ABSTRACT
The transcription factor SOX2 is a well-established and important stem cell marker. Its role in cancer biology remains unclear, but it has been proposed to also be a marker of cancer stem cells. We investigated the role of SOX2 protein expression in women with high-grade serous ovarian cancer (HGSOC) to determine its potential prognostic and treatment predictive value. We constructed a tissue microarray of 130 advanced stage HGSOC tumors with an average of 6 cores each, stained for SOX2 protein expression and evaluated survival outcomes. We also treated two HGSOC cell lines with carboplatin and paclitaxel and measured SOX2 expression by RT-PCR and immunoblotting at different doses and time-points. Among patients with non-radical debulking surgery overall and progression-free survival were shorter for patients with SOX2 positive tumors (mean 26 vs. 39 months, log-rank test: p = .0076, and mean 14 vs. 19 months, p = .055, respectively). Knockdown of SOX2 in cell lines did not affect growth inhibition following chemotherapy treatment. Our results show that SOX2 has a strong prognostic potential among HGSOC patients with residual tumor tissue after debulking surgery and suggest that SOX2 expressing cells remaining after non-radical debulking surgery may constitute a subpopulation of cancer stem cells with greater tumor-initiating potential.
KEYWORDS: Cancer stem cells, SOX2, high-grade serous ovarian cancer, survival, radical surgery
Introduction
High-grade serous ovarian carcinoma (HGSOC) is an aggressive form of epithelial ovarian cancer, which is the most common type of ovarian cancer. It mainly metastasizes locally and is generally not diagnosed until late stages, with wide-spread disease1,2 Most patients receive debulking surgery with as much as possible of the tumor removed,3 but given the fashion in which the tumor and metastases grow, with tissue often spread over a large area of the peritoneal lineage, full resection is often not achieved.3 Even with post-operative chemotherapy tumor tissue often remains, consisting of cells potentially capable of initiating new tumor bulks. Women with residual tumor tissue after surgery have a poor prognosis, while patients with no macroscopic tissue remaining have a significant survival benefit.1,4 Surgery is generally followed by platinum-based chemotherapy, and despite most women initially responding to the treatment, as HGSOC tumors are often susceptible to DNA damaging agents,5 most will eventually develop resistance, and suffer relapse/s. The relative 5-year survival rate is <50%.6 HGSOC is most likely derived from transformed secretory cells originating from the fallopian tube epithelium,7–9 which spread to the ovary at an early stage in cancer development. TP53 mutations constitute a precursor event which occurs in the fallopian tube epithelium years before the cancer establishes. Cells harboring this mutation may later develop into serous tubal intraepithelial carcinoma (STIC) lesions. Already in these STICs, several cancer specific alterations have been found, including mutations in TP53, BRCA1, BRCA2 and PTEN.8 In recent years, there has been growing interest in a group of cells referred to as cancer stem cells (CSC). These cells are thought to be of the same origin as the rest of the tumor cells, but they express genes associated with stem cell-ness and have developed certain stem cell-like characteristics. These cells may potentially have an increased ability to initiate metastasis and also cause relapse, if still viable after treatment.10,11
The study of CSCs could potentially answer questions concerning tumor aggressiveness and the occurrence of relapse. One of the key gene regulators in stem cells is the transcription factor SOX2.12,13 This gene belongs to the SOXB1 group of the SOX family. The SOX proteins are known to be involved in the regulation of embryonic development and stem cell maintenance, and inactivating mutations are known to cause certain developmental diseases; most well-known is the occurrence of anophthalmia. SOX2 has been described as one of the key factors needed to transform a fully differentiated cell into a pluripotent state.12 Considering its importance in stem cells SOX2 has also been studied in cancer to determine its potential role in tumor initiation and maintenance. Studies have detected increased levels of SOX2 and other stem cell markers in premalignant fallopian tube tissue,9,14,15 which could indicate that they may be involved in the initial steps of HGSOC development.
Studies of castration-resistant prostate cancer cell lines have shown that the combination of mutated RB1 and p53 lead to a marked increase in SOX2 expression, and SOX2 expression in turn has been linked to increased lineage plasticity.16,17 These mutations are also common in HGSOC and perhaps mutations in other DNA repair genes could serve as triggers for SOX2 expression. If SOX2 in turn can induce lineage plasticity it may be responsible for the development of eventual drug resistance and relapse.
SOX2 gene amplification and/or SOX2 upregulation have been found in a range of different cancer types, including small cell lung cancer,18 lung squamous cell carcinoma (SCC),19 esophageal SCC,20 rectal cancer21 and glioblastoma.22 It has also been shown to predict survival in lung adenocarcinoma,23 with high expression associated with poor prognosis. SOX2 has been found to be upregulated in cutaneous SCC and has even been proven crucial for cancer maintenance,24 tumor initiation, angiogenesis and tumor growth in established tumors.25
In epithelial ovarian cancers, the expression of SOX2 and other stem cell markers has been found to be more common in high-grade cancers compared to normal ovarian tissue, borderline tumors and low-grade serous carcinomas.26–28 Indumathi et al. found that SOX2 expression was present in the normal fallopian tube of healthy women, with a mean of 8.6% SOX2 positive cells.29 It has also been reported that high SOX2 expression is nearly ubiquitous in the normal-appearing fallopian tube epithelium of HGSOC patients, as opposed to the fallopian tubes of benign cases where it is less frequent, indicating that SOX2 could be a potential premalignant marker for high-grade serous tubal-ovarian carcinoma.9,14 SOX2 has also been found to increase migration and invasion in ovarian cancer cell lines,30,31 and in vivo studies have revealed a large increase in tumor size when injecting mice with SOX2 overexpressing HGSOC cells compared to control.11 A few studies have addressed the relation of SOX2 to treatment response and survival, but the results have been varying and somewhat inconclusive.30,32,33
In this study, our main focus was to investigate the prognostic value of SOX2 expression in relation to survival and relapse in women diagnosed with HGSOC. We also investigated its connection to chemotherapy treatment by a series of in vitro experiments.
Materials and methods
Patients
Ethical approval for this study was granted by the Ethics Committee at Lund University, Sweden, waiving the requirement for informed consent. A total of 156 consecutive cases of HGSOC were selected at the Gynecology Department in the southern Swedish healthcare region between 2011–2015, a cohort previously described.34 All cases were reviewed by a gynecologic pathologist (SWF) according to the World Health Organization Classification 201435 and staged according to the International Federation of Gynecology and Obstetrics,36 with 141 cases remaining after evaluation. For this study only patients with advanced stage HGSOC (III–IV) were included (n = 130, Figure 1). All but five patients underwent primary cytoreductive surgery while the remaining patients underwent either bowel obstruction surgery or had only biopsies taken. These 5 patients were excluded from the survival analyses (patients remaining n = 125). Platinum-based chemotherapy combinations were administered post-surgery to all but four of the 130 patients.
Figure 1.

Prisma chart of the inclusion process
Tissue microarray (TMA) construction and immunohistochemistry
The TMA was constructed from the 141 cases of tubal, ovarian, and peritoneal HGSOC described above. Viable tumor areas from multiple sites were selected from formalin-fixed paraffin-embedded tissue blocks. For cases where it was possible four cores from the primary site, two cores from lymph node metastases and 2 or 4 cores from peritoneal metastases were selected. Thus, between 3–10 (median 6) 1 mm core needle biopsies from varying sites were available from each patient in our cohort. Metastatic tissue was defined as tissue collected from any anatomic location in the abdomen except for ovary and fallopian tube, which instead was defined as primary tumor tissue. Sections, 3–4 mm in thickness, were deparaffinized, rehydrated, and stained with SOX2 antibody D6D9 (Cell Signaling, Cat. Nr: # 3579S) using the DAKO envision flex system (Agilent, Cat. Nr: K8010). The sections were incubated with the primary antibody (1:150 dilution) for 30 minutes at room temperature after antigen retrieval at pH 9. Samples of appendix, pancreas and fallopian tube were used as control tissue. The number of cancer cells with nuclear SOX2 staining was assessed by two blinded, independent examiners (MB and AE). Each core was scored into one of four categories: 0% stained cells, ≤10%, 11–50% or >50% (Figure 2, 10x magnification). Patients were then grouped according to the core with the highest score. In the subsequent analyses, patients were grouped as either SOX2+ or SOX2-, and a cutoff of >0% stained cells in the core with the highest score was used.
Figure 2.

Representative images of SOX2 protein expression levels
(A) 0% (SOX2-), (B) ≤10% (SOX2+), (C) 11–50% (SOX2+), (D) >50% (SOX2+).
Cell lines
The HGSOC cell lines OVCAR3 (ATCC, Cat. Nr; HTB-161) and COV362 (ECACC, Cat. Nr; 07071910) were cultured under standard conditions; OVCAR3 in RPMI-1640 medium supplemented with 20% FBS, 0.01 mg/mL insulin and 1% Penicillin-Streptomycin (P/S) and COV362 in DMEM supplemented with 10% FBS and 1% P/S.
mRNA and protein analyses
Cells were seeded at 30,000 cells/cm2, treated with a range of carboplatin or paclitaxel doses, and incubated for 1, 3 or 5 days for carboplatin and 1 or 3 days for paclitaxel. For mRNA analyses cells were lysed and RNA extracted using the RNeasy mini kit (QIAGEN, Cat. Nr: 74104). Reverse transcriptase reactions were performed using High Capacity cDNA Reverse Transcription Kits (Life Technologies, Cat. Nr: 4368814), and for the qPCR reaction SYBR green master mix (Life Technologies, Cat. Nr: 4309155) was used. For protein analyses cells were lysed and protein content was measured by the Bradford protein assay (Thermo Fisher Scientific, Cat. Nr: 23200). Samples were separated on TGX stain-free gels (Bio-Rad Laboratories, Cat. Nr: 4568083) and Western blotting was performed using SOX2 antibody D6D9 (Cell Signaling Cat. Nr; #3679), with ß-tubulin (Cell Signaling Cat. Nr; #2128) as loading control. Protein levels were quantified using Image LabTM 6.0 software (Bio-Rad Laboratories) and normalized to total protein content quantified from the stain-free blot images.
siRNA knockdown
To determine the effect of SOX2 down-regulation on chemotherapy response, cell lines were transfected with SOX2 siRNA SMARTpool (GE Healthcare, Cat. Nr: L-011778-00-0005) using reverse transfection with DharmaFECT reagent 1 (GE Healthcare, Cat. Nr: T-2001-02). Concentrations of siRNA and transfection reagent for both cell lines were determined based on the manufacturer’s recommendation for OVCAR3 cells. Growth inhibition following treatment was assessed in a 96-well format. The cells were treated with a range of carboplatin or paclitaxel doses (Selleckchem, Cat. Nr: S1215 & S1150) and proliferation was assessed after 7 days using the SRB assay (Sigma Aldrich, Cat. Nr: S1402). Confirmation of SOX2 knock-down on the protein level was performed by Western blotting, as described earlier.
Statistical analyses
Statistical models used to investigate correlations between SOX2 expression and age at diagnosis, surgical outcome, stage, treatment outcome and drug regimen used are specified in Table 1. The log-rank test was used to analyze the effect of SOX2 on overall survival (OS) and progression free survival (PFS) in the whole cohort as well as on a subgroup level. Cox regression models were used for both univariable and multivariable analyses, the latter of which included an interaction term for SOX2 and radical surgery. OS and PFS were defined as time from diagnosis to death or last follow-up, or time from diagnosis to relapse, or last follow-up, respectively. A maximum of 5 years follow-up was used as cutoff for OS, and 3 years for PFS. Age and stage were included as covariates in the multivariable analyses, with age as a continuous variable, and stage as a binary variable (stage III vs. IV). SOX2 was used as a binary variable, with SOX2- defined as no expression in any TMA core, and SOX2+ as 1 or more positive cancer cells in at least 1 core. The variable residual tumor was defined as any macroscopically visible tumor tissue remaining upon completed surgery. A comparison of models for growth curves was conducted using analysis of variance (ANOVA). Other tests used are defined in the Results section. The R statistical environment was used for all calculations and analyses.37
Table 1.
Clinico-pathological variables in relation to SOX2 expression
| All patients |
Patients with residual disease |
|||||||
|---|---|---|---|---|---|---|---|---|
|
All (n = 130) |
SOX2+ (n = 75) |
SOX2- (n = 55) |
p |
All (n = 54) |
SOX2+ (n = 31) |
SOX2- (n = 23) |
p | |
| Age, median (range) | 67 (43–86) | 66 (51–86) | 67 (43–83) | 0.30 T | 68 (45–86) | 69 (53–86) | 66 (45–83) | 0.08 T |
| Residual disease, n (%) 1 | 0.9 M | |||||||
| 0 | 76 (58) | 44 (59) | 32 (58) | - | - | - | ||
| 1 | 30 (23) | 17 (23) | 13 (24) | - | - | - | ||
| 2 | 13 (10) | 9 (12) | 4 (7) | - | - | - | ||
| 3 | 6 (5) | 3 (4) | 3 (5) | - | - | - | ||
| 4 | 5 (4) | 2 (3) | 3 (5) | - | - | - | ||
| Treatment response, n (%) | 0.88 M | 0.27 M | ||||||
| Complete response | 86 (72) | 49 (71) | 37 (74) | 27 (58) | 13 (48) | 14 (70) | ||
| Partial response | 30 (25) | 20 (29) | 10 (20) | 18 (38) | 14 (52) | 4 (20) | ||
| Progressive disease | 3 (2.5) | 0 (0) | 3 (6.0) | 2 (4) | 0 (0) | 2 (10) | ||
| Undetermined | 11 | 6 | 5 | 7 | 4 | 3 | ||
| Stage, n (%) | 1.0 F | 1.0 F | ||||||
| III | 100 (77) | 58 (77) | 42 (76) | 37 (69) | 21 (68) | 16 (70) | ||
| IV | 30 (23) | 17 (23) | 13 (24) | 17 (31) | 10 (32) | 7 (30) | ||
| Performance status, n (%) | 0.52 F | 0.62 F | ||||||
| ≤ 1 | 119 (92) | 67 (91) | 52 (95) | 49 (92) | 27 (90) | 22 (96) | ||
| > 1 | 10 (8) | 7 (9) | 3 (5) | 4 (8) | 3 (10) | 1 (4) | ||
| NA | 1 | 1 | 0 | 1 | 1 | 0 | ||
| Chemotherapy, n (%) | 0.77 F | 0.28 F | ||||||
| Carboplatin + Paclitaxel | 105 (81) | 61 (81) | 44 (80) | 42 (78) | 23 (74) | 19 (83) | ||
| Carboplatin only | 12 (9) | 8 (11) | 4 (7) | 6 (11) | 5 (16) | 1 (4) | ||
| Carboplatin + Doxorubicin | 6 (5) | 3 (4) | 3 (5) | 1 (2) | 0 (0) | 1 (4) | ||
| Other | 3 (2) | 1 (1) | 2 (4) | 1 (2) | 1 (3) | 0 (0) | ||
| No chemo | 4 (3) | 2 (3) | 2 (4) | 4 (7) | 2 (6) | 2 (9) | ||
10, No macroscopic disease; 1, <1 cm disease; 2, ≥1 cm disease but most of the tumor bulk resected; 3, most of the tumor bulk remaining; 4, tumor bulk intact (only bowel obstruction surgery or biopsy performed).
TStudent’s two sample t-test; FFisher’s exact test; MMann-Whitney-Wilcoxon test.
Results
SOX2 and clinico-pathological variables
Using the cutoff of >0%, 75/130 (58%) cases were SOX2 positive (SOX2+) and 55/130 (42%) were SOX2 negative (SOX2-). No differences between the SOX2+ and SOX2- groups were observed in relation to standard clinico-pathological variables including age at diagnosis, stage, treatment response or performance status (Table 1). There were also no significant differences observed between the SOX2+ and SOX2- groups for these variables in sub-analyses of patients with residual tumor after primary surgery.
SOX2 and survival
When considering the whole cohort of 125 patients with advanced stage HGSOC who underwent cytoreductive debulking surgery, using the Kaplan-Meier estimator and performing a log-rank test for SOX2+ vs. SOX2-, there was no differences in OS or PFS (Figure 3A-B). However, within the subgroup of patients with non-radical debulking surgery, defined as any macroscopically visible tumor tissue (49/125 patients), we observed a large difference between the SOX2+ and SOX2- groups in both OS where the mean survival times were 26 and 39 months respectively and in PFS where the mean was 14 and 19 months respectively (Figure 3C-D, OS: p = .0076; PFS: p = .055, n = 49). This was in contrast to the analyses of patients defined as macroscopically tumor-free, where there was no difference in OS or PFS between patients with SOX2+ and SOX2- tumors (Figure 3E-F). There was no difference in survival between the four levels of SOX2 expression in the whole cohort of 125 women (Supplementary Figure S1). In contrast, there was a difference between the four expression levels within the subgroup of women with residual tumor, with >50% SOX2+ cells resulting in the shortest survival, and 0% the longest (OS: p = .025; PFS: p = .013). We next used a univariable Cox regression model to investigate the impact of the variables age, stage, SOX2, and residual tumor on OS and PFS individually as well as a multivariable Cox regression model with an interaction term for the two variables SOX2 and residual tumor, together with age and stage as separate covariates (Table 2). We found an interaction effect of SOX2 and debulking surgery on survival (OS: β = 1.1, 95% CI: [0.21; 2.1], p = .017; PFS: β = 0.82, 95% CI: [−0.016; 1.7], p = .055). To investigate the hazard ratios (HR) for patients with different combinations of risk factors, coefficients were added, and HR calculated (see Supplementary Table S1). By stratifying the patients by amount of tumor burden remaining after surgery, we observed that the connection between SOX2 and survival was most evident among patients with minimal tumor burden (1–10 mm). Kaplan-Meier estimations and results from log-rank tests are displayed in Supplementary Figure S2.
Figure 3.
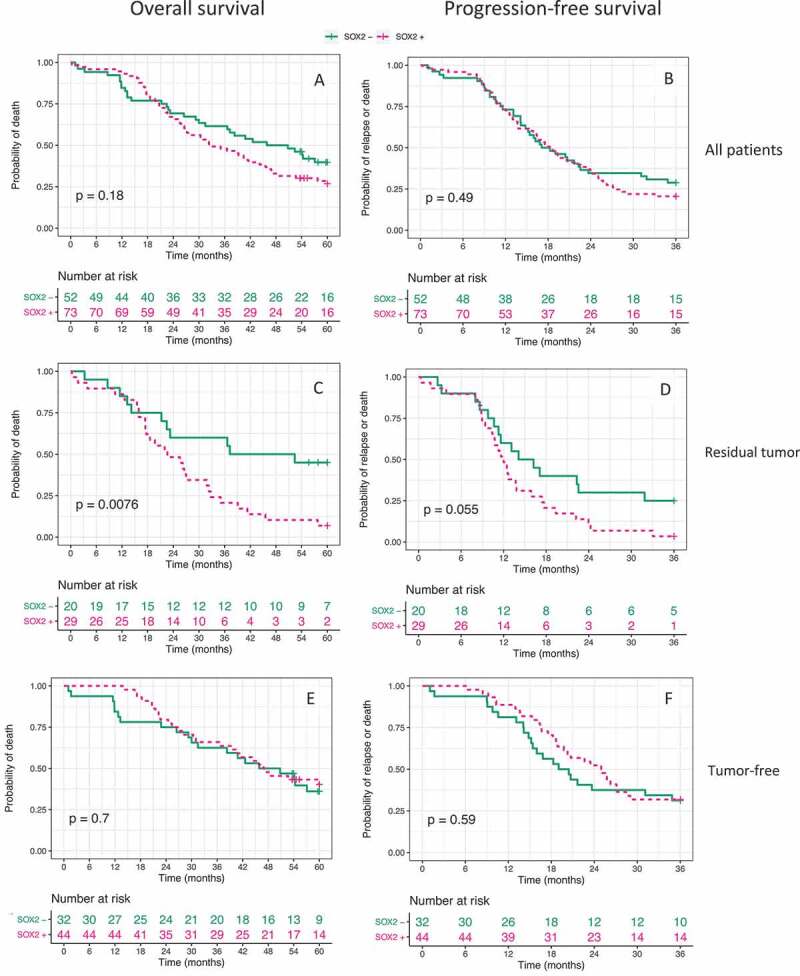
Kaplan-Meier estimates for OS and PFS. (A, B)
All 125 patients; (C, D) Patients with residual tumor after surgery (n = 49); (E, F) Macroscopically tumor-free patients (n = 76). P-values displayed represent results from log-rank tests.
Table 2.
Univariable and multivariable COX proportional hazard analysis of OS and PFS1.
| Univariable analysis |
Multivariable analysis |
||||||
|---|---|---|---|---|---|---|---|
| Risk factor | β | HR [95% CI] | p-value | β | [95% CI] | p-value | |
| OS | Stage IV | 0.99 | 2.7 [1.7; 4.3] | < 0.001 | 0.96 | [0.48; 1.5] | < 0.001 |
| Age | 0.047 | 1.1 [1.0; 1.1] | < 0.001 | 0.056 | [0.028; 0.084] | < 0.001 | |
| SOX2+ | 0.31 | 1.4 [0.87; 2.1] | 0.18 | −0.16 | [−0.74; 0.43] | 0.60 | |
| Residual tumor | 0.61 | 1.8 [1.2; 2.8] | <0.01 | −0.20 | [−0.94; 0.54] | 0.59 | |
| SOX2: Residual tumor | - | - | - | 1.1 | [0.21, 2.1] | 0.017 | |
| PFS | Stage IV | 1.2 | 3.3 [2.1; 5.3] | < 0.001 | 1.2 | [0.7; 1.7] | < 0.001 |
| Age | 0.023 | 1.0 [1.0;1.1] | 0.055 | 0.023 | [−0.0012; 0.047] | 0.061 | |
| SOX2+ | 0.14 | 1.2 [0.76; 1.8] | 0.49 | −0.27 | [−0.82; 0.29] | 0.34 | |
| Residual tumor | 0.78 | 2.2 [1.5; 3.3] | < 0.001 | 0.22 | [−0.44; 0.88] | 0.52 | |
| SOX2: Residual tumor | - | - | - | 0.82 | [−0.016; 1.7] | 0.055 | |
1The variables ‘Stage IV’, ‘SOX2+’ and ‘residual tumor’ are binary with the baseline counterparts ‘Stage III’, ‘SOX2-”’and ‘macroscopically tumor-free’, respectively. Age is a continuous variable with the HR and β representing the change for each year older a patient is. An interaction term for residual tumor and SOX2+ is included in the multivariable analysis.
SOX2 expression in primary tumors and metastases
Tissue from both the primary tumor and metastases was available from 114 (88%) patients. We observed no within-patient difference in SOX2 expression in primary tumors compared to metastases; 84 patients (74%) displayed no difference in expression between the highest scored core from the primary tumor and the highest scored core from metastatic sites. The remaining 30 patients displayed discordant expression, with 19 patients (17%) having a SOX2+ primary tumor core and a SOX2- metastasis core and 11 patients (9%) having a SOX2- primary tumor core and a SOX2+ metastasis core (McNemar’s exact test: OR = 0.58, 95%CI: [0.25; 1.28], p = .2). There was also no overall difference in SOX2 expression between primary tumors and metastases; 30% of 469 primary tumor cores were SOX2+ and 28% of 361 metastasis cores were SOX2+ (Pearson Chi-squared test: p = .46).
Chemotherapy treatment and SOX2 levels in HGSOC cell lines
The effect of carboplatin treatment on SOX2 expression was explored in vitro using the OVCAR3 and COV362 cell lines. For both lines there was a dose-, and time-dependent decrease in SOX2 mRNA expression as well as in SOX2 protein expression measured on days 1, 3 and 5 following treatment with carboplatin (Figure 4A,C). Quantification of Western blot images and variation over replicates can be found in Supplementary Figure S4. Following paclitaxel treatment, no effect on SOX2 protein or mRNA could be seen for OVCAR3 cells, however for COV362 cells a decrease on both mRNA and protein levels was observed on day 3 (Figure 4B,D).
Figure 4.
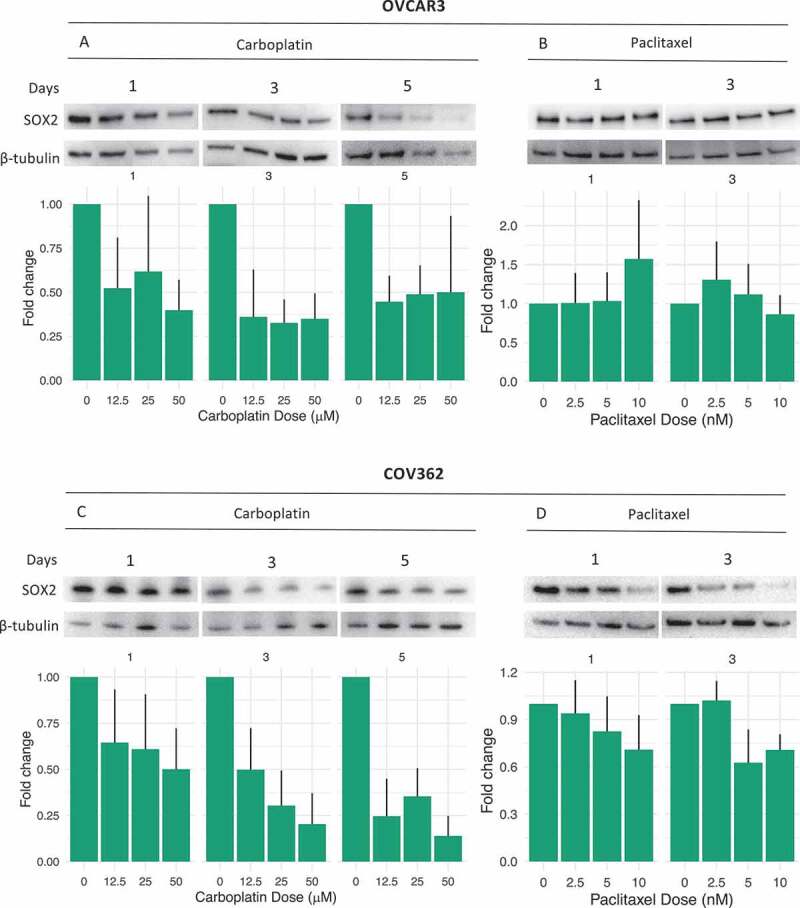
SOX2 expression following chemotherapy treatment
RT-PCR and Western blots displaying SOX2 mRNA and SOX2 protein expression following chemotherapy treatment. (A, C) SOX2 protein/mRNA levels following 1,3 and 5 days of exposure to carboplatin. (B, D) SOX2 protein/mRNA levels following 1 and 3 days of exposure to paclitaxel. mRNA fold-changes are normalized to the reference gene RPS10. Data are expressed as mean ± SEM of three independent experiments. Corresponding stain-free blots for the Western blotting can be found in Supplementary Figure S3 and barplots of quantified data are available in Supplementary Figure S4.
siRNA knockdown and growth inhibition
siRNA knockdown of SOX2 was performed to explore the role of SOX2 in relation to treatment response. Knockdown of SOX2 did not affect the growth inhibitory response to either carboplatin or paclitaxel to any significant extent in either cell line (Figure 5).
Figure 5.

Response to chemotherapy following SOX2 knockdown
Growh inhibition following chemotherapy treatment with and without SOX2 siRNA knockdown. (A, B) OVCAR3 cells, (C, D) COV362 cells. Knockdown (KD), non-targeting siRNA (NT). P-values represent results of analysis of variance tests for dose-response curves (ANOVA).
Discussion
The main focus of this study was to investigate the prognostic value of SOX2 in a consecutive and comprehensive cohort of women diagnosed with HGSOC. Given the crucial role of SOX2 in stem cells we hypothesized that even a small fraction of SOX2+ cells in the tumor tissue may be sufficient to affect the risk of relapse or death. The TMA used in the present study contained multiple cores per patients, selected from representative parts of both the primary and metastatic tumor, providing a good sample of the whole tumor bulk. When considering the whole cohort, we did not observe an effect of SOX2 expression on survival or relapse, even in the group with more than 50% SOX2+ cells. This was unexpected considering the importance of SOX2 as a driver of stem cells, which at least when present in an abundant mass of cells should increase the risk of relapse and result in faster-growing tumors.11 In contrast, however, when exploring the effect of SOX2 in relation to surgical outcome, we observed that SOX2 expression had a significant effect on outcome among patients with non-radical surgery, whereas there was no effect among patients who were determined macroscopically tumor-free after surgery. This appears reasonable considering that the composition of the tumor should not matter if complete tumor resection is achieved, whereas even a small population of remaining cells may be sufficient to initiate a new tumor bulk if some of these cells possess stem cell-like qualities. Also the fact that the result was seen only in the group of patients with minimal tissue left (1–10 mm) is in line with our hypothesis, as it is reasonable to assume that a large bulk of remaining tumor would create a high risk of relapse in itself, irrespective of SOX2 expression, while for patients with less tissue remaining the composition of tumor cells would have a more significant impact. Bareiss et al.11 showed upregulation of SOX2 upon sphere formation and a significant increase in sphere forming capacity in SOX2+ cell fractions compared to SOX2- cells. This indicates increased self-renewal capacity of SOX2+ cells and suggest that SOX2 may be connected to relapse formation, which is in line with our results. Several studies have raised the issue about residual disease after surgery, pointing to the importance of absolute resection.38,39 Du Bois et al. reported that there was a large difference in both OS and PFS between patients with 0 mm macroscopic tumor and those with 1–10 mm. However, the difference in survival if the women had 1–10 mm or >10 mm remaining tumor was small.38 Another study found a large survival benefit of complex primary surgery, even if limited residual tumor remained (≤ 0.5 cm) and argued that primary surgery should always be recommended if the patient could tolerate it.40 Based on this it is clear that even a small amount of remaining disease greatly decreases the survival rates, and therefore for this study we used a strict cutoff of no macroscopic tumor left to group patients, while also including sub-group analyses based on different tumor burden remaining. We did not observe any difference in SOX2 expression between primary tumors and metastases, indicating that aberrant SOX2 expression is not acquired during metastatic transformation, but may rather play a role in the early establishment of the tumor, as previously suggested.11,14
Our findings suggest that SOX2 may be an important prognostic factor for HGSOC patients with tumor tissue remaining after surgery. It is worth noting that the relapse rate is high for all patients with macroscopic residual tumor after primary surgery during the first 12 months after diagnosis, but that the groups separate after this point. This may be explained by the fact that patients who did not respond to treatment died shortly after surgery due to complications of the treatment or the disease itself, regardless of SOX2 expression. Conversely, patients who responded to the initial chemotherapy may have survived the first critical year, but if they did not not achieve complete resection, a SOX2+ cell population may have survived chemotherapy treatment, giving rise to a relapse.
A new prognostic tool would be valuable for HGSOC patients for whom complete tumor resection was not achieved as these patients may be eligible for alternative treatment options. Also, as many patients die during the first 6 months following surgery, the level of complexity and aggressiveness warranted in surgery could be determined with this biomarker in mind. If a SOX2- tumor were to be considered posing a smaller risk of relapse than a SOX2+ tumor, the surgeon may consider less aggressive surgery for these patients in cases when the risk of complications from complex surgery is expected to be high.
An even more appealing goal of studying SOX2 in this context would be to target these SOX2+ cells before relapses occur. To this end we performed in vitro experiments to investigate the relationship between SOX2 and response to treatment. SOX2 knockdown did not affect the response of HGSOC cell lines to either carboplatin or paclitaxel. Accordingly, we observed no difference in treatment response between women with SOX2+ and SOX2- tumors in the patient data, indicating that SOX2 expression itself does not protect the cells from DNA-damaging agents. This is in line with the report by Bareiss et al., who found that SOX2 over-expression did not affect proliferation despite inducing a CSC phenotype.11
We also found that SOX2 expression decreased in a dose and time-dependent manner following carboplatin treatment. This may indicate that SOX2 levels are initially suppressed during chemotherapy, but might increase again once chemotherapy is completed, as indicated by a study which found that cells collected immediately after primary chemotherapy treatment had increased levels of the stem cell marker CD133.41 Exploring the effect of chemotherapy treatment on stem cell markers both short and long term would be of great interest to optimize treatment.
Previous studies have reported on the association of SOX2 and clinico-pathological variables in ovarian cancer. However, given the differences in clinical presentation and prognosis between the histological subtypes of epithelial ovarian cancer, results from these studies are inconclusive, and not applicable specifically to advanced stage HGSOC.
Several studies have reported on shorter survival among serous ovarian carcinoma patients with SOX2+ tumors.28,30,32,33 It is important to note however, that the size and composition of these cohorts have not always been ideal, and antibodies as well as cutoffs used for SOX2 expression have varied. Only one study reported the opposite result, i.e. a favorable effect of SOX2 expression on survival.42 The authors argued that tumors may have a lower tolerance to treatment if the cells are relying on SOX2 than if they rely on other pathways. This study however did not take debulking outcome into account, and no covariates were included in the analysis.
A major strength of our study is the use of a current, comprehensive and consecutive cohort of pure advanced HGSOC. Moreover, the large number of cores from both primary and metastatic lesions collected in the TMA from each patient alleviates the potential issues associated with tumor heterogeneity.
Our results show that SOX2 is a strong prognostic predictor for advanced stage HGSOC patients with macroscopic residual tumor after primary surgery, where SOX2 expression has an impact on both progression-free, and overall survival. In contrast, there is no prognostic impact for patients with advanced stage disease following radical surgery, highlighting the need to include this variable in studies of different markers and survival following surgery. SOX2 levels are affected by carboplatin treatment in vitro, but SOX2 does not appear to affect the response to carboplatin in patients or in HGSOC cell lines.
Supplementary Material
Funding Statement
This work was supported by grants from the Swedish Cancer Society (Cancerfonden) [CAN 2018/613]; the Cancer and Allergy Foundation [2017/132]; the Fru Berta Kamprad Foundation [FBKS 2017-22]; the Lund University Hospital Research Foundation [2017-033]; BioCARE and Governmental funding of clinical research within the National Health Services (ALF) [2018/40615].
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Declaration of interest statement
The authors declare no competing interests.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):1–10. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Vaughan S, Coward JI, Bast RC, Berchuck A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R, Etemadmoghadam D, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11(10):719–725. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harter P, Muallem ZM, Buhrmann C, Lorenz D, Kaub C, Hils R, Kommoss S, Heitz F, Traut A, Du Bois A, et al. Impact of a structured quality management program on surgical outcome in primary advanced ovarian cancer. Gynecol Oncol. 2011;121(3):615–619. doi: 10.1016/j.ygyno.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 4.Pignata S, C Cecere S, Du Bois A, Harter P, Heitz F.. Treatment of recurrent ovarian cancer. Ann Oncol. 2017;28(suppl_8):viii51–viii56. doi: 10.1093/annonc/mdx441. [DOI] [PubMed] [Google Scholar]
- 5.Bowtell DD, Böhm S, Ahmed AA, Aspuria P-J, Bast RC, Beral V, Berek JS, Birrer MJ, Blagden S, Bookman MA, et al. Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer. 2015;15(11):668–679. doi: 10.1038/nrc4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cress RD, Chen YS, Morris CR, Petersen M, Leiserowitz GS.. Characteristics of long-term survivors of epithelial ovarian cancer. Obstet Gynecol. 2015;126(3):491–497. doi: 10.1097/AOG.0000000000000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurman R, Shih I. The Origin and pathogenesis of epithelial ovarian cancer-a proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labidi-Galy SI, Papp E, Hallberg D, Niknafs N, Adleff V, Noe M, Bhattacharya R, Novak M, Jones S, Phallen J, et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun. 2017;8:1. doi: 10.1038/s41467-017-00962-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirohashi Y, Torigoe T. Non-neoplastic fallopian tube epithelium carrying gene mutations of a novel SOX2 repressor region is soil of high-grade serous ovarian cancer. EBioMedicine. 2016;10:17–18. doi: 10.1016/j.ebiom.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vanner RJ, Remke M, Gallo M, Selvadurai H, Coutinho F, Lee L, Kushida M, Head R, Morrissy S, Zhu X, et al. Quiescent Sox2+ cells drive hierarchical growth and relapse in sonic hedgehog subgroup medulloblastoma. Cancer Cell. 2014;26(1):33–47. doi: 10.1016/J.CCR.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bareiss PM, Paczulla A, Wang H, Schairer R, Wiehr S, Kohlhofer U, Rothfuss OC, Fischer A, Perner S, Staebler A, et al. SOX2 expression associates with stem cell state in human ovarian carcinoma. Cancer Res. 2013;73(17):5544–5555. doi: 10.1158/0008-5472.CAN-12-4177. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, Muhlestein W, Melton DA. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26(11):1269–1275. doi: 10.1038/nbt.1502. [DOI] [PubMed] [Google Scholar]
- 14.Hellner K, Miranda F, Fotso Chedom D, Herrero-Gonzalez S, Hayden DM, Tearle R, Artibani M, KaramiNejadRanjbar M, Williams R, Gaitskell K, et al. Premalignant SOX2 overexpression in the fallopian tubes of ovarian cancer patients: discovery and validation studies. EBioMedicine. 2016;10:137–149. doi: 10.1016/j.ebiom.2016.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chene G, Ouellet V, Rahimi K, Barres V, Meunier L, De Ladurantaye M, Provencher D, Mes-Masson AM. Expression of stem cell markers in preinvasive tubal lesions of ovarian Carcinoma. Biomed Res Int. 2015;2015:1–5. doi: 10.1155/2015/808531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kregel S, Kiriluk KJ, Rosen AM, Cai Y, Reyes EE, Otto KB, Tom W, Paner GP, Szmulewitz RZ, Vander Griend DJ, et al. Sox2 is an androgen receptor-repressed gene that promotes castration-resistant prostate cancer. PLoS One. 2013;8:1. doi: 10.1371/journal.pone.0053701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen -C-C, Wongvipat J, Ku S-Y, Gao D, Cao Z, et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53 – and RB1 -deficient prostate cancer. Science (80-). 2017;355(6320):84–88. doi: 10.1126/science.aah4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudin CM, Durinck S, Stawiski EW, Poirier JT, Modrusan Z, Shames DS, Bergbower EA, Guan Y, Shin J, Guillory J, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet. 2012;44(10):1111–1116. doi: 10.1038/ng.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussenet T, Dali S, Exinger J, Monga B, Jost B, Dembelé D, Martinet N, Thibault C, Huelsken J, Brambilla E, et al. SOX2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. Idnurm A, ed. PLoS One. 2010;5(1):e8960. doi: 10.1371/journal.pone.0008960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, Kim SY, Wardwell L, Tamayo P, Gat-Viks I, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41(11):1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.You L, Guo X, Huang Y. Correlation of cancer stem-cell markers OCT4, SOX2, and NANOG with clinicopathological features and prognosis in operative patients with rectal cancer. Yonsei Med J. 2018;59(1):35. doi: 10.3349/ymj.2018.59.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alonso MM, Diez-Valle R, Manterola L, Rubio A, Liu D, Cortes-Santiago N, Urquiza L, Jauregi P, de Munain AL, Sampron N, et al. Genetic and epigenetic modifications of SOX2 contribute to the invasive phenotype of malignant gliomas. Futscher BW, ed. PLoS One. 2011;6(11):e26740. doi: 10.1371/journal.pone.0026740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sholl LM, Barletta JA, Yeap BY, Chirieac LR, Hornick JL. Sox2 protein expression is an independent poor prognostic indicator in stage i lung adenocarcinoma. Am J Surg Pathol. 2010;34(8):1193–1198. doi: 10.1097/PAS.0b013e3181e5e024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, Le Mercier M, Delatte B, Caauwe A, Lenglez S, Nkusi E, et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature. 2014;511(7508):246–250. doi: 10.1038/nature13305. [DOI] [PubMed] [Google Scholar]
- 25.Siegle JM, Basin A, Sastre-Perona A, Yonekubo Y, Brown J, Sennett R, Rendl M, Tsirigos A, Carucci JA, Schober M, et al. SOX2 is a cancer-specific regulator of tumour initiating potential in cutaneous squamous cell carcinoma. Nat Commun. 2014;5:9339–9344. doi: 10.1038/ncomms5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye F, Li Y, Hu Y, Zhou C, Hu Y, Chen H. Expression of Sox2 in human ovarian epithelial carcinoma. J Cancer Res Clin Oncol. 2011;137(1):131–137. doi: 10.1007/s00432-010-0867-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Chen K, Li L, Li R, Zhang J, Ren W. Overexpression of SOX2 is involved in paclitaxel resistance of ovarian cancer via the PI3K/Akt pathway. Tumor Biol. 2015;36(12):9823–9828. doi: 10.1007/s13277-015-3561-5. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Chang DY, Mercado-Uribe I, Liu J. Sex-determining region Y-box 2 expression predicts poor prognosis in human ovarian carcinoma. Hum Pathol. 2012;43(9):1405–1412. doi: 10.1016/j.humpath.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Indumathi S, Harikrishnan R, Rajkumar JS, Sudarsanam D, Dhanasekaran M. Prospective biomarkers of stem cells of human endometrium and fallopian tube compared with bone marrow. Cell Tissue Res. 2013;352(3):537–549. doi: 10.1007/s00441-013-1582-1. [DOI] [PubMed] [Google Scholar]
- 30.Wang, X., Ji, X., Chen, J., Yan, D., Zhang, Z., Wang, Q., Xi, X., & Feng, Y. SOX2 enhances the migration and invasion of ovarian cancer cells via Src kinase. PLoS One. 2014;9:6. doi: 10.1371/journal.pone.0099594. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Lou X, Han X, Jin C, Tian W, Yu W, Ding D, Cheng L, Huang B, Jiang H, Lin B, et al. SOX2 targets fibronectin 1 to promote cell migration and invasion in ovarian cancer: new molecular leads FOR THERAPEUTIC INTERVENTIOn. Omi A J Integr Biol. 2013;17(10):510–518. doi: 10.1089/omi.2013.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du J, Li B, Fang Y, Liu Y, Wang Y, Li J, Zhou W, Wang X. Overexpression of Class III β-tubulin, Sox2, and nuclear survivin is predictive of taxane resistance in patients with stage III ovarian epithelial cancer. BMC Cancer. 2015;15(1):536. doi: 10.1186/s12885-015-1553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belotte J, Fletcher NM, Alexis M, Morris RT, Munkarah AR, Diamond MP, Saed GM. Sox2 gene amplification significantly impacts overall survival in serous epithelial ovarian cancer. Reprod Sci. 2015;22(1):38–46. doi: 10.1177/1933719114542021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin de la Fuente, L., Westbom-Fremer, S., Arildsen, N. S., Hartman, L., Malander, S., Kannisto, P., Måsbäck, A., & Hedenfalk, I. (2020). PD-1/PD-L1 expression and tumor-infiltrating lymphocytes are prognostically favorable in advanced high-grade serous ovarian carcinoma. Virchows Archiv: an international journal of pathology, 477(1), 83–91. 10.1007/s00428-020-02751-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurman, RJ. (2014). WHO classification of tumours of female reproductive organs. Lyon: IARC. [Google Scholar]
- 36.Prat J. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynecol Obstet. 2014;124(1):1–5. doi: 10.1016/J.IJGO.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 37.R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/
- 38.Du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: A combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials. Cancer. 2009;115(6):1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 39.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 40.Wallace S, Kumar A, Mc Gree M, Weaver A, Mariani A, Langstraat C, Dowdy S, Bakkum-Gamez J, Cliby W. Efforts at maximal cytoreduction improve survival in ovarian cancer patients, even when complete gross resection is not feasible. Gynecol Oncol. 2017;145(1):21–26. doi: 10.1016/j.ygyno.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 41.Steg AD, Bevis KS, Katre AA, Ziebarth A, Dobbin ZC, Alvarez RD, Zhang K, Conner M, Landen CN. Stem cell pathways contribute to clinical chemoresistance in ovarian cancer. Clin Cancer Res. 2012;18(3):869–881. doi: 10.1158/1078-0432.CCR-11-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pham DL, Scheble V, Bareiss P, Fischer A, Beschorner C, Adam A, Bachmann C, Neubauer H, Boesmueller H, Kanz L, et al. SOX2 expression and prognostic significance in ovarian carcinoma. Int J Gynecol Pathol. 2013;32(4):358–367. doi: 10.1097/PGP.0b013e31826a642b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


