Abstract
As more meetings become virtual, the impact of “live” meetings is being reevaluated. Here one example of how a chance meeting at a national pharmacy meeting led to the development of a new drug therapy that reinvented how parenteral nutrition is provided to infants and children is described. Along the way, many lessons were learned both in the lab and at home. Addressing the challenges raised by others, understanding how the FDA works, and the power of parental involvement are all considered. Until 2013, the only FDA-approved lipid emulsions were those composed of pure soybean oils. Starting with compassionate use protocol in 2004, it took 18 years and hundreds of patients to bring a pure fish oil lipid emulsion to the US market. First used off label to treat a soy-allergic patient dependent on parenteral nutrition, researchers at Boston Children's Hospital later conducted animal studies on its role in treating and preventing intestinal failure associated with liver injury and later translated it into clinical trials that led to the drug's approval in 2018. This is a recount of those efforts.
Keywords: essential fatty acids; fish oil, lipid emulsion; mentoring; networking; Omegaven; parenteral nutrition; pharmacy; resilience
Introduction
Even though I have known about being honored with this award for more than 2 years, I am still very humbled and speechless. I was on the Pediatric Pharmacy Association (PPA) board of directors when Stephanie Phelps first suggested this award and I had the privilege of serving on the first selection committee with Dr Richard Helms. So much has happened since that time and the recent crises in the world have given me the unique opportunity to really reflect on my career and life in general.
Originally, I had titled this lecture “A Whale of a Tale,” but I had used that before and it didn't really cover what I wanted to share. The story of drug discovery is always an exciting one, but how we got from bench to bedside is really the more important story. It is the power of networking and how the chance encounters you make at professional meetings become life-changing experiences (Figure). This year's Covid-19 pandemic has really affected us and may make people think twice before they travel to meetings. I hope my experiences will cause you to maintain a commitment to continue to attend live meetings when we can safely travel again.
Figure.
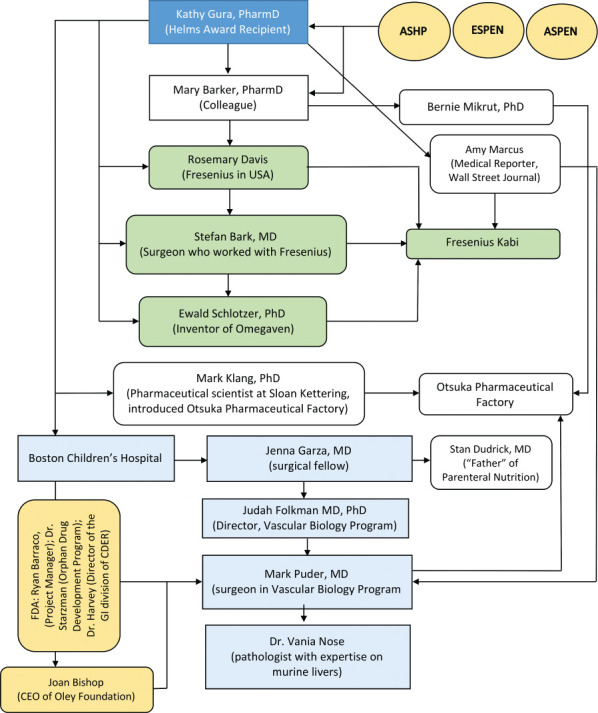
The power of networking.
This story begins with Dr Mary Baker, whom I have known for more years than I can remember. That said, I do vividly remember how we met. Back in the old days, poster sessions were a big part of the American Society of Hospital Pharmacists meetings. It was traditional to schedule enough time to do a thorough walk-through of the posters and met Mary when I was checking out compatibility and parenteral nutrition (PN)–related posters. We ran into each other again at the American Society of Parenteral and Enteral (ASPEN) meetings. We would commiserate on various problems and a friendship began to develop. It was during one of our conservations that I asked her a question that changed my career and ultimately the way many of us provide PN to infants and children today.
Sometimes You Need to Take a Chance
Chapter 1. How Does a Soy Allergy Relate to a Treatment for Liver Disease? In June 2002, we had a 16-year-old male in our bone marrow transplant unit who had a severe soy allergy. He was very ill and his parents had requested a do-not-resuscitate/do-not-intubate order, but they were passionate about continuing enteral and/or PN. Originally, he only received enteral feedings, but a severe GI bleed prevented reliable and consistent administration and a decision was made to begin PN. Following a 0.25-mL test dose of Intralipid (Fresenius Kabi, Uppsala, Sweden) he had a full-blown anaphylactic reaction. He also had a nasty rash that had been mistaken for acne. An attending physician on the nutrition team determined immediately that it was not acne but was in fact an essential fatty acid deficiency, which likely occurred because he wasn't receiving the prescribed enteral formula. This posed a challenge—how to provide essential fatty acids as his GI bleeding precluded enteral administration and topical oils are known to be unreliable. The nutrition team asked me for suitable options; hence, I contacted all the big nutrition support teams in Boston and put the question on various nutrition listserves. All but one person said the same thing: topical oil; don't bother with desensitization because it is impossible to do with food allergies. A drug information pharmacist (unfortunately his name escapes me) within the medical professional division at Baxter International (Deerfield, IL) didn't have any new solutions but did tell me how to complete an emergency investigational new drug (eIND) application along with the necessary FDA paperwork should I find a suitable alternative. The one person who came up with a different answer was Mary Baker. She is a frequent attendee at the European Society of Parenteral and Enteral Nutrition (ESPEN) meetings, where she learned of pure fish oil–based (i.e., soy-free) lipid emulsion (Omegaven, Fresenius Kabi, Bad Homburg, Germany). She faxed me a brochure containing product information and I shared with the primary team (oncology) what I had learned. Although the patient had several contraindications listed in the brochure (i.e., bleeding, hyperglycemia, elevated liver function tests) the team felt they could deal with any complications should they arise and asked me to determine if and how we could get Omegaven.
Chapter 2. The Challenge of Importing Omega-ven. The US division of Fresenius Kabi only dealt with dialysis-type products; hence, they did not even know Omegaven existed. Eventually, Mary Baker, who had some connections with Fresenius, introduced me to Rosemary Davis, who worked at a small Fresenius plant in North Carolina. In turn, Rosemary was able to connect me with Dr Stefan Bark, a surgeon based in Uppsala, Sweden, who also worked with Fresenius.
Pedi amino acid solutions contain less methionine in their formulations, but the downstream metabolites cysteine and taurine accommodate for this. The logic for using fish oil is similar to that for pedi amino acid products. Although fish oil has less linoleic acid (LA) and alpha–linolenic acid, the downstream metabolites arachidonic acid (ARA) and docosahexaenoic acid (DHA) may be able to reverse the essential fatty acid deficiency (EFAD) in our patient. After discussions, Dr Bark agreed it was worth a chance. Interestingly, I learned many years later when I talked to Ewald Schlotzer, PhD, the inventor of Omegaven, that had he called me back first he would have talked us out of using the product.
So, with all this information in hand, we drafted a protocol. Omegaven was originally designed as a supplement to be added to Intralipid and not used as monotherapy. If there was a list for the use of off-label products, surely our use would top that list. Our protocol used Omegaven as monotherapy, used 5 times the labeled dose, and we planned to administer it in a pediatric patient with numerous contraindications to its use. We submitted the protocol to the FDA as an eIND and were granted permission to use the fish oil within 48 hours. Dr Bark arranged an emergency shipment, which we received within days. In retrospect, it was probably best that an oncologist was the principal investigator on this project—they were willing to take the risks; moreover, they were able to think outside the box. Like me, they knew little about EFAD. The nutrition attendings, after hearing me out, were willing to try given there were no other suitable options.
The first dose of Omegaven was infused on a Saturday morning and proved to be a big non-event. Given the patient's earlier anaphylaxis triggered by a test dose of Intralipid, we were relieved that he not only tolerated the test dose, but also received the full infusion without any adverse effects. He remained on Omegaven for almost 50 days, and his EFAD corrected within the first 3 weeks of treatment. Despite having many of the contraindications, he did not experience worsening of any condition (i.e., bleeding, hyperglycemia, elevated liver function test); in fact, the bleeding resolved. Although I wasn't focusing on it at the time, his liver function greatly improved. All we focused on was his rash and the triene to tetraene ratios. By August, he was transitioned back to some enteral feedings and by September he was discharged home and was off PN shortly thereafter. When he went home, I emailed Mary to say if anyone ever gave her a hard time about international travel, to look back on this experience; had she not gone to ESPEN, she wouldn't know about Omegaven and wouldn't have been able to provide me with the much needed information. Simply put, a young man who had been under a do-not-resuscitate/do-not-intubate order in June went home 3 months later because she shared the knowledge she gained at a meeting.
I learned a lot from that experience. First, I learned about the whole eIND process and how to import drugs from Europe. I learned to write a full clinical protocol and developed dosing from what I knew about amino acids, not essential fatty acids. I learned that Omegaven could be safely used as monotherapy and could correct EFAD despite being low in linoleic acid and alpha–linolenic acid. Out of habit I kept all the paperwork associated with this whole process. In fact, it is still in my file cabinet 18 years later—just in case. I also kept a case of the unused drug in my office—just in case.
In September 2002, I attended my first ESPEN meeting in Scotland and learned more about lipid emulsions. It seemed that the Europeans were light years ahead of the US market in regard to nutritionals. Also at this meeting, through my colleague Mark Klang, PhD, a pharmaceutical scientist at Sloan Kettering, I connected with the folks from Otsuka Pharmaceutical Factory. They asked me to attend select sessions and to write them up for subsequent translation to Japanese. Several months later I presented the soy allergy case report at the ASPEN meeting; it was the first time Omegaven had been used as monotherapy and the first time it was presented at a meeting. The only people who stopped by were Mark Klang, Mary Baker, Dr Bark, Dr Schlotzer, and a few scientists from Otsuka Pharmaceutical Factory. Unfortunately, no one else cared. Why I mention all these people that I met over 18 years ago will become more apparent later.
Chapter 3. Summer of 2002—A Time for Many Other Connections. While I was dealing with our soy allergy patient on the oncology floor, I was also involved with our newly established Center for Advanced Intestinal Failure (CAIR) program—aka “short gut” team. We were busy, but that summer was an especially sad one for us. Six infants on the surgical ward had succumbed to PN-associated cholestasis. Dr Jenna Garza, a surgical fellow who trained in Dr Dudrick's (the inventor of PN) program, was with us for her research years and wanted to tackle the one thing Dr Dudrick was unable to solve: why some kids died of PN-associated liver disease (PNALD). I already knew Jenna through our newly established CAIR program, which followed up infants and children with intestinal failure. Coincidentally, Judah Folkman MD, PhD, also had a similar passion for discovering the cause of PNALD. Dr Folkman thought it was either a toxin or something missing in PN formulation. Interestingly, he had found that he was able to correct orally induced PN-associated cholestasis in rats by giving them a single pellet of rat chow. I used to compound the PN used in those studies but didn't really pay much attention to what he was doing. Moreover, in 1996, when Dr Folkman learned that I was returning to get my PharmD, he asked me to dialyze the rat chow to see if I could determine what was the missing link, but I passed on the opportunity like everyone before me.…I still didn't see the point.
As Dr Garza branched out into translational research, she began to look at how to approach the PNALD problem. During this time, Dr Folkman had a young surgeon (Mark Puder, MD) in his Vascular Biology Program who was doing basic science research on angiogenesis. She needed a laboratory-based physician mentor and Dr Puder was asked to serve in that role. Dr Puder had no real interest in nutrition research, but he was into matrix metalloproteinases and angiogenesis inhibitors. Fortunately, he wasn't just a scientist; he was a clinician who had trained as a pediatrician before he became a surgeon.
Dr Garza took Dr Folkman's rat model of using oral PN to induce liver disease and with Dr Puder's help, converted it into a murine model. Because I had previously made the PN used in Dr Folkman's rat studies, I was asked to make the same PN for the mice that we had used 10 years earlier. Dr Garza was able to get the model operational. Because the medical literature at the time of Dr Folkman's initial research suggested that IV fat emulsions were not implicated in PNALD, Dr Folkman did not incorporate them into his model. At Dr Puder's suggestion the model was changed to replicate what is done in clinical practice. Dr Garza changed the model slightly and gave the mice injections of fat emulsions. Surprisingly, she noted that liver disease occurred in the mice with and without the use of IV fat. Soon thereafter, Dr Garza completed her training at Boston Children's Hospital (BCH) and returned to Dr Dudrick's program at St. Mary's Hospital (Waterbury, CT), leaving Dr Puder without a laboratory person to continue any further experiments.
Dr Puder and Dr Folkman discussed possible explanations for why mice would develop liver disease in both the presence and absence of IV lipid emulsion. Although Dr Puder had no expertise in nutrition apart from what he had learned during residency training, he realized that 2 different mechanisms might explain this finding. In the fat-free Folkman model, the mice became deficient in essential fatty acids and got steatosis from the high carbohydrate diet they received. When the mice were given a concomitant injection of IV fat, their liver disease was attributed to a different mechanism. When the fat was give enterally via gavage, similar to what happened when Dr Folkman gave the rats a pellet of rat chow, the liver disease was avoided. Had we dialyzed the rat chow when Dr Folkman asked me to, we would have determined that the fat in the chow was actually protective. We later published the importance of the lipid administration route in 2005.1 In that article, we showed that IV administration of soybean oil was hepatoxic but when given orally, was hepatoprotective.
You might ask “How does this relate to Omegaven?” I had just returned from the ESPEN meeting in Scotland where I had gained new knowledge about lipids. I asked Dr Puder to humor me and use the leftover Omegaven in my office for the mouse model. We repeated all the experiments using Omegaven (oral and IV) as the lipid source along with oral chow and injectable soybean oil controls. Without Dr Garza, I became the new lab assistant helping with the experiments. Dr Puder and I continued to do our regular jobs during the day and conducted mice research on weekends and evenings. In June 2003, we took several boxes of liver histology slides to Dr Vania Nose, a pathologist at Brigham and Women's Hospital who happened to be an expert on murine livers. She was blinded to what formula or diet the mice got—it was all coded in our lab book. My job was to record her observations about the presence of liver pathology. We were gathered around the teaching stereo microscopes in the pathology department as she called out the results. All of the Omegaven-treated mice were read as normal, the same as chow-fed controls, while the soybean oil–treated mice had severe steatosis and hepatic necrosis. Dr Puder and I looked at each other and did a silent high five as we knew we were onto something. We then took it to the next level and caused liver disease in the mice by giving them fat-free PN for 19 days, and then gave them either IV soybean or fish oil IV lipid emulsion. Again, we sacrificed the mice and took the livers to Dr Nose who read the histology slides. The results were consistent with what we previously saw: IV Omegaven reversed the PNALD, while the preexisting liver disease was exacerbated in mice given IV soybean oil. We published these findings in Pediatric Research.2
We were confident we were onto something and began to discuss our findings with others at BCH. The response was not what we expected. We were told “Everyone knows it's not the lipids because liver disease happened with them and without them.” Some individuals would even pull me aside to ask “Are the results real?” Dr Folkman was totally supportive of our research methodology and our findings and said we should ignore the naysayers and needed to expand our research to other animal models. We wrote almost 20 different grant proposals to a variety of funding sources including ASPEN, American College of Clinical Pharmacy, and the Gerber Foundation. The response was always the same…. “Everyone knows it's not the lipids.” Even Fresenius, who held the rights to Omegaven, was not interested in sponsoring our research. One rejection letter from a pharmacy organization noted that I wasn't qualified to do translational research, Dr Folkman (the father of angiogenesis) was not a suitable mentor, and Harvard was not the proper place to train a pharmacist in scientific research! Luckily, BCH, the Departments of Pharmacy and Surgery, and the Vascular Biology Program (Dr Folkman's' lab) saw value in our work and funded us when no one else would. That is one of the reasons BCH is so special and why I believe that this discovery could not have happened anywhere else.
Chapter 4. September 2004…From Mouse to Man. While we continued our “day” jobs, we persisted with the mouse studies and with writing grant proposals that never got funded. My oldest daughter, Alessandra (aka Sandy) decided to volunteer at BCH where she took care of a little guy named Charlie who was born with gastroschisis and was PN dependent. He had PNALD, but was too small to be considered for a liver transplant. His surgeon, Dr Rusty Jennings, isn't one to follow the status quo. Some may call him a “cowboy,” but he is one of those guys who just won't give up. He came to Dr Puder and me one afternoon and asked us to do the mouse experiment using our “fish squeezings” on his patient Charlie. It was during this time that we couldn't get funding for additional animal studies. In fact, in our last submission, we stated, “if these experiments prove to be successful, they will provide additional evidence to translate this work into clinical studies in humans.” It was our hope to buy Charlie time to gain the necessary weight to make him eligible to undergo a multivisceral transplant.
Although Dr Puder was hesitant about doing this, because the only other case of fish oil monotherapy was that of my soy allergy patient 2 years' prior,3 he agreed. I retrieved all of my old soy allergy paperwork and began the process of developing a protocol that included informed consent. I also began to prepare the required FDA materials. Additionally, I contacted Fresenius and spoke with Dr Schlotzer, the inventor of Omegaven. Although initially hesitant, they ultimately agreed to provide a new supply of Omegaven, especially for Charlie for as long as he should need it. Charlie's parents understood that this was a long shot, but they felt they had no other options. They knew Charlie was the first baby, and the first patient with liver disease, to receive Omegaven. The only request they made was that if it worked we would offer it to other children just like Charlie.
Charlie started Omegaven on a Saturday night in September. Dr Puder and I stayed at the hospital in the event issues would arise. Like the original patient with soy allergy, it was a big non-event. Every day Dr Puder and I rounded on Charlie. I kept massive spreadsheet of all sorts of data—anything related to liver disease, inflammation, fats. Anyone observing this would attest to the fact that I overcollected data! About 10 weeks after Omegaven was begun, Dr Puder and I reviewed Charlie's laboratory values and finally saw the bilirubin levels go below 2 mg/dL. By definition he was no longer considered cholestatic—his PNALD had reversed while he continued to receive full PN! By now Charlie was home and growing. Unfortunately, the other babies who were on the ward with him had died of PNALD. To this day, 16 years later, Charlie's mom recalls their names and remembers it could have been her son who succumbed. We called Dr Schlotzer to inform him of the good news, thinking we might get some research support. It didn't work.
Chapter 5. From 1 to 10 to 300. Over time, more cholestatic babies were treated with Omegaven at BCH by the dynamic duo of Puder and Gura. The results were fairly consistent, as those seen with Charlie. During this time, administration of Omegaven in each patient was done under an eIND request. Eventually, the FDA suggested we apply for an IND. Having never done an IND application we turned to our project manager at the FDA, Ryan Barraco. He suggested we begin by making an appointment with the FDA for a pre-IND meeting. So team Puder-Gura traveled to Bethesda, MD, to meet with individuals at the FDA. Enter Mary Baker, who mentored us on how to prepare for the 1-hour meeting at which we would make our case. We arrived to find a room full of FDA people including pediatricians, statisticians, and Dr Starzman of the Orphan Drug Development Program who was brought in at the request of Dr Harvey, the division director of the GI division of the Center for Drug Evaluation and Research (CDER). As expected we were initially petrified, but by the end of session, they were thanking us for doing this research. They explained to us the available grant funding opportunities that were consistent with our research. One of the FDA representatives suggested that we find a company that would aid us in bringing fish oil to the US market. Dr Harvey told us to contact his brother, the president of the Massachusetts Medical Society to see if he could be of assistance.
At that time, with the exception of Charlie, the Surgical Foundation had paid for the Omegaven. When other non-surgical departments asked that their patients receive this therapy, the costs were assumed by BCH endowment, saying it was part of its mission. This made it possible for any patient at BCH who qualified to get Omegaven.
At first we didn't really publicize this work. What if it was a flash in the pan and could not be reproduced? We already had some pushback within our hospital by those who said the effects we saw were totally due to the smaller dose of Omegaven than of soybean oils. We published our first clinical paper in 2005, the soy allergy case report.3 That only happened because Dr Puder pushed me to do so. Others who had been involved in the child's care didn't think it was a significant or unique event. In my mind it was only important because it was the first case report of EFAD being reversed by fish oil. It also provided an alternative to topical oils for patients unable to use soybean oil IV lipid emulsion.
After 13 patients had received fish oil, we decided it was time to publish our experience as we could demonstrate that the results were in fact reproducible. I had presented 2 cases from our Omegaven experience earlier that year as a platform session at the Pediatric Intestinal Failure Consortium (PIFCON) conference in Pittsburgh, PA. Again it seemed that no one cared except for Paul Wales, a surgeon from Toronto's Sick Kids, and no one else asked a single question. From our experience in publishing the case report in Clinical Nutrition, we thought subsequent publication involving more patients would be easy. Since we were in Boston, Dr Puder contacted the editor of the New England Journal of Medicine for advice and they suggest we go for it; however, the editor rejected the paper and did not even send it for review. Similar to the difficulties we experienced with grant applications, we submitted our paper to at least 10 different journals. It was a full year before our first paper, discussing 2 patient cases, was published in Pediatrics in 2006.4 Even then, it was only published electronically and was not included in the printed journal. We had no idea what to expect as a response. The day after online publication, another dynamic duo, Dr Bob Poole (the first Helms Award recipient and Director of Pharmacy) and Dr John Kerner (Director of the home PN program) at Lucille Packard Children's Hospital contacted us and asked if we would share our protocol and all accompanying information. We continue to work together to this day on various projects—they are our West Coast compatriots and “got us.”
With the publication of the Pediatrics article, we began to see referrals from other centers and were contacted by motivated pharmacists and dieticians (rarely physicians) on how to implement the protocol at their institution. You would think the inquiries would have come from well-endowed academic medical centers like Stanford, but that was the exception and not the rule. It was small hospitals that inquired, like those in South Dakota (PPA legend Dr Helen Fiechtner was another early adopter) or the community hospital in Minnesota that had its local chapter of the Red Cross cover the costs of the emulsion. We made relationships with trainees like Dr Kara Calkins, a young medical student at University of California, Los Angeles (UCLA), whose team assigned her to write their own protocol for use. She later became an attending neonatologist at UCLA and her research became part of the new drug application (NDA) for Omegaven. We continue to collaborate to this day. In another case, the US Public Health Service flew a pharmacist couple, who had a child with short-bowel syndrome and PNALD, to Boston to learn how to implement the protocol themselves in Alaska. Since I was the literature and people person of the team, I made sure that we aggressively pursued any connections made at national meetings. One such example was at the Pediatric Academic Society meeting in Toronto in 2007. Dr Steve Abrams had a poster close to mine. I knew of him from his numerous publications as the neonatal nutrition/bone expert. After discussing our work, he asked for the protocol, and soon Texas Children's became the second largest site after Boston to treat kids with PNALD by using Omegaven. They later became the second site included in the NDA application.
Our trainees began to graduate and relocate and use Omegaven at their new home institutions. We asked few things of those given our protocol—to let us know how their patients were doing and to publish what they found. Some did; unfortunately, most didn't. The number of requests began to exceed our ability to respond. Luckily, because of my work with the clinical nutrition service, I knew of the Oley Foundation and asked if we could house our information on their website. It would be a way to “get the word out” and drive more traffic to their website, and perhaps increase Oley membership. It was a win-win for both of us. When I went to my first annual Oley meeting in Cape Cod in 2006, the only kids at the meeting were our “Omegaven Families.” Joan Bishop, the CEO of Oley, said that meeting changed the face of the organization. From that time forward, a large influx of pediatric consumers and their families began to attend the Oley meeting. They even had to create separate pediatric programing for the kids.
Chapter 6. We're in the Money…Sorta. In the midst of treating patients, we were criticized, and rightfully so, as we had not done any well-designed randomized clinical trials. We had submitted protocols to numerous funding agencies, but similar to our experience with the translational grant applications, we were always rejected. Finally, in 2006, the March of Dimes and the FDA Orphan Drug Development Program funded us to conduct a prevention study comparing Omegaven to the standard of care (i.e., soybean oil lipid emulsions). The FDA also funded us for a treatment protocol using Omegaven. These grants helped treat approximately 30 children who met specific inclusion/exclusion criteria. The rest who were being treated for PNALD continued to have their study costs covered by BCH. We published the results of the prevention trial in the Journal of Parenteral and Enteral Nutrition5 and the treatment trial in Annals of Surgery.6
Media wars can be helpful. After all of our success, the manufacturer of Omegaven still wasn't planning on bringing the product into the United States. Hospitals that did use it had to do so on their own. This required that each institution have its own INDs and funding source. Eventually, the FDA created its own website and had its team address requests. In 2006, Amy Marcus, a Wall Street Journal medical reporter who had followed the work in the Folkman lab and knew about the study decided to write about it. She interviewed us about the challenges we had faced, the successes we had experienced, and the significance of our finding.7
All of this was an attempt to “encourage” Fresenius to release Omegaven in the United States. In 2007, after Dr Schlotzer and Fresenius CEO met with some of our families, they asked for a media truce. The FDA also got caught in the crossfire; however, this was misguided because they had no NDA to evaluate. If a company doesn't submit, they can't approve or deny a drug. Finally, after 5 very long years of work the manufacturer was on board.
In addition to the Wall Street Journal piece, the FDA held workshops on PNALD and a second on lipid emulsions. Surprisingly, no one from our group of researchers was asked to speak—what is it they say about a prophet in their own home? Years later in 2016, Andrew Mulberg, a pediatric gastroenterologist and deputy director of GI CDER invited me to speak at the FDA's liver workshop to highlight the differences in pediatric and adult biomarkers of drug-induced liver disease.8 Dr Puder even did a piece on NBC's Rock Center, highlighting the challenges associated with Omegaven therapy.9 In 2012, Fresenius began the process of submitting an NDA for Omegaven as a treatment for PNALD. Through FDA guidance, the NDA was later changed to a nutrition indication. Had the NDA remained a therapeutic indication, then liver biopsies would have been necessary, which could have proven fatal to a neonate with PNALD.
Chapter 7. Everyone Loves a “Winner”…Not. No one likes the new kid on the block who brings ideas that go against convention. The fact that we demonstrated that IV soybean oil was detrimental, but IV fish oil was not, created controversy in the medical community. Papers and commentaries were published that suggested that Omegaven caused growth failure, bleeding, and progression of hepatic fibrosis. These criticisms led to us to do more translational and clinical studies. We began to have fellows join the Puder lab from the area hospitals and even from the Netherlands. This meant that I was no longer the principal lab technician. Eventually I was eased out of my pseudo study coordinator role as the hospital hired a research nurse and study coordinator. I continued to oversee the day-to-day Omegaven dispensing, but assigning some activities to other individuals enabled me to focus on clinical trial design and manuscript preparation. My pharmacist's training caused me to run everything like a sponsored trial in the event someone in industry would take over the protocol. Finally, that day happened in 2012. I'm proud to say our records were pristine and that the FDA mentioned it during their site visit as part of the NDA review!
With every paper we published or lecture one of us gave, we had to address limitations of our work. Originally we had every intention of doing a double-blind, randomized, controlled trial comparing Omegaven to Intralipid but we never received the necessary funding. When we did finally acquire funding, it arrived after we had accrued years of data of successfully treating patients. To randomly assign a patient to an arm of the study that administered a product thought to cause disease would in our judgment have been unethical. At the FDA PNALD workshop, others in the room told the FDA there was no longer equipoise; Omegaven was now becoming standard of care.
I was reviewing manuscripts for a variety of journals at that time and was sent one from Hong Kong. The authors conducted the very study we couldn't do…a randomized, controlled trial comparing equal doses of Omegaven and Intralipid in infants with PNALD. The authors stopped the trial early, but failed to explain why. I inquired about this and was told that parents didn't want their child to be part of a study where they might get randomly assigned to receive soybean oil. They wanted their baby to get Omegaven. I insisted the authors include that in the revised manuscript.10 This paper was later accepted and convinced the FDA that a randomized, controlled trial was not necessary. The FDA would instead accept pair-matched historical controls. In 2018, I had the pleasure of lecturing at a meeting of the Hong Kong Surgical Society, where I acknowledged Dr Lam and his team for their important work that influenced the FDA.
Omegaven Doesn't Cause Bleeding. Whenever you talk about large doses of fish oil, someone always asks about bleeding. The evidence for this concern came from studies done by Danish researchers studying cardiovascular disease in Greenland Inuits who had a diet high in omega-3 fatty acids.11 In addition to having coronary artery disease in comparison to Danes, the Inuits had a high incidence of bleeding gums. The authors postulated it was due to the high omega-3 content of their diet. For decades, many practitioners accepted this and instructed patients to hold doses of fish oil preoperatively lest they have bleeding complications. I wasn't familiar with these data and we had given Omegaven to the patient with soy allergies who had GI bleeding severe enough to require parenteral estrogen at one point.
During this time, I was also serving as the codirector of the Harvard Nutrition Course with Dr George Blackburn. He introduced me to Artemis P. Simopoulos, MD, the physician who popularized the Mediterranean diet.12 She taught me that the omega-3's did not cause bleeding; the body was able to regulate itself so that although the blood cells became more pliable, bleeding did not occur. She also directed me to the American Heart Association guidelines and the work of Bill Harris who did considerable work with the omega-3 index and heart health.13 Each provided more evidence that fish oil did not cause bleeding. Likewise, we knew from our clinical experience that bleeding didn't occur in our patients and we weren't holding doses of fish oil preoperatively. In fact, platelet counts rose as the liver recovered. None of this was enough to stop the detractors from claiming that it did. We decided to do a study on bleeding risk in patients treated with Omegaven.14 We looked at all patients who underwent surgery and their bleeding events and compared them to published normal groups. Bleeding was the same or less.
Omegaven Doesn't Cause Essential Fatty Acid Deficiency. Despite the fact that Omegaven had been successfully used to reverse preexisting EFAD and although the FDA accepts it as a source of essential fatty acids, concerns continue to be raised that it isn't a complete fat source. When we first started hearing these concerns one of our fellows took the lead to review and summarize our data, which showed that it did not cause EFAD.15,16 Another fellow designed a murine study in which the mice were only fed the downstream essential fatty acids DHA and ARA.17 The mice did fine and were able to keep the line going for 9 generations. Additional neurodevelopmental and fertility studies arose from that work.18,19 Others, who felt the need to mix Omegaven with soybean oil, showed that the combination did not reverse PNALD.20
Omegaven Doesn't Cause Growth Failure. Another frequent concern was that Omegaven would stunt infant growth. None of our patients had the traditional appearance of a “short-gut kid” to us. In full street clothes, they looked “normal.” Studies involving enteral formula had shown that DHA alone (i.e., no ARA) resulted in growth failure.21 Omegaven has both. We published 2 articles on the topic.22,23 The first was done with colleagues Chris Duggan and Bram Raphael and it compared our patients treated with Omegaven to children with PNALD in the PIFCON database.22 The other was the growth data that were used in the NDA submission to the FDA.23 In both cases, growth was similar or better than what was in the control groups.
Omegaven Isn't a Wonder Drug. With every patient treated, we always asked families to look for anything amiss. We would tell them “You know your baby better than we do and you will be the first to know when they are getting better and if there are any side effects.” No one mentioned any side effects, only that their once sleepy babies were now more active. In several instances, the patients continued to get worse. We had one of our fellows take a deep dive into our database to identify factors that predisposed patients to an Omega-ven treatment failure.24 We learned that patients whose PNALD was left untreated for too long had the highest risk of treatment failure. In another case, a severely malnourished infant with both EFAD and PNALD did not respond to Omegaven until the dose was increased to 1.5 g/kg/day.25 In response to questions from others about the benefits of Omegaven in other forms of drug-induced hepatic dysfunction, we did a murine study to determine the benefits of Omegaven following acetaminophen toxicity.26 We found that it didn't help and might even exacerbate the hepatic damage. As Dr Puder once said, “Omegaven may not be a wonder drug, it may simply not be as bad as the others.”
Chapter 8. Other Lessons Learned. Show Gratitude. No one believed me when I said it takes 10 years for a drug to get from bench to market…it took a total of 14 years from the time we treated Charlie until the Omega-ven received FDA approval. Many people could have stopped us at various times along the way but didn't: the FDA who continued to allow us to treat patients under eINDs and made the information available to others; Sandi Fenwick, the CEO at Children's Hospital who approved the pharmacy department's exceeding its operating budget for more than a decade to cover the cost of the drug; and Eileen Sporing, the VP of patient services at BCH who successfully advocated for me when the IRB [institutional review board] would not let me consent patients for my own drug study. I would be remiss not to acknowledge the Clinical Nutrition Service at BCH that helped optimize nutrition for these kids. At any point, had one of these groups said no, the study would have stopped. The pharmacy staff at BCH was extremely important to this entire project. This study ran 365 days a year for over 14 years. The buyers, the inventory clerks, sterile products technicians, and pharmacists were each involved in making sure the kids got their Omegaven when needed. To Al Patterson, director of pharmacy, thank you for allowing me the gift of time to do this study and all the related ones. Like the others, you could have said no, but you never have. You have always been one of my biggest supporters and I am again grateful for your continued support over the years.
Of course much is owed to the more than 300 families who trusted Dr Puder and me with this therapy. We weren't sure if it would work, and as mentioned previously, it is not a miracle drug. Some children died and when that happened we cried with their parents. We continue to keep in touch with many of the success stories, attending birthday parties, bat mitzvahs, and the like. Many of the babies are now in high school, soon going off to college. I am grateful you were willing to take a chance on us and wanted us to stay in your lives for the happy times.
I'd like to also thank my other mentors, starting with PPA founders Doug Smith and Steve Allen who gave me my first job as a pharmacist at the then Children's Hospital National Medical Center (CHNMC). Doug's calcium phosphorous curves continue to be used to this day. Sam Roberts, CHNMC's IV room supervisor, who instilled in me his passion for compounding and PN in general. Thanks also to Joe Sceppa, my first boss at BCH, who had the strength to call it as he saw it and told me I was clinically, not managerially, focused. Lastly, the original members of the Clinical Nutrition Service at BCH: Clifford Lo, MD, PhD; Sharon Collier, RD; Denise Richardson, BSN; and Bill Eichelberger, RPh. They taught me the basics and brought me into the fold. I am now the last man standing at BCH from that team, and I am now training the next generation of nutrition support pharmacists such as Dr Margaret Murphy.
Thank you also to my partner in crime, Dr Mark Puder. We are a true team, each with our own strengths and weaknesses. He taught me how to be a scientist and gave me the confidence to advocate for myself. We've become the elder statesmen in the lab and the fellows are now younger than our children. Thanks for continuing to work with me after all these years.
It goes without saying that I need to acknowledge and thank Dr Helms for his contributions to the profession. I knew of his work in nutrition before we met. I later learned that both he and I were both Massachusetts College of Pharmacy alumni. Dr Helms became a remote distance mentor for me as I had no pharmacist mentor of his caliber in Boston. Thank you, Rich, for your guidance and mentorship over these many years.
Take Advantage of Opportunities When They Present. Over time, we started lecturing and meeting researchers around the world regarding omega-3 fatty acids and fish oil. I was asked to work with Hanoi Medical University to help establish a clinical nutrition program as a result of my work with Carine Lenders, the physician who first identified EFAD in the patient with soy allergies. Through those efforts we have decreased morbidly and mortality in patients receiving specialized nutrition.27 Through my work with fish oil I have been able to collaborate with Drs Philip Calder, Sheila Innis, and Gordon Jensen, all preeminent scholars and me the only pharmacist in the gang. It led me to be part of the pre-B project with the National Institutes of Health to optimize neonatal nutrition, and then to partner with the Academy of Nutrition and Dietetics and Dr Tannis Fenton to come up with new nutrition guidelines for extremely low-birth-weight infants.28 Again, I'm the only pharmacist at the party and it was only through networking that I got the invite.
In 2012, Dr Puder and I started working with a Norwegian fish oil company who helped us set up a lab so we could make our emulsions. This enabled us to tease out all facets of the lipid emulsion story—the influence of phytosterols, vitamin E, and natural triglycerides versus re-esterified oils. Making lipid emulsions is not a skill taught in pharmacy school. Once again, I turned to Mary Baker. I knew she had worked with Bernie Mikrut, PhD, when they both were at Abbott and I had attended several ESPEN meetings with them in 2002 and 2004. He made lipid emulsions in the lab at Abbott and had even made a generic version of Omegaven. He was now retired, so we asked him to help us set up the lab. He taught us the whole process and soon we were able to formulate USP 729 quality emulsions that we could use in our animal models. That led us to the work we are doing today—how to design better emulsions for different patient populations.29–31 I had kept in touch with the Japanese scientists that I met in Glasgow through Mark Klang. They had been following our research and wanted to partner with us on lipid emulsion product development. They are essentially taking our lab work and moving it to the next level. We have come full circle since 2002. We would never have connected had it not been for Drs Baker and Klang and that ESPEN meeting in Glasgow in 2002. That Glasgow meeting was also important because my nutrition mentor, Dr Clifford Lo, was a visiting professor at Yorkhill Children's Hospital in Glasgow and introduced me to Dr Tom Diamantidis. This nutrition support pharmacist owned a home care company and was well connected with many of the leading nutrition teams throughout the world, and we are now about to embark on several new projects to improve the lives of home PN patients.
Chapter 9. Life Still Goes on Outside the Lab. When all this stuff was going on, life continued outside the lab. I could have talked about much of what is to come in the section entitled “Show Gratitude,” but these comments go beyond gratitude. Starting with the patient who had soy allergies, many aspects of my family's life have been impacted in some manner by my work. When the decision was made to pursue Omegaven for that first patient, I was attempting to juggle work and family and was trying to get my 2 young daughters off to sleep-away camp. I still remember driving down to the Cape while talking to a variety of people from around the world with lousy cell phone service and horrific traffic and telling the girls to be quiet.
It was a very long time from the initial mouse experiments to achieving FDA approval—14 years in fact. My oldest daughter, Sandy, was a high-school sophomore when this started, went through nursing school, immigrated to Israel, got a master of public health degree, married, and had her first child, Shoshana, all before Omegaven was approved in 2018. My other daughter, Samantha, was only in 7th grade and had to compete for my time, and often lost, as she went through high school and college at the Art Institute in Philadelphia. Along the way I missed some important milestones in their lives. That is one of my biggest regrets. Samantha summed it by one time telling me that “I loved the kids at BCH more than her,” which isn't true, but given all the time I spent on this, it was totally understandable. In 2014, she gave birth to twins, Kelli and Cassidy, the first of my 4 grandchildren. They were born prematurely. When they were in the NICU, they told her the twins were lucky as they didn't need PN. She told the team their grandmother would be doing the happy dance because of that. They are now entering the first grade and Cassie wants to be like grandma—I hope not.
While Sandy lived in Israel, we got to meet one of the teams using Omegaven at Soroka Medical Center in Beersheba. This was the first time my husband Scott saw the efforts of my work—when they gave us a tour of their NICU and introduced us to several patients who were receiving Omegaven. He had never seen such sick, yellow babies, which I took for granted. Sandy is now a nurse at Children's Hospital Los Angeles and often administers Omegaven. Having lived through the whole experience, it was an unusual feeling for her at first. All the while Scott held down the fort while I did Omegaven work after my shift at Children's was over. Some days ended into the wee hours. Thank you all. I know it wasn't easy nor was it fair but thank you for letting me pursue my dream.
My mom said that as a child I told her I was going to invent a drug to treat Kozak's disease…well, that's not exactly what I did but it is pretty darn close. It's a shame Dad wasn't there to see it. My mother Joyce Kozak, my father Phil, and my brother Steve were all big supporters, for me and for one another. My mom continues to be my role model for what it takes to be a successful working mom; my dad, he instilled in me the importance of treating your whole staff as equals; and my little brother just for being there and reminding me when I was going off the rails to think of the big picture. Now I am trying to enjoy my family with the same passion that I gave to the research years.
In addition to our blood families, many of us have professional families. My former office mate, Dr Shannon Manzi, was there when this all unfolded. You are truly amazing and I appreciate your encouragement and un-dying support. PPA and its members were always a part of this story, whether it was speaking at a meeting, talking to a pharmacy resident like Emma Tillman about her research project, or serving on its board. When things with Omegaven started to take off, I had to step down as Vice President of Finance because I knew I wouldn't have time to do the job that the PPA requires, do this study, and be a wife and mother. Fortunately, I served with a great board that stepped in to get it done. I was proud to be part of the PPA board during one of the biggest transition periods of PPA and during the time we hired Matt Helms as PPA CEO. It has been wonderful to watch its membership expand exponentially and to see PPA mature into the preeminent pediatric pharmacy organization in the world. It was truly an honor to be part of the leadership of this organization.
Conclusions
I'm not sure what to call it. Perseverance, grit, resilience, stubbornness, or simply an inability to say no seem to be a recurrent theme as I look back at this time of my life. We didn't know what to expect or how to behave, we just wanted to get this drug into the US market and for people to take us seriously. Things have changed over the years, and I started to feel we were becoming mainstream when Omegaven was on the GI boards. To those new practitioners hearing the “back story” for the first time, just remember if you have a passion for something, expect to do a lot of work and don't let the naysayers hold you back. It is hard to balance and to learn to manage imbalance, but don't forget your family. Learn from my mistakes. Like Dr Dudrick did for us, when he gave us his personal phone number and said it was going to get ugly, call if you need to talk…. Please feel free to reach out to me. It takes a village. No one can do this alone. As you can see I did nothing alone, it was always with others with different skills—that's what made things move along faster (Figure). Don't throw away any paperwork, you may need it later and if you don't know something, ask. Don't be afraid to go back and review earlier literature, sometimes those old papers were right all along. Most importantly, keep in touch with all those contacts you make at those conferences—you never know how they will change your life. Similarly, if you get contacted by someone who is stuck, make sure to share your knowledge and experience, you never know where it will lead next.
Epilogue
So, you wonder what happened to our first 2 trailblazing patients. The patient with soy allergies is now in his 30's, married, and has a little girl. A member of the Fresenius team met him in a restaurant in Cambridge and he told her the story of how Omegaven saved his life when he was in high school. I later confirmed it with his physician who had treated him back in 2002. Charlie is now 16 and in high school and wants to do something with marine engineering. He lives down on the Cape and is big on boats. I still make it a point to hang with him during his clinic visits. Both guys lead full, rich lives that may not have been possible had we not thought outside the box. It was only possible because their parents were willing to take a leap of faith by allowing their child to be the first. Thank you for believing in me.
Acknowledgment
We thank Stephanie Phelps, PharmD, BCPS, for her sage editorial advice during the course of writing this manuscript.
ABBREVIATIONS
- ARA
arachidonic acid
- ASPEN
American Society for Parenteral and Enteral Nutrition
- BCH
Boston Children's Hospital
- CAIR
Center for Advanced Intestinal Failure
- CDER
Center for Drug Evaluation and Research
- CEO
chief executive officer
- CHNMC
Children's Hospital National Medical Center
- DHA
docosahexaenoic acid
- EFAD
essential fatty acid deficiency
- eIND
emergency investigational new drug
- ESPEN
European Society of Parenteral and Enteral Nutrition
- FDA
US Food and Drug Administration
- GI
gastrointestinal
- IND
investigational new drug
- IV
intravenous
- NDA
new drug application
- NICU
neonatal intensive care unit
- PIFCON
Pediatric Intestinal Failure Consortium
- PN
parenteral nutrition
- PNALD
parenteral nutrition–associated liver disease
- PPA
Pediatric Pharmacy Association
- UCLA
University of California, Los Angeles
Footnotes
Disclosure Kathleen Gura is a consultant for Pronova/BASF, Northsea Therapuetics, Xellia Pharmaceuticals, Pfizer Pediatric Center of Excellence, Baxter; received research support from Northsea Therapuetics, Otsuka Pharmaceutical Company, Alcresta, and Fresenius Kabi. Patent/Royalties for Omegaven are forthcoming.
Editor's note: The Richard A. Helms Award of Excellence in Pediatric Pharmacy Practice was established in 2006 by the Pediatric Pharmacy Association Board of Directors. The award is made possible by the generous donations of colleagues, friends, and past postdoctoral trainees of Dr Helms. The Award recognizes 1) exemplary pediatric patient care, 2) sustained and meritorious contributions to pediatric pharmacy practice, 3) sustained and meritorious contributions to PPA, 4) contributions to education, precepting, and/or mentorship, and 5) contributions of new knowledge through scholarship. For more information and list of past recipients see: https://www.ppag.org/index.cfm?pg=HelmsAward.
REFERENCES
- 1.Javid PJ, Greene AK, Garza J et al. The route of lipid administration affects parenteral nutrition-induced hepatic steatosis in a mouse model. J Pediatr Surg. 2005;40(9):1446–1453. doi: 10.1016/j.jpedsurg.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 2.Alwayn IP, Gura KM, Nosé V et al. Omega-3 fatty acid supplementation prevents hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Pediatr Res. 2005;57(3):445–452. doi: 10.1203/01.PDR.0000153672.43030.75. [DOI] [PubMed] [Google Scholar]
- 3.Gura KM, Parsons SK, Bechard LJ et al. Use of a fish oil-based lipid emulsion to treat essential fatty acid deficiency in a soy allergic patient receiving parenteral nutrition. Clin Nutr. 2005;24(5):839–847. doi: 10.1016/j.clnu.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 4.Gura KM, Duggan CP, Collier SB et al. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management. Pediatrics. 2006;118(1):e197–e201. doi: 10.1542/peds.2005-2662. [DOI] [PubMed] [Google Scholar]
- 5.Nehra D, Fallon EM, Potemkin AK et al. A comparison of 2 intravenous lipid emulsions: interim analysis of a randomized controlled trial. JPEN J Parenter Enteral Nutr. 2014;38(6):693–701. doi: 10.1177/0148607113492549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puder M, Valim C, Meisel JA et al. Parenteral fish oil improves outcomes in patients with parenteral nutrition associated liver injury. Ann Surg. 2009;250(3):395–402. doi: 10.1097/SLA.0b013e3181b36657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcus AD. A doctor's push for drug pits him against its maker. Accessed July 3, 2020. https://www.wsj.com/articles/SB116338867216321332.
- 8.Gura KM, Mulberg AE, Mitchell PD et al. Pediatric intestinal failure associated liver disease: challenges in identifying clinically relevant biomarkers. JPEN J Parenter Enerteral Nutr. 2018;42(2):455–462. doi: 10.1177/0148607116671781. [DOI] [PubMed] [Google Scholar]
- 9.NBCNews Rock Center Experimental drug saves babies, but FDA hurdle remains. Accessed July 3, 2020. http://www.nbcnews.com/video/rock-center/52137171.
- 10.Lam HS, Tam YH, Poon TCW et al. A double-blind randomised controlled trial of fish oil-based versus soy-based lipid preparations in the treatment of infants with parenteral nutrition-associated cholestasis. Neonatology. 2014;105(4):290–296. doi: 10.1159/000358267. [DOI] [PubMed] [Google Scholar]
- 11.Jørgensen KA, Høj Nielsen A, Dyerberg J. Hemostatic factors and renin in Greenland Eskimos on a high eicosapentaenoic acid intake: results of the Fifth UmanaK Expedition. Acta Med Scand. 1986;219(5):473–479. doi: 10.1111/j.0954-6820.1986.tb03342.x. [DOI] [PubMed] [Google Scholar]
- 12.Allport S. The Queen of Fats Why Omega–3s Were Removed From the Western Diet and What We Can Do to Replace Them. University of California Press; 2006. pp. 108–112. [Google Scholar]
- 13.Harris WS. Fish oils and bleeding: where is the evidence? JAMA Intern Med. 2016;176(9):1405–1406. doi: 10.1001/jamainternmed.2016.3968. [DOI] [PubMed] [Google Scholar]
- 14.Nandivada P, Anez-Bustillos L, O'Loughlin AA. Risk of post-procedural bleeding in children on intravenous fish oil. Am J Surg. 2017;214(4):733–737. doi: 10.1016/j.amjsurg.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Meijer VE, Lee HD, Meisel JA et al. Parenteral fish oil as monotherapy prevents essential fatty acid deficiency in parenteral nutrition dependent patients. J Pediatr Gastroenterol Nutr. 2010;50(2):e212–e218. doi: 10.1097/MPG.0b013e3181bbf51e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le HD, de Meijer VE, Zurakowski D et al. Parenteral fish oil as monotherapy improves lipid profiles in patients with parenteral nutrition-associated liver disease. JPEN J Parenter Enteral Nutr. 2010;46(1):122–127. doi: 10.1177/0148607110371806. [DOI] [PubMed] [Google Scholar]
- 17.Le HD, Meisel JA, de Meijer VE et al. The essentiality of arachidonic acid and docosahexaenoic acid. Prostaglandins Leukot Essent Fatty Acids. 2009;81(2–3):165–170. doi: 10.1016/j.plefa.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson SJ, O'Loughlin A, Anez-Bustillos L et al. A Diet with docosahexaenoic and arachidonic acids as the sole source of polyunsaturated fatty acids is sufficient to support visual, cognitive, motor, and social development in mice. Front Neurosci. 2019;2513:72. doi: 10.3389/fnins.2019.00072. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nehra D, Le HD, Fallon EM et al. Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging Cell. 2012;11(6):1046–1054. doi: 10.1111/acel.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diamond IR, Sterescu A, Pencharz PB et al. Changing the paradigm: Omegaven for the treatment of liver failure in pediatric short bowel syndrome. J Pediatr Gastroenterol Nutr. 2009;48(2):209–215. doi: 10.1097/MPG.0b013e318182c8f6. [DOI] [PubMed] [Google Scholar]
- 21.Carlson SE. Lessons learned from randomizing infants to marine oil-supplemented formulas in nutrition trials. J Pediatr. 1994;125(5 pt 2):S33–S38. doi: 10.1016/s0022-3476(06)80734-1. [DOI] [PubMed] [Google Scholar]
- 22.Raphael BP, Mitchell PD, Gura KM et al. Growth in infants and children with intestinal failure associated-liver disease treated with intravenous fish oil. J Pediatr Gastroenterol Nutr. 2020;70(2):261–268. doi: 10.1097/MPG.0000000000002551. [DOI] [PubMed] [Google Scholar]
- 23.Gura KM, Premkumar MH, Calkins KL, Puder M. Intravenous fish oil monotherapy as a source of calories and fatty acids promotes age-appropriate growth in pediatric patients with intestinal failure-associated liver disease. J Pediatr. 2020;219:98–105.e4. doi: 10.1016/j.jpeds.2019.12.065. doi. [DOI] [PubMed] [Google Scholar]
- 24.Nandivada P, Baker MA, Mitchell PD et al. Predictors of failure of fish-oil therapy for intestinal failure-associated liver disease in children. Am J Clin Nutr. 2016;104(3):663–670. doi: 10.3945/ajcn.116.137083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Riedy M, DePaula B, Puder M et al. Higher doses of fish oil-based lipid emulsions used to treat inadequate weight gain and rising triene:tetraene ratio in a severely malnourished infant with intestinal failure-associated liver disease. JPEN J Parenter Enteral Nutr. 2017;41(4):667–671. doi: 10.1177/0148607116661031. [DOI] [PubMed] [Google Scholar]
- 26.de Meijer VE, Kalish BT, Meisel JA et al. Dietary fish oil aggravates Paracetamol-induced liver injury in mice. JPEN J Parenter Enteral Nutr. 2013;37(2):268–273. doi: 10.1177/0148607112450735. [DOI] [PubMed] [Google Scholar]
- 27.Young LS, Huong PTT, Lam NT et al. Nutritional status and feeding practices in gastrointestinal surgery patients at Bach Mai Hospital, Hanoi, Vietnam. Asia Pac J Clin Nutr. 2016;25(3):513–520. doi: 10.6133/apjcn.092015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raiten DJ, Steiber AL, Carlson SE et al. Working group reports: evaluation of the evidence to support practice guidelines for nutritional care of preterm infants-the Pre-B Project. Am J Clin Nutr. 2016 Feb;103(2):648S–78S. doi: 10.3945/ajcn.115.117309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baker MA, Cho BS, Anez-Bustillos L et al. Fish oil-based injectable lipid emulsions containing medium-chain triglycerides or added α-tocopherol offer anti-inflammatory benefits in a murine model of parenteral nutrition-induced liver injury. Am J Clin Nutr. 2019;109(4):1038–1050. doi: 10.1093/ajcn/nqy370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fell GL, Anez-Bustillos L, Dao DT et al. Alpha-tocopherol in intravenous lipid emulsions imparts hepatic protection in a murine model of hepatosteatosis induced by the enteral administration of a parenteral Nutrition Solution. PLoS One. 2019;14(7):e0217155. doi: 10.1371/journal.pone.0217155. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fell GL, Cho BS, Pan A et al. A comparison of fish oil sources for parenteral lipid emulsions in a murine model. JPEN J Parenter Enteral Nutr. 2017;41(2):181–187. doi: 10.1177/0148607116640275. [DOI] [PMC free article] [PubMed] [Google Scholar]


