ABSTRACT
Medulloblastoma (MB) represents the most common malignant pediatric brain tumor and is defined by four molecular subgroups with WNT MB having the most favorable prognosis. Our work provides a rational therapeutic option in which the protective effects of WNT-driven MBs may be augmented in Group 3 and 4 MB.
KEYWORDS: Medulloblastoma, WNT, beta-catenin, brain tumor-initiating cell
Contemporary frameworks for studying tumorigenesis consider the process as development gone awry. The aberrant activation of evolutionarily conserved signal transduction pathways has traditionally provided a mechanism for the transition of normal homeostatic cellular cascades toward malignancy. Such processes, beginning in embryonic development and continuing throughout adulthood, maintain the balance between cellular potency and differentiation. While the canonical WNT signaling pathway regulates the development and maturation of several tissues including those of the colon, blood, and central nervous system (CNS), its activation may also promote the neoplastic transformation of these tissues.1 The complex context-dependent outcomes of activated WNT signaling are well illustrated in the CNS where WNT activation promotes the expansion of neural stem cells (NSCs) in the forebrain,2 while inhibiting their self-renewal in the hindbrain.3 Such regional differences are also represented by the propensity for WNT pathway mutations in tumors of the forebrain and hindbrain. While recurrent WNT mutations have yet to be identified in forebrain malignancies, they have been well described in the pediatric hindbrain tumor, medulloblastoma (MB).4
MB is the most common malignant pediatric brain tumor, representing 20% of newly diagnosed childhood CNS cancers. Consensus molecular subgroups of MB define four distinct groups (WNT, sonic hedgehog (Shh), Group 3, Group 4) based on transcriptional and epigenetic profiles with unique prognostic and predicted therapeutic responses.4,5 WNT MB accounts for 10% of cases with the majority harboring somatic CTNNB1 (catenin beta 1) mutations and chromosomal alterations for monosomy 6.4 Clinically, WNT MBs have the most favorable prognosis with a > 95% 5-year survivorship.5 By contrast, non-WNT MBs (Shh, Group 3, and Group 4) are characterized by metastatic disease, increased rates of recurrence, and intermediate-poor overall survivorship.5 Given that WNT MBs rarely metastasize and represent the only subgroup in which metastasis is not indicative of a poor prognosis, it has been suggested that WNT signaling may contribute to their remarkable response to standard therapy.
By using primary patient-derived MB cell lines, our work initially characterized intrinsic differences in the tumor-initiating capacity of WNT, Group 3, and Group 4 MBs.6 We found WNT MB xenografts to generate smaller, less invasive tumors, that resulted in a survival advantage when compared to Group 3 xenografts. We then demonstrated xenografts generated from Group 3 MB cells with ectopic expression of β-CATENIN, the downstream WNT effector protein, to result in an improved overall survivorship and reduced tumor burden when compared to control mice. Since RNA-seq profiling of the bulk tumor cell population has dominated the MB genomics literature, the presence of rare clonal populations unique to each subgroup have not been adequately described. We used single cell RNA-seq to determine the clonal architecture of patient-derived MB cell lines reflective of all four subgroups. Our analysis identified the presence of WNT-active cells in non-WNT MB subgroups. The clinical utility of these findings was seen with a survival advantage in patients with Group 3 MB containing an enriched WNT gene expression profile. We further validated the impaired tumorigenic profile of endogenous WNT active cells in non-WNT MBs by developing stable Group 3 and 4 MB lines containing a WNT reporter and isolating WNT-active (TGP+) and WNT-inactive (TGP-) cells using flow cytometric cell sorting. TGP+ xenografts resembled WNT MB xenografts in that they formed small circumscribed tumors and maintained a survival advantage over TGP- xenografts, which resembled their native Group 3 or 4 MB (Figure 1). In order to develop a rational therapeutic option, we treated Group 2, 3, and 4 xenografts with L807mts, a substrate-competitive peptide inhibitor of the WNT inhibitor GSK3 (glycogen synthase kinase 3). Xenografts from all non-WNT MBs had a robust response to L807mts treatment resulting in impaired tumor formation and prolonged survival compared to control xenografts. Overall, our data support a context-specific tumor suppressive function for the WNT/β-CATENIN pathway and establishes activated WNT signaling as a mechanism for potentially targeting Group 3 and 4 MB.
Figure 1.
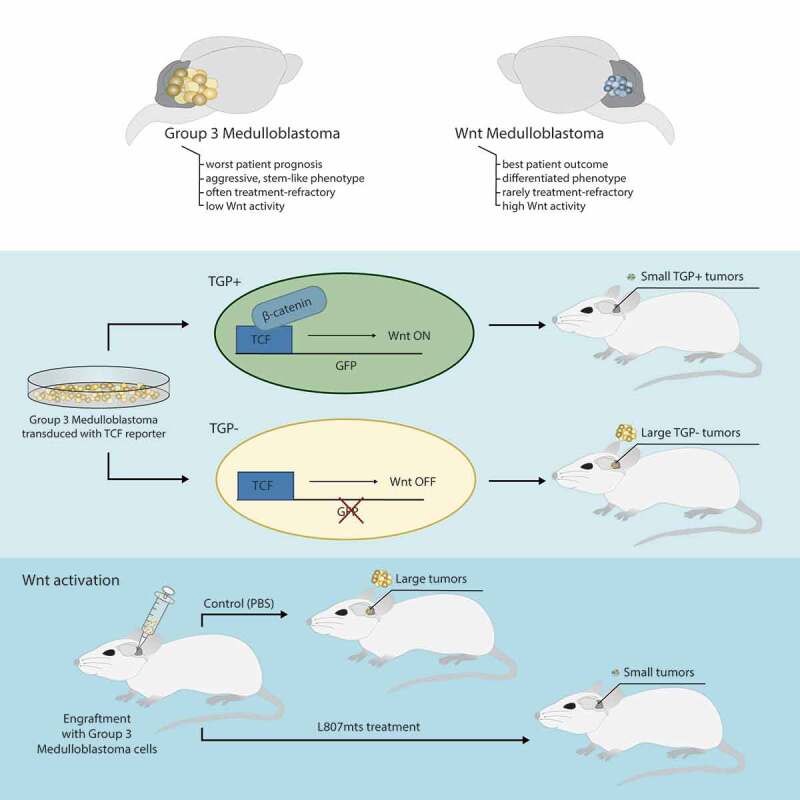
WNT activation impairs tumor growth in Group 3 and 4 medulloblastoma (MB). Endogenous WNT-active cells were isolated from Group 3 and 4 MB samples after generating stable Group 3 and 4 MB brain tumor-initiating cell (BTIC) lines containing the WNT TCF reporter, TGP. TGP+ cells (WNT active, GFP+) and TGP- cells (WNT inactive, GFP-) were isolated using flow cytometric cell sorting. TGP+ xenografts generated smaller tumors with a prolonged survival compared to TGP- xenografts. In a separate experiment, Group 2, 3, and 4 xenografts were treated with small molecule WNT agonist, L807mts, which resulted in impaired tumor formation and survival advantage in treated mice compared to controls. (GFP: green fluorescent protein, TCF: T-cell factor)
Although changes in risk-adapted therapy have greatly improved overall survival in pediatric oncology across the globe, the advent of molecular diagnostics and large-scale integrated genomic analyses of tumors have reconceptualized our understanding of the biology of many pediatric tumors, including MB. Accordingly, current clinical trials in MB have aimed to base risk-adapted therapy regimens on the molecular subgroup affiliation of tumors. Treatment options for recurrent MB patients who remain refractory to current risk-adapted therapy regimens are limited to palliation alone and this therefore represents a uniformly fatal cohort of patients. Given the limited targeted treatment options for non-WNT MBs, our work highlights a rational therapeutic option in which the protective effects of WNT-driven MBs may be augmented in non-WNT MBs through targeted activation of the canonical WNT pathway. Although WNT signaling has historically been associated with tumor growth and the maintenance of stemness, emerging data in melanoma,7,8 neuroblastoma,8 and triple negative breast cancer9 have implicated a novel context-dependent tumor suppressive function for the pathway. While such disparate outcomes in response to WNT activation may be attributed to differences in each tumor’s molecular phenotype, an alternative explanation may account for differences in gene dosage.10 Since different tissues have been shown to have different vulnerabilities to β-CATENIN-mediated tumorigenesis, gene dosage effects may provide an additional mechanism by which activated WNT signaling functions to promote or impede tumorigenesis. Although WNT activation has been shown to block tumor formation in various tissues, a stemness-dependent mechanism as defined in our work has yet to be described. As molecularly-based clinical oncology trials continue to expand, novel approaches to overcome the dependence of tumors on malignant pathways are warranted and as such we provide a therapeutic rationale that may alter the way in which we approach the management of malignant tumors – converting them to less aggressive lesions through targeted inhibition of the determinants of stemness.
Acknowledgments
B.M. was supported by a Canadian Institutes of Health Research Vanier Canada Graduate Scholarship, American Brain Tumor Association Medical Student Summer Fellowship, Alex’s Lemonade Stand Foundation for Childhood Cancer Pediatric Oncology Student Training Program (POST), Mac-Gaensslen Foundation of Canada Medical Student Research Grant, and Brain Tumour Foundation of Canada Medical Student Research Scholarship. S.K.S. is supported by the Canadian Institutes of Health Research Operating Grant, Neurosurgical Research and Education Foundation and American Association of Neurological Surgeons, Pediatric Section, the Ontario Institute for Cancer Research, Brain Tumour Foundation of Canada and McMaster University Department of Surgery.
Disclosure statement
The authors declare that they have no conflict of interest.
References
- 1.Nusse R, Clevers H.. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:1–3. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 2.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 3.Pei Y, Brun SN, Markant SL, Lento W, Gibson P, Taketo MM, Giovannini M, Gilbertson RJ, Wechsler-Reya RJ. WNT signaling increases proliferation and impairs differentiation of stem cells in the developing cerebellum. Development. 2012;139:1724–1733. doi: 10.1242/dev.050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Northcott PA, Buchhalter I, Morrissy SA, Hovestadt V, Weischenfeldt J, Ehrenberger T, Gröbner S, Segura-Wang M, Zichner T, Rudneva VA, et al. The whole-genome landscape of medulloblastoma subtypes. Nature. 2017;547(7663):311–317. doi: 10.1038/nature22973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramaswamy V, Remke M, Bouffet E, Bailey S, Clifford SC, Doz F, Kool M, Dufour C, Vassal G, Milde T, et al. Risk stratification of childhood medulloblastoma in the molecular era: the current consensus. Acta Neuropathol. 2016;131(6):821–831. doi: 10.1007/s00401-016-1569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manoranjan B, Venugopal C, Bakhshinyan D, Adile AA, Richards L, Kameda-Smith MM, Whitley O, Dvorkin-Gheva A, Subapanditha M, Savage N, et al. Wnt activation as a therapeutic strategy in medulloblastoma. Nat Commun. 2020;11(1):4323. doi: 10.1038/s41467-020-17953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chien AJ, Moore EC, Lonsdorf AS, Kulikauskas RM, Rothberg BG, Berger AJ, Major MB, Hwang ST, Rimm DL, Moon RT. Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proc Natl Acad Sci U S A. 2009;106:1193–1198. doi: 10.1073/pnas.0811902106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biechele TL, Kulikauskas RM, Toroni RA, Lucero OM, Swift RD, James RG, Robin NC, Dawson DW, Moon RT, Chien AJ, et al. Wnt/beta-catenin signaling and AXIN1 regulate apoptosis triggered by inhibition of the mutant kinase BRAFV600E in human melanoma. Sci Signal. 2012;5:ra3. doi: 10.1126/scisignal.2002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green JL, La J, Yum KW, Desai P, Rodewald L-W, Zhang X, Leblanc M, Nusse R, Lewis MT, Wahl GM, et al. Paracrine Wnt signaling both promotes and inhibits human breast tumor growth. Proc Natl Acad Sci U S A. 2013;110(17):6991–6996. doi: 10.1073/pnas.1303671110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakker ER, Hoekstra E, Franken PF, Helvensteijn W, van Deurzen CHM, van Veelen W, Kuipers EJ, Smits R. beta-Catenin signaling dosage dictates tissue-specific tumor predisposition in Apc-driven cancer. Oncogene. 2013;32:4579–4585. doi: 10.1038/onc.2012.449. [DOI] [PubMed] [Google Scholar]


