Number of patients with perioperative COVID-19 infection | 46 (6.7%) |
| COVID-19 infection surveillance | |
| Outpatient | 34 (73.9%) |
| Inpatient | 12 (26.1%) |
| Hospital stay duration, median (range) | 6.5 days (1−21) |
| ICU admission⁎ | 12 (33.3%) |
| Need for mechanic ventilation† | 2 (16.6%) |
| ICU stay duration, median (range) | 3.0 days (1−11) |
| Timing of diagnosis | |
| Preoperative | 1 (2.2%) |
| Postoperative | 45 (97.8%) |
| Location of diagnosis | |
| During hospitalization | 4 (8.7%) |
| After discharge | 42 (91.3%) |
| Time to SARS-CoV-2 infection after the operation, median (range) | 16.5 (3−30) days |
| Time to SARS-COV-2 infection after discharge, median (range) | 8.0 (2–28) |
| Diagnosed >14 days later after discharge | 10 (23.8%) |
| Diagnosed ≤14 days later after discharge | 32 (76.2%) |
Abbreviations: ICU, intensive care unit.
Among hospitalized patients.
Among ICU patients.
However, there were 3 (0.43%) deaths in the early-postoperative period among all gynecologic cancer patients who had undergone major surgery. None of those were associated with COVID-19. Two of them were due to intraabdominal hemorrhage requiring relaparotomy, whereas one patient was dead in the early postoperative period because of pulmonary thromboembolism.
In univariate analysis, we were not able to define any correlation between perioperative SARS-CoV-2 infection and the factors those were investigated such as age, comorbidities, type of surgery, type of gynecologic cancer diagnosed, the surgical procedure applied (major or complex major), execution of lymphadenectomy, type of anesthesia used and length of hospital stay (Table 3 ). Therefore, a multivariate analysis was not performed.
Table 3.
Univariate analyses of possible co-factors related to COVID-19 infection.
| Characteristic (n = 688) | COVID-19 (−) | COVID-19 (+) | p |
|---|---|---|---|
| Age, years, median (range) | 59 (15–88) | 57.5 (21–81) | 0.251 |
| Age, years | 642 (100%) | 46 (100%) | 0.398 |
| <65 | 436 (67.9%) | 34 (73.9%) | |
| ≥65 | 206 (32.1%) | 12 (26.1%) | |
| Co-morbidity | 642 (100%) | 46 (100%) | 0.698 |
| Absent | 199 (93.9%) | 13 (6.1%) | |
| Present | 443 (93.1%) | 33 (6.9%) | |
| Co-morbidity types | 642 (100%) | 46 (100%) | |
| Hypertension | 398 (62.0%) | 29 (63.0%) | 0.887 |
| Diabetes Mellitus⁎ | 245 (38.1%) | 16 (34.7%) | 0.648 |
| Chronic pulmonary disease† | 189 (29.4%) | 11 (23.9%) | 0.425 |
| Coronary heart disease | 81 (12.6%) | 6 (13.0%) | 0.993 |
| Chronic renal disease | 13 (2.0%) | 1 (2.1%) | 0.945 |
| Chronic liver disease | 1 (0.1%) | 0 (0%) | 0.789 |
| Severity of co-morbidities | 642 (100%) | 46 (100%) | |
| Presence of ≥2 co-morbidities | 304 (47.3%) | 21 (45.6%) | 0.823 |
| Presence of ≥3 co-morbidities | 121 (18.8%) | 7 (15.2%) | 0.541 |
| Presence of ≥4 co-morbidities | 25 (3.7%) | 1 (2.1%) | 0.555 |
| Type of surgeries | 642 (100%) | 46 (100%) | 0.703 |
| Major surgery | 85 (13.2%) | 7 (15.2%) | |
| Complex-major surgery | 557 (86.8%) | 39 (84.8%) | |
| Type of anesthesia | 642 (100%) | 46 (100%) | |
| General anesthesia | 621 (96.7%) | 44 | |
| Regional anesthesia | 21 (3.3%) | 2 | |
| Surgical approach | 642 (100%) | 46 (100%) | 0.628 |
| Open surgery | 590 (91.9%) | 44 (95.6%) | |
| MIS | 48 (7.4%) | 2 (4.4%) | |
| Conversion from MIS to open | 4 (0.6%) | 0 (0%) | |
| Lymphadenectomy | 642 (100%) | 46 (100%) | 0.786 |
| Performed | 520 | 37 | |
| Not performed | 122 | 9 | |
| Length of hospital stay, days, median (range) | 5 (1–37) | 5 (1–28) | 0.177 |
| Length of hospital stay | 642 (100%) | 46 (100%) | 0.299 |
| <5 days | 302 (47.0%) | 18 (39.1%) | |
| ≥5 days | 340 (53.0%) | 28 (60.9%) | |
Abbreviations: MIS, minimally invasive surgery.
Need for insulin or oral anti diabetic medication,
Including chronic obstructive pulmonary disease, chronic asthma, chronic bronchitis, other chronic obstructive and restrictive pulmonary diseases.
4. Discussion
To our knowledge, this is the first nationwide, retrospective, cohort study describing the clinical characteristics and short-term outcomes of gynecologic cancer patients undergoing major surgery in the COVID-19 era. The principal findings of our study indicate that 6.7% of gynecologic cancer patients undergoing major surgery developed perioperative SARS-CoV-2 infection. Among women with perioperative SARS-CoV-2 infection, the rates of ICU admission and invasive mechanical ventilation were 8.7% and 4.3%, respectively. The 30-day mortality was 0% among gynecologic cancer patients who had perioperative SARS-CoV-2 infection.
The preparedness of the national and local healthcare system is crucial for fighting against a pandemic. It was reported that gynecologic cancer cases with strong personal protection measures may be candidates for surgery in the COVID-19 era if appropriate precautions such as social distancing, facemask, and self-isolation are taken [16]. In Turkey, a national policy of precautions was strictly adhered nationwide according to the declarations of the Turkish Ministry of Health throughout the study period. These precautions were prohibition of attendees at an in-patient setting, prohibition of visitors at all hospitals, mandatory facemask for all staff and patients, social distancing and never more than one patient in a hospital room. As a national health policy, flexible working schedules for the staff were organized at each hospital, and every surgical team worked with the minimum number of persons in order to prevent the potential for spread to other staff and patients. Additionally, preoperative self-isolation was recommended routinely for all scheduled surgeries as a strategy to reduce the probability of surgical intervention during the incubation period of the novel coronavirus.
Withholding or postponing cancer treatment during the COVID-19 pandemic was mainly founded on the Liang study [3], reporting an increased risk of adverse events in patients who received chemotherapy or underwent recent surgery prior to SARS-CoV-2 infection. These data should be met cautiously since there are four main pitfalls associated with that study. First, the sample size was small; there were only 18 patients with cancer. Second, most of the patients had either lung cancer or hematological malignancies. Third, only four patients had a disease that is actively treated, whereas 12 were in complete remission. Fourth, patients with cancer had a significantly older median age compared to their controls as well as a more significant history of smoking. Finally, there was no case of gynecologic cancer in that cohort. It is obvious that the reported susceptibility and poor outcome of cancer patients are limited by the small number of patients and lack of generalizability. Therefore, those data are obviously insufficient to make a specific recommendation.
The safety of performing surgery in SARS-CoV-2-exposed hospitals has to be determined [6] as potential exposure of COVID-19 is a major threat for cancer patients; that could result in mortality [21]. Admission to the hospital has been reported as an independent risk factor to acquire SARS-CoV-2 infection in two retrospective studies [22,23]. The authors emphasized that most of the cancer patients with SARS-CoV-2 infection received in-hospital treatments such as surgery, radiotherapy or chemotherapy, whereas the remaining had a hospital-associated transmission [22,23]. In the current study, we were not able to demonstrate an association between the length of hospital stay and perioperative SARS-CoV-2 infection in gynecologic cancer patients undergoing major surgery (Table 3). However, of women with postoperative SARS-CoV-2 infection after hospital discharge, 76.2% were diagnosed within 14 days after discharge from the hospital. This finding implies that most of the postoperative SARS-CoV-2 infections in our cohort were hospital-acquired as the incubation periods of COVID-19 is approximately two weeks [24]. Nevertheless, 23.8% of the postoperative SARS-CoV-2 infections seem to be community-acquired as they were diagnosed >14 days later after hospital discharge. It is clear that all gynecologic cancer patients as well as all gynecologic cancer surgeons are worried about the risk of hospital-acquired COVID-19 [21]. However, as the pandemic continues to persist; the risk of disease progression associated with treatment delay will become a more critical issue [21].
The data associated with gynecologic cancer surgery during the COVID-19 pandemic are very limited in the literature [18,19]. Yang et al. [18] reported the rate of developing SARS-CoV-2 infection among women undergoing gynecologic cancer surgery as 6.1%. However, this was a small case series with a total of 33 women undergoing gynecologic cancer surgery of which two were found to contract SARS-CoV-2. In the current study, we found out the rate of perioperative SARS-CoV-2 infection as 6.7% among 688 gynecologic cancer patients undergoing major surgery. In a recent study investigating the outcomes of patients with gynecologic cancer at three affiliated New York City hospitals, 57 women with symptoms related to COVID-19 among 302 gynecologic cancer patients were tested and 19 of them (6.3%) had positive COVID-19 test [5]. Our finding of 6.7% is in accordance with 6.1% of Yang et al. [18] and 6.3% of Frey et al. [5]. It should be emphasized that preoperative COVID testing was not routinely performed during the period of the current study. Considering the high prevalence of COVID-19 among asymptomatic cases [25] as well as the false-negative rate of RT-PCR around 30%, we might have presented the rate of perioperative SARS-CoV-2 infection lower than its actual rate. On the other hand, the total number of COVID-19 testing was 1,033,617 whereas the number of SARS-CoV-2 infected individuals was 120,204 nationwide during the study period [26]; leading to a rate of 11.6% of SARS-CoV-2 infection among all people who had been tested in our country. The established indications for COVID-19 testing were the same for all Turkish citizens and gynecologic cancer patients undergoing major surgery during the study period. As mentioned above, those indications were individuals with suspicious symptoms, or an ascertained suspicious contact with a verified COVID-19 patient. The rate of SARS-CoV-2 infection was 11.6% among all people who had been tested throughout the country, whereas the corresponding figure was 6.7% among all gynecologic cancer patients undergoing major surgery during the same period. This finding can be attributed to the predisposition of the novel coronavirus to infect males. Nevertheless, gynecologic cancer patients undergoing major surgery in Turkey seem to be at relatively low risk of contracting SARS-CoV-2 compared to the general population. We would like to notify that routine preoperative screening for COVID-19 has been mandatory in Turkey since June 1, 2020. Routine preoperative COVID-19 testing with RT-PCR for SARS-CoV-2 is currently recommended to screen all potential surgical candidates globally [16].
The outcome of women with perioperative SARS-CoV-2 infection was perfect in the current study with a 30-day mortality rate of 0%. The rates of ICU admission and mechanical invasive ventilation among women with perioperative SARS-CoV-2 infection were 8.7% and 4.3%, respectively. Those rates seem to be acceptable when the total number of gynecologic cancer patients undergoing surgery (n = 688) in this series is considered.
Dai et al. [27] reported no difference between the cancerous and noncancerous populations in terms of COVID-19 related death rate or severity of COVID-19 if the cancer is diagnosed in early-stage with no metastases. Based on this information, it is plausible to speculate that operable gynecologic cancer patients with no distant metastases seem to experience similar outcomes to noncancerous population in terms of COVID-19 related adverse events. However, it should be reminded that surgery and invasive mechanical ventilation may result in immunosuppressive responses and increased pro-inflammatory cytokines leading to vulnerability and increased risk of SARS-CoV-2 exposure at the hospital [28,29]. However, improved surgical outcomes can be achieved by strictly adhering to restricted infectious triage protocols in addition to comprehensively applied preventive measures as self-isolation in the preoperative setting [15]. Abdelrahman et al. [30] reported that preoperative self-isolation provides an opportunity to evaluate the silent phase of SARS-CoV-2 infection when the patients are asymptomatic but contagious. In the current study, preoperative self-isolation was recommended routinely for all scheduled gynecologic cancer surgeries throughout the study period. Chen and Li [31] have recently reported the transmission capacity to be controllable under strict control measures for gynecologic cancer patients. The relatively low prevalence of perioperative SARS-CoV-2 infection as well as the perfect outcome of women who contracted SARS-CoV-2 in our study highlights the importance of adherence to the established infection control measures as well as the importance of preoperative self-isolation.
The reader should pay attention to some limitations of the current study including its retrospective nature based on an official database. For example, we could not present the number of preoperative low-dose chest computed tomography scans because of missing data although this practice is common in our country. However, we consider that our study contributes to the limited body of knowledge on this topic with many gynecologic cancer patients undergoing major surgery in the COVID-19 era.
We could not perform routine screening for SARS-CoV-2 infection as a part of preoperative work-up during the early phase of the COVID-19 pandemic; only women with suspicious symptoms or those with suspicious contact with an individual diagnosed with SARS-CoV-2 infection were tested. This might have led under-estimation of the real number of positive women for SARS-CoV-2 infection. However, the Turkish Ministry of Health first announced the daily test number applied nationwide and the number of positive tests on March 27 with 2069 positives out of 7533 tests, which led to a 27.4% positive rate among people with suspicious symptoms or suspicious contact. During the following days, the daily test number gradually increased and on the last day of our study, 30th April 2020, 2615 new patients were detected out of 42,004 tests indicating a prevalence of 6.2% [32]. That's why we assume the prevalence of 6.7% in our cohort as acceptable compared to the national results during the same time period.
COVID-19 pandemic is a unique dynamic process. Many societies [[9], [10], [11], [12], [13], [14]] declared recommendations, particularly on how to manage surgical cases. However, most of these recommendations were expert opinions without any solid evidence. A significant delay in primary surgery may result in upstaging the disease as well as increasing the burden of advanced and inoperable cases in the gynecologic cancer centers [16]. Vaccines for SARS-CoV-2 and effective therapies are not expected to be developed in 2020 [25]. All gynecologic cancer surgeons should face the reality of learning how to navigate COVID-19 safely. Nevertheless, we should try to provide the best possible treatment for gynecologic cancer patients as long as the national and local health sources permit.
According to the findings of this retrospective, nationwide, cohort study, we conclude that gynecologic cancer surgery may be performed in the COVID-19 era with an acceptable rate of perioperative SARS-CoV-2 infection if the staff and the patients strictly adhere to the established infection control measures. The gynecologic cancer surgeons should pay attention to the available sources, area census of COVID-19 cases, and COVID-19 associated risks for each patient before making the decision to perform surgery.
Declaration of Competing Interest
Authors declare that there is neither financial nor academic support of relationships that may pose potential conflict of interest.
Acknowledgements
None.
References
- 1.Clinical Management of Severe Acute Respiratory Infection (SARI) when COVID-19 Disease is Suspected. c2020 [cited 2020 30.06.2020] 2020. https://apps.who.int/iris/rest/bitstreams/1278777/retrieve Available from.
- 2.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang W., Guan W., Chen R., Wang W., Li J., Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W.J., Liang W.H., Zhao Y., Liang H.R., Chen Z.S., Li Y.M. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frey M.K., Fowlkes R.K., Badiner N.M., Fishman D., Kanis M., Thomas C. Gynecologic oncology care during the COVID-19 pandemic at three affiliated New York City hospitals. Gynecol. Oncol. 2020;159:470–475. doi: 10.1016/j.ygyno.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collaborative C.O. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrag D., Hershman D.L., Basch E. Oncology practice during the COVID-19 pandemic. JAMA. 2020;323:2005–2006. doi: 10.1001/jama.2020.6236. [DOI] [PubMed] [Google Scholar]
- 8.Moletta L., Pierobon E.S., Capovilla G., Costantini M., Salvador R., Merigliano S. International guidelines and recommendations for surgery during Covid-19 pandemic: a systematic review. Int. J. Surg. 2020;79:180–188. doi: 10.1016/j.ijsu.2020.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramirez P.T., Chiva L., Eriksson A.G.Z., Frumovitz M., Fagotti A., Gonzalez Martin A. COVID-19 global pandemic: options for management of gynecologic cancers. Int. J. Gynecol. Cancer. 2020;30:561–563. doi: 10.1136/ijgc-2020-001419. [DOI] [PubMed] [Google Scholar]
- 10.COVID-19 Resources for Health Care Practitioners. c2020 [cited 2020 30.06.2020] 2020. https://www.sgo.org/practice-management/covid-19/ Available from.
- 11.Joint Statement on Minimally Invasive Gynecologic Surgery During the COVID-19 Pandemic c2020 [cited 2020 30.06.2020] https://www.aagl.org/news/covid-19-joint-statement-on-minimally-invasive-gynecologic-surgery/ Available from. [DOI] [PMC free article] [PubMed]
- 12.Kimmig R., Verheijen R.H.M., Rudnicki M. Robot assisted surgery during the COVID-19 pandemic, especially for gynecological cancer: a statement of the Society of European Robotic Gynaecological Surgery (SERGS) J. Gynecol. Oncol. 2020;31 doi: 10.3802/jgo.2020.31.e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.COVID-19 Guidelines for Triage of Gynecology Patients. c2020 [cited 2020 30.06.2020] 2020. https://www.facs.org/covid-19/clinical-guidance/elective-case/gynecology Available from.
- 14.Lavoue V., Akladios C., Gladieff L., Classe J.M., Lecuru F., Collinet P. Onco-gynecologic surgery in the COVID-19 era: risks and precautions-a position paper from FRANCOGYN, SCGP, SFCO, and SFOG. J. Gynecol. Obstet. Hum. Reprod. 2020;49 doi: 10.1016/j.jogoh.2020.101787. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wainstein A.J.A., Drummond-Lage A.P., Ribeiro R., de Castro Ribeiro H.S., Pinheiro R.N., Baiocchi G. Risks of COVID-19 for surgical cancer patients: The importance of the informed consent process. J. Surg. Oncol. 2020 Jun doi: 10.1002/jso.26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatla N.S.S. The COVID-19 pandemic and implications for gynaecologic cancer care. Indian J. Gynecol. Oncolog. 2020;18:1. doi: 10.1007/s40944-020-00395-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weber LeBrun E.E., Moawad N.S., Rosenberg E.I., Morey T.E., Davies L., Collins W.O. Coronavirus disease 2019 pandemic: staged management of surgical services for gynecology and obstetrics. Am. J. Obstet. Gynecol. 2020;223:85 e1–85 e19. doi: 10.1016/j.ajog.2020.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang S., Zhang Y., Cai J., Wang Z. Clinical characteristics of COVID-19 after gynecologic oncology surgery in three women: a retrospective review of medical records. Oncologist. 2020;25:e982–e985. doi: 10.1634/theoncologist.2020-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J., Peng P., Li X., Zha Y.F., Xiang Y., Zhang G.N. Management strategies for three patients with gynecological malignancies during the outbreak of COVID-19. Zhonghua Fu Chan Ke Za Zhi. 2020;55:221–226. doi: 10.3760/cma.j.cn112141-20200302-00168. [DOI] [PubMed] [Google Scholar]
- 20.Bupa Schedule of procedures. c2020 [cited 2020 30.06.2020] 2020. https://codes.bupa.co.uk/procedures Available from.
- 21.Moujaess E., Kourie H.R., Ghosn M. Cancer patients and research during COVID-19 pandemic: a systematic review of current evidence. Crit. Rev. Oncol. Hematol. 2020;150:102972. doi: 10.1016/j.critrevonc.2020.102972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu J., Ouyang W., Chua M.L.K., Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6:1108–1110. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020;31:894–901. doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fader A.N., Huh W.K., Kesterson J., Pothuri B., Wethington S., Wright J.D. When to operate, hesitate and reintegrate: Society of Gynecologic Oncology Surgical Considerations during the COVID-19 pandemic. Gynecol. Oncol. 2020;158:236–243. doi: 10.1016/j.ygyno.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.TC Sağlık Bakanlığı Halk Sağlığı Genel Müdürlüğü. c2020 [cited 2020 30.06.2020] 2020. https://hsgm.saglik.gov.tr/tr/ Available from.
- 27.Dai M., Liu D., Liu M., Zhou F., Li G., Chen Z. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kirmeier E., Eriksson L.I., Lewald H., Jonsson Fagerlund M., Hoeft A., Hollmann M. Post-anaesthesia pulmonary complications after use of muscle relaxants (POPULAR): a multicentre, prospective observational study. Lancet Respir. Med. 2019;7:129–140. doi: 10.1016/S2213-2600(18)30294-7. [DOI] [PubMed] [Google Scholar]
- 30.Welsh Surgical Research Initiative C Surgery during the COVID-19 pandemic: operating room suggestions from an international Delphi process. Br. J. Surg. 2020;107:1450–1458. doi: 10.1002/bjs.11747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y., Li G. Gynecological malignancies with asymptomatic SARS-CoV-2 infection during the convalescence of outbreak. Gynecol. Oncol. 2020;158:44–46. doi: 10.1016/j.ygyno.2020.04.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.T.C. Sağlık Bakanlığı COVID-19 Bilgilendirme Sayfası. c2020 [cited 2020 28.10.2020] 2020. https://covid19.saglik.gov.tr/TR-66935/genel-koronavirus-tablosu.html Available from.