ABSTRACT
The oxygen-evolving complex is integrated into photosystem (PSII). An essential part of oxygenic photosynthetic apparatus, embedded in the thylakoid membrane of chloroplasts. The OEC is a super catalyst to split water into molecular oxygen in the presence of light. The OEC consist of four Mn atoms, one Ca atom and five oxygen atoms (CaMn4O5) and this cluster is maintained by its surrounding proteins viz., PsbQ, PsbP, PsbO, PsbR. The function of this super catalyst with a high turnover frequency of 500 s−1 in standard condition. Chlorophyll a fluorescence (OJIP transients) are used to understand structural and functional cohesion of photosynthetic apparatus. A further K-peak in OJIP curve reflects damage at the OEC donor site in response to salinity, drought, and high temperature. The decline in performance indices (PI, SFI) also revealed structural damage of photosynthetic apparatus that leads to disruption of electron transport rate under abiotic conditions. This review discusses the structural and function cohesion of the OEC in plant against variable abiotic conditions.
Keywords: PSII, OEC, OJIP, Salinity, molecular oxygen, water
1. Introduction
The photosynthesis is an essential physiological process common to cyanobacteria, algae, and plants. That provides the molecular oxygen and source of foods for sustaining life on earth. The photosynthesis is an energy-conversion process by which the light energy converts into chemical energy. The organic compounds are synthesized by reducing atmospheric CO2 with the reducing equivalents (electron and protons) obtained by splitting water molecule, and molecular oxygen is released as a by-product in the atmosphere. The photosynthetic apparatus consists of three major complexes of protein pigments: Photosystem II (PSII), cytochrome b6f complex (Cytb6f), and Photosystem I (PSI). They are embedded in the thylakoid membrane of oxygenic-photosynthetic organisms. PSII breaks the water molecule and releases the protons in the lumen side while passing electrons into the plastoquinone pool and the Cytb6f complex. This complex pumps protons from the stroma to the lumen side of the thylakoid membrane while transferring electrons to plastocyanin and PSI, and the electrons are transferred to ferredoxin a final acceptor and NADP(H) 1 (Figure 1). PSII is an engine of photosynthetic apparatus, produces an oxidant with high redox potential to oxidize H2O, ensuring life on earth has an infinite source of the electron.2,3 The multisubunit complex of PSII includes the oxygen-evolving complex (OEC) or water-oxidizing complex (WOC), reaction centres (RCs), and the light-harvesting antenna complex (LHC).4 The OEC consist of four Mn atoms, one Ca atom and five oxygen atoms (CaMn4O5) and this cluster maintained by its surrounding proteins 10 kDa, 18 kDa, 23 kDa, and/or 33 kDa (PsbR, PsbQ, PsbP, PsbO).5–7 The structure of this “super catalyst with a high turnover frequency of 500 s−1” is evolutionary preserved and virtually identical from cyanobacteria to various algae and higher plants, and dates back to 2.4 billion years ago.8,9 Understanding the structure and function of the OEC catalytic center is often believed to be one of the key steps in producing efficient catalysts for the synthesis of molecular oxygen.
Figure 1.
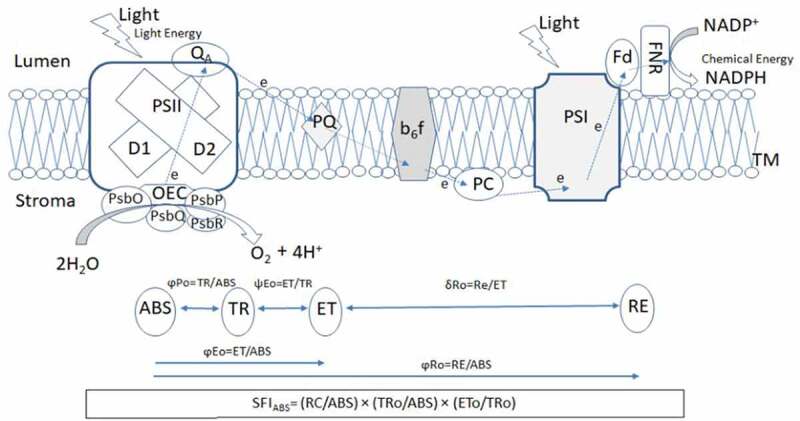
Distribution of different proteins of photosynthetic apparatus embedded in the thylakoid membrane. Systematic scheme of conversion of light energy to chemical energy through photosynthetic electron transport chain between PSII and PSI (based on Gupta et al. 2020 and Huang et al., 2020)
Here PSII – Photosystem II, OEC- the oxygen-evolving complex, PsbO, PsbP, PsbQ, and PsbR – are extrinsic proteins stabilize the structure of OEC, QA- primary and secondary quinone electron acceptors of photosystem II, PQ – plastoquinone; b6f -cytochrome b6f complex; PC – plastocyanin; PSI – photosystem I, Fd-soluble ferredoxin; FNR-ferredoxin-NADP+-reductase. ABS – absorption flux; ET-electron transport flux; TR-trapped excitation flux; RE-electron transport flux until PSI acceptors; φPo–maximum quantum yield of primary PSII photochemistry; ψ Eo–the efficiency/probability that an electron moves further than QA; δ Ro – efficiency with which an electron from QB is transferred until PSI acceptors; φEo–quantum yield of the electron transport flux from QA to QB; φRo – the quantum yield for the reduction of end electron acceptors at the PSI and SFIABS- structural and functional index on absorption basis.
Chl a fluorescence provides valuable information on the basic understanding of the structure and function of the photosynthetic apparatus.10 When a dark-adapted leaf is exposed to light, fast dynamic changes in Chl a fluorescence occur and induction can be used to extract information about the efficiency of electron transport through PSII.11–13 During fluorescence induction, a polyphasic pattern (O-J-I-P transients) is formed when plotted on a logarithmic time scale.14,15 These phases of fluorescence induction are linked with different energy fluxes, starts from light absorption (ABS), trapping (TR), dissipation (D1), electron transport (ET), and ends with the electron acceptor side at the PSI (RE).15,16 Some additional parameters viz., the quantum yields of primary PSII photochemistry (φPo), electron transport from QA to PQ (ψEo), and electron transport from QA to final PSI acceptor (φRo) and the overall performance indices are characterized by O-J-I-P transients (Figure 1).15,17,18 Various attempts have made to understand the impact of different abiotic stresses, i.e., high and low temperature, salinity, droughts, high-intensity light, on the photosynthetic apparatus of the plants.3,7,16,19,20 Many workers reviewed the structure and function of PSII under abiotic stresses; however, progress in understanding the structure and function of mysterious super catalyst, i.e., OEC of PSII is faraway. The present review aims to summarise the structural and functional cohesion of the oxygen-evolving complex in response to various abiotic stresses.
2. Chlorophyll a fluorescence and the oxygen-evolving complex
Chlorophyll a fluorescence (OJIP transients) is a non-destructive technique based on the theory of energy flow in the thylakoid membrane.21 In OJIP transients ‘O’ represents the origin, i.e., minimal fluorescence (Fo), J and I for intermediate inflexions (Fj and Fi) and ‘P’ for maximal (Fm).13 The structure and function of electron transport apparatus could be accessed by chlorophyll a fluorescence. In the OJIP transients, the OJ corresponds to the reduction of primary electron acceptor quinone (QA) of PSII, JI means the reduction of secondary electron acceptors viz., quinone (QB), plastoquinone (PQ), cytochrome (Cyt b6f), and plastocyanin (PC), and IP represents the reduction of electron transporters of PSI ferredoxin (fd), intermediate acceptors, and NADP.22 Exposure of abiotic stresses forms an addition K level peak in OJIP transients at 300 to 350 µs that shows a disruption in the water-splitting complex or OEC 21,23 (Figure 2A). Whenever the electron flow to the acceptor side exceeds the electron flow from the donor side, an additional K-step occurs. It leads to reaction center oxidation with a photosystem shift towards the P680 which have a lower fluorescence yield. Henceforth, the OEC dissociation inhibits efficient electron donation to the reaction center resulted in an additional K-phase in OJIP curve.24 Relative variable fluorescence at phase K of the fluorescence induction curve determines as 21,25
Figure 2.
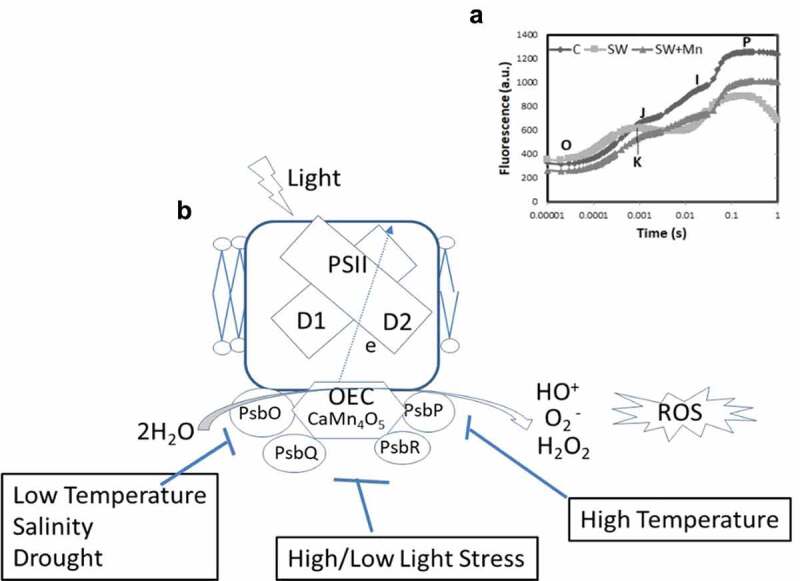
a: The OJIP transients of maize seedlings under seawater with or without exogenous manganese. Here O is for origin (minimal fluorescence Fo), J and I for two different inflexions (Fj and Fi) and P for peak (maximum fluorescence Fp or Fm). An additional K step in salinity treated maize seedlings observed after seven days of seawater exposure (based on Gupta, 2020). b: Effect of various abiotic factors viz., salinity, high and low temperature and high light intensity on extrinsic proteins i.e, PsbO, PsbP, PsbQ, and PsbR in higher plants. Instability of the OEC caused production of reactive oxygen species (ROS) that adversely affect photosynthetic efficiency in plants
, FK is fluorescence at the K-step (300 μs)
and the damage to oxygen-evolving complex OEC represents as
Performance indices (PIs) are proposed to combine information on the performance of PSII and efficiencies of specific electron transport reactions in the thylakoid membrane during the OJIP transients.17 PIs are calculated with mathematical formulae that capture (and integrate) information contained in 3–4 fluorescence parameters in one number, which is then used to rank different samples according to their PSII and electron transport performance.15 Following four parameters are used to calculate the PIs (1) RC/ABS- the ratio of the total number of active PSII reaction centers (RC) per absorption flux (ABS); (2) TRo/ABS (equivalent to φP0) – the maximum quantum yield of PSII photochemistry that leads to QA reduction; it was estimated by FV/FM; (3) ETo/TRo (equivalent to ψE0) – the efficiency (ψ) with which a trapped exciton by PSII reaction center leads to electron transfer (E0) from QA− to PQ, in the PQ pool and (4) REo/ETo (equivalent to δRo) – the efficiency (δ) of the electron transport (R0) from plastoquinol (PQH2), in the PQ pool, to the final electron acceptors of PSI (Figure 1). Following performance indices were proposed on the basis of parameters obtained from the OJIP curve.
SFIABS – “structure-function index” on absorption basis: which characterize structural and functional characteristics of PSII 26,27
where (RC/ABS): the amount of active PSII reaction centers, (TR0/ABS): a higher quantum yield of PSII photochemistry, (ET0/TR0): higher efficiency of the electron transport from QA- to the PQ pool. An increase in SFIABS suggests reflecting changes that “favor” photosynthesis
- (2) PIABS – performance index on absorption basis: PIABS is the most widely used PI, and was proposed by Strasser et al. 28 as a product of RC/ABS.
(3) PIABS,total – total performance index on absorption basis: proposed by Tsimilli-Michael and Strasser 17
PIABS, the total can have positive or negative values, with negative values expressing a “loss” of ability for energy conservation.29
Since PIABS, the total is related to the function of the “whole” linear electron transport, whereas PIABS is related only to the electron transport to the PQ pool,
Therefore, performance indices have been used to understand structural and functional cohesion of the photosynthetic apparatus in higher plants.15
3. Effect of abiotic stresses on the OEC
Photosystem II complex (PSII) is one of the most vulnerable parts in plant photosynthesis system, which is often disrupted by different abiotic stresses such as heat, chilling, salinity, and intense visible light.7,19,30,31 The oxygen-evolving complex (OEC) of PSII is protected by extrinsic proteins viz., PsbO, PsbP, PsbQ, and PsbR located at the luminal side, encoded by multiple gene families in pea, tomato, tobacco, and arabidopsis 32 (Figure 2B). These extrinsic proteins are an easy target to different stresses. Instability of extrinsic proteins facilitates the generation of reactive oxygen species (ROS) molecules leads to damage the OEC.33 Consequently, the decreases in PSII activity and over-reduction in the electron transport chain (ETC) results in the photooxidation 34,35 (Figure 2B). Molecular mechanism of photoinhibition of PSII explains in two proposed schemes. First is the excess-energy scheme, in which ROS cause direct oxidative damage to PSII complexes. Second, two steps scheme demonstrates that the primary photodamage to PSII occurs at the oxygen-evolving complex (OEC) resulted in the release of manganese ions (Mn2+).36 Following photodamage to OEC, the supply of electrons from water to the primary electron donor of PSII (P680) is blocked that might damage the PSII reaction centers.37,38 Furthermore, ROS inhibits the repair of photodamaged PSII.39 Net PSII photoinhibition occurs only when the rate of PSII photodamage exceeds than the speed of recovery.40
Under optimal conditions, an intact manganese cluster (Mn4CaO5) at the OEC promotes electron donation from water to PSII-RC with a low constant. It reduces the accessibility of non-water electron donation. However, non-water electron donors, i.e., Asc and Pro compete with the water-splitting complex/oxygen-evolving complex (OEC), where the reaction constant for non-water electron donors (kD) is much higher than that of splitting water (kW). PSII is adversely affected under various abiotic stresses and the OEC then presumably favors donation of electrons from non-water electron donors with a high rate constant. As the OEC activity decreases, the total electron transport increases, due to the easy accessibility of non-water electrons. Hence, the fraction of electrons donated by water is lower in the stressed condition.41 Different performance indices determine damage and repair to oxygen-evolving complex (OEC) against various abiotic stresses.15
4. Salinity
Salt stress has deleterious effects on the Mn cluster of OEC resulted in a reduction of PSII activity.42 Surrounding extrinsic proteins that protect the OEC detach during high salinity by which OEC releases two or three manganese ions and leads to a permanent cessation of oxygen evolution.43,44 PsbO appears to prime importance in stabilizing the OEC.3,7,45 The PsbP protein plays a role in optimizing Ca2+ and Cl− availability for maintaining the Mn–Ca2+–Cl− cluster of OEC. At the same time, the PsbQ is required at low Cl−concentrations (<3 mM) for oxygen evolution.46,47 The PsbR locates at PSII luminal side, but the activity of this is not yet experimentally tested.7 Seawater exposed maize seedlings form a pronounced K step in 0.3 ms reveals the damage of oxygen-evolving complex (OEC) of PSII.21 A gradual increase in VK and WK under salinity exposure indicated that photodamage to oxygen-evolving complex.21,26,48 A sharp decline Fv/Fo ratio in response to salinity represents damage in the donor site of the OEC.21,49 Declined performance indices (SFIABS and PIABS) under high salinity indicates instability of the photosynthetic apparatus. That leads to disruption of electron transport rate and overall photosynthetic activity of many plants.21,50,51
5. Temperature
In both high and low temperature, Photosystem II complex is the most susceptible part of the photosynthetic apparatus.19,20,52 The extrinsic proteins viz., PsbO, PsbP, PsbQ, and PsbR disassociates from the OEC complex of PSII.3 The donor site of the OEC is primary target site to damage under a gradual increase in temperature, causing an appearance of K peak at 0.3 ms on chlorophyll a fluorescence induction curve.7,20,53,54 Before K-peak discovery, the FO increase was generally used for screening plants for high-temperature sensitivity/resistance.55,56 PSII repair mechanism inhibited after exposure of low-temperature while no evident for photodamage to PSII.30,57 Several studies reveal the response of performance indices to temperature. PIABS decreased was reported in response to the high-temperature in wheat,58 sorghum,59 barley 60 and pigeon pea.20 However, PIABS total showed an increase in response to high temperature.51 A linear relationship between log(PIABS) to temperature in Crofton weed reflects the normalized level of the K-peak.61 The PIs or log(PIs) have indicated a tendency to decrease in response to chilling and freezing tolerance.15,52,62–64
6. High Light intensity
Generally, leaves receive significantly more light than can be processed by photosynthesis, which leads to photoinhibition of PSII.65 During this inactivation of PSII reaction centers, damage to the OEC and/or decreased turnover of D1 protein observed in many plants.3,16,30,66,67 The balance between optimal utility of light and thermal dissipation during high light intensity is of key impedance for plants.68 Also, high light produces ROS, which inhibits the repair of PSII mainly through suppressing the de novo synthesis of proteins,69 thus damaging the photochemical reaction center of PSII, the OEC in particular. The extrinsic protein PsbR protects the damage of OEC in the presence of high light and maintain the standard rate of oxygen evolution.70 Several attempts have been made to measured PIABS after prolonged exposure to excessive light. PIABS was found to be strongly affected by the high-light treatment, much more so than FV/FM.71–73
7. Drought
Water deficit in plants is often accompanied by high-light and salinity stress.3,24,74–76 Drought damages of the OEC may be observed and assessed through the increase in relative variable fluorescence at 300 µs (K-step).24,77,78 Several performance indices (PIs) have been tested to quantify responses to drought stress. PIABS was shown to decrease in response to drought stress.15,79,80 Drought factor index (DFI) based on PIABS measured was used to quantify the response of barley and sesame varieties to drought.81,82
8. Conclusion and prospectives
Plants are subjected to various abiotic factors and need to maintain stasis between environment and plant functionality. The photosynthetic apparatus is more vulnerable to abiotic stresses, PSII in particular. In the presence of light water split into molecular oxygen catalyzed by a super catalyst, the OEC. The OEC is protected and stabilized by four different extrinsic proteins viz., PsbO, PsbP, PsbQ, and PsbR located at the luminal side. Chlorophyll a fluorescence (OJIP transients) are used to understand the structural and functional integrity of photosynthetic apparatus. An addition K-peak in OJIP curve reflects damage at the OEC donor side. Performance indices are also used for better understanding of structural and functional cohesion of photosynthetic apparatus as a whole. Over the year, much information has been gathered about PSII response in various abiotic conditions. However, despite the extensive efforts put into studying PSII, a huge gap exist especially with respect to the structure and function cohesion of the OEC is still a mystery in response to various abiotic factors. Now researchers should think and put more efforts into translating this knowledge for a better understanding of dynamics of the OEC of plants in response to variable climatic conditions.
Acknowledgments
Fiji National University supported the work.
Conflict of Interest
The author declares that he has no conflict of interest.
References
- 1.Rochaix J-D. The Dynamics of the Photosynthetic Apparatus in Algae. In: Najafpour MM, editor. Applied Photosynthesis–New Progress, In Tech. London (UK): Intech Open Limited; 2016. p. 1–7. doi: 10.5772/62261. [DOI] [Google Scholar]
- 2.Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S.. Architecture of the photosynthetic oxygen-evolving center. Science. 2004. doi: 10.1126/science.1093087. [DOI] [PubMed] [Google Scholar]
- 3.Huang S, Zuo T, Ni W.. Important roles of glycinebetaine in stabilizing the structure and function of the photosystem II complex under abiotic stresses. Planta. 2020;251:36. doi: 10.1007/s00425-019-03330-z. [DOI] [PubMed] [Google Scholar]
- 4.Allahverdiyeva Y, Suorsa M, Rossi F, Pavesi A, Kater MM, Antonacci A, Tadini L, Pribil M, Schneider A, Wanner G, et al.. Arabidopsis plants lacking PsbQ and PsbR subunits of the oxygen-evolving complex show altered PSII super-complex organization and short-term adaptive mechanisms. Plant J. 2013;75:671–684. doi: 10.1111/tpj.12230. [DOI] [PubMed] [Google Scholar]
- 5.Cao P, Xie Y, Li M, Pan X, Zhang H, Zhao X, Su X, Cheng T, Chang W.. Crystal structure analysis of extrinsic PsbP protein of Photosystem II reveals a manganese-induced conformational change. Mol Plant. 2015;8:664–666. doi: 10.1016/j.molp.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Najafpour MM, Zaharieva I, Zand Z, Hosseini SM, Kouzmanova M, Hołyńska M, Tranca I, Larkum AW, Shen JR, Allakhverdiev SI. Water-oxidizing complex in Photosystem II: Its structure and relation to manganese-oxide based catalysts. Coord Chem Rev. 2020:409. doi: 10.1016/j.ccr.2020.213183. [DOI] [Google Scholar]
- 7.Sasi S, Venkatesh J, Daneshi RF, Gururani MA. Photosystem II Extrinsic Proteins and Their Putative Role in Abiotic Stress Tolerance in Higher Plants. Plants. 2018;7:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinyard DJ, Ananyev GM, Dismukes GC. Photosystem II: The Reaction Center of Oxygenic Photosynthesis. Annu Rev Biochem. 2013;82:577–606. [DOI] [PubMed] [Google Scholar]
- 9.Blankenship (2014). Molecular Mechanisms of Photosynthesis. John Wiley & Sons
- 10.Papageorgiou GC, Govindjee, editors. Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration, Vol. 19. Dordrecht: Springer; 2004. p. 820 [Google Scholar]
- 11.Strasser RJ, Tsimilli-Michael M, Srivastava A. Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou GC, Govindjee, editors. Chlorophyll a Fluorescence: A Signature of Photosynthesis, Advances in Photosynthesis and Respiration, Vol. 19. Dordrecht: Springer; 2004. p. 321–362 [Google Scholar]
- 12.Stirbet A, Govindjee G. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: Basics and applications of the OJIP fluorescence transient. . Journal of Photochemistry and Photobiology B: Biology. 2011;104(1–2):236–257. doi: 10.1016/j.jphotobiol.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Stirbet A, Govindjee . Chlorophyll a fluorescence induction: a personal perspective of the thermal phase, the J-I-P rise. Photosynth. Res. 2012;113:15–61. [DOI] [PubMed] [Google Scholar]
- 14.Lazár D. The polyphasic chlorophyll a fluorescence rise measured under high intensity of exciting light. – Funct. Plant Biol. 2006;33:9–30. [DOI] [PubMed] [Google Scholar]
- 15.Stirbet A, Lazár D, Kromdijk J; Govindjee . Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses?. Photosynthetica. 2018;56(1):86–104. doi: 10.1007/s11099-018-0770-3. [DOI] [Google Scholar]
- 16.Gupta R, Sharma RD, Singh M. Energy dissipation and photosynthetic electron flow during the transition from juvenile red to mature green leaves in mango (Mangifera indica L.). Plant Biosystems - An International Journal Dealing with all Aspects of Plant Biology. 2020. doi: 10.1080/11263504.2020.1810807. [DOI] [Google Scholar]
- 17.Tsimilli-Michael M, Strasser RJ. In vivo assessment of stress impact on plant’s vitality: applications in detecting and evaluating the beneficial role of mycorrhization on host plants. In: Varma A. editor. Mycorrhiza, Springer. 2008. p. 679–703. [Google Scholar]
- 18.Kalaji HM, Rackova L, Paganova V, Swoczyna T, Rusinowski S, Sitko K. Can chlorophyll-a fluorescence parameters be used as bio-indicators to distinguish between drought and salinity stress in Tilia cordata Mill?. Environ Exp Bot. 2018;152:149–157. [Google Scholar]
- 19.Gupta R. Tissue specific disruption of photosynthetic electron transport rate in pigeonpea (Cajanuscajan L.) under elevated temperature. Plant Signal Behav. 2019. doi: 10.1080/15592324.2019.1601952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta R. Manganese Repairs the Oxygen-Evolving Complex (OEC) in Maize (Zea mays L.) Damage During Seawater Vulnerability. J Soil Sci Plant Nutr. 2020. doi: 10.1007/s42729-020-00220-2. [DOI] [Google Scholar]
- 21.Force L, Critchley C, JJS VR. New fluorescence parameters for monitoring photosynthesis in plants. 1. The effect of illumination on the fluorescence parameters of the JIP-test. Photosynth Res. 2003;78:17–33. [DOI] [PubMed] [Google Scholar]
- 22.Kalaji HM, Jajoo A, Oukarroum A, Brestic M, Zivcak M, Samborska IA, Magdalena DC, Izabela L, Vasilij G, Richard JL. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol Plant. 2016;38:102. [Google Scholar]
- 23.Guissé B, Srivastava A, Strasser RJ. The polyphasic rise of the chlorophyll a fluorescence (O–K–J–I–P) in heat stressed leaves. Arch. Sci. Genève. 1995;48:147–160. [Google Scholar]
- 24.Urban L, Aarrouf J, Bidel LPR. Assessing the Effects of Water Deficit on Photosynthesis Using Parameters Derived from Measurements of Leaf Gas Exchange and of Chlorophyll a Fluorescence. Front. Plant Sci. 2017;8:2068. doi: 10.3389/fpls.2017.02068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parmoon G, Ebadi A, Jahanbakhsh S, Hashemi M, Moosavi SA. Assessing photosynthetic performance of fennel (Foeniculum vulgare mill) influenced by plant growth regulators and drought stress imposed at vegetative and reproductive stages. Ital J Agron. 2019;14:93–100. [Google Scholar]
- 26.Tsimilli-Michael M, Pêcheux M, Strasser RJ. Vitality and stress adaptation of the symbionts of coral reef and temperate foraminifers probed in hospite by the fluorescence kinetics OJ-I-P. – Arch. Sci. Genève. 1998;51:1–36. [Google Scholar]
- 27.Srivastava A, Govindjee SRJ. Greening of peas: parallel measurements on 77 K emission spectra, OJIP chlorophyll a fluorescence transient, period four oscillation of the initial fluorescence level, delayed light emission, and P700. – Photosynthetica. 1999;37:365–392. [Google Scholar]
- 28.Strasser RJ, Srivastava A, Tsimilli-Michael M. Screening the vitality and photosynthetic activity of plants by fluorescence transient. In: Behl RK, Punia MS, Lather BPS, editors. Crop Improvement for Food Security. Hisar, India: SSARM; 1999. p. 72–115. [Google Scholar]
- 29.Yusuf MA, Kumar D, Rajwanshi R, Strasser RJ, Tsimilli-Michael M, Govindjee SNB. Overexpression of gamma-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: Physiological and chlorophyll fluorescence measurements. Biochim Biophys Acta. 2010;1797:1428–1438. [DOI] [PubMed] [Google Scholar]
- 30.Murata N, Takahashi S, Nishiyama Y, Allakhverdiev SI. Photoinhibition of photosystem II under environmental stress. Biochim Biophys Acta Bioenerg. 2007;1767:414–421. 10.1016/j.bbabio.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 31.Xu Z, Jiang Y, Zhou G. Response and adaptation of photosynthesis, respiration, and antioxidant systems to elevated CO2 with environmental stress in plants. Front Plant Sci. 2015. doi: 10.3389/fpls.2015.00701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pérez-Bueno ML, Barón M, García -Luque I. PsbO,PsbP,andPsbQofphotosystemIIareencodedbygene families in Nicotiana benthamiana. Structure and functionality of their isoforms. Photosynthetica. 2011;49:573–580. [Google Scholar]
- 33.Choudhury FK, Rivero RM, Blumwald E, Mittler R. Reactive oxygen species. abiotic stress and stress combination. Plant J. 2017;90:856–867. [DOI] [PubMed] [Google Scholar]
- 34.Nishiyama Y, Yamamoto H, Allakhverdiev SI, Inaba M, Yokota A, Murata N. Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J. 2001;20:5587–5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gururani MA, Upadhyaya CP, Strasser RJ, Woong YJ, Park SW. Physiological and biochemical responses of transgenic potato plants with altered expression of PSII manganese stabilizing protein. Plant Physiol Biochem. 2012;58:182–194. [DOI] [PubMed] [Google Scholar]
- 36.Murata N, Allakhverdiev SI, Nishiyama Y. The mechanism of photoinhibition in vivo: Re-evaluation of the roles of catalase, α-tocopherol, non-photochemical quenching, and electron transport. – BBA-Bioenergetics. 2012;1817:1127–1133. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi S, Murata N. How do environmental stresses accelerate photoinhibition?. – Trend Plant Sci. 2008;13:178–182. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi S, Badger MR. Photoprotection in plants: a new light on photosystem II damage. – Trends. Plant Sci. 2011;16:53–59. [DOI] [PubMed] [Google Scholar]
- 39.Nishiyama Y, Allakhverdiev SI, Murata N. Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem II. – Physiol. Plantarum. 2011;142:35–46. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y-J, Liu T, Zhang S-B, Huang W. Special issue in honour of Prof. Reto J. Strasser – Photoinhibition of oxygen-evolving complex and photosystem II at chilling stress in the tropical tree species Dalbergia odorifera.. Photosynthetica. 2020;58(special issue):245–252. doi: 10.32615/ps.2019.138. [DOI] [Google Scholar]
- 41.Gururani MA, Venkatesh J, Tran L-SP. Regulation of Photosynthesis during Abiotic StressInduced Photoinhibition. Mol. Plant. 2015:1–17. doi: 10.1016/j.molp.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Allakhverdiev SI, Murata N. Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage–repair cycle of photosystem II in Synechocystis sp. PCC 6803 Biochem. Biophys. Acta Bioenerg. 2004;1657:23–32. [DOI] [PubMed] [Google Scholar]
- 43.Papageorgiou GC, Murata N. The unusually strong stabilizing effects of glycine betaine on the structure and function of the oxygen-evolving Photosystem II complex. Photosynth Res. 1995;44:243–252. doi: 10.1007/bf00048597. [DOI] [PubMed] [Google Scholar]
- 44.Rivas JDL, Heredia P, Roman A. Oxygen-evolving extrinsic proteins (PsbO, P, Q, R): bioinformatic and functional analysis. Biochim Biophys Acta Bioenerg. 2007;1767:575–582. 10.1016/j.bbabio.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 45.Bondarava N, Un S, Krieger-Liszkay A. Manganese binding to the 23 kDa extrinsic protein of Photosystem II. Biochim Biophys Acta Bioenerg. 2007;1767:583–588. doi: 10.1016/j.bbabio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Miyao M, Murata N. The Cl− effect on photosynthetic oxygen evolution: Interaction of Cl− with WkDa, 24-kDa and 33-kDa proteins. FEBS Lett. 1985;180:303–308. [Google Scholar]
- 47.Tomita M, Ifuku K, Sato F, Noguchi T. FTIR evidence that the PsbP extrinsic protein induces protein conformational changes around the oxygen-evolving Mn cluster in photosystem II. Biochemistry. 2009;48:6318–6325. [DOI] [PubMed] [Google Scholar]
- 48.Rastogi A, Kovar M, He X, Zivcak M, Kataria S, Kalaji HM, … Brestic M. Special issue in honour of Prof. Reto J. Strasser – JIP-test as a tool to identify salinity tolerance in sweet sorghum genotypes. Photosynthetica. 2020;58(SPECIAL ISSUE):518–528. doi: 10.32615/ps.2019.169. [DOI] [Google Scholar]
- 49.Zhang H, Liu N, Zhao J, Ge F, Xu Y, Chen Y. Disturbance of photosystem II-oxygen evolution complex induced the oxidative damage in Chlorella vulgaris under the stress of cetyltrimethylammonium chloride. Chemosphere. 2019;223:659–667. [DOI] [PubMed] [Google Scholar]
- 50.Mehta P, Jajoo A, Mathur S. Chlorophyll a fluorescence study revealing effects of high salt stress on photosystem II in wheat leaves. – Plant Physiology and Biochemistry. 2010;48:16–20. [DOI] [PubMed] [Google Scholar]
- 51.Stefanov D, Petkova V, Denev ID. Screening for heat tolerance in common bean (Phaseolus vulgaris L.) lines and cultivars using JIP-test. Sci Hortic. 2011;128:1–6. [Google Scholar]
- 52.Vilas JM, Corigliano MG, Clemente M, Javier MS, Rodríguez AA. Close relationship between the state of the oxygen evolving complex and rice cold stress tolerance. Plant Science. 2020:296. doi: 10.1016/j.plantsci.2020.110488. [DOI] [PubMed] [Google Scholar]
- 53.Brestic M, Zivcak M, Kalaji HM, Carpentier R, Allakhverdiev SI. Photosystem II thermostability in situ: environmentally induced acclimation and genotype-specific reactions in Triticum aestivum L. Plant Physiol Biochem. 2012;57:93–105. 10.1016/j.plaphy.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 54.Brestic M, Zivcak M, Kunderlikova K, Allakhverdiev SI. High temperature specifically affects the photoprotective responses of chlorophyll b-deficient wheat mutant lines. Photosynth Res. 2016;130:251–266. doi: 10.1007/s11120-016-0249-7. [DOI] [PubMed] [Google Scholar]
- 55.Nauš J, Kuropatwa R, Klinkovský T, Ilík P, Lattová J, Pavlová Z. Heat injury of barley leaves detected by the chlorophyll fluorescence temperature curve. – Biochim. Biophys. Acta. 1992;1101:359–362. [Google Scholar]
- 56.Lazár D, Ilík P. High-temperature induced chlorophyll fluorescence changes in barley leaves. Comparison of the critical temperatures determined from fluorescence induction and from fluorescence temperature curve. – Plant Sci. 1997;124:159–164. [Google Scholar]
- 57.Allakhverdiev SI, Murata N. Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage–repair cycle of Photosystem II in Synechocystis sp. PCC 6803. Biochim Biophys Acta Bioenerg. 2004;1657:23–32. doi: 10.1016/j.bbabio.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Mathur S, Jajoo A, Mehta P,Bharti S. Analysis of elevated temperature-induced inhibition of photosystem II using chlorophyll a fluorescence induction kinetics in wheat leaves (Triticum aestivum). – Plant Biol. 2011;13:1–6. [DOI] [PubMed] [Google Scholar]
- 59.Yan K, Chen P, Shao H, Zhao S, Zhang L, Zhang L, Xu G, Sun J. Responses of photosynthesis and photosystem II to higher temperature and salt stress in sorghum. – J. Agron. Crop Sci. 2012;198:218–226. [Google Scholar]
- 60.Jedmowski C, Brüggemann W. Imaging of fast chlorophyll fluorescence induction curve (OJIP) parameters, applied in a screening study with wild barley (Hordeum spontaneum) genotypes under heat stress. – J. Photoch. Photobio. B. 2015;151:153–160. [DOI] [PubMed] [Google Scholar]
- 61.Chen S, Yang J, Zhang M. Classification and characteristics of heat tolerance in Ageratina adenophora populations using fast chlorophyll a fluorescence rise O-J-I-P. – Environ. Exp. Bot. 2016;122:126–140. [Google Scholar]
- 62.Rapacz M, Sasal M, Wójcik-Jagła M. Direct and indirect measurements of freezing tolerance: advantages and limitations. – Acta Physiol. Plant. 2015a;37:157–173. [Google Scholar]
- 63.Rapacz M, Sasal M, Kalaji HM. Is the OJIP test a reliable indicator of winter hardiness and freezing tolerance of common wheat and triticale under variable winter environments? PLoS ONE. 2015b;10:e0134820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adamski JM, Cargnelutti D, Sperotto RA. Identification and physiological characterization of two sister lines of indica rice (Oryza sativa L.). with contrasting levels of cold tolerance. – Can. J. Plant Sci. 2016;96:197–214. [Google Scholar]
- 65.Adams III WW, Zarter CR, Mueh KE. Energy dissipation and photoinhibition: A continuum of photoprotection. In: Demmig-Adams B, Adams III WW, Mattoo AK, editors. Photoprotection, Photoinhibition, Gene Regulation, and Environment. Dordrecht (Netherland): Springer Science+Business Media B.V; 2008. p. 49–64. [Google Scholar]
- 66.Hakala M, Tuominen I, Keränen M, Tyystjärvi T, Tyystjärvi E. Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of Photosystem II.. Biochim Biophys Acta Bioenerg. 2005;1706:68–80. doi: 10.1016/j.bbabio.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 67.Kale R, Hebert AE, Frankel LK. Amino acid oxidation of the D1 and D2 proteins by oxygen radicals during photoinhibition of Photosystem II. – Proc. Natl. Acad. Sci. USA. 2017;114:2988–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dai J, Gao H, Dai Y, Zou Q. Changes in activity of energy dissipating mechanisms in wheat fl ag leaves during senescence. Plant Biol. 2004;6:171–177. [DOI] [PubMed] [Google Scholar]
- 69.Nishiyama Y, Allakhverdiev SI, Murata N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim Biophys Acta Bioenerg. 2006;1757:742–749. doi: 10.1016/j.bbabio.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 70.Suorsa M, Sirpio S, Allahverdiyeva Y, Paakkarinen V, Mamedov F. PsbR,aMissingLinkintheAssembly of the Oxygen-evolving Complex of Plant Photosystem II. J Biol Chem. 2006;281:145–150. [DOI] [PubMed] [Google Scholar]
- 71.Kalaji HM, Carpentier R, Allakhverdiev SI. Fluorescence parameters as early indicators of light stress in barley. – J. Photoch. Photobio. B. 2012;112:1–6. [DOI] [PubMed] [Google Scholar]
- 72.Wang XY, Xu XM, Cui J. The importance of blue light for leaf area expansion, development of photosynthetic apparatus, and chloroplast ultrastructure of Cucumis sativus grown under weak light. – Photosynthetica. 2015;53:213–222. [Google Scholar]
- 73.Dinis L-T, Ferreira H, Pinto G. Kaolin-based, foliar reflective film protects photosystem II structure and function in grapevine leaves exposed to heat and high solar radiation. – Photosynthetica. 2016;54:47–55. [Google Scholar]
- 74.Morales F, Abadía A, Abadía J. Photoinhibition and photoprotection under nutrient deficiencies, drought and salinity. In: Demmig-Adams B, Adams III WW, Mattoo AK, editors. Photoprotection, Photoinhibition, Gene Regulation, and Environment. Dordrecht (Netherland): Springer Science+Business Media B.V; 2008. p. 65–85. [Google Scholar]
- 75.Chaves MM, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot.-London. 2009;103:551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 77.Kotakis C, Kyzeridou A, Manetas Y. Photosynthetic electron flow during leaf senescence: evidence for a preferential maintenance of photosystem I activity and increased cyclic electron flow. Photosynthetica. 2014;52:413–420. doi: 10.1007/s11099-014-0046-5. [DOI] [Google Scholar]
- 78.Arslan Ö, Nalçaiyi ASB, Erdal Ş, Pekcan V, Kaya Y, Çiçek N, Ekmekçi Y. Special issue in honour of Prof. Reto J. Strasser – Analysis of drought response of sunflower inbred lines by chlorophyll a fluorescence induction kinetics. Photosynthetica. 2020;58(special issue):348–357. doi: 10.32615/ps.2019.171. [DOI] [Google Scholar]
- 79.Jedmowski C, Bayramov S, Brüggemann W. Comparative analysis of drought stress effects on photosynthesis of Eurasian and North African genotypes of wild barley. – Photosynthetica. 2014;52:564–573. [Google Scholar]
- 80.Jedmowski C, Ashoub A, Momtaz O. Impact of drought, heat, and their combination on chlorophyll fluorescence and yield of wild barley (Hordeum spontaneum). – J. Bot. 2015;2015:120868. [Google Scholar]
- 81.Boureima S, Oukarroum A, Diouf M. Screening for drought tolerance in mutant germplasm of sesame (Sesamum indicum) probing by chlorophyll a fluorescence. Environ. Exp. Bot. 2012;81:37–43. [Google Scholar]
- 82.Oukarroum A, El Madidi S, Schansker G. Probing the responses of barley cultivars (Hordeum vulgare L.). by chlorophyll a fluorescence OLKJIP under drought stress and re-watering. – Environ. Exp. Bot. 2007;60:438–446. [Google Scholar]


