Abstract
OBJECTIVES
Some drugs need to be compounded by the pharmacist before being administered to the patient. A study was conducted to determine the stability of acetazolamide suspensions in 2 different vehicles (Oral Mix and Oral Mix Sugar Free [SF]) from bulk drug and tablets at 2 different temperatures and in 2 different containers (amber plastic bottles and clear plastic syringes).
METHODS
Acetazolamide suspensions (25 mg/mL) were prepared from bulk drug or tablets. Each suspension, using Oral Mix or Oral Mix SF, was split between 2 types of containers—amber plastic bottles and clear plastic syringes—and stored either at room temperature (23°C–27°C) or under refrigeration (3°C–7°C). Samples were drawn from the suspensions right after preparation and on days 7, 14, 30, 45, 60, 75, and 90. They were then analyzed by high-performance liquid chromatography (HPLC) using a reverse-phase column. A validated stability-indicating HPLC with ultraviolet detection method was developed. A visual inspection and a pH measurement were also completed at each time point. Stability was defined as retention of at least 90% of the initial concentration of acetazolamide suspension.
RESULTS
At least 91.2% of the initial acetazolamide concentration in suspensions remained throughout the 90-day study period for both vehicles, both containers, and both temperatures. Assays varied between 91.2% and 105.0% of the initial concentration for all 112 tested conditions but 2 (105.2% and 109.0%). Linear regression was calculated for each time profile and remained above 95.0% at the end of the study in all cases. Similarly, pH remained within 0.1 unit of the initial pH, which was 4.2 for Oral Mix and 4.3 for Oral Mix SF. Furthermore, no changes in organoleptic properties were observed because the preparations remained as white fluid suspensions without sedimentation.
CONCLUSIONS
Acetazolamide suspensions were stable for at least 90 days in all tested conditions because the average drug concentration was not less than 90% of the initial concentration. The beyond-use date could be extended from 60 to 90 days.
Keywords: acetazolamide, drug stability, extemporaneous compounding, HPLC, pharmaceutical preparations
Introduction
Pharmacists are often confronted with patients for whom solid drug forms are not suitable, such as pediatric and geriatric patients, patients unable to swallow solid oral forms, or patients with specific dosage needs. Pharmacists need to prepare compounded drugs to overcome this shortcoming. To compound a liquid dosage form, they often need to add new excipients, which could have a chemical or a physical impact on the drug.1,2
Chapter 795 of the US Pharmacopeia (USP)3 indicates that if a stability study has not been conducted for a compounded aqueous oral formulation, the beyond-use date is 14 days. It also specifies that the drug should be stored between 2°C and 8°C. However, these conditions are not ideal for hospital pharmacies and, in reality, they can vary depending on the products used. Because the number of compounded preparations is increasing, it is beneficial to conduct studies that can establish the stability of the drug in its compounded form.
According to the USP monograph for acetazolamide oral suspension, the stability for a compounded preparation of acetazolamide at 25 mg/mL is 60 days if stored in a light-resistant container in a controlled temperature room.4 This formulation is prepared by mixing acetazolamide powder (from tablets or pure bulk drug) with an oral vehicle.
In the United States, acetazolamide is available as tablets (125 and 250 mg),5 as powder for injection (500 mg)6 or as extended-release capsules (500 mg).7 In Canada, it is available as immediate-release tablets (250 mg of acetazolamide)8 or injection (acetazolamide sodium equivalent to 500 mg of acetazolamide per vial)9.
Acetazolamide is a carbonic anhydrase inhibitor10 that is used to treat glaucoma,11 epilepsy,12 mountain sickness,13 and fluid retention in adults and children. Even if it is used by both adults and children, no equivalent commercial oral liquid forms currently exist on the market.
Recently, the stability of acetazolamide oral suspensions (20 mg/mL) was evaluated by Santoveña et al14 using different suspending and wetting agents; however, the authors were not successful in preparing homogeneous suspensions using these vehicles. Two decades ago, Allen and Erickson15 evaluated the stability of acetazolamide oral suspensions (25 mg/mL) in a 1:1 mixture of Ora-Sweet and Ora-Plus (Paddock Laboratories, Minneapolis, MN), a 1:1 mixture of Ora-Sweet Sugar Free (SF) and Ora-Plus (Paddock Laboratories), and cherry syrup. Stability was demonstrated for at least 60 days when stored at 5°C and 25°C in plastic bottles. In 1991, Alexanders et al16 demonstrated the stability of acetazolamide suspensions (25 mg/mL) in a 70% sorbitol solution for at least 79 days when stored at 5°C, 22°C, and 30°C in amber glass bottles. A preformulation study of acetazolamide also concluded that a pH of approximately 4 was required to maintain the chemical stability of this compound in aqueous preparations.17
Cherry syrup is prepared from cherry juice, but even if both products are listed in the USP-National Formulary, they are rarely used nowadays because of the variability that can be observed with products of natural origin. Similarly, sorbitol is a known laxative. Therefore, the only current option is to prepare acetazolamide suspensions using Paddock vehicles. Stability is known for 60 days in vehicles containing sugar (1:1 mixture of Ora-Sweet and Ora-Plus) and those that are SF (1:1 mixture of Ora-Sweet SF and Ora-Plus) when stored at 5°C and 25°C in plastic bottles. Based on discussions with the hospital community, there is a need to establish the stability of acetazolamide suspensions in readily available dye-free vehicles in bottles, as well as in oral syringes because these are often used for storage purposes as well. The need for dye-free vehicle stability results are critical for the sensitive patient population, particularly in pediatrics.
The aim of this study was to establish the physical and chemical stability of extemporaneously prepared acetazolamide formulations using Oral Mix and Oral Mix SF (lot I185/A and lot H1136, Medisca Pharmaceutique Inc, Montréal, QC, Canada) from commercial tablets (MH9405, Pharma Inc) and bulk drug (lot 602553/B, Medisca). Oral Mix and Oral Mix SF are 2 oral vehicles with preservatives and buffering agents that help to minimize degradation. Both have the advantage of being alcohol and dye free.
This stability study was developed by following the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guidelines,18 Trissel principles, and several published articles.19–27 Stability studies for oral extemporaneous solutions were performed in general during a period of 3 months, with variance in the number of time points at refrigerated and controlled room temperatures. The current study design included replication of time points, which was performed by the preparation of 1 batch of samples divided in several containers (i.e., 6 bottles and 48 syringes [experimental replicates]) and duplicated injections for high-performance liquid chromatography (HPLC) analysis (technical replicates).
Materials and Methods
In this study, 4 extemporaneous formulations of acetazolamide were prepared using 2 vehicles (Oral Mix and Oral Mix SF) from either bulk drug or tablets. Each of these preparations was conditioned in 6 bottles and 48 syringes. The bottles and syringes were incubated at 5°C and 25°C, and samples were pulled out after 7, 14, 30, 45, 60, 75, and 90 days to be analyzed. At each time point, for each temperature and each preparation, a sample was analyzed from the 3 bottles; samples from 3 syringes were also pulled for analysis. This sampling ensured that all the results were supported from data collected from at least 3 separately aged containers. To perform the analytic evaluation, a stability indicating HPLC with an ultraviolet (UV) detector method was developed and validated.28
Oral Vehicles. Two different vehicles were tested in this study: Oral Mix and Oral Mix SF. These vehicles have been formulated especially for the preparation of extemporaneous forms and are both alcohol and dye free. Their pH is slightly acidic and they contain preservatives and buffering agents to prevent degradation.
Extemporaneous Preparations. Preparations were developed based on formulation described in the Acetazolamide Oral Suspension USP monograph.4 Four preparations of 230 mL were made, one from bulk drug with Oral Mix, one from tablets with Oral Mix, one from bulk drug with Oral Mix SF, and one from tablets with Oral Mix SF. Each solution was divided into 6 amber plastic bottles and 48 clear plastic syringes. Half of them were stored at 5°C ± 2°C and the other half at 25°C ± 2°C at 60% ± 5% RH (relative humidity).
Suspensions compounded from bulk drug were prepared by geometric addition of acetazolamide (5.75 g) with Oral Mix or Oral Mix SF (quantity sufficient to make 230 mL). A similar procedure was followed for suspensions prepared from tablets (23 × 250-mg tablets), which were first pulverized prior to geometric incorporation of Oral Mix or Oral Mix SF (quantity sufficient to make 230 mL). The final concentration of the 4 solutions was 25 mg/mL. The pH was measured with an Accumet pH meter (model AP61, Thermo Fisher Scientific, Saint-Laurent, QC, Canada). The initial pH measurements were 4.16 ± 0.01 for the bulk drug in Oral Mix suspension and 4.17 ± 0.01 for the tablets using the same vehicle, and 4.25 ± 0.02 for the bulk drug in Oral Mix SF and 4.26 ± 0.01 with the tablets in the same vehicle. The suspensions were all white in visual appearance.
All 4 preparations were packaged in 50-mL amber plastic bottles (30-mL fill volume, 6 bottles per preparation) and 3-mL clear plastic syringes (i.e., 1-mL fill volume, 48 syringes per preparation). The formulations were incubated at 5°C ± 2°C and 25°C ± 2°C at 60% ± 5% RH (relative humidity) (Form 3911 Environmental Chamber, Thermo Scientific) for up to 90 days. Three bottles of each preparation and 3 syringes for each time point were stored at both conditions. At predetermined time points (0, 7, 14, 30, 45, 60, 75, and 90 days) an aliquot (1 mL) was retrieved from each bottle, and 3 syringes were retrieved from each temperature condition. Bottles and syringes were shaken prior to sampling. The organoleptic properties of each test sample were inspected, pH was measured, and acetazolamide was assayed using a stability-indicating HPLC-UV method.28
Sample Analysis. Concentration of samples was measured using a stability-indicating HPLC-UV method previously developed and validated by our group for this study.28 Before sampling, each suspension was visually inspected for color change, and pH was measured. Before HPLC injection, each sample was vortexed and prepared as specified by the HPLC-UV method. Ten microliters of each sample was injected into the HPLC-UV system in duplicate. To validate each day run, a calibration curve was injected before the sample.
Statistical Analysis. Each condition was tested 3 times to obtain an SD for each result. All values are presented as mean ± SD. Results were expressed as percentages of the initial concentration (day 0 assay) of the corresponding suspension. Linear regression was calculated for all time profiles to highlight degradation trends.
Specifications. The stability was monitored by reporting the concentration of the drug at different time points as the percentage of the initial concentration. Stability was defined as the absence of noticeable changes in the organoleptic properties, a pH variation of no more than 1.0 unit of pH relative to the initial pH, and a concentration of acetazolamide of not less than 90% of the initial concentration.
Results
After 90 days, the concentration of acetazolamide was not less than 90.0% of the initial concentration for all suspensions at each test condition (Figures 1–4). The different conditions (vehicle used, storage temperature, container, and form of the drug used) did not have a significant impact on the stability of acetazolamide suspensions for 90 days.
Figure 1.
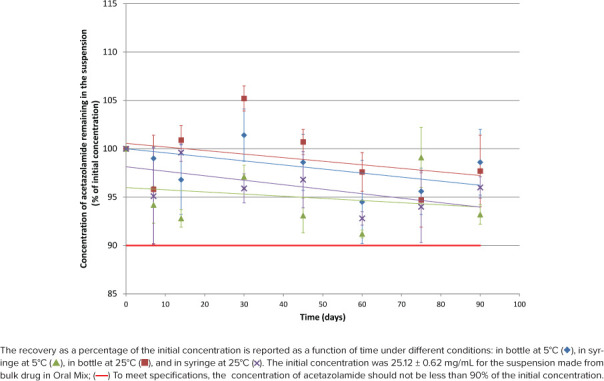
Acetazolamide concentration remaining according to time for suspension from bulk drug in Oral Mix.
Figure 4.
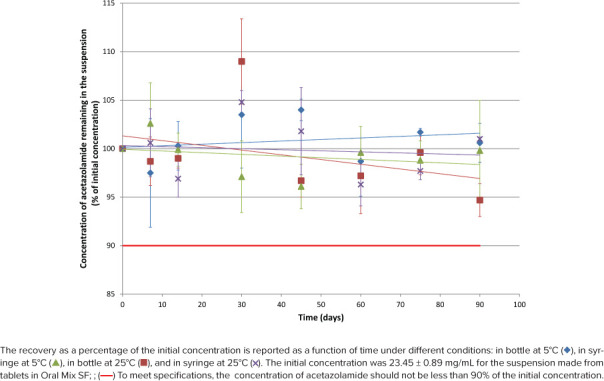
Acetazolamide concentration remaining according to time for suspension from tablet in Oral Mix SF.
Figure 2.
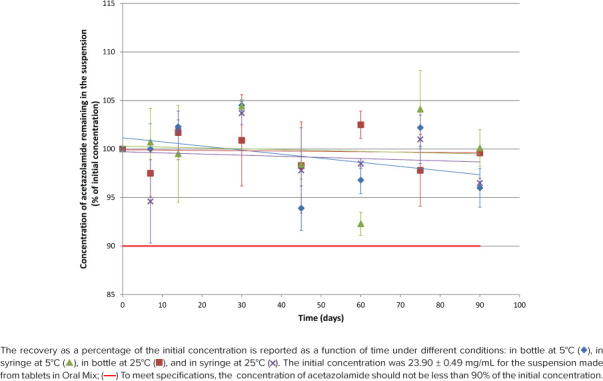
Acetazolamide concentration remaining according to time for suspension from tablet in Oral Mix.
Figure 3.
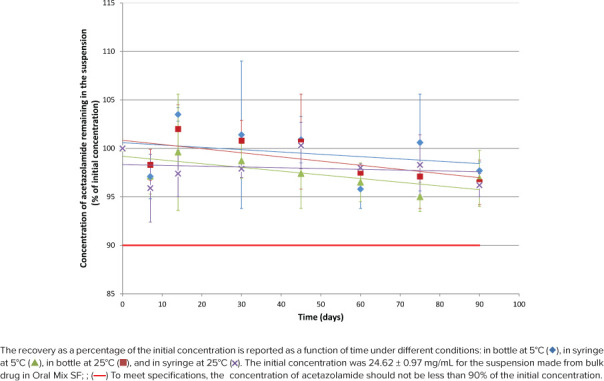
Acetazolamide concentration remaining according to time for suspension from bulk drug in Oral Mix SF.
As shown in Figures 1 through 4, initial concentrations of acetazolamide in preparations comprised between 23.45 and 25.12 mg/mL or within 93.8% to 100.5% of the target concentration (25 mg/mL). Recoveries during the stability study varied between 91.2% and 105.0% for all 112 tested conditions but 2 (105.2% and 109%).
Linear regression was calculated for the 16 stability profiles as illustrated in Figures 1 through 4. The y-intercepts comprised between 94.0% and 101.6%, whereas the slopes were all negative between −0.0488%·day−1 and −0.0034%·day−1, but 1 slope was slightly positive (+0.0161%·day−1, acetazolamide suspension prepared from tablets in Oral Mix SF and stored in bottles at 5°C). The coefficients of determination (r2) comprised between 0.0061 and 0.5666 for the negative slopes, and the coefficient was 0.0569 for the positive slope. Noteworthy were 13 regression slopes between 95.0% and 100.0% after 90 days. The regression slopes for the acetazolamide suspension prepared from bulk drug in Oral Mix and stored in syringes at 5°C and 25°C both passed by 94.0% after 90 days. The positive regression slope for the acetazolamide suspension prepared from tablets in Oral Mix SF and stored in bottles at 25°C passed by 101.6% after 90 days.
The difference between the initial pH and the pH measured at 90 days is less than 0.10 unit of pH in all cases. After 90 days there was no change in the organoleptic properties of the suspension. They remained white without sedimentation.
Discussion
Based on the results, all acetazolamide suspensions were stable at least 90 days when stored in amber plastic bottles or clear syringes at 5°C and 25°C. Stability was demonstrated because the measured concentration of acetazolamide at each time point remained above 90.0% of the initial concentration, no changes in organoleptic properties were observed, and pH did not vary by more than 1.0 unit.
The stability of acetazolamide was also demonstrated in a few other aqueous vehicles using similar conditions.14–16 It was also known that a pH around 4 was required to avoid degradation.17 Acetazolamide in Oral Mix and Oral Mix SF was never tested before, and this study was required to prove its stability in these vehicles. Nonetheless, the observed results were expected based on prior reports on the stability of this drug. The current study was exhaustive because it systematically evaluated all of the 16 possible permutations of the following variables: drug source (tablets vs bulk drug), vehicle (Oral Mix vs Oral Mix SF), packaging (bottle vs syringes), and temperature (5°C vs 25°C).
Because very limited degradation was observed during the time course of this study, poor coefficients of correlation were calculated for the linear regression analyses. The analytic method has its own variability, and in some cases may be larger than the actual degradation. It was still possible to observe the expected negative trends. In one case, a non-expected but very slightly positive trend was observed, which is most likely explained by the normal analytic error.
Although no formal microbial analysis was performed in this study, according to the information provided by the manufacturer, Oral Mix and Oral Mix SF both contain methylparaben as a preservative and both conform to the USP microbial challenge test.29 The addition of pharmaceutical-grade acetazolamide should not be detrimental to their microbial stability as long as the preparation conforms to good compounding practices. Furthermore, no organoleptic evidence of microbial contamination was observed during this study.
Conclusions
This study demonstrated the stability of acetazolamide suspensions (25.0 mg·mL−1) prepared from bulk drug and tablets in Oral Mix and Oral Mix SF when stored in amber plastic bottles and clear plastic syringes at 5°C ± 2°C and 25°C ± 2°C for up to 90 days. This provides an extension to the 60-day beyond-use date presented in the USP Acetazolamide Oral Suspension monograph.4 Acetazolamide oral suspensions using Oral Mix and Oral Mix SF can be used for those who are looking for a dye-free alternative.
Acknowledgments
Results presented in this paper were partially presented as posters at the 2016 Annual Meeting of the American Association of Pharmaceutical Scientists; Denver, CO; November 2016, and the 2017 Annual Meeting of the Canadian Society for Pharmaceutical Sciences; Montreal, QC; May 2017. The authors acknowledge the contributions of Rabeb Mouna Derbali for revising the manuscript.
ABBREVIATIONS
- HPLC
high-performance liquid chromatography
- SF
sugar free
- USP
US Pharmacopeia
- UV
ultraviolet
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data and take responsibility for the integrity and accuracy of the data analysis.
Ethical Approval and Informed Consent Given the nature of this study, the project was exempt from institution review board/ethics committee review.
REFERENCES
- 1.Glass B, Haywood A. Stability considerations in liquid dosage forms extemporaneously prepared from commercially available products. J Pharm Pharm Sci. 2006;9(3):398–426. [PubMed] [Google Scholar]
- 2.Cutaia K, Chablani L, Zhao F. Basics of compounding: vehicles for compounded oral liquid medications: a review. Int J Pharm Compd. 2018;22(6):480–489. [PubMed] [Google Scholar]
- 3.United States Pharmacopeia 42–National Formulary 37 General Chapter <795> Pharmaceutical Compounding–Nonsterile Preparations. Rockville, MD: US Pharmacopeial Convention; 2018. [Google Scholar]
- 4.United States Pharmacopeia 42–National Formulary 37 Acetazolamide Oral Suspension. Rockville, MD: US Pharmacopeial Convention; 2018. [Google Scholar]
- 5.Drugs@FDA FDA approved drug products ANDA 040195. Silver Spring, MD: US Food and Drug Administration; Updated January 9, 2016. Accessed May 3, 2019. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=040195. [Google Scholar]
- 6.Drugs@FDA Silver Spring, MD: US Food and Drug Administration; FDA approved drug products. ANDA 202693. Updated December 19, 2014. Accessed May 3, 2019. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=202693. [Google Scholar]
- 7.Drugs@FDA Silver Spring, MD: US Food and Drug Administration; FDA approved drug products. ANDA 40904. Updated December 10, 2008. Accessed May 3, 2019. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=040904. [Google Scholar]
- 8.Drug Product Database (DPD) Ottawa, ON, Canada: Health Canada; DIN 00545015. Updated March 19, 2019. Accessed May 3, 2019. https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=4899. [Google Scholar]
- 9.Drug Product Database (DPD) Ottawa, ON, Canada: Health Canada; DIN 02358328. Updated March 19, 2019. Accessed May 3, 2019. https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=84336. [Google Scholar]
- 10.Silver Spring, MD: US Food and Drug Administration; FDA professional drug information for acetazolamide. Updated May 1, 2019. Accessed May 3rd, 2019. https://www.drugs.com/pro/acetazolamide.html. [Google Scholar]
- 11.Grant WM, Trotter RR. Diamox (acetazolamide) in treatment of glaucoma. AMA Arch Ophthalmol. 1954;51(6):735–739. doi: 10.1001/archopht.1954.00920040745001. [DOI] [PubMed] [Google Scholar]
- 12.Resor SR, Resor LD. Chronic acetazolamide monotherapy in the treatment of juvenile myoclonic epilepsy. Neurology. 1990;40(11):1677–1681. doi: 10.1212/wnl.40.11.1677. [DOI] [PubMed] [Google Scholar]
- 13.Forwand SA, Landowne M, Follansbee JN, Hansen JE. Effect of acetazolamide on acute mountain sickness. N Engl J Med. 1968;279(16):839–845. doi: 10.1056/NEJM196810172791601. [DOI] [PubMed] [Google Scholar]
- 14.Santoveña A, Suárez-González J, Martín-Rodríguez C, Fariña JB. Formulation design of oral pediatric Acetazol-amide suspension: dose uniformity and physico-chemical stability study. Pharm Dev Technol. 2017;22(2):191–197. doi: 10.1080/10837450.2016.1175475. [DOI] [PubMed] [Google Scholar]
- 15.Allen LV, Erickson MA. Stability of acetazolamide, allopurinol, azathioprine, clonazepam, and flucytosine in extemporaneously compounded oral liquids. Am J Health Syst Pharm. 1996;53(16):1944–1949. doi: 10.1093/ajhp/53.16.1944. [DOI] [PubMed] [Google Scholar]
- 16.Alexander KS, Haribhakti RP, Parker GA. Stability of acetazolamide in suspension compounded from tablets. Am J Hosp Pharm. 1991;48(6):1241–1244. [PubMed] [Google Scholar]
- 17.Parasrampuria J, Gupta VD. Preformulation studies of acetazolamide: effect of pH, two buffer species, ionic strength, and temperature on its stability. J Pharm Sci. 1989;78(10):855–857. doi: 10.1002/jps.2600781015. [DOI] [PubMed] [Google Scholar]
- 18.Geneva, Switzerland: International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use; Q1A(R2) Stability testing of new drug substances and products; 2003. Accessed October 14, 2020. https://www.ich.org/page/quality-guidelines. [Google Scholar]
- 19.Trissel LA, Flora KP. Stability studies: five years later. Am J Hosp Pharm. 1988;45(7):1569–1571. [PubMed] [Google Scholar]
- 20.Li Q, Liu Z, Kolli S et al. Stability of extemporaneous erlotinib, lapatinib, and imatinib oral suspensions. Am J Heal Syst Pharm. 2016;73(17):1331–1337. doi: 10.2146/ajhp150581. [DOI] [PubMed] [Google Scholar]
- 21.Ensom MH, Décarie D. Dexamethasone 1 mg/mL suspension prepared from crushed tablets: stability in glass and plastic bottles and plastic syringes. Can J Hosp Pharm. 2016;69(1):49–51. doi: 10.4212/cjhp.v69i1.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nahata M. Long-term stability of zonisamide, amitriptyline, and glycopyrrolate in extemporaneously prepared liquid-dosage forms at two temperatures. Int J Pharm Compd. 2016;20(2):164–166. [PubMed] [Google Scholar]
- 23.Ferreira AO, Polonini HC, Silva SL et al. Feasibility of amlodipine besylate, chloroquine phosphate, dapsone, phenytoin, pyridoxine hydrochloride, sulfadiazine, sulfasalazine, tetracycline hydrochloride, trimethoprim and zonisamide in SyrSpend(®) SF PH4 oral suspensions. J Pharm Biomed Anal. 2016;118:105–112. doi: 10.1016/j.jpba.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 24.Vrignaud S, Briot T, Launay A et al. Design and stability study of a pediatric oral solution of methotrexate 2mg/ml. Int J Pharm. 2015;487(1–2):270–273. doi: 10.1016/j.ijpharm.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 25.Prohotsky DL, Juba KM, Zhao F. Formulation and stability of an extemporaneously compounded oral solution of chlorpromazine HCl. J Pain Palliat Care Pharmacother. 2014;28(4):367–370. doi: 10.3109/15360288.2014.969874. [DOI] [PubMed] [Google Scholar]
- 26.Stanisz BJ, Paszun SK, Zalewska A. Stability of cilazapril in pediatric oral suspensions prepared from commercially available tablet dosage forms. Acta Pol Pharm. 2014;71(4):661–666. [PubMed] [Google Scholar]
- 27.Kommanaboyina B, Rhodes CT. Trends in stability testing, with emphasis on stability during distribution and storage. Drug Dev Ind Pharm. 1999;25(7):857–868. doi: 10.1081/ddc-100102246. [DOI] [PubMed] [Google Scholar]
- 28.Gillium C, Friciu M, Abatzoglou N, Leclair G. Stability-indicating HPLC-UV assay for acetazolamide in Oral Mix and Oral Mix SF vehicles. MethodsX. 2020;7:100844. doi: 10.1016/j.mex.2020.100844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.United States Pharmacopeia 42–National Formulary 37 General Chapter <51> Antimicrobial effectiveness testing. Rockville, MD: US Pharmacopeial Convention; 2018. [Google Scholar]


