Abstract
OBJECTIVES
Enoxaparin has been studied for prophylaxis and treatment of thromboembolism in the pediatric population. Dose-finding studies have suggested higher mean maintenance dose requirements in younger children; however, the current recommended dosing schema endorsed by the American College of Chest Physicians remains conservative, likely secondary to limited data on the safety and efficacy of escalated starting doses. Primary objectives of this study included the identification of patient characteristics and risk factors with associations to anti–factor Xa (anti-Xa) values. The secondary objective was to determine an association between the initial anti-Xa value and thrombus resolution. Safety outcomes related to bleeding were also assessed.
METHODS
This retrospective cohort study reviewed records of all pediatric patients ≤18 years of age who were initiated on therapeutic subcutaneous enoxaparin between October 1, 2008, and October 1, 2018, at Children's Hospitals and Clinics of Minnesota for an indication of incident thrombus (N = 283).
RESULTS
Successful resolution of thrombus was directly associated with attaining a therapeutic anti-Xa concentration upon first laboratory evaluation. Other characteristics with associations to initial anti-Xa values included age, body mass index, and certain diagnoses. The rate of composite bleeding was consistent across concentrations of anti-Xa (p = 0.4944).
CONCLUSIONS
Despite adherence to protocol, the current enoxaparin dosing nomogram is only successful at achieving a therapeutic anti-Xa concentration (0.5–1.0 unit/mL) 55.8% of the time. A more aggressive enoxaparin dosing nomogram is warranted, as delaying time to therapeutic anti-Xa values impacts clinical outcomes, specifically thrombus resolution. Further investigation into characteristics with association to anti-Xa concentrations is needed.
Keywords: anticoagulation, CHEST, drug monitoring, enoxaparin, low-molecular-weight heparin, pediatric, thrombus
Introduction
Low-molecular-weight heparins (LMWHs) are often used for the treatment of thromboembolic events in the pediatric population. Enoxaparin (Lovenox, Fresenius Kabi USA, LLC, Lake Zurich, IL) is the most commonly used LMWH in this population within the United States. Therapeutic values of subcutaneous enoxaparin are monitored via anti–factor Xa (anti-Xa) values with a target reference range between 0.5 and 1 unit/mL. A blood sample should be obtained between 4 and 6 hours post dose. This range is extrapolated from previously established therapeutic ranges in adult patients.1 Considering the differences between adult and pediatric coagulation systems, this extrapolation poses significant concerns regarding validation in the pediatric population.2–4 The American College of Chest Physicians (CHEST) echoes this sentiment: “the hemostatic system is a dynamic, evolving entity that likely affects not only the frequency and natural history of thromboembolic events in children, but also the response to therapeutic agents.”1
The current starting dose of enoxaparin recommended by the CHEST is based on age: 1.5 mg/kg per dose for infants <2 months of age and 1 mg/kg per dose for children ≥2 months.1 This dose was derived from a single study completed in 1996 that contained a sample of 9 neonates and 23 children between 2 months to 17 years of age.5 Subsequent studies have suggested higher average maintenance doses are required in certain pediatric populations.6,7 A meta-analysis found doses upwards of 2.27 mg/kg were required in premature neonates to attain anti-Xa values between 0.5 and 1 unit/mL.6 Based on a combination of these findings, a more aggressive, alternative dosing schema is used by some institutions.6 The concern for varying pharmacokinetics within the pediatric population warrants large-scale evaluation of CHEST dosing recommendations for enoxaparin.
There are concerns about using the CHEST dosing nomogram because it may delay time to therapeutic values of anticoagulation. A study determined that pediatric patients starting enoxaparin needed on average 3 dose adjustments and 11 days before attaining target anti-Xa values.8 Delaying the time to therapeutic anti-Xa values results in multiple laboratory draws and extra manipulations in enoxaparin dosing. Excessive laboratory draws cause unnecessary pain for children, risk of anemia in very small patients, and additional costs for health care organizations. Most importantly, the delay in achieving therapeutic anti-Xa values results in a theoretical risk for thrombosis propagation. This study evaluated the clinical outcomes of pediatric patients receiving treatment dosing of enoxaparin per pharmacist-driven protocol (synonymous with CHEST) for an incident thrombus at Children's Hospitals and Clinics of Minnesota. The first objective was to describe characteristics associated with non-therapeutic anti-Xa values. A second objective was to determine association between initial anti-Xa values and thrombus resolution. This focus on the clinical outcome of thrombus resolution distinguishes this work from previously published literature, typically consisting of dose-finding studies.
Materials and Methods
Study Design. This retrospective cohort study reviewed records of all pediatric inpatients who were initiated on therapeutic subcutaneous enoxaparin (Lovenox) for an incident thrombus at Children's Hospitals and Clinics of Minnesota between October 1, 2008, and October 1, 2018. The therapeutic enoxaparin protocol follows the 2012 recommendations from CHEST.1 That is, initial treatment dosing of 1.5 mg/kg per dose for infants <2 months of age and 1 mg/kg for children ≥2 months, with a target therapeutic anti-Xa range of 0.5 to 1.0 unit/mL. Titrations of doses and frequency of repeated laboratory testing are based on anti-Xa values (Table 1). Data were collected via chart review and electronic medical record query.
Table 1.
Enoxaparin Treatment Dosing Adjustment and Monitoring Nomogram
| Anti-Xa Value (units/mL) | Hold Next Dose | Dose Change | Repeated Anti-Xa Value |
|---|---|---|---|
| <0.35 | No | Increase 25% | 4 hr after second dose given |
| 0.35–0.49 | No | Increase 10% | 4 hr after second dose given |
| 0.51 | No | No change | Repeat once within 24–48 hr, then once weekly |
| 1.01–1.5 | No | Decrease 20% | 4 hr after second dose given |
| 1.51–2 | 3 hr | Decrease 30% | 4 hr after second dose given |
| >2 | All further doses should be held and anti-Xa values measured every 12 hr until value is <0.5 unit/mL. Restart at 60% of last dose. | ||
anti-Xa, anti–factor Xa
The cohort included hospitalized patients ≤18 years of age whose anticoagulation therapy was managed by pharmacy and who had at least 1 anti-Xa value drawn during the hospital admission. Exclusion criteria were use of prophylactic, once-daily, or intravenous enoxaparin; indication for use for non-incident thrombus; concomitant grade III or IV intracranial hemorrhage; or concomitant chylous effusion. Administration of antithrombin III during enoxaparin therapy was reported but was not an exclusion criterion.
Patient demographics (i.e., age, sex, and race) and information on the clinical presentation—including primary diagnosis, body mass index (BMI), creatinine clearance (CrCL), concurrent use of nephrotoxic medications, and thrombus type—were collected. Diagnoses were grouped via clinical classification software,9 which categorizes the International Classification of Disease primary diagnosis codes (versions 9 and 10). For children older than 2 years, BMI was standardized for age and sex by using growth charts from the Centers for Disease Control and Prevention and categorized as underweight, normal weight, overweight, and obese (corresponding to <5th percentile, 5th–85th percentile, 85th–95th percentile, and >95th percentile, respectively). Renal function was evaluated by using the modified or “bedside” Schwartz equation, and impairment was defined as an estimated glomerular filtration rate of less than 30 mL/min/1.73 m2. See Supplemental Table 1 for a list of nephrotoxic medications.
Attainment of therapeutic value of enoxaparin was determined from the first anti-Xa value drawn after starting enoxaparin. The first anti-Xa value had to be within a 14- to 32-hour window following the start of enoxaparin. This window was selected to allow for clinical judgment and optimal laboratory draw times; an additional 2-hour window on either side of a value assessed 4 to 6 hours after the second or third enoxaparin dose was permitted. Patients with a first anti-Xa value outside of this time window were excluded. Values of anti-Xa were classified as either therapeutic (0.5–1.0 unit/mL), subtherapeutic (<0.5 unit/mL), or supratherapeutic (>1 units/mL). The secondary outcome was resolution of thrombus as assessed via follow-up imaging (i.e., computed tomography scan, magnetic resonance imaging, or ultrasonography) after 7 days for arterial line–associated thrombus, 6 to 8 weeks for venous line–associated thrombus, 3 months for venous and cerebral venous sinus thrombi, and 6 months (if at all) for pulmonary embolism (refer to CHEST guidelines for other types).1 The safety endpoint evaluated was clinically relevant bleeding following the definition from the International Society of Thrombosis and Haemostasis criteria of major bleed.10 This composite outcome was defined as any bleed with a symptomatic fall in hemoglobin of 2 g/dL or more, any bleed located in a critical area or organ, any bleed requiring surgical intervention or transfusion, or any bleed resulting in death.
Statistical Analysis. Associations of therapeutic values with patient characteristics and outcomes were assessed by using chi-squared test (or Fisher exact test if indicated). Analysis of the primary outcome of resolution of thrombus was also stratified by age group (younger than or older than 60 days) and type of thrombus by using the Cochran-Mantel-Haenszel test. Statistical significance for either test was defined as p < 0.01.
Results
Study Population. During a 10-year period, 686 children received enoxaparin while hospitalized, of which 367 met inclusion criterion. The most common reason for ineligibility was a lack of a pharmacist consult. The final sample size was 283 patients because many patients did not have a first anti-Xa value recorded within 14 to 32 hours of starting enoxaparin. Almost half of patients (48%) were receiving enoxaparin to treat a line-associated thrombus. The sample included mostly patients older than 60 days (73%). Starting doses generally followed (96.6%) the protocol with an average starting dose of 1.4 mg/kg (Q1–Q3: 1.3–1.5; minimum-maximum: 0.9–2) for patients <60 days of age and 1.0 mg/kg (Q1–Q3: 0.9–1.1, minimum-maximum: 0.5–2.3) for patients ≥60 days of age. Age was unadjusted and documented as postnatal age. Only 89.4% of patients obtained a therapeutic value before discharge from the hospital. A total of 158 (55.8%), 57 (20.1%), and 38 (13.4%) patients required 1, 2, and 3 or more laboratory draws, respectively, before being discharged or obtaining a therapeutic anti-Xa. Four patients (1.4%) had antithrombin III administration during the course of anticoagulation.
Association With Therapeutic Values. Overall, 56% of patients had anti-Xa values within the therapeutic range upon first laboratory evaluation (95% CI, 50–62), and 26% were supratherapeutic (95% CI, 21–32). Age, classified on the basis of dosing recommendations, was associated with non-therapeutic first anti-Xa values. Children <60 days of age were more likely to have subtherapeutic values than those ≥60 years of age (32% versus 13%, Table 2). A higher BMI was also associated with non-therapeutic values; overweight and obese patients tended to have supratherapeutic values more often than normal or underweight children (Table 2). Neither renal dysfunction nor concomitant nephrotoxic medications appeared to be related to initial anti-Xa values.
Table 2.
Patient Demographics
| Characteristics | Total | Anti-Xa Values (units/mL)* | p value | ||
|---|---|---|---|---|---|
| Subtherapeutic (<0.5) | Therapeutic (0.5–1) | Supratherapeutic (>1) | |||
| Age | |||||
| <60 days | 76 | 24 (31.6) | 48 (63.2) | 4 (5.3) | <0.001 |
| ≥60 days | 207 | 27 (13) | 110 (53.2) | 70 (33.8) | |
| Sex | |||||
| Male | 149 | 32 (21.5) | 84 (56.4) | 33 (22.1) | 0.134 |
| Female | 134 | 19 (14.2) | 74 (55.2) | 41 (30.6) | |
| Race | |||||
| White | 179 | 38 (21.2) | 95 (53.1) | 46 (25.7) | 0.400 |
| Black | 46 | 4 (8.7) | 29 (63) | 13 (28.3) | |
| Hispanic | 15 | 4 (26.7) | 7 (46.7) | 4 (26.7) | |
| Other | 43 | 5 (11.6) | 27 (62.8) | 11 (25.6) | |
| BMI† | |||||
| Underweight | 8 | 1 (12.5) | 6 (75) | 1 (12.5) | 0.033 |
| Normal | 72 | 2 (2.8) | 45 (62.5) | 25 (34.7) | |
| Overweight | 22 | 2 (9.1) | 7 (31.8) | 13 (59.1) | |
| Obese | 35 | 2 (5.7) | 13 (37.1) | 20 (57.2) | |
| CrCL‡ | |||||
| <30 mL/min/1.73 m2 | 9 | 0 (0) | 7 (77.8) | 2 (22.2) | 0.284 |
| ≥30 mL/min/1.73 m2 | 273 | 51 (18.7) | 151 (55.3) | 71 (26) | |
| Concurrent nephrotoxic medications | |||||
| No | 137 | 24 (16.4) | 78 (53.4) | 44 (30.2) | 0.277 |
| Yes | 146 | 27 (19.7) | 80 (58.4) | 30 (21.9) | |
| Thrombus type | |||||
| Arterial line–associated | 14 | 4 (28.6) | 8 (57.1) | 2 (14.3) | 0.005 |
| Cerebral venous sinus thrombosis | 29 | 3 (10.3) | 16 (55.2) | 10 (34.5) | |
| Pulmonary embolism | 30 | 0 (0) | 14 (46.7) | 16 (53.3) | |
| Venous | 67 | 11 (16.4) | 35 (52.2) | 21 (31.3) | |
| Venous line–associated | 123 | 29 (23.6) | 72 (58.5) | 22 (17.9) | |
| Other | 20 | 4 (20) | 13 (65) | 3 (15) | |
anti-Xa, anti–factor Xa; CrCL, creatinine clearance
* All data are presented as n (row %).
† BMI based on the CDC test cutoff for 2019, which excludes patients <2 years of age (total sample size 137). Fischer exact test probability <0.0001, p = 0.0126.
‡ CrCL per modified Schwartz equation. Missing 1 patient, Fisher exact test probability 0.041, p = 0.33. Renal dysfunction requiring dose adjustment of enoxaparin is defined as CrCL <30 mL/min.
Of 18 pooled diagnosis codes, 8 diagnoses were significantly associated with non-therapeutic anti-Xa values upon first evaluation (Supplemental Table 2). Diagnoses of congenital anomalies, conditions originating in the perinatal period, and injury and poisoning were associated with subtherapeutic anti-Xa values.
Thrombus Resolution. A total of 240 patients had follow-up imaging for their thrombus. Among these patients, resolution of the incident thrombus was more likely to occur with higher initial anti-Xa values (p = 0.014). Specifically, for subtherapeutic, therapeutic, and supratherapeutic anti-Xa values, resolution occurred in 50%, 60%, and 73% of patients, respectively (Table 3, Figure; respective 95% CIs: 34–63, 37–67, and 61–85). This trend was consistent within the 2 age groups used for dosing (Table 3, Figure). After controlling for the type of thrombus, the association was also statistically significant (p = 0.009).
Table 3.
Thrombus Resolution by Anti-Xa Values, Overall and Within Age Groups and Thrombus Types
| Cohort | Anti-Xa Values (units/mL)* | p value | ||
|---|---|---|---|---|
| Subtherapeutic (<0.5) | Therapeutic (0.5–1) | Supratherapeutic (>1) | ||
| All patients |
23/48 (48) | 78/136 (57) | 42/56 (75) | 0.014 |
| Age | 0.031† | |||
| <60 days | 10/22 (45) | 24/46 (52) | 4/4 (100) | |
| ≥60 days | 13/26 (50) | 54/90 (60) | 38/52 (73) | |
| Thrombus type | 0.009† | |||
| Arterial line–associated | 2/4 (50) | 4/8 (50) | 2/2 (100) | |
| Cerebral venous sinus thrombosis | 2/3 (66.7) | 6/15 (40) | 4/7 (57.1) | |
| Pulmonary embolism | 0 (0) | 3/6 (50) | 8/10 (80) | |
| Venous | 4/10 (40) | 17/29 (58.6) | 13/19 (68.4) | |
| Venous line–associated | 14/28 (50) | 44/67 (65.7) | 12/15 (80) | |
| Other | 1/3 (33.3) | 4/11 (36.4) | 3/3 (100) | |
Anti-Xa, Anti–factor Xa
* Data presented as n resolved/total (%).
† Stratified Cochran-Mantel-Haenszel test.
Safety. Among all patients, the rate of composite bleeding was consistent across values of anti-Xa (p = 0.4944). Among patients with subtherapeutic, therapeutic, and supratherapeutic values, rates of bleeding were 5.9%, 8.2%, and 4.1%. There was 1 death thought to be related to bleeding. Concomitant medications known to impact bleeding were not associated with bleeding (Supplemental Table 3).
Discussion
Evaluation of the initial dosing in these patients identified excellent protocol adherence by pharmacists at our institution. The average starting doses closely mirrored the protocol dosing of 1.5 mg/kg and 1 mg/kg for those <60 days of age and patients ≥60 days of age, respectively. Despite excellent adherence to protocol (96.6%), the current enoxaparin dosing nomogram was only successful 56% of the time; only half of patients had therapeutic anti-Xa values upon first analysis. Traditionally, a clinical practice guideline should encompass the majority of a target population, or conceivably 80% of the population. Therefore, the current protocol is insufficient for a significant proportion of our population. These results are comparable to those of a similar study that determined the success rate for achieving therapeutic anti-Xa values via the CHEST protocol to be 59%.11 To further support the insufficiency, thrombus resolution was determined to be directly associated with attaining a therapeutic (or supratherapeutic) anti-Xa value upon first laboratory evaluation. In other words, delaying time to therapeutic values is associated with worsened clinical outcomes.
Of no surprise, younger patients were more likely to be subtherapeutic, whereas older patients were more likely to be supratherapeutic. By extrapolation, the current protocol at our institution tends to underdose younger patients; modification of our guideline to a higher starting dose (>1.5 mg/kg) could increase the percentage of younger patients that reach therapeutic anti-Xa values upon first check. This result supports previous work that identified maintenance dose requirements of up to 2 mg/kg in premature neonates.6
Unfortunately, the very small number of patients younger than 60 days who had a supratherapeutic anti-Xa value was a limitation with potential to impact power. Heparin is often preferred over enoxaparin for anticoagulation in neonatal patients owing to limited subcutaneous tissue, which may explain the limitation. Although unlikely, subtherapeutic anti-Xa values in younger patients could be due to inconsistent administration and needle priming. In an attempt to control for these safety and quality risks, the minimum measurable volume for a sterile medication at Children's Hospitals and Clinics of Minnesota is 0.1 mL, and all subcutaneous and intramuscular medications have 0.1 mL of overfill included during preparation for the purpose of needle priming. Furthermore, there are 3 concentrations of enoxaparin available, which reduces the risk of medication loss during priming.
Final enoxaparin weight-based doses were not evaluated in this study, as it was not the primary aim. Additional confounders making the outcome difficult to assess included transitioning to prophylactic enoxaparin dosing or discharging prior to attaining a therapeutic anti-Xa value. In fact, 10% of patients never reached their final maintenance dose before discharge. Often these patients will follow-up with external facilities on an outpatient basis for further titration of enoxaparin; the evaluators would not have access to the data for these future encounters. This is also why the average number of dose titrations was difficult to evaluate and compare to that of previous studies.8
At first glance, adherence to protocol is excellent, but it also appears that some types of thrombosis may be more aggressively treated upfront. As seen in Table 2, cerebral venous sinus thrombosis and pulmonary embolism were rarely subtherapeutic upon first evaluation of anti-Xa values. These types of thrombi can be more life-threatening than a line-associated thrombosis, and may be subject to clinical judgement regarding initial weight-based dosing. They also occur more commonly in older pediatric patients, which could help to explain the age gap or decreased prevalence in younger children. Higher anti-Xa values in this population could be in part due to increased age, and therefore decreased clearance of enoxaparin, or from higher, more aggressive starting doses related to the clinical severity of these thrombus types.
A few diagnoses holding significant associations with anti-Xa values warrant further discussion (Supplemental Table 2). Congenital anomaly was a diagnosis code found to be highly associated with non-therapeutic anti-Xa values. In general, patients without a congenital defect were more likely to be supratherapeutic than patients with a congenital defect. Explanations for this finding could be due to increased clearance of enoxaparin related to high diuretic use in the cardiac unit, a younger patient population in the cardiac unit, or a different inherent hemostatic balance related to critical illness and surgery. Conditions originating in the perinatal period were also anticipated to be strongly associated with subtherapeutic anti-Xa values, especially prematurity, which may be related to drug clearance and comorbidities. Injury and poisoning were also found to be associated with subtherapeutic anti-Xa values; this is likely related to vigorous hydration and chelation to prevent acute organ damage. Further investigation into these diagnoses is necessary to determine the absolute reason for non-therapeutic anticoagulation.
Figure.
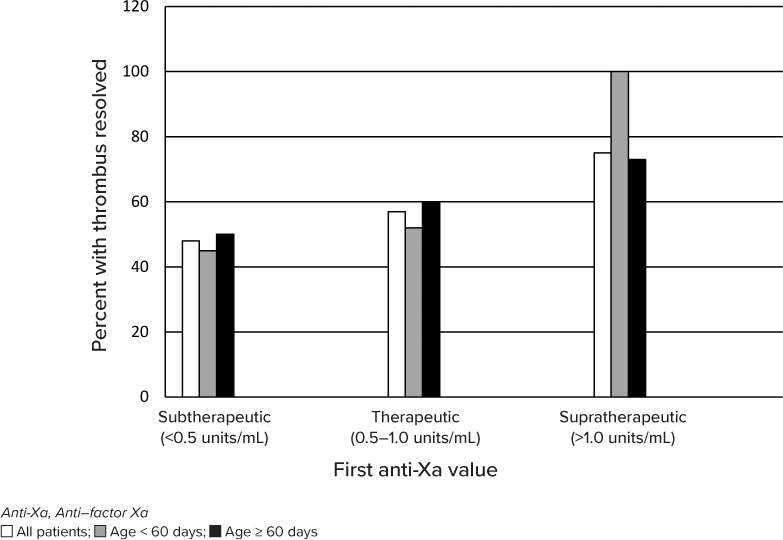
Thrombus resolution overall and by age group.
The institutional protocol does permit dosing adjustments for renal impairment, which may or may not have impacted initial dosing. Very few patients in this study had clinically significant renal dysfunction, which would traditionally mandate smaller enoxaparin dosing per pharmacotherapy references, such as Micromedex and Lexi-Comp. The limited preponderance of renal dysfunction also supports previously studied alternative dosing schemas. Evidently, pediatric patients tend to have good renal function and therefore may tolerate higher initial doses owing to better baseline drug clearance. Concomitant nephrotoxic medications, like vancomycin, also did not appear to impact initial anti-Xa values.
While the CHEST guideline advises to adjust initial enoxaparin dosing for renal function, it does not permit body weight dosing adjustments. Many other pediatric and adult institutions use ideal body weight or a modified dosing nomogram for overweight and obese patients for fear of inducing over anticoagulation in this population.12 Our study confirmed this relationship between BMI and anti-Xa values. Overweight and obese patients, based on BMI, had higher anti-Xa values when using the CHEST dosing recommendations. The clinical impact of this relationship will require further investigation in order to warrant a nomogram adjustment at Children's Minnesota.
The low rate of bleeding in this study (4.1%–8.2%) also supports use of a larger starting dose than the current CHEST schema recommends. Hematologic adverse effects are considered common incidences during treatment with enoxaparin. Occurrence of minor bleeding, including anemia, has been reported in previous studies up to 16%, whereas major bleeding has been documented in up to 4% of patients.13 One patient had life support withdrawn owing to bilateral intracranial hemorrhage, which was found approximately 12 hours after the fourth dose of enoxaparin. The patient had been on therapeutic heparin prior to transitioning to enoxaparin, and the cause of intracranial hemorrhage was unknown. Of note, anti-Xa values were all within therapeutic range for the patient, so the dose would not have been reduced before identification of the bleed.
Despite positive efficacy and safety findings, some limitations to this study should be noted. Overall, a large number of patients were excluded from the study. The primary reason was due to no pharmacist consult. Providers often practice with more clinical discretion than pharmacists, and this exclusion criterion was chosen to ensure adequate adherence to the institutional protocol. Another exclusion criterion that decreased the potential study population was for a missing anti-Xa value within 14 to 32 hours of the first enoxaparin administration in the hospital. Laboratory draws may be delayed or drawn early for many reasons. For example, anti-Xa may be timed with an existing culture to avoid excess blood loss or drawn after the third dose to prevent peripheral needle pokes during the middle of the night while the patient is sleeping. Unfortunately, the selected window was still narrow and resulted in exclusion of study subjects.
Additional limitations included lack of follow-up imaging on chart review and subjective radiology reporting and interpretation of various imaging reports (ultrasonography, magnetic resonance imaging, and computed tomography). To provide consistency, all imaging reports were manually reviewed by 1 investigator and thrombus outcomes were documented as unresolved if the thrombus was reported as “present” or “partially resolved” in the diagnostic note. Unfortunately, not all types of thrombus require confirmation of resolution to determine the anticoagulation plan of therapy. Even considering missing data, the trend was consistent amongst all thrombus types and patient ages.
Lastly, antithrombin III administration was recorded but not considered an exclusion criterion in this study. While this may seem controversial after a 2018 retrospective chart review found that exogenous antithrombin administration increased anti-Xa values by 0.2 unit/mL, it did not impact the results of our study.14 Less than 2% of all study patients received antithrombin during enoxaparin therapy, and only 1 patient had administration that may have impacted the anti-Xa value (between starting enoxaparin and the first anti-Xa value). This patient had an initial supratherapeutic value of 1.29 units/mL, which would have remained supratherapeutic even with an augmentation of 0.2 unit/mL owing to antithrombin.
Conclusions
In summary, a more aggressive starting dose for patients using therapeutic subcutaneous enoxaparin for thromboembolism is necessary. Not only is a higher initial dose clinically required for thrombus resolution, but also it is likely safe considering lower-than-average bleed rate with traditional CHEST dosing. Other patient characteristics require further investigation to determine appropriate dosing requirements, especially BMI, age, and certain diagnoses.
Supplementary Material
Acknowledgments
The authors acknowledge pharmacy staff Danica Smith, Melisa Lu, Jack Beckman, and Merisa Tricic for their assistance with data collection.
ABBREVIATIONS
- anti-Xa
anti–factor Xa;
- BMI
body mass index;
- CDC
Centers for Disease Control and Prevention;
- CHEST
American College of Chest Physicians;
- CrCL
creatinine clearance;
- IV
intravenous;
- LMWH
low-molecular-weight heparin
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. Kayla Wiltrout and Dave Watson have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Ethical Approval and Informed Consent The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and have been approved by the appropriate committees at our institution (Children's Minnesota, Minneapolis, MN). All patients and/or parents/caregiver(s) provided written informed consent and/or assent (as applicable) at enrollment.
Supplemental Material
DOI: 10.5863/1551-6776-25.8.689.S1
REFERENCES
- 1.Monagle P, Chan AKC, Goldenberg NA et al. Antithrombotic Therapy in Neonates and Children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 suppl):e737S–e801S. doi: 10.1378/chest.11-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lippi G, Franchini M, Montagnana M, Guidi GC. Coagulation testing in pediatric patients: the young are not just miniature adults. Semin Thromb Hemost. 2007;33(8):816–820. doi: 10.1055/s-2007-1000373. [DOI] [PubMed] [Google Scholar]
- 3.Rojnuckarin P, Akkawat B, Juntiang J. Stability of plasma anti-Xa activity in low-molecular-weight heparin monitoring. Clin Appl Thromb Hemost. 2010;16(3):313–317. doi: 10.1177/1076029609336853. [DOI] [PubMed] [Google Scholar]
- 4.Kuhle S, Eulmesekian P, Kavanagh B et al. Lack of correlation between heparin dose and standard clinical monitoring tests in treatment with unfractionated heparin in critically ill children. Haematologica. 2007;92(4):554–557. doi: 10.3324/haematol.10696. [DOI] [PubMed] [Google Scholar]
- 5.Massicotte P, Adams M, Marzinotto V et al. Low-molecular-weight heparin in pediatric patients with thrombotic disease: a dose finding study. J Pediatr. 1996;128(3):313–318. doi: 10.1016/s0022-3476(96)70273-1. [DOI] [PubMed] [Google Scholar]
- 6.Malowany JI, Monagle P, Knoppert DC et al. Enoxaparin for neonatal thrombosis: a call for a higher dose for neonates. Thromb Res. 2008;122(6):826–830. doi: 10.1016/j.thromres.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Streif W, Goebel G, Chan AK, Massicotte MP. Use of low molecular mass heparin (enoxaparin) in newborn infants: a prospective cohort study of 62 patients. Arch Dis Child Fetal Neonatal Ed. 2003;88(5):F365–F370. doi: 10.1136/fn.88.5.F365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrade-Campos MM, Montes-Limon AE, Fernandez-Mosteirin N et al. Dosing and monitoring of enoxaparin therapy in children: experience in a tertiary care hospital. Blood Coagul Fibrinolysis. 2013;24(2):194–198. doi: 10.1097/MBC.0b013e32835b72b8. [DOI] [PubMed] [Google Scholar]
- 9.HCUP CCS Healthcare Cost and Utilization Project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality. 2017 Mar; Accessed September 19, 2019. www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. [PubMed]
- 10.Wells G, Coyle D, Cameron C Safety Effectiveness and CostEffectiveness of New Oral Anticoagulants Compared with Warfarin in Preventing Stroke and Other Cardiovascular Events in Patients with Atrial Fibrillation. Ottawa, Canada: Canadian Agency for Drugs and Technologies in Health; 2012. Table 14, Major Bleeding Definitions. Accessed September 21, 2019. https://pubmed.ncbi.nlm.nih.gov/24279001/ [PubMed] [Google Scholar]
- 11.Nguyen Dinh C, Moffett BS, Galati M et al. A critical evaluation of enoxaparin dose adjustment guidelines in children. J Pediatr Pharmacol Ther. 2019;24(2):128–133. doi: 10.5863/1551-6776-24.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nutescu EA, Spinler SA, Wittkowsky A, Dager WE. Low-molecular weight heparins in renal impairment and obesity: available evidence and clinical practice recommendations across medical and surgical settings. Ann Pharmacother. 2009;43(6):1064–1083. doi: 10.1345/aph.1L194. [DOI] [PubMed] [Google Scholar]
- 13.Green-wood Village, CO: Truven Health Analytics; Enoxaparin. DRUGDEX. IBM Micromedex. Updated 2018. Accessed November 5, 2019. https://www.micromedexsolutions.com/micromedex2/librarian/CS/44ABB9/ND_PR/evidencexpert/ND_P/evidencexpert/DUPLICATIONSHIELDSYNC/21DD3F/ND_PG/evidencexpert/ND_B/evidencexpert/ND_AppProduct/evidencexpert/ND_T/evidencexpert/PFActionId/evidenc-expert.DoIntegratedSearch?SearchTerm=enoxaparin&UserSearchTerm=enoxaparin&SearchFilter=filterNone&navitem=searchALL#. [Google Scholar]
- 14.Logston BB, Rodman EA, Dinh KL et al. Effect of exogenous antithrombin administration on anti-xa levels in infants treated with enoxaparin. J Pediatr Pharmacol Ther. 2018;23(4):315–319. doi: 10.5863/1551-6776-23.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


