ABSTRACT
In this research, the lettuce high-temperature-sensitive variety Beisan San 3 was used as a test material. The effects of exogenous spermidine (Spd) on membrane lipid peroxidation, the antioxidant system, the ascorbic acid-glutathione (AsA-GSH) system and the glyoxalase (Glo) system in lettuce seedlings under high-temperature stress were studied by spraying either 1 mM spermidine or ionized water as a control. The results showed that, under high-temperature stress, the growth of lettuce seedlings was weak, and the dry weight (DW) and fresh weight (FW) were reduced by 68.9% and 82%, respectively, compared with those of the normal-temperature controls. In addition, the degree of membrane lipid peroxidation increased, and the reactive oxygen species (ROS) level increased, both of which led to a significant increase in malondialdehyde (MDA) content and lipoxygenase (LOX) activity. Under high-temperature stress, the activity of superoxide dismutase (SOD) decreased, the activities of peroxidase (POD) and catalase (CAT) increased first but then decreased, and the activity of ascorbic acid peroxidase (APX) decreased first but then increased. Glutathione reductase (GR) activity, ascorbic acid (AsA) and glutathione (GSH) content showed an upward trend under high-temperature stress. The activities of glyoxalase (GloI and GloII) in the lettuce seedling leaves increased significantly under high-temperature stress. In contrast, the application of exogenous Spd alleviated the oxidative damage to the lettuce seedlings, which showed a decrease in MDA content and LOX activity and an increase in SOD, POD, CAT, APX, GR, GloI, and GloII activities. In addition, the antioxidant AsA and GSH contents also increased to varying degrees. It can be seen from the results that high temperature stress leads to an increase in the level of ROS and cause peroxidation in lettuce seedlings, and exogenous Spd can enhance the ability of lettuce seedlings to withstand high temperature by enhancing the antioxidant system, glyoxalase system and AsA-GSH cycle system.
Keywords: Antioxidant system, exogenous substance, high temperature stress, methylglyoxal, oxidative stress
Introduction
With global warming, high-temperature stress has become a major constraint on crop production and has become more frequent.1 Physiological and biochemical processes in plants are sensitive to high temperature stress,2,3 and high-temperature stress varies with temperature, duration and plant type.4 Extreme high temperature can cause cell damage or death within minutes, as can prolonged exposure to moderate high temperature.5 High temperature hinders the growth, phenology and physiological processes of plants and can substantially decrease the productivity of many plant species.6,7 High-temperature stress can produce excessive reactive oxygen species (ROS) as a result of oxidation, such as singlet oxygen(1O2), O2 superoxide (O2−), hydrogen peroxide (H₂O₂) and hydroxyl radical (OH.).8 Increased ROS concentration will cause oxidative damage to cell membranes (lipid peroxidation), proteins, RNA and DNA molecules, which will cause oxidative damage to the cells and eventually lead to plant death.9,10 Plants are able to resist oxidative stress to some extent by regulating potential enzymatic and nonenzymatic antioxidant defense systems.11 Enzymatic components include superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione peroxidase (GPX), glutathione reductase (GR), glutathione S-transferase (GST), hydrogen monooxide ascorbate reductase (MDHAR) and dehydroascorbate reductase (DHAR), among which APX, MDHAR, DHAR and GR participate in the ascorbate glutathione cycle and can remove harmful H₂O₂, which can protect plants from ROS-induced damage.12,13 Glutathione S-transferase (GST) is another antioxidant enzyme that catalyzes the binding of toxic isomers, electrophiles, and hydrophobic substances to GSH.14 Nonenzymatic antioxidants include glutathione (GSH), ascorbic acid (AsA), phenolic compounds, and tocopherols, which can regulate the functions of main cells and participate in the removal of ROS produced by plants during stress.15, 16–17, 18 In addition to ROS, the cytotoxic compound methylglyoxal (MG) also accumulates during high-temperature stress.19,20 Overproduction of MG, a cytotoxic compound, can damage the ultrastructure of cells; increase the degradation of carbohydrates, proteins and lipids; and cause mutations and even cell death.21,22 The plant has a glyoxalase system that effectively eliminates MG. The system consists of glyoxalase I (GloI) and glyoxalase II (GloII). It can cooperate with GSH to catalyze the conversion of over-produced MG to D- Lactic acid.23,24
Polyamines (PAs), including spermine (Spm), spermine (Spd) and putrescine (Put), are low-molecular-weight aliphatic amines commonly found in plants. In higher plants, PAs are involved in physiological regulatory and developmental processes, such as seed germination, flower and fruit development, and biotic and abiotic stress responses.25–26, 27 Spd is a common PA in plants and is involved in adaptations to various abiotic stresses, such as salinity,28,29 drought,30,15 cold,31 high temperature32,33 and heavy metals.34 The accumulation of Spd under high-temperature stress may be related to the improvement of heat resistance.
Exogenous Spd improves the heat tolerance of rice seeds by regulating the metabolism of endogenous starch and polyamines.35 Spd pretreatment plays a key role in regulating the antioxidant and glyoxal enzyme systems and enhancing the heat resistance of rice seedlings.8 In addition, Spd also regulates antioxidant enzyme activity and the expression of related genes in tomato seedlings exposed to high temperatures.36
Lettuce grows well under cool temperatures but is not resistant to high temperature, lettuce hearts and leaf edges burn easily under high temperature. Fluctuations in temperature can affect the physiological and biochemical properties of lettuce, resulting in reduced lettuce yields. Previous studies on the role of spermine in response to adverse conditions have mainly focused on rice, tomato, cucumber and other crop species,37–38, 39 while research on lettuce is relatively limited, and the research content has mostly focused on the antioxidant system,15,40 with little understanding of the glyoxal enzyme system. Therefore, to better understand the role of antioxidant and glyoxalase systems in heat resistance, we used the method of treating lettuce for consecutive days, focused on the effects of high-temperature stress on the antioxidant enzyme, non-enzymatic antioxidant and glyoxalase activities in lettuce were investigated, and how Spd might be involved in the heat resistance of lettuce by regulating the antioxidant and glyoxalase systems was determined.
Materials and methods
Plant materials, growth conditions and experimental treatments
The lettuce variety used in this experiment was provided by the Beijing University of Agriculture lettuce research group and was the high-temperature-sensitive variety Beisan San. Two hundred plump seeds that uniform and disease free were selected. The seeds were germinated by being immersed in 9-cm-wide petri dishes filled with filter paper and moistened with distilled water, after which the dishes were placed in a lighted incubator to germinate. The photoperiod was 12 h/12 h (light/dark), the light intensity was maintained at 200 μmol.m−2s−1, the temperature was 20 °C/15 °C (day/night), and the relative humidity was 70% to 75%. After the seeds developed a white radical, they were transplanted into a plug tray and placed in a light incubator for cultivation. When the lettuce seedlings reached the three-leaf-center stage, they were transplanted into a nutrition bowl of 11 cm × 12 cm (diameter × height), which were still cultivated in the abovementioned light incubator; plants displaying consistent growth conditions were selected for testing. When the seedlings reached the six-leaf-center stage, they were subjected to a day/night temperature setting of 35 °C/30 °C, a photoperiod of 12 h/12 h, a light intensity of 200 μmol.m−2s−1, and a relative humidity of 70%~75% for high-temperature treatment. After starting the heat treatment, deionized water and 1 mM Spd were sprayed onto the plants with a small sprayer at 9:00 a.m. The spray was applied while wearing gloves, and it was applied evenly on both the abaxial and abaxial leaf surfaces; the solutions were sprayed until the leaf surfaces were wet but not dripping. Spd was obtained from Sigma Co. as a white solid.
There were four treatments in this experiment: a control (CK) treatment, which involved normal-temperature controls, a day/night temperature of 22 °C/17 °C, and foliar sprays of deionized water; a Spd treatment, which involved a day/night temperature of 22 °C/17 °C and foliar sprays of 1 mM Spd; an H treatment, which involved a day/night temperature of 35 °C/30 °C and foliar sprays of deionized water; and an H + Spd treatment, which involved a day/night temperature of 35 °C/30 °C and foliar sprays of 1 mM Spd.
The experiment was carried out for 8 days at 0, 2, 4, 6 and 8 days after treatment, the indexes of the main functional leaves were determined.
Measurement indexes and methods
Plant height, which was the distance from the soil surface to the top of the main stem, was measured with a ruler. The selected lettuce seedlings with then cleaned with deionized water, and any impurities were removed from the surface. Any water on the plant surface was dried with absorbent paper, and the fresh weight was measured. The samples were then placed in an electric thermostatic drying oven (DHG-9245, Shanghai Yiheng Scientific Instrument Co., Ltd.) set to 105 °C for 30 min. After adjusting the temperature to 75 °C and drying the material to constant weight, its dry weight was obtained.
The lipid peroxidation level was measured by estimating malondialdehyde (MDA) content using thiobarbituric acid (TBA).41
Lipoxygenase (LOX) activity42 was estimated by monitoring the increase in absorbance at 234 nm, with linoleic acid used as a substrate.
Using a supercooled mortar and pestle, a lettuce sample (0.5 g) was ground in 5 mL of phosphate buffer (pH 7.0). The homogenate was centrifuged twice at 4°C (11,000 × g) for 10 min and the collected supernatant was then used for the determination of enzyme activity. Superoxide dismutase (SOD) activity was determined using the NBT (nitro blue tetrazolium) photochemical reduction method,43 and 50% inhibition of the NBT photochemical reduction was considered one enzyme activity unit (U). Peroxidase (POD) activity was measured by the guaiacol method,44 with an increase of 1 OD470nm·min−1 considered one unit of enzyme activity (U). Catalase (CAT) activity was measured by hydrogen peroxide ultraviolet spectrophotometry,45 with an increase of 0.1 OD240nm·min−1 considered one enzyme activity unit (U). Ascorbate peroxidase (APX) activity was determined by measuring the rate of ascorbic acid oxidation at 290 nm (ε = 2.8 mM−1·cm−1).46 Glutathione reductase [GR) activity was measured according to the methods of.47
The ascorbic acid [AsA] content was determined according to the methods of,48 and the contents of GSH and GSSH were determined by the,49 method.
The activity of GloI and GloII was estimated according to the methods of.50 The extraction buffer and steps of these enzymes were the same as those of the antioxidant enzymes. The absorbances of GloI and GloII were 240 nm and 412 nm, respectively, and the enzyme activity was expressed as moles per minute per gram of fresh weight.
Statistical analysis
All the measurements were replicated at least 3 times. Duncan’s multiple range test was used for statistical analysis with SPSS (version 26, SPSS Inc., USA], and the significance level was P < .05. The data in the tables and figures are the means ± standard deviations (SDs). The means followed by different letters are significantly different from each other.
Results
Effects of exogenous spermidine on the growth of lettuce leaves under high-temperature stress
Figure 1 shows the growth of lettuce seedlings for 8 days. It can be seen from the figure that the lettuce seedlings under high-temperature stress display weak growth, slow leaf growth, and small leaves, which are significantly different from those of the normal-temperature control seedlings. Thus, spraying Spd under high-temperature stress could significantly alleviate the growth of lettuce seedlings.
Figure 1.
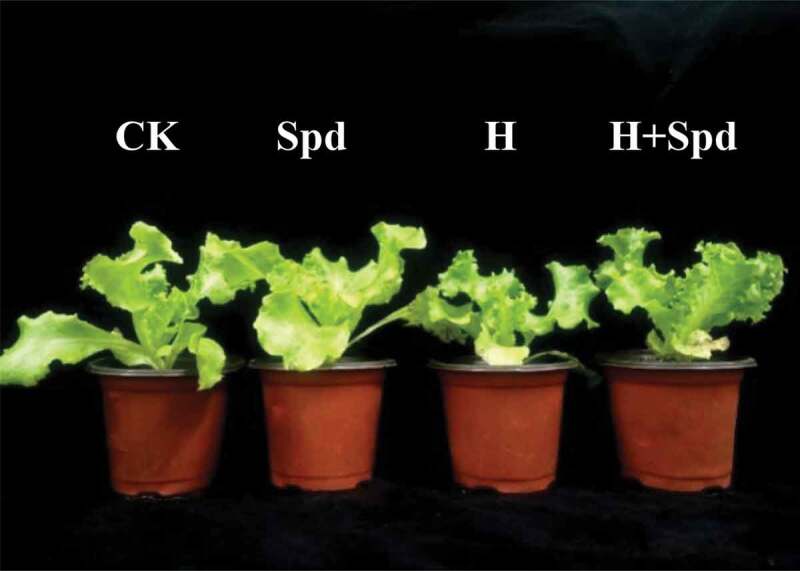
Effects of exogenous spd on the growth of lettuce seedlings under high-temperature stress (8 d)
Table 1 shows the growth of lettuce seedlings for 8 days. Spraying Spd at room temperature had no significant effect on seedling growth, and the treated seedlings did not significantly differ from the normal-temperature control seedlings. Under high-temperature stress, the growth of the lettuce seedlings significantly decreased. Compared with those the normal-temperature control seedlings, the plant height, fresh weight and dry weight of the treated lettuce seedlings significantly decreased by 89%, 82% and 68%, respectively; spraying exogenous Spd under high-temperature stress significantly increased the growth indexes of the lettuce seedlings by 1.03 times, 1.47 times and 1.55 times, respectively. Thus, spraying Spd kept lettuce seedlings growing at normal levels.
Table 1.
Effects of exogenous spd on the growth of lettuce seedlings under high-temperature stress
Effects of exogenous spermidine on membrane lipid damage in lettuce leaves under high-temperature stress
Under high-temperature stress, the MDA content increased significantly. After 4 days of stress treatment, high-temperature stress led to a significant difference from the controls, and the seedling leaf MDA content in the other high-temperature stress and Spd treatments were 1.12 times and 1.03 times that of the control, respectively. In contrast, after 8 days of stress treatment, the seedling leaf MDA content in response to high-temperature stress was 1.24 times that of the control (Figure 2(a)). The results show that high-temperature stress aggravates the degree of membrane lipid peroxidation and that exogenous spermine treatment can effectively inhibit the generation of MDA under high-temperature stress to alleviate membrane lipid peroxidation.
Figure 2.
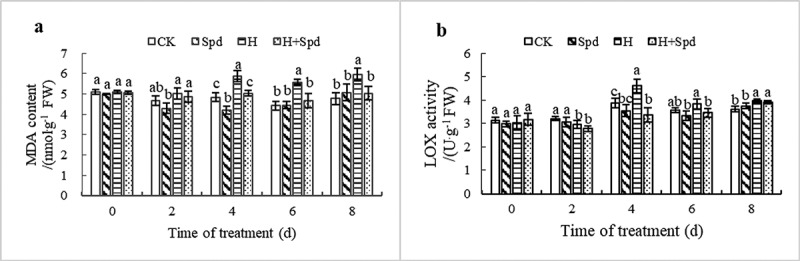
Effects of exogenous spd on the malondialdehyde content (a) of and LOX activity (b) in lettuce seedlings under high-temperature stress. The vertical bars represent the SDs of the means (n = 3). The different letters indicate significant differences at P < .05 according to Duncan’s multiple range test
Under high-temperature stress, LOX activity in leaves first increased but then decreased. The LOX activity in leaves peaked at 4 days after stress treatment, the value of which was 1.07 times higher than that of the control treatment. However, exogenous Spd treatment significantly reduced LOX activity in the seedling leaves (Figure 2(b)). Thus, under high-temperature stress, the MDA content gradually increased, and LOX activity first increased but then decreased; however, exogenous Spd significantly reduced the MDA content and LOX activity.
Effects of exogenous spermidine on antioxidant enzyme activity in lettuce seedling leaves under high-temperature stress
Under normal temperature, antioxidant enzyme activity in lettuce seedling leaves remained stable without a wide range of variation (Figure 3). When Spd was sprayed at room temperature, SOD activity decreased first, increased and then decreased again, all of which were lower than the control levels (Figure 3(a)). In contrast, POD activity increased first, decreased and then increased again (Figure 3(b)); the CAT activity and POD activity were consistently higher than the control levels. However, APX activity decreased first and then increased during the high-temperature treatment (Figure 3(d)). SOD activity continued to decrease for 4 days and was only 60% of that of the control group. However, exogenous Spd significantly inhibited the decrease in SOD activity under high-temperature stress. At the fourth day, the SOD activity was 82% of that of the control group (Figure 3(a)), effectively improving the ability of lettuce seedlings to remove O2.- under high-temperature stress. Moreover, under high-temperature stress, POD activity first increased but then decreased, reaching its maximum value at 2 days after stress, with an activity 1.14 times higher than that of the control group. Under high-temperature stress, exogenous Spd significantly increased the POD activity (Figure 3(b)), thus relieving the effects of the high-temperature stress. CAT activity increased first but then decreased with the prolonging of the high-temperature treatment. Although exogenous Spd could increase CAT activity under high-temperature stress, the difference was not significant (Figure 3(c)). APX activity first decreased and then reached its lowest value after 4 days of stress treatment, and its activity was 0.84 times that of the control group. Afterward, the APX activity began to increase again (Figure 3(d)). Exogenous Spd under stress conditions can improve APX activity, thus alleviating the damage of H2O2 to plants.
Figure 3.
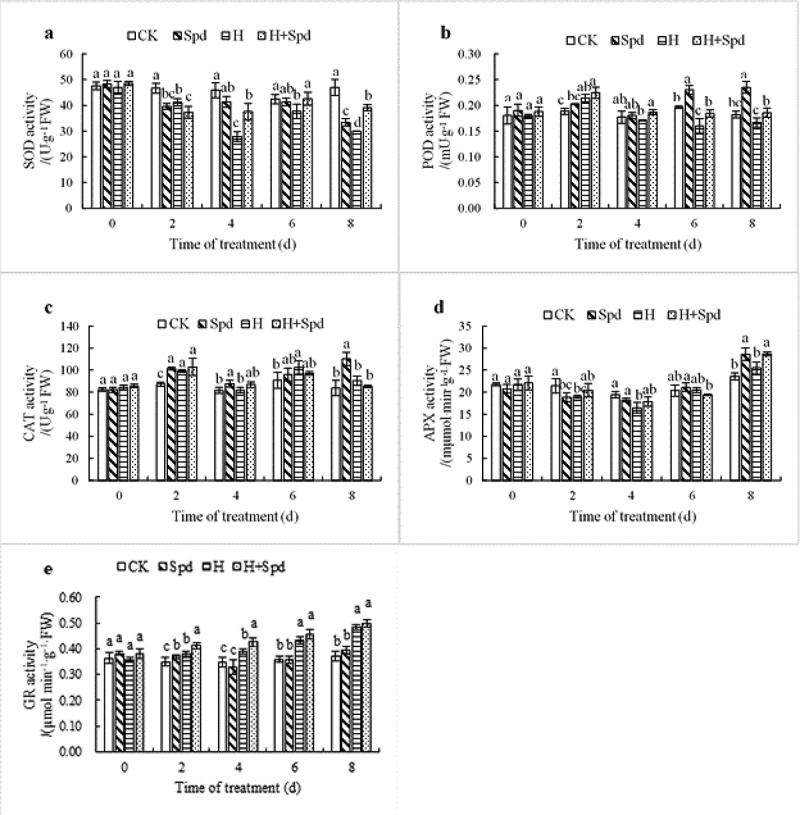
Effects of exogenous spd on the activities of the antioxidant enzymes SOD (a), POD (b), CAT (c), APX (d) and GR(e) in lettuce seedlings under high-temperature stress. The vertical bars represent the SDs of the means (n = 3). The different letters indicate significant differences at P < .05 according to Duncan’s multiple range test
Under the high-temperature stress treatment, the GR activity in the lettuce seedling leaves tended to first decrease and then increase, reaching the minimum value 4 days after high-temperature treatment. However, under high-temperature stress treatment, spraying Spd significantly increased the GR activity, the differences in which were significant (Figure 3(e)). The above results show that spraying Spd under high-temperature stress could effectively improve antioxidant enzyme activity.
Effects of exogenous spermidine on the ascorbic acid content in lettuce seedling leaves under high-temperature stress
Spraying spermine under normal temperature reduced the AsA content, albeit with little change, and there was no significant difference in AsA content compared with that in the control treatment. Under high-temperature stress, the AsA content tended to increase after initially decreasing, reaching the lowest content at 4 day, which was 0.91 times that of the normal-temperature controls (Figure 4(a)). Spraying Spd under high-temperature stress increased the content of AsA, and there was a significant difference between the two days of treatment and high-temperature stress treatment, the former of which was 1.08 times higher than that of high-temperature stress treatment. Other treatment durations also increased the content of AsA, but the difference was not significant compared with that of the high-temperature stress treatment (Figure 4(a)). Under high-temperature stress, the DHA content tended to increase. The maximum value was reached at 8 days after treatment, which was 1.34 times that of the control treatment. Spd spraying under high-temperature stress significantly reduced the DHA content and restored the DHA content to the control level (Figure 4(b)). High-temperature stress had a significant effect on the ratio of reduced AsA and oxidized DHA, which tended to increase first and then decrease, reaching the lowest value at 8 day, which was 0.83 times that of the control treatment (Figure 4(c)). Spraying Spd under high-temperature stress can increase the ratio of AsA/DHA and promote an increase in the proportion of oxidized DHA. In conclusion, the application of exogenous Spd under high-temperature stress can increase the AsA content, reduce the DHA content, and reduce the AsA/DHA ratio.
Figure 4.
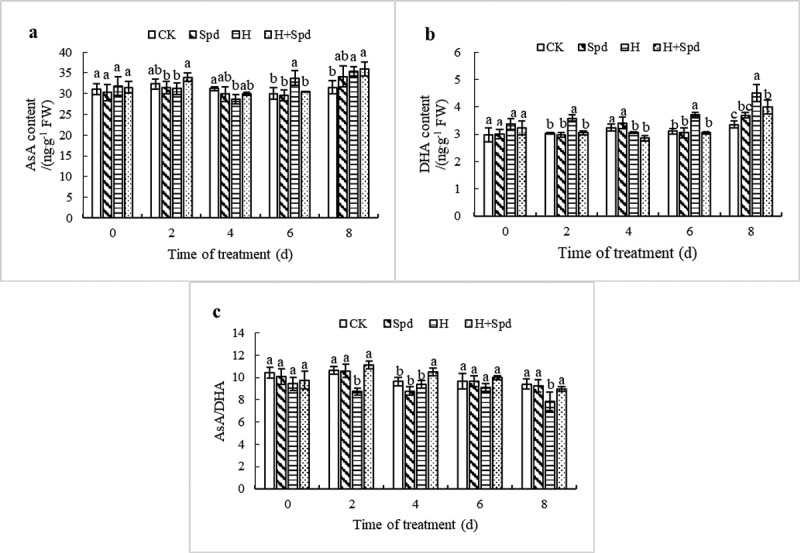
Effects of exogenous spd on the AsA content (a), the DHA content (b), and the AsA/DHA ratio (c) of lettuce seedlings under high-temperature stress. The vertical bars represent the SDs of the means (n = 3). The different letters indicate significant differences at P < .05 according to Duncan’s multiple range tests
Effects of exogenous spermidine on the glutathione content in leaves of lettuce seedlings under high-temperature stress
Figure 5 shows the variation trend of glutathione (GSH) content in lettuce seedling leaves under high-temperature stress and in response to exogenous Spd spraying, which is consistent with the variation in ascorbic acid (AsA) content. Under normal temperature, spraying Spd had no significant effect on the change in GSH content in the lettuce seedlings. Under high-temperature stress, the GSH content tended to first decrease but then slowly increased, reaching the maximum value at 8 days after the stress treatment, and the content was 1.11 times higher than that of the normal-temperature controls. As the days of high-temperature stress treatment increased, exogenous Spd spraying further increased the GSH content, with the maximum value occurring at 8 days of stress treatment, which was 1.12 times higher than that of high-temperature stress treatment. In contrast to that which occurred for AsA, spraying Spd significantly increased the GSH content under high-temperature stress (Figure 5(a)). Moreover, under high-temperature stress conditions, the GSSH content first decreased but then increased. Although the application of exogenous Spd could reduce the GSSH level, the difference was not significant (Figure 5(b)). High-temperature stress reduced the GSH/GSSH ratio, which was related to the increase in GSSH content (Figure 5(c)). In conclusion, the application of exogenous Spd under high-temperature stress significantly increased GSH levels and the GSH/GSSH ratio.
Figure 5.
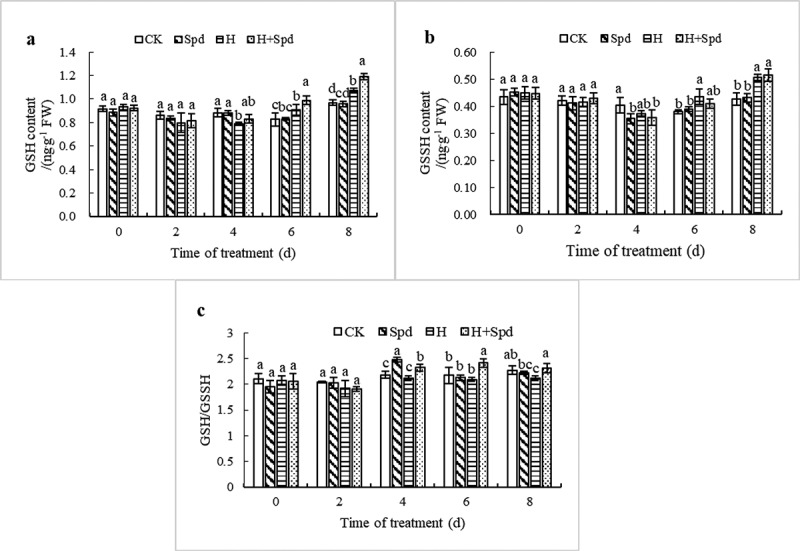
Effects of exogenous spd on the GSH content (a), GSSH content (b), and the GSH/GSSH ratio (c) of lettuce seedlings under high-temperature stress. The vertical bars represent the SDs of the means (n = 3). The different letters indicate significant differences at P < .05 according to Duncan’s multiple range test
Effects of exogenous spermidine on glyoxalase activity in the leaves of lettuce seedlings under high-temperature stress
The effect of spraying Spd on glyoxalase I (GloI) activity at room temperature was not significant, and there was no significant difference in this activity compared with the room temperature control activity (Figure 6(a)). Under high-temperature stress, GloI activity showed a gradual increasing trend and reached the maximum value after 8 days of treatment, which was 1.18 times that of the normal-temperature controls. Spraying Spd under high-temperature stress further increased the GloI activity (Figure 6(a)). Under nonstress conditions, there was no significant difference in GloII activity between the control seedlings and the lettuce seedlings sprayed with exogenous Spd. Under high-temperature stress, GloII activity tended to first decrease but then increase. It reached the lowest value at 4 day, which was 0.79 times that of the normal-temperature control seedlings, and spraying Spd under high-temperature stress significantly increased the GloII activity (Figure 6(b)). In conclusion, spraying Spd under high-temperature stress can significantly increase glyoxalase activity.
Figure 6.
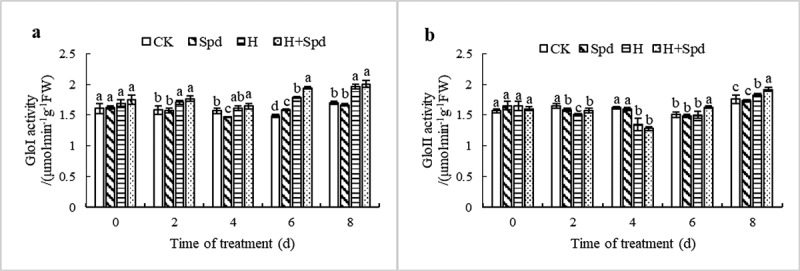
Effects of exogenous spd on the activities of GloI (a) and GloII (b) in lettuce seedlings under high-temperature stress. The vertical bars represent the SDs of the means (n = 3). The different letters indicate significant differences at P < .05 according to Duncan’s multiple range test
Discussion
High-temperature stress has adverse effects on plant growth and morphology, and exogenous Spd has been known to effectively alleviate the inhibitory effect of abiotic stress on plant growth.51 In this study, lettuce seedling growth and dry weight decreased significantly under high-temperature stress (Table 1), while spraying Spd alleviated the negative effects on plant growth (Figure 1), which was consistent with previous studies in tomato.32 Excess reactive oxygen species (ROS), such as O2.-, H2O2, OH., and(1O2), are produced under high-temperature stress. An increase in these ROS can cause oxidative damage to the membrane (membrane lipid peroxidation), which can lead to oxidative damage to the cell.52 MDA, a lipid peroxidation product, was used as an indicator of oxidative stress in lettuce seedlings under high-temperature stress in this study. With the increase in heat treatment days, the MDA content increased gradually and showed that the oxidation of lettuce seedlings was occurring under prolonged stress; spraying Spd significantly reduced the MDA level under high-temperature stress (Figure 2(a)), which is consistent with rice research results,35 suggesting that Spd is an effective ROS scavenger that can effectively inhibit membrane lipid peroxidation and help plants establish lipid balance. Lipoxygenase is an oxidase involved in lipid peroxidation via its catalysis of the oxidation of membrane lipids to H2O2. In this study, the LOX activity and MDA content showed the same change trend, and both increased under high-temperature stress (Figure 2), indicating that the degree of membrane lipid peroxidation in lettuce seedlings was severe. Under high-temperature stress, spraying Spd could reduce the LOX activity, which was consistent with the results of a rice study,13 which further indicated that Spd functions in membrane stability.
The active oxygen that accumulated under abiotic stress needs to be removed by antioxidant enzyme systems, including those comprising SOD, POD, and CAT, which work together to remove ROS produced under high-temperature stress in plants. SOD is the first line of defense to remove ROS. It catalyzes the generation of superoxide anion radicals (O2.-) to form H2O2 and 1O2 under high-temperature stress.53 In this study, a significant reduction in SOD activity under high-temperature stress may be due to the removal of O2.- in the plant tissue (Figure 3(a)). O2.- is present at relatively low levels, but high-temperature stress produces excess O2.-, resulting in a decline in SOD removal efficiency and thus reducing enzyme activity. Research has shown that the SOD activity in different rice varieties under high-temperature stress tended to decrease and that exogenous spraying of Spd can significantly inhibit the reduced SOD activity caused by thermal stress,54 which is consistent with our results. Under high-temperature stress, Spd spraying can significantly increase SOD activity, which can improve the O2.- removal efficiency and alleviate the lipid peroxidation of lettuce seedlings. POD, CAT and APX can remove H2O2 produced under high-temperature stress. In addition to removing H2O2, POD can also remove phenols, amines, aldehydes, etc., which have dual functions.52 Our results show that the POD activity under high-temperature stress decreased after first increasing (Figure 3(b)), and the CAT activity occurred thereafter, which tended to increase and then decrease (Figure 3(c)). Similarly, the APX activity showed an upward trend after decreasing (Figure 3(d)). Therefore, we suspect that the CAT, POD and APX enzymes may work synergistically. Under high temperature stress, GR activity showed an upward trend (Figure 3(e)), which was used to synthesize more GSH to improve the ASA-GSH circulatory system, while spraying Spd under high temperature stress could further improve GR activity and accelerate the ASA-GSH circulatory system. Spd spraying under high-temperature stress significantly increased the activity of POD, CAT APX and GR; accelerate the circulation of antioxidant enzyme system and ascorbic acid glutathione system to a certain extent, so as to improve the scavenging efficiency of ROS and reduce the oxidative damage caused by ROS.
The ascorbic acid-glutathione cycle (AsA-GSH cycle) is an important nonenzymatic system in plants that maintains the oxidative environment in cells by regulating the content of both AsA and GSH and plays a protective role in plants.55 GSH is widely distributed in plant tissues and can be directly or indirectly involved in ROS detoxification.56 In addition to ROS detoxification, GSH is also involved in methylglyoxal (MG) detoxification.56 Studies have shown that high-temperature stress can cause oxidative damage to wheat seedlings, while exogenous substances increase the heat tolerance of rice by increasing GSH levels, the GSH/GSSH ratio, and other components of the AsA-GSH cycle (e.g., APX and GR activities).57 Other studies have shown that pretreatment with GSH can improve both the antioxidant system and the glyoxalase system of Vigna radiata L. seedlings, resulting in better growth conditions and lower levels of MG and oxidative stress.58 AsA is an effective active oxygen scavenger.59 In this study, the AsA and GSH contents of lettuce tended to increase under high-temperature stress (Figures 4 and 5), which was consistent with the results of the study on the effect of heat stress on wheat,60 and the levels of these two antioxidants further increased after the application of exogenous Spd. The AsA/DHA ratio tended to decrease under high-temperature stress (Figure 4(c)), probably because DHA increased faster than AsA, and reduced AsA was consumed too quickly. Spd under high-temperature stress alleviated this situation and significantly increased the AsA/DHA ratio (Figure 4(c)). Similar to the change in ascorbic acid content, the GSH/GSSH ratio decreased under high-temperature stress (Figure 5(c)), but the difference was not significant because AsA is more sensitive to high temperature than to normal temperature. Our results show that exogenous Spd can increase the heat resistance of lettuce seedlings under high-temperature stress by increasing AsA and GSH levels and the AsA/DHA and GSH/GSSH ratios.
Methylglyoxal (MG) is a product of abiotic stress and can further increase oxidative stress. The glyoxalase system is the most effective system for removing methylglyoxal. MG produced during high temperature stress is first removed by GloⅠ, and at the same time, reducing glutathione is used as a cofactor to produce the intermediate product SD-lactoylglutahione (SLG), GloⅡ catalyzes the hydrolysis of SLG to form D-lactic acid, and in the meantime regenerates GSH.61 In this study, the activity of GloI and GloII tended to increase under high-temperature stress (Figure 6), which is consistent with the results of previous reports.62,63 Our results show that spraying Spd under high-temperature stress can increase the activity of GloI and GloII, by increasing the activities of the two glyoxalases, the utilization and regeneration of GSH were increased indirectly, and the ascorbic acid glutathione (ASA GSH) circulation system was improved to some extent. Finally, accelerated the scavenging rate of ROS and MG, reduced the damage of lettuce, and enhanced the tolerance of lettuce to high temperature stress.
In summary, MDA and LOX levels, antioxidant systems, nonenzymatic antioxidants, and the glyoxalase system were used to determine whether lettuce was experiencing oxidative stress due to high-temperature stress. Spraying Spd reduced the MDA and LOX levels, increased the activity of enzymes in the oxidative system, promoted the ascorbic acid-glutathione cycle, and increased glyoxalase activity, which could thus remove reactive oxygen species and the toxic substance methylglyoxal more quickly, providing a protective mechanism for lettuce.
Funding Statement
This work was supported by the National Key Research and Development Program of China (2016YFD0201010), the Beijing Innovation Consortium of Agriculture Research System (BAIC07-2020) and the Science and Technology Program of the Beijing Municipal Education Commission (KM201910020012).
Disclosure of Potential Conflicts of Interest
The authors report no conflict of interest.
Author contributions
Chengjie Li carried out the experiments, collected and analysed the results, and wrote the manuscript. Chaojie Liu designed the experiments. Yingyan Han, Jinghong Hao, Xiaoxiao Qin, and Shuangxi Fan helped analyse the results and edit manuscript. All authors have contributed signifcantly, and all authors are in agreement with the content of the manuscript.
References
- 1.Shah F, Huang J, Cui K, Nie L, Shah T, Chen C, Wang K.. Impact of high-temperature stress on rice plant and its traits related to tolerance. J Agric Sci. 2011;149:1–9. [Google Scholar]
- 2.Asthir B. Protective mechanisms of heat tolerance in crop plants. Biol Plant. 2015;10(1):202–210. doi: 10.1080/17429145.2015.1067726. [DOI] [Google Scholar]
- 3.Fragkostefanakis S, Röth S, Schleiff E, Scharf K-D.. Prospects of engineering thermotolerance in crops through modulation of heat stress transcription factor and heat shock protein networks. Plant Cell Environ. 2014;38:1881–1895. doi: 10.1111/pce.12396. [DOI] [PubMed] [Google Scholar]
- 4.Mathur S, Agrawal D, Jajoo A.. Photosynthesis: response to high temperature stress. J Photochem Photobiol B. 2014;137:116–126. doi: 10.1016/j.jphotobiol.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 5.Wahid A, Gelani S, Ashraf M, Foolad MR. Heat tolerance in plants: an overview. Environ Exp Bot. 2007;61(3):199–223. doi: 10.1016/j.envexpbot.2007.05.011. [DOI] [Google Scholar]
- 6.Barros, Vicente, Stocker, Thomas F. Managing the risks of extreme events and disasters to advance climate change adaptation: special report of the intergovernmental panel on climate change. J Clin Endocrinol Metab. 2012;18:586–599. [Google Scholar]
- 7.Bita CE, Gerats T. Plant tolerance to high temperature in a changing environment: scientific fundamentals and production of heat stress-tolerant crops. Front Plant Sci. 2013;4:373. doi: 10.3389/fpls.2013.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mostofa MG, Yoshida N, Fujita M. Spermidine pretreatment enhances heat tolerance in rice seedlings through modulating antioxidative and glyoxalase systems. Plant Growth Regul. 2014;73:31–44. doi: 10.1007/s10725-013-9865-9. [DOI] [Google Scholar]
- 9.Ke Q, Ye J, Wang B, Ren J, Yin L, Deng X, Wang S. Melatonin mitigates salt stress in wheat seedlings by modulating polyamine metabolism. Front Plant Sci. 2018;9:914. doi: 10.3389/fpls.2018.00914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 11.Kamran M, Xie K, Sun J, Wang D, Shi C, Lu Y, Gu W, Xu P. Modulation of growth performance and coordinated induction of ascorbate-glutathione and methylglyoxal detoxification systems by salicylic acid mitigates salt toxicity in choysum (Brassica parachinensis L.). Ecotoxicol Environ Saf. 2020;188:109877. doi: 10.1016/j.ecoenv.2019.109877. [DOI] [PubMed] [Google Scholar]
- 12.Jahan MS, Shu S, Wang Y, Chen Z, He M, Tao M, Sun J, Guo S. Melatonin alleviates heat-induced damage of tomato seedlings by balancing redox homeostasis and modulating polyamine and nitric oxide biosynthesis. BMC Plant Biol. 2019;19:414. doi: 10.1186/s12870-019-1992-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mostofa MG, Hossain MA, Siddiqui MN, Fujita M, Tran LS. Phenotypical, physiological and biochemical analyses provide insight into selenium-induced phytotoxicity in rice plants. Chemosphere. 2017;178(JUL.):212–223. doi: 10.1016/j.chemosphere.2017.03.046. [DOI] [PubMed] [Google Scholar]
- 14.Roxas VP, Lodhi SA, Garrett DK, Mahan JR, Allen RD. Stress tolerance in transgenic tobacco seedlings that overexpress glutathione s-transferase/glutathione peroxidase. Plant Cell Physiol. 2000;41:1229–1234. doi: 10.1093/pcp/pcd051. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Gu W, Li C, Li W, Li C, Li J, Wei S. Exogenous spermidine improves drought tolerance in maize by enhancing the antioxidant defence system and regulating endogenous polyamine metabolism. Crop Pasture Sci. 2018a;69:1076–1091. doi: 10.1071/cp18271. [DOI] [Google Scholar]
- 16.Mir MA, John R, Alyemeni MN, Alam P, Ahmad P. Jasmonic acid ameliorates alkaline stress by improving growth performance, ascorbate glutathione cycle and glyoxylase system in maize seedlings. Sci Rep. 2018;8:2831. doi: 10.1038/s41598-018-21097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 18.Yadav S, Singla-Pareek S, Reddy M, Sopory S. Methylglyoxal detoxification by glyoxalase system: a survival strategy during environmental stresses. Physiol Mol Biol Plants. 2005;11:1–11. [Google Scholar]
- 19.Hasanuzzaman M, Nahar K, Anee TI, Fujita M. Glutathione in plants: biosynthesis and physiological role in environmental stress tolerance. Physiol Mol Biol Plants. 2017a;23:249–268. doi: 10.1007/s12298-017-0422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turóczy Z, Kis P, Török K, Cserháti M, Á L, Dudits D, Horváth GV. Overproduction of a rice aldo–keto reductase increases oxidative and heat stress tolerance by malondialdehyde and methylglyoxal detoxification. Plant Mol Biol. 2011;75:399–412. doi: 10.1007/s11103-011-9735-7. [DOI] [PubMed] [Google Scholar]
- 21.Hoque TS, Hossain MA, Mostofa MG, Burritt DJ, Fujita M, Tran L-SP. Methylglyoxal: an emerging signaling molecule in plant abiotic stress responses and tolerance. Front Plant Sci. 2016;7:1341. doi: 10.3389/fpls.2016.01341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li L, Gu W, Li J, Li C, Xie T, Qu D, Meng Y, Li C, Wei S. Exogenously applied spermidine alleviates photosynthetic inhibition under drought stress in maize (Zea mays L.) seedlings associated with changes in endogenous polyamines and phytohormones. Plant Physiol Biochem. 2018b;129:35–55. doi: 10.1016/j.plaphy.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Rahman A, Mostofa MG, Nahar K, Hasanuzzaman M, Fujita M. Exogenous calcium alleviates cadmium-induced oxidative stress in rice (Oryza sativa L.) seedlings by regulating the antioxidant defense and glyoxalase systems. Braz J Bot. 2016a;39:393–407. doi: 10.1007/s40415-015-0240-0. [DOI] [Google Scholar]
- 24.Rahman A, Nahar K, Hasanuzzaman M, Fujita M. Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front Plant Sci. 2016b;7:609. doi: 10.3389/fpls.2016.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta K, Dey A, Gupta B. Plant polyamines in abiotic stress responses. Acta Physiol Plant. 2013;35:2015–2036. doi: 10.1007/s11738-013-1239-4. [DOI] [Google Scholar]
- 26.Liu J-H, Kitashiba H, Wang J, Ban Y, Moriguchi T. Polyamines and their ability to provide environmental stress tolerance to plants. Plant Biotechnol. 2007;24:117–126. doi: 10.5511/plantbiotechnology.24.117. [DOI] [Google Scholar]
- 27.Wang Z, Xu Y, Wang J, Yang J, Zhang J. Polyamine and ethylene interactions in grainfilling of superior and inferior spikelets of rice. Plant Growth Regul. 2012;66:215–228. doi: 10.1007/s10725-011-9644-4. [DOI] [Google Scholar]
- 28.ElSayed AI, Rafudeen MS, El-hamahmy MAM, Odero DC, Hossain MS. Enhancing antioxidant systems by exogenous spermine and spermidine in wheat (Triticum aestivum) seedlings exposed to salt stress. Funct Plant Biol. 2018;45:745–759. doi: 10.1071/fp17127. [DOI] [PubMed] [Google Scholar]
- 29.Saha J, Giri K. Molecular phylogenomic study and the role of exogenous spermidine in the metabolic adjustment of endogenous polyamine in two rice cultivars under salt stress. Gene. 2017;609:88–103. doi: 10.1016/j.gene.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Amri E, Shahsavar AR. Response of lime seedlings (Citrus aurantifolia L.) to exogenous spermidine treatments under drought stress. Aust J Basic Appl Sci. 2010;4:4483–4489. [Google Scholar]
- 31.Sun X, Xie L, Han L. Effects of exogenous spermidine and spermine on antioxidant metabolism associated with cold-induced leaf senescence in Zoysiagrass (Zoysia japonica Steud.). Hortic Environ Biotechnol. 2019;60:295–302. doi: 10.1007/s13580-018-0089-9. [DOI] [Google Scholar]
- 32.Sang QQ, Shu S, Shan X, Guo SR, Sun J. Effects of exogenous spermidine on antioxidant system of tomato seedlings exposed to high temperature stress. Russ J Plant Physiol. 2016a;63:645–655. doi: 10.1134/s1021443716050113. [DOI] [Google Scholar]
- 33.Sang T, Shan X, Li B, Shu S, Sun J, Guo S. Comparative proteomic analysis reveals the positive effect of exogenous spermidine on photosynthesis and salinity tolerance in cucumber seedlings. Plant Cell Rep. 2016b;35:1769–1782. doi: 10.1007/s00299-016-1995-x. [DOI] [PubMed] [Google Scholar]
- 34.Xu X, Shi G, Ding C, Xu Y, Zhao J, Yang H, Pan Q. Regulation of exogenous spermidine on the reactive oxygen species level and polyamine metabolism in Alternanthera philoxeroides (Mart.) Griseb under copper stress. Plant Growth Regul. 2011;63:251–258. doi: 10.1007/s10725-010-9522-5. [DOI] [Google Scholar]
- 35.Fu Y, Gu Q, Dong Q, Zhang Z, Lin C, Hu W, Pan R, Guan Y, Hu J. Spermidine enhances heat tolerance of rice seeds by modulating endogenous starch and polyamine metabolism. Molecules. 2019;24:1395. doi: 10.3390/molecules24071395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sang Q, Shan X, An Y, Shu S, Sun J, Guo S. Proteomic analysis reveals the positive effect of exogenous spermidine in tomato seedlings’ response to high-temperature stress. Front Plant Sci. 2017;8:120. doi: 10.3389/fpls.2017.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu L, Xiang L, Zhang L, Zhou X, Zou Z, Hu X. The photoprotective role of spermidine in tomato seedlings under salinity-alkalinity stress. PLoS One. 2014;9:e110855. doi: 10.1371/journal.pone.0110855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pouramir F, Mohammad K-H, Masoud E. Alleviating harmful effects of chilling stress on rice seedling via application of spermidine as seed priming factor. Afr J Agric Res. 2014;9:1412–1418. doi: 10.5897/ajar2014.8709. [DOI] [Google Scholar]
- 39.Wu J, Shu S, Li C, Sun J, Guo S. Spermidine-mediated hydrogen peroxide signaling enhances the antioxidant capacity of salt-stressed cucumber roots. Plant Physiol Biochem. 2018;128:152‐162. doi: 10.1016/j.plaphy.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Hussain A, Nazir F, Fariduddin Q. Polyamines (spermidine and putrescine) mitigate the adverse effects of manganese induced toxicity through improved antioxidant system and photosynthetic attributes in Brassica juncea. Chemosphere. 2019;236:124830. doi: 10.1016/j.chemosphere.2019.124830. [DOI] [PubMed] [Google Scholar]
- 41.Heath RL, Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- 42.Doderer A, Kokkelink I, van der Veen S, Valk BE, Schram A, Douma AC. Purification and characterization of two lipoxygenase isoenzymes from germinating barley. Biochim Biophys Acta. 1992;1120:97–104. doi: 10.1016/0167-4838(92)90429-h. [DOI] [PubMed] [Google Scholar]
- 43.Giannopolitis CN, Ries SK. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hammerschmidt R, Nuckles EM, Kuć J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol Plant Pathol. 1982;20:73–82. doi: 10.1016/0048-4059(82)90025-x. [DOI] [Google Scholar]
- 45.Chance B, Maehly AC. [136] Assay of catalases and peroxidases. Methods Enzymol. 1955;2:764–775. doi: 10.1016/s0076-6879(55)02300-8. [DOI] [PubMed] [Google Scholar]
- 46.Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. doi: 10.1093/oxfordjournals.pcp.a076232. [DOI] [Google Scholar]
- 47.Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/bf00386001. [DOI] [PubMed] [Google Scholar]
- 48.Arakawa N, Tsutsumi K, Sanceda NG, Kurata T, Inagaki C. A rapid and sensitive method for the determination of ascorbic acid using 4,7-diphenyl-l,10-phenanthroline. Agric Biol Chem. 1981;45:1289–1290. doi: 10.1080/00021369.1981.10864697. [DOI] [Google Scholar]
- 49.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 50.Li Z-G, Nie Q, Yang C-L, Wang Y, Zhou Z-H. Signaling molecule methylglyoxal ameliorates cadmium injury in wheat (Triticum aestivum L) by a coordinated induction of glutathione pool and glyoxalase system. Ecotoxicol Environ Saf. 2018c;149:101–107. doi: 10.1016/j.ecoenv.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 51.Sun J, Lu N, Xu H, Maruo T, Guo S. Root zone cooling and exogenous spermidine root-pretreatment promoting Lactuca sativa L. growth and photosynthesis in the high-temperature season. Front Plant Sci. 2016;7:368. doi: 10.3389/fpls.2016.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choudhury FK, Rivero RM, Blumwald E, Mittler R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017;90:856–867. doi: 10.1111/tpj.13299. [DOI] [PubMed] [Google Scholar]
- 53.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 54.Tang S, Zhang H, Li L, Liu X, Chen L, Chen W, Ding Y. Exogenous spermidine enhances the photosynthetic and antioxidant capacity of rice under heat stress during early grain-filling period. Funct Plant Biol. 2018;45:911–921. doi: 10.1071/fp17149. [DOI] [PubMed] [Google Scholar]
- 55.Bartoli CG, Buet A, Gergoff Grozeff G, Galatro A, Simontacchi M. Ascorbate-glutathione cycle and abiotic stress tolerance in plants. In Hossain M, Munné-Bosch S, Burritt D, Diaz-Vivancos P, Fujita M, Lorence A, editors. Ascorbic acid in plant growth, development and stress tolerance. Cham: Springer International Publishing; 2017. p. 177–200. doi: 10.1007/978-3-319-74057-7_7. [DOI] [Google Scholar]
- 56.Hasanuzzaman M, Nahar K, Hossain M, Mahmud J, Rahman A, Inafuku M, Oku H, Fujita M. Coordinated actions of glyoxalase and antioxidant defense systems in conferring abiotic stress tolerance in plants. Int J Mol Sci. 2017b;18:200. doi: 10.3390/ijms18010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hasanuzzaman M, Nahar K, Alam M, Fujita M. Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology. 2013;22:584–596. doi: 10.1007/s10646-013-1050-4. [DOI] [PubMed] [Google Scholar]
- 58.Nahar K, Hasanuzzaman M, Alam MM, Fujita M. Exogenous glutathione confers high temperature stress tolerance in mung bean (Vigna radiata L.) by modulating antioxidant defense and methylglyoxal detoxification system. Environ Exp Bot. 2015;112:44–54. doi: 10.1016/j.envexpbot.2014.12.001. [DOI] [Google Scholar]
- 59.Foyer CH, Noctor G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 2011;155:2–18. doi: 10.1104/pp.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kocsy G, Szalai G, Galiba G. Effect of heat stress on glutathione biosynthesis in wheat. Acta Biol Szeged. 2002;46:71–72. [Google Scholar]
- 61.Li Z-G. Methylglyoxal and glyoxalase system in plants: old players, new concepts. Bot Rev. 2016;82:183–203. doi: 10.1007/s12229-016-9167-9. [DOI] [Google Scholar]
- 62.Hossain MA, Mostofa MG, Fujita M. Heat-shock positively modulates oxidative protection of salt and drought-stressed mustard (Brassica campestris L.) seedlings. J Plant Sci Mol Breed. 2013;2:1–13. doi: 10.7243/2050-2389-2-2. [DOI] [Google Scholar]
- 63.Wang R, Mei Y, Xu L, Zhu X, Wang Y, Guo J, Liu L. Differential proteomic analysis reveals sequential heat stress-responsive regulatory network in radish (Raphanus sativus L.) taproot. Planta. 2018;247:1109–1122. doi: 10.1007/s00425-018-2846-5. [DOI] [PubMed] [Google Scholar]


