ABSTRACT
Limited therapeutic options impede the clinical outcome of triple-negative breast cancer (TNBC). Our recent study uncovered a novel signaling pathway implicating gamma-butyrobetaine hydroxylase 1 (BBOX1) in the control of cell growth in TNBC, via inositol 1, 4, 5-trisphosphate receptor type 3 (IP3R3) mediated calcium signaling which is essential for cellular energy metabolism.
KEYWORDS: TNBC, 2-OG dependent enzyme, therapeutic target
Triple-negative breast cancer (TNBC) is a clinically aggressive, heterogeneous and highly lethal disease. TNBC is a subtype immunohistochemically characterized by lacking hormone receptors (ER and PR) or HER2 expression, which accounts for 15–20% of breast cancers.1 It is imperative to identify new therapeutic targets in this subtype of breast cancer.
The important role of 2-oxoglutarate (2-OG)-dependent enzymes in utilizing oxygen to regulate a broad spectrum of cellular processes (histone/DNA/RNA demethylation, protein hydroxylation, small-molecule oxidation, etc.) caught numerous attentions.2 Notably, emerging literature uncovers their critical roles in various diseases such as cancer,3 while systematic investigation to their role in cancer is lacking. We thus sought to identify novel oncogenic targets in TNBC among 2-OG-dependent enzymes through unbiased loss-of-function screening.
To this end, we performed a focused phenotypic screen using a customized siRNA library, which targeting the currently known 65 members of 2-OG-dependent enzyme genes. We were interested to identify enzymes essential for the TNBC cell growth. Genes scored both in the 2-D cell proliferation growth and 3-D anchorage-independent growth were selected for further investigation. Gamma-butyrobetaine hydroxylase 1 (BBOX1) was one of top candidates that displayed consistent phenotypes in multiple TNBC cell lines with siRNA knockdown. We confirmed the results using different BBOX1 shRNAs in TNBC cell lines, whereas BBOX1 depletion did not grossly affect the growth of normal breast epithelial cells or some other subtypes (ER+/HER2+) of breast cancer cells. Notably, BBOX1 protein levels are relatively highly expressed in the basal-like TNBC cells, which was furtherly strengthened by the finding that higher BBOX1 mRNA level predicted worse prognosis shown in the basal-like breast cancer patients. In vivo, BBOX1 depletion suppressed TNBC xenograft tumor growth either by direct or doxycycline induced shRNA BBOX1 knock-down.4 Altogether, these findings suggest BBOX1 plays an oncogenic role in TNBC.
BBOX1 is the key enzyme for carnitine biosynthesis while very few studies focused on its biological function in the setting of cancer. We asked whether carnitine, which is critical for long-chain fatty acid (FA) transporting to mitochondria for β-oxidation,5 contributes to TNBC cell growth. We performed metabolic profiling and confirmed that the carnitine synthesis pathway was largely affected, and the carnitine level was decreased upon BBOX1 depletion.4 However, our follow-up experiments suggested that carnitine is not the determinant for TNBC cell growth. We found that the carnitine supplement failed to rescue the FA-based mitochondrial oxidation and the growth defect in BBOX1-depleted cells, suggesting that BBOX1 contributes to TNBC in a non-canonical manner.
Intriguingly, the enzymatic ability of BBOX1 is still required for TNBC cell growth. We generated a catalytically inactive version of BBOX1 by double mutating the two key amino acid residues (Asn191, Asn292) which are required for the BBOX1 substrate γ-butyrobetaine binding. Overexpressing wild-type BBOX1, but not the catalytically inactive mutant can promote cell growth. To elucidate the underlying mechanism of BBOX oncogenic function, we performed TAP-TAG purification coupled with mass spectrometry analysis. Inositol 1, 4, 5-trisphosphate receptor type 3 (IP3R3) was identified as a new binding partner of BBOX1, and their interaction relies on BBOX1 catalytic binding sites. Indeed, we found that BBOX1 protects IP3R3 from being ubiquitinated and degraded by E3 ubiquitin ligase f-box and leucine-rich repeat protein 2 (FBXL2). BBOX1 depletion decreases IP3R3 protein level in TNBC cells. On the contrary, BBOX1 overexpression leads to IP3R3 accumulation. Furthermore, this regulation was confirmed in the clinical datasets as IP3R3 protein level positively correlates with BBOX1 in breast cancer patient samples.4
The oncogenic role of IP3R3 is well documented in multiple cancers, which largely depends on its role for calcium release from the endoplasmic reticulum (ER).6 Calcium, as a versatile messenger, controls various cellular processes such as cell proliferation, metabolism, and gene expression. Cancer cells rewire calcium signaling to meet their growth needs.7 We speculated that TNBC cells are addictive to the IP3R3-mediated calcium signaling. Either depleting BBOX1 or IP3R3 significantly reduces calcium flux from the ER, as a consequence, blocks mitochondrial respiration and cell bioenergetics which requires IP3R3-mediated calcium release.8 In addition, the metabolomics study also showed that the TCA cycle was impaired in BBOX1-depleted cells. On the other hand, previous studies showed that calcium signaling also stimulates the mammalian target of rapamycin complex 1 (mTORC1) activity.9 Our RNA-sequencing and metabolomics data showed that mTORC1 activity as well as glycolysis were consistently impaired upon BBOX1 depletion. Therefore, we speculated the BBOX1-IP3R3-calcium signaling may regulate mTORC1 mediated glycolysis. We first confirmed that mTORC1 was down-regulated by knocking down either BBOX1 or IP3R3, while the downregulation was rescued by inducing intercellular calcium release pharmacologically.4 Consequently, we observed decreased glycolysis in BBOX1 or IP3R3 depleted TNBC cells. Taking together, BBOX1 plays a vital role in cellular energetics by controlling the IP3R3-mediated calcium signaling (Figure 1).
Figure 1.
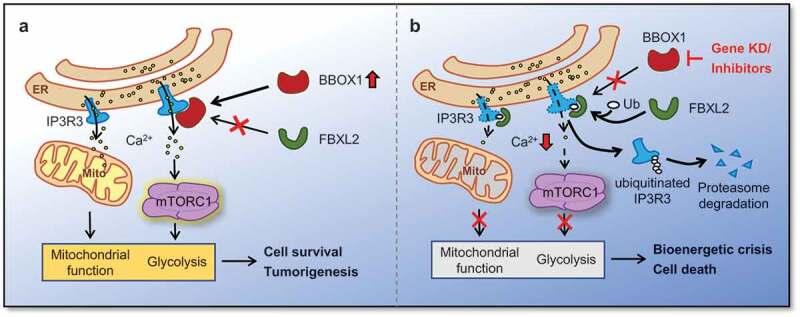
Potential mechanism by which gamma-butyrobetaine hydroxylase 1 (BBOX1) promotes cell growth and tumorigenesis in triple-negative breast cancer (TNBC). (a) In BBOX1 elevated TNBC cells, inositol-1, 4, 5-trisphosphate receptor type 3 (IP3R3) is stabilized via binding with BBOX1. Therefore, calcium is constitutively released from the endoplasmic reticulum (ER). The calcium signal, on one hand, sustains mitochondrial activity for energy production; on the other hand, stimulates mammalian target of rapamycin complex 1 (mTORC1) mediated glycolysis and other biosynthetic processes. (b) Genetic ablation or pharmacological inhibition blocks BBOX1-IP3R3 interaction. Decreased IP3R3 protein level mediated by FBXL2 disturbs the calcium-dependent metabolic processes, which eventually causes TNBC cell death. Ca2+ = calcium; Mito = mitochondria; Ub = ubiquitin
Therapeutically, we applied several BBOX1 inhibitors to block the BBOX1-mediated cancer-specific calcium signaling and energy homeostasis in TNBC cells. Among them, Meldonium (trade name Mildronate) which has been developed 50 years ago as an approved anti-ischemia medication.10 The others are still in development with improved specificity and efficacy. Mechanistically, these inhibitors can block the catalytic pocket of BBOX1, preventing its binding with IP3R3 which eventually triggers the same cascade as by BBOX1 depletion. In mouse xenograft model, Meldonium successfully suppresses tumorigenesis without noticeable toxicity even though at a high dose up to 400 mg/kg body weight was used.4 Therefore, BBOX1 inhibitor could be a potential therapeutic option for TNBC. In summary, targeting the BBOX1-IP3R3-calcium oncogenic signaling may offer new therapeutic avenues for this lethal disease.
Funding Statement
This work was supported by Cancer Prevention and Research Institute of Texas (Q. Zhang, CPRIT, RR190058).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L.. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13:1–3. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Islam MS, Leissing TM, Chowdhury R, Hopkinson RJ, Schofield CJ.. 2-Oxoglutarate-Dependent Oxygenases. Annu Rev Biochem. 2018;87:585–620. doi: 10.1146/annurev-biochem-061516-044724. [DOI] [PubMed] [Google Scholar]
- 3.Liao C, Zhang Q. Understanding the oxygen-sensing pathway and its therapeutic implications in diseases. Am J Pathol. 2020;190:1584–1595. doi: 10.1016/j.ajpath.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liao C, Zhang Y, Fan C, Herring LE, Liu J, Locasale JW, Takada M, Zhou J, Zurlo G, Hu L, et al. Identification of BBOX1 as a therapeutic target in triple-negative breast cancer. Cancer Discov. 2020;CD-20-0288. doi: 10.1158/2159-8290.CD-20-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bremer J. Carnitine–metabolism and functions. Physiol Rev. 1983;63:1420–1480. doi: 10.1152/physrev.1983.63.4.1420. [DOI] [PubMed] [Google Scholar]
- 6.Mangla A, Guerra MT, Nathanson MH. Type 3 inositol 1,4,5-trisphosphate receptor: A calcium channel for all seasons. Cell Calcium. 2020;85:102132. doi: 10.1016/j.ceca.2019.102132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchi S, Giorgi C, Galluzzi L, Pinton P. Ca(2+) fluxes and cancer. Mol Cell. 2020;78:1055–1069. doi: 10.1016/j.molcel.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 8.Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 2008;7:456–465. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simkhovich BZ, Shutenko ZV, Meirena DV, Khagi KB, Mezapuke RJ, Molodchina TN, Kalvins IJ, Lukevics E. 3-(2,2,2-Trimethylhydrazinium)propionate (THP)–a novel gamma-butyrobetaine hydroxylase inhibitor with cardioprotective properties. Biochem Pharmacol. 1988;37:195–202. doi: 10.1016/0006-2952(88)90717-4. [DOI] [PubMed] [Google Scholar]


