Abstract
Placebo treatments reliably reduce pain in the clinic and in the lab. Because pain is a subjective experience, it has been difficult to determine whether placebo analgesia is clinically relevant. Neuroimaging studies of placebo analgesia provide objective evidence of placebo-induced changes in brain processing and allow researchers to isolate the mechanisms underlying placebo-based pain reduction. We conducted formal meta-analyses of 25 neuroimaging studies of placebo analgesia and expectancy-based pain modulation. Results revealed that placebo effects and expectations for reduced pain elicit reliable reductions in activation during noxious stimulation in regions often associated with pain processing, including the dorsal anterior cingulate, thalamus, and insula. In addition, we observed consistent reductions during painful stimulation in the amygdala and striatum, regions implicated widely in studies of affect and valuation. This suggests that placebo effects are strongest on brain regions traditionally associated with not only pain, but also emotion and value more generally. Other brain regions showed reliable increases in activation with expectations for reduced pain. These included the prefrontal cortex (including dorsolateral, ventromedial, and orbitofrontal cortices), the midbrain surrounding the periaqueductal gray, and the rostral anterior cingulate. We discuss implications of these findings as well as how future studies can expand our understanding of the precise functional contributions of the brain systems identified here.
Keywords: Placebo effect, Placebo response, Expectancy, Pain, fMRI, PET, Opioid, Prefrontal cortex, Periaqueductal gray, Amygdala, Meta-analysis, MKDA, Neuroimaging
For decades, the public and scientific community have been well aware of the “powerful placebo effect” (Beecher 1955). However, many scientists and laypeople alike still think placebo effects represent false improvement, or changes in subjective reports without “real” (viz., clinically or functionally meaningful) changes in objective symptoms. Placebo analgesia, or placebo-based pain reduction, provides a particularly unique challenge to researchers seeking to determine whether placebos cause functionally and/or neurobiologically significant changes, as pain itself is subjective and psychological. When someone says she is in pain, how do we evaluate whether she is telling the truth? To do this, we need reliable biological markers linked to pain processing. Brain imaging techniques have provided a powerful way to assess placebo effects, and to understand how they influence pain reports. Today, researchers can conduct carefully controlled studies of placebo-related changes in the brain, and test whether placebos cause changes in early nociceptive pathways (Eippert et al. 2009b; Geuter and Buchel 2013), understand the neurochemical bases underlying placebo effects (Scott et al. 2008; Wager et al. 2007b; Zubieta et al. 2005), and determine the brain changes that are associated with placebo-induced changes in subjective pain (Wager et al. 2011).
To date, over 40 neuroimaging studies have been published on placebo effects. Here, we provide a summary of the most consistent findings across studies in relation to theories of the biological causes and effects of placebo treatment. We present a formal meta-analysis of 25 studies that measured placebo effects and related expectancy effects on brain responses using functional magnetic resonance imaging (fMRI) and positron emission tomography (PET). We report the brain regions that show consistent placebo-induced reductions in pain-related processing during noxious stimulation, which provides information on how placebos affect the systems thought to generate and regulate pain, and may provide clues about how psychological context informs the construction of pain in the central nervous system. We also summarize brain circuits that show increases in activation with placebo treatment, which can inform us both about pain-modulatory mechanisms and about the neurobiological underpinnings of expectations and beliefs more broadly. We then discuss limitations in our current knowledge and how to address some of the outstanding questions in future work.
1. Biological Mechanisms of Placebo Analgesia
The first evidence that placebo analgesia depends on biological mechanisms was published in 1978. Levine et al. (1978) showed that the opioid antagonist naloxone abolished placebo effects on pain, suggesting that placebo analgesia depends on endogenous opioid release. In 2002, Petrovic et al. (2002) published the first neuroimaging study of placebo analgesia. Using PET imaging, they compared placebo analgesia with opioid analgesia produced by the μ-opioid agonist remifentanil. They showed that the effects of endogenous placebo-based opioids and exogenous drug-based opioids overlapped during pain processing: Both caused increases in glucose metabolism in the same brain region, the rostral anterior cingulate cortex (rACC). The first fMRI study of placebo analgesia was conducted in 2004 (Wager et al. 2004). This study showed that placebo administration caused increases during pain anticipation in the lateral and medial prefrontal cortex, including rACC, and also that it caused activity decreases during pain in a subset of regions traditionally associated with pain processing, including the dorsal anterior cingulate cortex (dACC), insula, and thalamus. Later studies measured fMRI responses in the spinal cord and found that spinal responses to pain are reduced with placebo (Eippert et al. 2009b) and increased with nocebo (a “negative placebo” associated with expectations of increased symptoms; Geuter and Buchel 2013). Spinal changes provide evidence for placebo effects on ascending nociceptive signals, before cortical processing. Together, these neuroimaging studies of placebo provide evidence that not only does placebo cause real biological changes, but that placebos actually change responses to noxious stimuli in the central nervous system in ways that are relevant to pain experience.
2. Advantages of Meta-analyses of Expectancy-Based Pain Modulation
Clearly, our ability to observe the neural processes associated with placebo analgesia provides a new, and potentially strong, test of whether placebo effects cause “real” changes. Many cortical and subcortical brain regions have been implicated in individual studies of placebo analgesia and other forms of expectancy-based pain modulation. But findings from a given study can reflect either (a) fundamental mechanisms that support all instances of placebo analgesia or (b) idiosyncratic effects of that study’s unique context and design. The best way to differentiate the former from the latter is to collapse across individual studies and identify commonalities using meta-analysis.
To elaborate, while placebo paradigms generally involve similar experimental paradigms (see Fig. 1), individual studies also vary substantially, not only as a function of technical details (e.g., sample size, fMRI scanner strength, acquisition parameters) but also in important experimental features. Studies vary in the type of pain they induce: Many apply noxious heat (Eippert et al. 2009a; Kong et al. 2009b; Wager et al. 2004, 2007b), some use lasers (Bingel et al. 2004; Lui et al. 2010), and others measure pain in patient populations (Harris et al. 2009; Lieberman et al. 2004; Price et al. 2007). Different pain modalities are associated with different effects in the brain (Baumgartner et al. 2010; Friebel et al. 2011), and different modalities may show different activity patterns and placebo responses (Liberman 1964). Likewise, studies differ in the type of pain they measure: Some ask participants to judge pain intensity (Keltner et al. 2006), while others also measure pain unpleasantness (Zubieta et al. 2005). Studies test different types of placebos, including topical ointments (Eippert et al. 2009a; Geuter et al. 2012; Wager et al. 2004), sham electrical stimulation (Lui et al. 2010), and sham acupuncture (Kong et al. 2006). Individuals hold different beliefs about the efficacy of various treatments as a result of cultural influences and previous experiences (Barrett et al. 2006), and different placebos can induce slightly different effects (de Craen et al. 2000; Kaptchuk et al. 2000); therefore, each type of placebo might even be linked to unique mechanisms. Many other experimental features vary across experiments: whether a study combines verbal suggestions and conditioning to induce expectations about the placebo treatment (Lee et al. 2012; Wager et al. 2004) or uses verbal suggestion alone (Price et al. 2007), whether noxious stimuli vary in intensity (Atlas et al. 2010, 2012; Study 1 in Wager et al. 2004) or remain constant during a test phase (Lui et al. 2010; Study 2 in Wager et al. 2004; Wiech et al. 2010), and whether test stimuli are cued (Lui et al. 2010; Wager et al. 2004) or uncued (Kong et al. 2006). These experimental differences are clearly substantial, and therefore we need a way to identify brain responses that are consistent across these different experimental choices. Ultimately, with more and larger studies, we will understand more about the impact of these choices on brain placebo responses; for now, however, we focus on commonalities across studies and one distinction that is particularly highly powered—manipulations of treatment expectancies vs. stimulus expectancies—because there are a number of studies of each type.
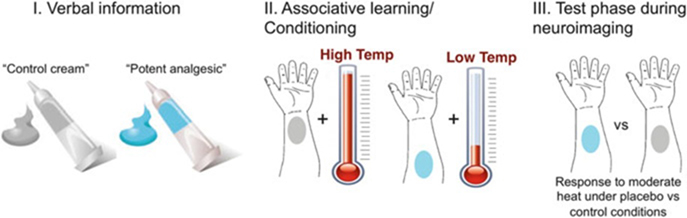
Fig. 1.
Typical neuroimaging placebo paradigm. In a typical placebo study, participants are given an inert treatment (e.g., a topical cream) along with verbal instructions (e.g., “This is a potent analgesic”) that induce expectations for pain relief. This is compared to a control condition—the same inert substance without expected pain relief. To reinforce verbal instructions, the placebo is paired with reduced stimulus intensity during an associative learning, or conditioning, phase. Finally, participants go through neuroimaging testing during a test phase during which the same stimuli are administered under both control and placebo conditions and experimenters test whether pain reports and brain responses are modulated by beliefs about treatment
Meta-analysis provides a way to combine individual experiments and determine which brain responses are consistently implicated across studies. Voxel-wise coordinates of individual contrasts from individual studies are added together and compared to random permutations to identify the regions that are consistently activated by a given psychological process (see Kober and Wager 2010 or Wager et al. 2007a for review). To our knowledge, three published meta-analyses of placebo analgesia exist to date. Amanzio et al. (2011) used Activation Likelihood Estimation (ALE; Eickhoff et al. 2009) to conduct a formal meta-analysis of 11 placebo studies (9 fMRI, 2 PET) and separately analyzed brain responses during pain anticipation and during noxious stimulation. We chose to combine placebo-induced increases in activation during pain anticipation with increases during noxious stimulation, as both reflect modulatory mechanisms. Our study also expands on this work by incorporating three more years of prolific neuroimaging research on placebo and expectancy—increasing the number of relevant studies from 11 to 25—and by using multilevel kernel density analysis (MKDA) instead of ALE. In brief, though ALE and MKDA now produce similar results, MKDA focuses on the distribution of statistical contrast maps rather than the distribution of peak coordinates alone, which ensures that studies that report many peaks in a location are not overrepresented (see Wager et al. (2007a) for a more thorough discussion of the relationship between MKDA and other meta-analytic approaches, including ALE). Two other more recent publications have reported qualitative meta-analyses, focusing on regions that are activated by three or more studies (Meissner et al. 2011; Wager and Fields 2013). These reports included both contrasts between placebo and control as well as brain–behavior correlations. Correlations are extremely useful in establishing links between brain activity and pain, but they do not isolate causal effects of placebo on brain responses. The meta-analysis presented here extends this work by applying quantitative meta-analysis, by focusing only on contrasts rather than correlations with behavior, and by acknowledging different forms of expectancy-based pain modulation.
We performed meta-analyses on 25 neuroimaging studies that manipulated and measured placebo analgesia and expectancy-based pain modulation during brain imaging with fMRI or PET (see Table 1). The studies varied in the experimental dimensions listed above, but all studies compared one condition that induced expectations for pain relief (e.g., placebo administration or a cue predictive of low intensity) with a second control condition, in which the physical treatment or stimulus was identical but there was no expectation for relief. We focused on contrasts between these conditions, rather than correlations with behavior or analyses of responders vs. nonresponders, as contrasts allow for stronger inferences on causal effects of placebo administration.
Table 1.
Experiments included in the meta-analyses.
| Study | Category | Imaging modality | Type of pain | Sample size | Contrasts associated with reduced activation | Contrasts associated with increased activation |
|---|---|---|---|---|---|---|
| (Petrovic et al. 2002) | Placebo analgesia | PET rCBF | Thermal | 9 | N/A | 1. (Pain + Placebo)-(Pain alone); 2.[(Pain + Placebo)-(Pain alone)]-[(Warm + Placebo)-(Warm alone]) |
| (Wager et al. 2004): Study 1 | Placebo analgesia | fMRI | Shock | 24 | 1. (Control-Placebo)-(Intense-Mild Shock) | N/A |
| (Wager et al. 2004): Study 2 | Placebo analgesia | fMRI | Thermal | 23 | 1. (Control-Placebo) during early pain; 2. (Control-Placebo) during late pain | 1. (Placebo-Control) during anticipation |
| (Zubieta et al. 2005) | Placebo analgesia | PET μOR binding | Muscle (sustained) | 14 | N/A | 1. (Placebo-Control) opioid increase; carfentanil binding decrease |
| (Koyama et al. 2005) | Stimulus expectancy | fMRI | Thermal | 10 | 1. Decreased pain-induced activation evoked by decreased expectations (expect 48C,actual 50C versus expect 50C,actual 50C) | N/A |
| (Bingel et al. 2006) | Placebo analgesia | fMRI | Laser | 19 | N/A | 1. (Placebo-Control) during laser stimulation |
| (Kong et al. 2006) | Placebo analgesia | fMRI | Thermal | 24 | N/A | 1. (After-Before placebo treatment)-(Placebo-Control site) |
| (Keltner et al. 2006) | Stimulus expectancy | fMRI | Thermal | 27 | 1. High Expectancy,High Temp-Low Expectancy,High temp | N/A |
| (Price et al. 2007) | Placebo analgesia | fMRI | Visceral (Rectal) in patients with irritable bowel syndrome | 9 | 1. Pre-placebo (B1)-Placebo during pain AND Post-placebo (B2)-Placebo; ROIs | N/A |
| (Wager et al. 2007b) | Placebo analgesia | PET μOR binding | Thermal | 15 | N/A | 1. (Placebo-Control) opioid increase,(Painful-Nonpainful Heat) |
| (Craggs et al. 2008) | Placebo analgesia | fMRI | Visceral (Rectal) in patients with irritable bowel syndrome | 9 | N/A | 1. Placebo-Baseline during pain |
| (Eippert et al. 2009a) | Placebo analgesia | fMRI | Thermal | 48 | 1. (Control-Placebo) during early pain; 2. (Control-Placebo) during late pain | 1. (Placebo-Control) early pain; 2. (Placebo-Control) during pain,brainstem-specific |
| (Lu et al. 2009) | Placebo analgesia | fMRI | Visceral (Esophageal) | 14 | 1. (Control-Placebo) during pain | N/A |
| (Watson et al. 2009) | Placebo analgesia | fMRI | Laser | 11 | N/A | 1. (Placebo-Control) during anticipation |
| (Kong et al. 2009b) | Placebo analgesia | fMRI | Thermal | 12 | 1. Control > High expectancy in Placebo group | 1. Placebo acupuncture group (N=12),(Pre-Post-placebo) x (Placebo-Control) site |
| (Kong et al. 2009a) | Expectancy effects during treatment | fMRI | Thermal | Different size for each contrast | 1. Verum acupuncture (High-Low expectancy) groups,(Pre-Post-treatment) n = 24; 2. ME of expectancy on high-expectancy site,(pre-post treatment) n = 48 | 1. Verum acup high-expectancy group,(Pre-Post-treatment)-(Expected-Control site) n = 12 |
| (Harris et al. 2009) | Placebo analgesia | PET μOR binding | Chronic (fibromyalgia) | 10 | N/A | 1. Opioid increase (binding decrease) After-Before sham acupuncture |
| (Lui et al. 2010) | Placebo analgesia | fMRI | Laser | 31 | N/A | 1. Placebo/Low exp vs Control/High exp during anticipation; 2. Placebo/Low exp vs Control/High exp during pain |
| (Atlas et al. 2010) | Stimulus expectancy | fMRI | Thermal | 18 | 1. Assimilation/Expectancy-induced reductions | 1. Contrast/Expectancy-induced incr |
| (Wiech et al. 2010) | Stimulus expectancy | fMRI | Laser | 16 | 1. High > Low threat,across pain and non; 2. Intxn: [Pain,High > Low threat] > [No pain,High > Low threat] | 1. Low > High threat,across pain and non |
| (Bingel et al. 2011) | Expectancy effects during treatment | fMRI | Thermal | 22 | 1. Placebo-Nocebo: Decreases | 1. Placebo-Nocebo: Increases |
| (Lee et al. 2012) | Placebo analgesia | fMRI | Visceral (Rectal) in patients with irritable bowel syndrome and healthy controls | 17 | 1. Placebo-control during rectal pain: healthy volunteers (decreases); 2. Placebo-control during rectal pain: IBS pts (decreases) | 1. Placebo-control during rectal pain: healthy volunteers (increases); 2. Placebo-control during rectal pain: IBS pts (increases) |
| (Geuter et al. 2012) | Placebo analgesia | fMRI | Thermal | 40 | 1. antic: (Control > strong placebo) > (Control > Weak placebo); 2. Early pain: Control > placebo; 3. Late pain: Control > placebo; 4. Late pain: Control > strong placebo; 5. Late pain: Control > weak placebo; 6. Late pain: (Control > strong placebo) > (Control > weak placebo) | 1. antic: Placebo > Control; 2. antic: Strong placebo > Control; 3. antic: Weak placebo > Control; 4. Early pain: placebo > control; 5. Early pain: strong placebo > control; 6. Early pain: weak placebo > control; 7. Early pain: (Strong placebo > control) > (weak placebo > control); 8. Late pain: Weak placebo > control; 9. Late pain: (Strong placebo > Control) > (weak placebo > control) |
| (Atlas et al. 2012) | Expectancy effects during treatment | fMRI | Thermal | 21 | 1. Instruction-related decreases; 2. Open-Hidden: Decreases | 1. Instruction-related increases; 2. Open-Hidden: Increases |
| (Kong et al. 2013) | Stimulus expectancy | fMRI | Thermal | 46 | 1. For subjs with large exp fx, HC > LC | 1. Low cue > High cue, pain; 2. Low cue > High cue,antic |
In our first meta-analysis, we combined (1) studies that manipulated expectations about treatments and tested responses to inert treatments (placebo and nocebo studies), (2) studies that manipulated expectations about treatments and tested responses during actual treatments (open-hidden paradigms), and (3) studies that manipulated expectations about noxious stimulus intensity (cue-based expectancy studies). All of these are types of placebo manipulations in that they manipulate the psychological context—usually a combination of instructions and prior experiences—and all elicit more positive expectations in the placebo condition than a matched control condition with the same physical testing conditions. This primary analysis isolates the brain mechanisms that show consistent increases with expectations about pain, and the brain regions whose pain-evoked activation is influenced by expectations. As a secondary analysis, we separated studies that manipulated expectations about treatments from those that measured expectations about stimuli. This analysis summarizes whether different types of expectations about pain rely on similar or different mechanisms.
3. Methods
3.1. Study Selection and Coordinate Identification
Forty neuroimaging studies of expectancy-based pain modulation were identified using literature searches in PubMed and Google Scholar, the authors’ personal libraries, and examining references of relevant papers. We included only studies that (1) used experimental manipulations to induce pain relief; (2) reported formal comparisons (i.e., subtraction-based contrasts) between experimental and control conditions; and (3) reported voxel-wise results in either Montreal Neurological Institute (MNI) or Talairach/Tournoux coordinates. Brain–behavior correlations and ROI-wise analyses were not included in the meta-analysis in order to isolate the direct effects of experimental manipulations on brain responses.
Of the 40 studies originally identified, 25 studies met our criteria (see Table 1). Seventeen of these studies manipulated expectations using placebo manipulations, five manipulated expectations using cue-based information about stimulus intensity, and three measured expectancy effects during drug treatment using open-hidden administration paradigms. We divided the analyses and results into expectancy-related reductions (e.g., reduced activation with placebo vs. control during pain) or expectancy-related increases (e.g., increased activation with placebo vs. control during anticipation or pain). Our analysis of expectancy-related reductions included only contrasts that focused on brain responses during noxious stimulation, as this identifies regions in which pain processing is modulated by expectancy. However, because pain is thought to be influenced by both preparatory processes and modulation during stimulation, our analysis of expectancy-related increases includes contrasts of activation during both anticipation and stimulation periods. Our meta-analysis of expectancy-related increases also included PET studies that reported reductions in μ-opioid receptor (MOR) tracer binding (consistent with increases in endogenous MOR binding), as MORs comprise one well-supported mechanism of expectancy-based pain modulation.
We extracted peak voxel coordinates from relevant contrasts, and used the Tal2MNI algorithm (Matthew Brett; http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach) to convert Talaraich coordinates to MNI space. We identified 358 peaks from 61 contrasts in 25 studies (see Fig. 2). Some studies reported both voxel-wise reductions and increases, while others reported effects in only one direction (see Table 1). Table 2 provides detail on the number of peaks, contrasts, and studies included in each meta-analysis.
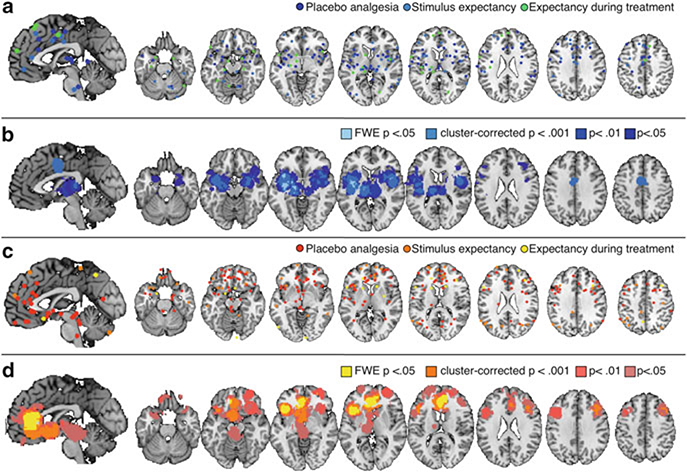
Fig. 2.
Meta-analysis 1: expectancy-based pain modulation. (a) Peaks included in a meta-analysis of expectancy-based reductions during pain. (b) Brain regions that showed reliable reductions during placebo administration and expectations for reduced pain (see Table 3). (c) Peaks included in meta-analysis of modulatory increases during pain. (d) Regions that showed consistent increases during anticipation or pain stimulation with expectations for reduced pain (see Table 4)
Table 2.
Meta-analysis details.
| Meta-analysis | Number of individual studies included | Number of contrasts | Number of peak coordinates |
|---|---|---|---|
| Decreases during pain: all paradigms | 16 | 27 | 171 |
| Decreases during pain: Placebo studies | 8 | 16 | 83 |
| Decreases during pain: Stimulus expectancy studies | 5 | 6 | 56 |
| Increases: all paradigms | 19 | 34 | 187 |
| Increases: Placebo studies | 13 | 26 | 122 |
| Increases: Stimulus expectancy studies | 3 | 4 | 43 |
3.2. Analysis
We performed meta-analyses with MKDA, which summarizes the number of contrasts that activated in the local vicinity (here, within 15 mm) of each voxel in the brain, and uses Monte Carlo simulations to identify regions that are activated more frequently than would be expected by chance. The MKDA approach is described in detail in Wager et al. (2007a, 2009). Peak activations for each contrast were convolved with a spherical smoothing kernel with a 15-mm radius and a weighted average (with weights based on the square root of the sample size) of these was used to generate an activation frequency map. The map was thresholded at p < 0.05 family-wise error rate corrected across the whole brain using the maximum null hypothesis activation frequency from each of 5,000 Monte Carlo simulations. The null hypothesis was a random uniform distribution of activation peaks throughout gray matter, which was simulated by permuting the peak locations for each contrast and recalculating the activation frequency map for each iteration. Voxels that survived correction are reported below. We conducted six separate meta-analyses: (1) expectancy-induced reductions in activity (combined across stimulus and treatment expectancy); (2) Expectancy-induced increases; (3) treatment expectancy-induced reductions (placebo analgesia); (4) treatment expectancy-induced increases; (5) stimulus expectancy-induced reductions (cue-based manipulations that show assimilation toward expectations); and (6) stimulus expectancy-induced increases (cue-based manipulations that show contrast away from expectations, e.g., increased activation with expectation for reduced pain).
4. Results
4.1. Meta-analyses 1 and 2: Expectancy-Based Pain Modulation
This analysis combines across three standard types of experimental manipulations that all induce expectations about pain, either through placebo manipulations, pain-predictive cues, or open information about drug delivery. Included studies and contrasts are listed in Tables 1 and 2. The results reported below and in Tables 3 and 4 incorporate both height-corrected results (FWE-corrected p < 0.05) and spatial extent-corrected results (cluster-corrected p < 0.001).
Table 3.
Meta-analysis 1: reductions during paina
| Name | x | y | z | Voxels | Studies active (%) | |
|---|---|---|---|---|---|---|
| Height-corrected FWE p < 0.05 | Left amygdala | −24 | −4 | −8 | 60 | 37.73 |
| Insula, L Middle | −38 | 8 | −2 | 63 | 37.25 | |
| Insula, L Posterior | −36 | −10 | 0 | 233 | 44.42 | |
| −38 | −6 | 10 | 26 | 44.42 | ||
| Insula, L dorsal posterior/Rolandic Operculum/OP4 | −48 | −10 | 12 | 5 | 25.64 | |
| SII, L (Rolandic Operculum/OP1) | −46 | −22 | 14 | 4 | 27.28 | |
| Insula, R Middle | 44 | 10 | −2 | 3 | 25.61 | |
| 44 | 6 | 10 | 48 | 29.13 | ||
| Putamen, L | −30 | −18 | 8 | 12 | 27.53 | |
| Thalamus, L (Premotor) | −14 | −18 | −2 | 109 | 40.1 | |
| Extent-corrected p < 0.001 | SMA, L | −8 | 0 | 46 | 137 | 25.07 |
| Cingulate, L Middle | 0 | −2 | 40 | 559 | 25.07 | |
| Thalamus, L | −12 | −22 | 12 | 346 | 32.44 | |
| Rolandic Operculum, L | −50 | 4 | 10 | 110 | 34.22 | |
| Thalamus, L | −6 | −10 | 6 | 41 | 27.35 | |
| Thalamus, Medial | 0 | −24 | 8 | 45 | 27.11 | |
| Superior temporal gyrus, L (TE 1.0) | −42 | −20 | 4 | 257 | 30.73 | |
| Insula, L Middle | −44 | −4 | −2 | 216 | 39.52 | |
| Pallidum, L | −26 | −12 | 0 | 176 | 50.18 | |
| Insula, L | −36 | −20 | −4 | 96 | 35.35 | |
| Hippocampus, L | −34 | −8 | −12 | 197 | 38.05 | |
| −22 | −14 | −16 | 68 | 24.62 | ||
| Superior temporal gyrus, L | −42 | 2 | −14 | 207 | 22.97 | |
| Insula, R Middle/Rolandic Operculum (OP4) | 52 | −6 | 12 | 88 | 21.14 | |
| 34 | 8 | 6 | 153 | 37.06 | ||
| 52 | 0 | 4 | 209 | 24.59 | ||
| 42 | −6 | 6 | 234 | 24.59 | ||
| Putamen, R | 30 | 0 | −6 | 138 | 41.42 | |
| Amygdala, R | 28 | −6 | −14 | 91 | 21.72 | |
| 30 | 4 | −16 | 120 | 19.6 |
This table reports clusters and contiguous subclusters corresponding to Fig. 2b
Table 4.
Meta-analysis 2: modulatory mechanisms/expectancy-induced increasesa
| Name | x | y | z | Voxels | Studies active (%) | |
|---|---|---|---|---|---|---|
| Height-corrected FWE p < 0.05 | mOFC/sgACC | −8 | 38 | −10 | 89 | 33.75 |
| pgACC, medial | −10 | 28 | 0 | 178 | 34.45 | |
| 4 | 40 | 0 | 96 | 49.56 | ||
| −2 | 36 | 10 | 524 | 52.58 | ||
| pgACC, R | −8 | 40 | 0 | 267 | 40.89 | |
| 6 | 44 | 14 | 362 | 39.19 | ||
| Insula, L anterior | −40 | 20 | 2 | 692 | 28.7 | |
| Anterior PFC/Superior orbital gyrus, R | 28 | 54 | −4 | 4 | 22.8 | |
| IFG pars triangularis, L (latPFC) | −46 | 18 | 18 | 4 | 22.33 | |
| Extent-corrected p < 0.001 | DLPFC, R (Middle Frontal Gyrus) | 42 | 20 | 36 | 409 | 27.07 |
| 36 | 26 | 30 | 71 | 20.3 | ||
| rdACC, R | 2 | 32 | 20 | 120 | 38.58 | |
| 12 | 40 | 22 | 48 | 28.02 | ||
| 12 | 26 | 12 | 102 | 24.97 | ||
| rdACC, L | −10 | 34 | 16 | 138 | 25.12 | |
| −2 | 24 | 14 | 102 | 28.43 | ||
| Insula, L Anterior | −28 | 24 | 6 | 130 | 30.07 | |
| −36 | 16 | −8 | 233 | 25.62 | ||
| −34 | 12 | 8 | 52 | 26.35 | ||
| pgACC | −14 | 44 | 8 | 63 | 32.84 | |
| Inferior frontal gyrus, pars Triangularis (BA 45) | −50 | 18 | 6 | 69 | 26.35 | |
| −42 | 32 | 2 | 125 | 26.35 | ||
| sgACC | 8 | 28 | 0 | 79 | 36.14 | |
| 2 | 16 | −6 | 160 | 22.79 | ||
| Caudate/Ventral striatum, L | −10 | 10 | −2 | 207 | 23.89 | |
| Ventral striatum, R | 2 | 0 | −4 | 86 | 18.91 | |
| Ventral striatum/globus pallidus, L | −8 | −2 | −4 | 157 | 24.85 | |
| Ventral Striatum, L | −14 | 10 | −12 | 209 | 31.67 | |
| Ventral Striatum/sgACC/Olfactory cortex | −2 | 8 | −12 | 336 | 26.39 | |
| Inferior frontal gyrus, pars Orbitalis | −32 | 28 | −6 | 82 | 31.2 | |
| −48 | 26 | −6 | 50 | 28.7 |
This table reports clusters and contiguous subclusters corresponding to Fig. 2d
4.1.1. Expectancy-Induced Reductions During Noxious Stimulation
As shown in Fig. 2b, experimentally manipulated expectations for reduced pain were associated with consistent decreases in activation during noxious stimulation in bilateral anterior insula, bilateral middle insula, left posterior insula, bilateral thalamus, bilateral amygdala, dorsal anterior cingulate (dACC), and bilateral lateral prefrontal cortex (see Table 3).
4.1.2. Expectancy-Induced Increases During Noxious Stimulation
Experimentally manipulated expectations for increased pain were associated with modulatory increases in medial and lateral orbitofrontal cortex (OFC), right anterior prefrontal cortex/superior orbital gyrus, pregenual/rostral ACC (pgACC), rostrodorsal ACC, left ventral striatum, left anterior insula, and midbrain surrounding the periacqueductal gray (PAG; see Fig. 2d and Table 4).
4.2. Meta-analyses 3 to 6: Stimulus Expectancies Versus Treatment Expectancies
We performed separate meta-analyses for studies that manipulated placebo-based expectations about treatments and those that manipulated expectations about stimuli on a trial-by-trial basis using conditioned cues (see Tables 1 and 2 for details). We then performed direct contrasts between these two forms of expectancy-based modulation to identify any regions that are differentially modulated by each form of expectancy. The results reported below and in Tables 5, 6, and 7 incorporate both height-corrected results (FWE-corrected p < 0.05) and spatial extent-corrected results (cluster-corrected p < 0.001).
Table 5.
Meta-analysis 3: placebo-induced reductions during paina
| Placebo-induced reductions | Name | x | y | z | Voxels | Studies active (%) |
|---|---|---|---|---|---|---|
| Height-corrected FWE p < 0.05 | Insula, L Anterior | −38 | −2 | −16 | 60 | 31.67 |
| −44 | −4 | −8 | 95 | 31.67 | ||
| −42 | −18 | 2 | 65 | 32.62 | ||
| Insula, L Middle | −36 | −10 | −4 | 153 | 46.35 | |
| −34 | 4 | −4 | 249 | 49.83 | ||
| −40 | −6 | 6 | 310 | 49.83 | ||
| Extent-corrected p < 0.001 | dpIns, L (OP4)/Heschl’s Gyrus | −48 | −16 | 10 | 141 | 36.1 |
| Insula, L Anterior (BA44) | −42 | 10 | 2 | 82 | 36.92 | |
| Putamen, L | −26 | −12 | 2 | 66 | 53.69 | |
| Putamen, L, contiguous with L Anterior Insula | −30 | 14 | −2 | 241 | 33.44 | |
| Amygdala, L | −26 | −6 | −10 | 159 | 39.01 | |
| Amygdala, L, contiguous with L Putamen | −26 | 4 | −12 | 161 | 26.1 | |
| Insula, L Anterior | −38 | 8 | −14 | 141 | 31.67 |
This table reports clusters and contiguous subclusters corresponding to Fig. 3a
Table 6.
Meta-analysis 4: placebo-induced increasesa
| Name | x | y | z | Voxels | Studies active (%) | |
|---|---|---|---|---|---|---|
| Height-corrected FWE p < 0.05 | pgACC, L | −8 | 34 | −6 | 399 | 43.47 |
| −2 | 40 | 0 | 415 | 56.31 | ||
| −12 | 28 | 4 | 108 | 37.84 | ||
| −4 | 32 | 10 | 340 | 50.09 | ||
| −4 | 42 | 12 | 223 | 50.68 | ||
| pgACC, R | 4 | 38 | 18 | 76 | 40.54 | |
| Ventral Striatum, L | −6 | 6 | −8 | 22 | 23.95 | |
| −8 | −2 | −2 | 33 | 28.78 | ||
| Inferior Frontal Gyrus, Pars Triangularis, L | −46 | 24 | 0 | 151 | 25.46 | |
| Insula, L Anterior | −38 | 18 | 2 | 498 | 25.46 | |
| rdACC, R | 6 | 28 | 24 | 10 | 23.1 | |
| Extent-corrected p < 0.001 | Midbrain surrounding the PAG | 0 | −32 | −12 | 482 | 16.76 |
| Midbrain, contiguous with thalamus | −6 | −20 | −4 | 313 | 21.6 | |
| mOFC (mid orbital gyrus) | −2 | 26 | −14 | 147 | 26.2 | |
| mOFC, L (rectal gyrus) | −6 | 36 | −16 | 60 | 28.5 | |
| sgACC | 0 | 20 | −6 | 202 | 26.02 | |
| 4 | 34 | −8 | 193 | 36.76 | ||
| rACC | 2 | 22 | 8 | 84 | 41.04 | |
| 12 | 24 | 12 | 38 | 16.3 | ||
| 10 | 44 | 12 | 259 | 44.21 | ||
| VMPFC, L | −12 | 46 | −10 | 14 | 16.42 | |
| Insula, L Anterior | −38 | 18 | −10 | 92 | 18.21 | |
| −40 | 10 | −4 | 68 | 18.21 | ||
| −30 | 28 | −2 | 99 | 25.46 | ||
| −40 | 32 | 2 | 103 | 25.46 | ||
| Inferior frontal gyrus, pars Triangularis, LL | −40 | 24 | 12 | 364 | 32.87 |
This table reports clusters and contiguous subclusters corresponding to Fig. 4a
Table 7.
Meta-analysis 6: stimulus expectancy-induced increasesa
| Stimulus-expectancy induced increases | Name | x | y | z | Voxels | Studies active (%) |
|---|---|---|---|---|---|---|
| Height-corrected FWE p < 0.05 | Angular Gyrus, L / IPC (PFm) | −40 | −60 | 46 | 319 | 81.66 |
| Angular Gyrus, L / IPC (PGa) | −48 | −60 | 36 | 158 | 81.66 | |
| Superior Frontal Gyrus, L (aPFC) | −16 | 66 | 10 | 131 | 81.66 | |
| Extent-corrected p < 0.001 | None |
This table reports clusters and contiguous subclusters corresponding to Fig. 4b
4.2.1. Placebo-Induced Reductions During Noxious Stimulation
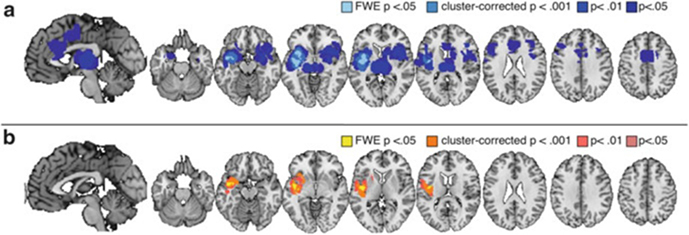
As shown in Fig. 3a and reported in Table 5, placebo-induced expectations for reduced pain were associated with consistent reductions during noxious stimulation in bilateral anterior insula, left middle insula, left posterior insula, dACC, bilateral medial thalamus, bilateral amygdala, and right lateral prefrontal cortex.
Fig. 3.
Decreases: treatment expectancies vs. stimulus expectancies. (a) Brain regions that showed reliable reductions during placebo analgesia (see Table 5). (b) Differences between placebo analgesia and stimulus expectancy-induced reductions (placebo analgesia > stimulus expectancies; see Table 8). Left anterior insula was significantly more likely to show reductions with placebo analgesia than with stimulus expectancies
4.2.2. Stimulus Expectancy-Induced Reductions During Noxious Stimulation
We found no regions that showed consistent increases in response to cue-based expectations for reduced pain. This is likely due to the small number of studies included in this analysis (5 contrasts from 3 studies; see Table 2).
4.2.3. Placebo-Induced Increases During Noxious Stimulation
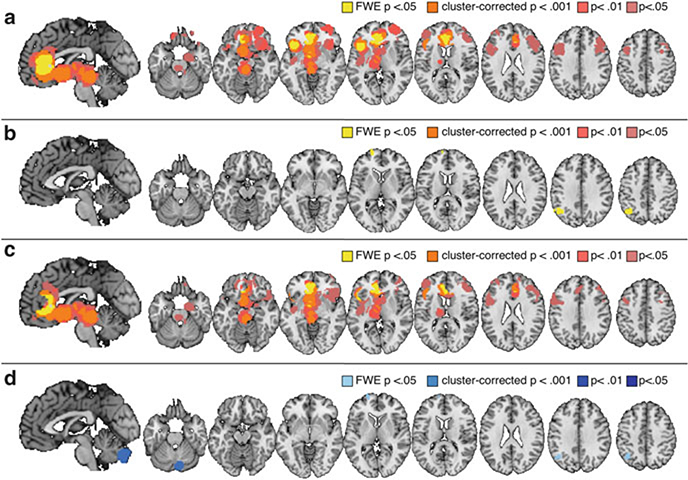
Placebo-induced expectations for reduced pain were associated with consistent increases in activation during noxious stimulation in medial OFC, right lateral OFC, pgACC, right anterior prefrontal cortex, bilateral dorsolateral prefrontal cortex, ventral striatum, left thalamus, and midbrain surrounding PAG (see Fig. 4a and Table 6).
Fig. 4.
Modulatory increases: treatment expectancies vs. stimulus expectancies. (a) Brain regions that showed reliable increases prior to or during placebo analgesia (see Table 6). (b) Brain regions that showed reliable increases as a function of stimulus expectancy (i.e., increased activity with expectation for reduced pain; see Table 7). (c) Differences between placebo analgesia and stimulus expectancy-induced increases (placebo analgesia > stimulus expectancies; see Table 8). (d) Regions that showed larger increases with stimulus expectancy than placebo analgesia (see Table 9)
4.2.4. Stimulus Expectancy-Induced Increases During Noxious Stimulation
Cue-based expectations for reduced pain were associated with consistent increases in left anterior prefrontal cortex and left superior parietal lobule/angular gyrus (see Fig. 4b and Table 7).
4.2.5. Treatment Expectancy Effects Versus Stimulus Expectancy Effects
A formal comparison of treatment expectancy studies and stimulus expectancies revealed that treatment expectancies were significantly more likely to reduce activation in left insula (anterior, middle, and posterior insula; see Fig. 3b and Table 8). There were no regions that showed reductions unique to stimulus expectancy, which is unsurprising given that the meta-analysis revealed no common reductions in the few studies included. We also examined differences in expectancy-related increases (regions that showed increases with placebo administration, showed greater activation following stimulus expectancy cues that predict low pain, or showed reductions with cues that predict high pain). We found that treatment expectancies were associated with larger increases in ventromedial prefrontal cortex (VMPFC), PAG, dorsomedial prefrontal cortex, left ventral striatum, pgACC, sgACC, left lateral prefrontal cortex, and left thalamus than stimulus expectancies (see Fig. 4c and Table 9). Stimulus expectancies were more likely to activate left anterior prefrontal cortex, left superior parietal lobule, and the cerebellum (see Fig. 4d). We note that these differences should be considered tentative and exploratory, as the number of placebo studies far outweighed the number of stimulus expectancy studies included.
Table 8.
Contrast A: expectancy-induced reductions during paina
| Placebo-induced reductions > Stimulus expectancy-induced reductions | Names | x | y | z | Voxels | Placebo studies active (%) | Stimulus expectancy studies active (%) |
|---|---|---|---|---|---|---|---|
| Height-corrected FWE p < 0.05 | Insula, L Anterior | −32 | 12 | −6 | 7 | 33.44 | 0 |
| Insula, L Posterior/Rolandic Operculum (OP3) | −42 | −10 | 12 | 76 | 36.1 | 0 | |
| Insula, L Posterior/Superior Temporal Gyrus (TE 1.0) | −44 | −18 | 0 | 38 | 32.62 | 0 | |
| Insula, L Middle | −42 | −8 | 2 | 173 | 49.83 | 27.04 | |
| Insula, L Posterior/Superior Temporal Gyrus | −42 | −2 | −12 | 151 | 39.01 | 0 | |
| Extent-corrected p < 0.001 | Rolandic Operculum, L (OP4) | −52 | −2 | 14 | 86 | 26.21 | 0 |
| Amygdala, L | −26 | 4 | −10 | 142 | 26.1 | 0 | |
| Stimulus expectancy-induced reductions > Placebo-induced reductions | |||||||
| Height-corrected FWE p < 0.05 | none | ||||||
| Extent-corrected p < 0.001 | none |
This table reports clusters and contiguous subclusters corresponding to Fig. 3b
Table 9.
Contrast B: expectancy-induced increasesa
| Placebo-induced increases > Stimulus expectancy-induced increases | Name | x | y | z | Voxels | Placebo studies active (%) | Stimulus expectancy studies active (%) |
| Height-corrected FWE p < 0.05 | DMPFC | 4 | 42 | 24 | 1 | 23.17 | 0 |
| rdACC | 6 | 28 | 24 | 10 | 23.1 | 0 | |
| Inferior Frontal Gyrus, Pars Triangularis, L (BA44) | −46 | 16 | 6 | 88 | 25.46 | 0 | |
| Ventral Striatum, L | −8 | −2 | −2 | 33 | 28.78 | 0 | |
| Ventral Striatum, L | −6 | 6 | −8 | 22 | 23.95 | 0 | |
| pgACC, L | −10 | 42 | 10 | 141 | 43.56 | 0 | |
| −4 | 32 | 10 | 360 | 54.35 | 0 | ||
| −12 | 26 | 2 | 153 | 37.84 | 0 | ||
| −6 | 38 | −4 | 474 | 52.05 | 0 | ||
| Extent-corrected p < 0.001 | Midbrain surrounding the PAG | 2 | −32 | −14 | 274 | 16.76 | 0 |
| −4 | −24 | −6 | 518 | 21.6 | 0 | ||
| mOFC (mid orbital gyrus, L) | 0 | 26 | −14 | 73 | 26.2 | 0 | |
| 0 | 46 | −6 | 33 | 32.38 | 0 | ||
| −10 | 48 | −10 | 9 | 16.42 | 0 | ||
| sgACC | 4 | 34 | −8 | 190 | 28.18 | 0 | |
| 0 | 20 | −6 | 195 | 26.02 | 0 | ||
| WM_rACC | 2 | 22 | 10 | 82 | 41.04 | 0 | |
| 12 | 24 | 12 | 27 | 16.3 | 0 | ||
| Inferior frontal gyrus, pars triangularis, L (BA 45) | −52 | 22 | 0 | 33 | 25.46 | 0 | |
| −44 | 26 | 12 | 272 | 32.87 | 0 | ||
| Stimulus expectancy-induced increases > Placebo-induced increases | Name | x | y | z | voxels | Placebo studies active (%) | Stimulus expectancy studies active (%) |
| Height-corrected FWE p < 0.05 | Inferior parietal lobule, L (IPC (Pga)) | −40 | −58 | 54 | 50 | 0 | 81.66 |
| Angular Gyrus, L (IPC (PFm)) | −38 | −60 | 44 | 192 | 0 | 81.66 | |
| Angular Gyrus, L (IPC (Pga)) | −46 | −62 | 34 | 84 | 0 | 81.66 | |
| Superior Frontal Gyrus, L (aPFC) | −16 | 66 | 10 | 117 | 0 | 81.66 | |
| Extent-corrected p < 0.001 | Cerebellum, R | 4 | −86 | −28 | 198 | 0 | 62.2 |
| 16 | −82 | −30 | 325 | 0 | 80.54 | ||
| 8 | −74 | −32 | 386 | 0 | 80.54 | ||
| 2 | −82 | −38 | 198 | 0 | 80.54 | ||
| 12 | −88 | −40 | 158 | 0 | 62.2 | ||
| 14 | −76 | −42 | 202 | 0 | 80.54 |
5. Discussion
We used formal meta-analysis to examine the brain mechanisms associated with placebo- and expectancy-based pain modulation, as identified by 25 neuroimaging studies published between 2002 and 2013. Relative to control conditions, expectations for pain reduction were associated with widespread reductions in brain responses during painful stimulation, with decreases in dACC, insula, thalamus, amygdala, striatum, and lateral prefrontal cortex. Expectations for reduced pain were also associated with increases in activation prior to and during noxious stimulation in a number of regions, including dorsolateral and ventromedial prefrontal cortex, rostral anterior cingulate cortex, the midbrain surrounding the PAG, left anterior insula, and the striatum. These regions reveal the most reliable neural mechanisms underlying placebo analgesia and expectancy-based pain modulation, and they can serve as regions of interest in future studies designed to directly isolate their specific contributions. In addition, we observed initial support for separate mechanisms underlying placebo-based treatment expectancy effects and stimulus expectancy effects, though results should be considered tentative. In this final section, we discuss these networks from the standpoint of cognitive and affective neuroscience, and we address outstanding questions that future studies can address.
5.1. Placebo Effects on Brain Regions Traditionally Associated with Pain Processing
We observed consistent reductions in dACC, bilateral insula, and thalamus, a subset of the regions that are most reliably activated by noxious stimulation (Duerden and Albanese 2011; Apkarian et al. 2005; Friebel et al. 2011; Peyron et al. 2000; Wager et al. 2013). Interestingly, secondary somatosensory cortex (SII) and dorsal posterior insula (dpIns) are conspicuously absent (although we saw left posterior insula when we collapsed across stimulus and treatment expectancy). SII and dpIns are most consistently and specifically activated by noxious stimuli in neuroimaging studies (Kross et al. 2011; Peyron et al. 2000), are thought to support pain’s sensory components (Maihöfner et al. 2006), and were recently shown to be the only cortical regions in which intracranial stimulation can produce a sensation of pain (Mazzola et al. 2011).
Why might we see this distinction? Is this evidence that placebo analgesia does not alter the earliest levels of processing? We believe that the absence of SII and dpIns reductions in our analysis does not imply that early nociceptive processing is unaltered during placebo analgesia. First, we know from individual studies that placebo analgesia and nocebo hyperalgesia can influence spinal nociceptive reflexes and spinal responses to noxious stimuli (Eippert et al. 2009b; Goffaux et al. 2007; Matre et al. 2006; Geuter and Buchel 2013) which reveals that placebo can alter pain processing before ascending nociceptive signals even reach cortex. Second, behavioral investigations indicate that placebo alters both pain intensity and unpleasantness ratings (De Pascalis et al. 2002; Price et al. 1999), although early psychophysical investigations that used signal detection theoretic analyses suggested that placebo altered response biases without altering sensory discrimination (Clark 1969). Third, our meta-analysis collapsed across both pain modalities (see Table 1) and site of noxious stimulation, and SII and mid-to-posterior insula are sensitive to different types of pain (Baumgartner et al. 2010; Friebel et al. 2011) and contain a somatotopic map (Baumgartner et al. 2010), thereby representing different body sites in different precise locations. Finally, it is possible that the strength of nociceptive modulation varies across studies and contexts, and that effects in these regions exist only for the strongest contexts and the strongest placebo responders. Thus the fact that these regions did not emerge in our meta-analyses is not definitive evidence that sensory processing is unaffected during expectancy-based pain modulation.
Despite the fact that we cannot say with certainty that SII and dpIns are unaffected by placebo, we must also acknowledge that there is a large literature supporting a distinction between these two regions and those that were consistently modulated by placebo and expectancy and identified in the meta-analysis presented here. Medial thalamus, dACC, and insula are all targets of the spinothalamic tract (Dum et al. 2009). They are functionally connected when individuals rate noxious stimuli but not when individuals make non-nociceptive magnitude estimations (Baliki et al. 2009). They have been traditionally thought to support pain’s affective (i.e., motivational and emotional) components (Peyron et al. 2000; Rainville 2002; Rainville et al. 1997, 1999; Tölle et al. 1999; Zubieta et al. 2001; Bushnell et al. 2013). The insula has also been implicated in interoception and thermosensory processing (Craig 2009; Craig et al. 2000).
If one interprets the results of the present meta-analysis and considers only the aforementioned pain literature, one might assume that our findings reveal that pain affect is reliably influenced by placebo administration. However, one glaring caveat must be acknowledged. The insula, thalamus, and ACC are the most widely activated regions across all task-based fMRI studies (Yarkoni et al. 2011). The dACC and insular cortices show intrinsic connectivity during resting state fMRI and have been referred to as a “salience network” (Seeley et al. 2007). They are implicated in many broad psychological processes, including simple maintenance of task sets (Dosenbach et al. 2006), interoception (Craig 2002; Critchley et al. 2004), conflict monitoring (Botvinick et al. 2004), and affective processing (Shackman et al. 2011). All of these psychological processes might be implicated in a standard placebo experiment, and so determining whether the presence of these brain regions reflects these nonspecific processes or reflects something unique about placebo requires more sophisticated analyses. In this regard, correlations between brain responses and the magnitude of placebo analgesia (or placebo “responder status,” a dichotomous version) are informative, as they establish a relationship between brain activity and pain. Correlations between placebo analgesia magnitude and reductions in dACC, anterior insula, thalamus, and SII have been reported in multiple studies (reviewed in Koban et al. 2013). Nonetheless, such correlations do not provide strong evidence that the brain processes that are affected by placebo are directly associated with nociception or pain, and stronger tests are needed. As the question of specificity applies to all of the regions identified in the meta-analyses presented here, we discuss this issue in greater detail—and propose several solutions—below (see Sect. 5.6).
With regard to the question of whether nociceptive processing is altered, we note that the vast majority of the studies included in the present analyses applied either a single level of noxious stimulation, or, if stimulus intensity varied, the paradigm included cues that gave information about upcoming stimulus intensity, thereby influencing stimulus expectancies (even in the context of placebo analgesia studies designed to test only treatment expectancy). A simple modification of the basic experimental paradigm depicted in Fig. 1 would greatly improve our ability to determine the extent to which pain-related processing is altered by placebo. If stimulus intensity varies during placebo and control conditions, researchers can examine intensity-related changes in the control condition to establish subject-specific regions of interest involved in pain processing, and then test for placebo effects on these responses. This would also allow for more sophisticated analyses, such as tests of placebo x intensity interactions (Wager et al. 2004). Another important direction is to test the effects of placebo on brain patterns that are validated to be sensitive and specific to pain across studies, such as that recently developed by Wager et al. (2013).
5.2. Reductions During Pain in the Amygdala
In addition to regions often implicated in pain processing, we observed placebo-and expectancy-induced reductions in bilateral amygdala. The amygdala does receive nociceptive input through the spinopontoamygdalar pathway (Bernard et al. 1992; Willis and Westlund 1997), and some fMRI studies have shown that it tracks changes in noxious input (Bornhovd et al. 2002) and is important for the modulation of nociception by behavioral context (Helmstetter 1992). Thus it is possible that amygdala modulation is consistent with a straightforward account expectancy-based reduction in nociception. However, the amygdala is also strongly implicated in cognitive and affective processes (Phelps 2006) such as vigilance (Davis and Whalen 2001), threat (LaBar et al. 1998; LeDoux 1995; Rogan et al. 1997), and uncertainty (Rosen and Donley 2006; Whalen 2007)—processes that often accompany or precede pain, but that also exist in the absence of noxious input, such as in response to salient cues (e.g., subliminal fear expressions; Whalen et al. 1998, 2004) that induce vigilance or change one’s motivational and attentional state. As with the regions reviewed in the prior section, future experiments should directly test whether placebo-induced changes in amygdala responses relate more closely to pain processing or vigilance and uncertainty, as might be expected if the treatment context causes patients to feel calm and protected.
5.3. Modulatory Mechanisms
In addition to expectancy- and placebo-based reductions, we saw widespread increases in activation with expectation for decreased pain. Increases were apparent in the pgACC/rACC and the periacqueductal gray, two brain regions that have been linked with endogenous opioid release in animal models (Fields 2000, 2004) and in prior studies of placebo analgesia in humans (Levine et al. 1978) (Eippert et al. 2009a; Wager et al. 2007b; Zubieta et al. 2005). In addition, we observed consistent increases in the ventral striatum, a region that has been linked with dopamine binding and reward processing (Scott et al. 2007, 2008). The ventral striatum is also involved in learning about affective value, and works in concert with the VMPFC/OFC—another region that showed reliable increases with expectations for pain reduction—to track expected value and update expectations (Liljeholm and O’Doherty 2012; Murray et al. 2007; Schoenbaum et al. 2009). Relating placebo and expectancy effects with models of reinforcement learning is likely to be a fruitful direction for future research. We also note that VMPFC might have a more general role in ascribing value and meaning to stimuli (Roy et al. 2012), perhaps linking to meaning-based conceptions of placebo analgesia (Moerman and Jonas 2002). Finally, we also observed consistent placebo-based increases in the DLPFC, a region broadly implicated in executive function, including cognitive control, working memory, and rule maintenance (Miller 2000; Miller and Cohen 2001). For a more thorough discussion of these systems and their involvement in cognitive and affective functions, see Atlas and Wager (2013).
5.4. Treatment Expectancies Versus Stimulus Expectancies
Expectations about stimuli and expectations about treatment are often discussed interchangeably in the literature. Our first meta-analysis adopts this perspective to identify the brain mechanisms associated with expectancy-based pain modulation. However, we and others have argued that the two should be thought of as distinct processes (Atlas et al. 2010; Atlas and Wager 2012, 2013; Kirsch 1985, 1997). Stimulus expectancies are predictions about discrete events in the environment. Thus, stimulus expectancies are likely to rely upon transient processes that can change from moment to moment depending on the content of expectation, and may even recruit preparatory antinociceptive responses. We have hypothesized that cue-based stimulus expectancies about pain intensity are likely to depend on phasic responses in dopamine neurons and related systems involved in processing expected value and prediction error (Atlas et al. 2010). Indeed, quantitative modeling supports this account (Seymour et al. 2004, 2005), but the relationship between such signals and perceived pain has not been formally tested. Treatment expectancies, on the other hand, involve knowledge about one’s overall context, and beliefs that one will be less affected by stimuli in the environment. Thus, we have hypothesized that treatment expectancies are likely to depend on sustained mechanisms, such as affective shifts and tonic opioid binding (Atlas et al. 2010; Atlas and Wager 2012).
The theoretical distinctions between these two types of expectancy motivated us to separate and formally compare them in our secondary set of meta-analyses. Placebo-based reductions and increases were nearly identical to our findings from the collapsed meta-analysis. This is because of the vast imbalance in the studies that were included: Our meta-analysis was heavily weighted toward studies of placebo-based treatment expectancy (17 treatment expectancy vs. 5 stimulus expectancy). The dearth of experiments relating stimulus expectancies with perceived pain is also likely responsible for the fact that our meta-analysis failed to identify any regions that showed reliable stimulus expectancy-induced reductions (assimilation with expectations) during pain. We did, however, observe consistent increases with expectations for low pain in the parietal cortex and anterior prefrontal cortex, which might be related to the frontoparietal network, a network involved in selective attention (Szczepanski et al. 2013). We note that these regions were not observed in our analysis of treatment expectancy-induced increases, providing at least some support for the notion of neural segregation, although we feel it would be premature to infer that attention networks are themselves altered by the two processes. We encourage researchers to consider these distinctions in the future, so we can better identify the similarities and differences between these two types of expectations.
5.5. Relationship to Prior Meta-analyses of Placebo Analgesia
It is important to consider the present findings in relation to a quantitative meta-analysis published previously (Amanzio et al. 2011). Both analyses examined placebo-induced reductions during noxious stimulation and found evidence for reductions in the cingulate cortex and insula/clustrum, although laterality and precise location differed [middle and posterior cingulate in Amanzio et al. (2011) vs. anterior and middle cingulate here; right posterior and left anterior insula in Amanzio et al. (2011) vs. bilateral anterior and left posterior insula here]. All other reductions reported in Amanzio et al. (2011) fell within the boundaries of the placebo-induced reductions reported here. We also observed reductions in bilateral amygdala and bilateral lateral prefrontal cortex, which were not observed in the previous meta-analysis.
Amanzio et al. (2011) separated placebo-induced increases during anticipation (“stage 1”) and noxious stimulation (“stage 2”). The “stage 1” anticipatory increases identified by Amanzio et al. all overlap with or are directly adjacent to increases identified here. There were some slight differences when it came to “stage 2” increases. We did not observe consistent increases in the pons, the inferior parietal lobule, the postcentral gyrus, or the medial frontal gyrus, and the dorsolateral prefrontal cortex activations we observed were bilateral and posterior to the left DLPFC activation reported by Amanzio et al. (2011). We also found evidence of consistent placebo-induced increases in the ventral striatum and bilateral anterior insula/inferior frontal gyrus, which were not observed in the earlier meta-analysis.
Some of these differences are likely due to differences in power: The present analysis included twice as many studies than Amanzio et al. (2011), which points to a growing scientific interest in the brain basis of placebo analgesia as neuroimaging studies of placebo continue to be published. In addition, we used MKDE rather than ALE, which might have accounted for subtle differences in exact location of peaks. However, other differences are likely to stem from explicit decisions based on theoretical considerations. Amanzio et al. separated increases during anticipation and stimulation, whereas we collapsed across both phases in our analysis of modulatory increases. We decided to collapse across these periods since not all experiments are designed to separate anticipation and pain, and both periods involve modulatory mechanisms. In addition, we incorporated analyses of cue-based stimulus expectancies (though the comparisons discussed here refer specifically to our analysis of placebo-based treatment expectancy effects) to isolate expectancy-based changes and to determine whether there are reliable differences in treatment and stimulus expectancies.
5.6. Unanswered Questions: Extending the Meta-analysis
Throughout this discussion, we have deliberately avoided reverse inferences about the specific processes supported by the regions identified in our meta-analysis. As we have pointed out, the regions identified here are reliably influenced during placebo, but are also implicated in many other psychological processes. Meta-analyses tell us where placebo effects occur, but not how these brain regions—either individually or as a network—contribute to placebo effects on pain. How can future studies establish specificity?
One simple way to understand the contributions of these commonly activated brain regions in any particular study is to link placebo effects on the brain with placebo effects on behavior. This has been accomplished by (1) correlating individual differences in placebo effects on activation with effects on pain reports (Kong et al. 2006; Wager et al. 2004) [see (Koban et al. 2013) for review and meta-analysis]; (2) mediation analyses that identify regions that dynamically mediate the effects of experimental manipulations on measured behavior (Atlas et al. 2010); and (3) machine learning techniques that identify spatially distributed patterns of brain responses predictive of the magnitude of the placebo response across individuals (Wager et al. 2011). These brain-behavior approaches can help individual studies differentiate between regions that are simply activated by elements of the treatment context or experimental context and those that actually correlate with or even cause changes in subjective pain. Further specificity can be attained by differentiating between various aspects of the pain experience, e.g., pain intensity versus pain unpleasantness (De Pascalis et al. 2002; Kulkarni et al. 2005; Price et al. 1999). For example, one could use any of these methods to test formally the hypothesis that placebo effects on dACC reflect placebo-based reductions in pain unpleasantness.
Another way to specify the precise functional contributions of these regions is to design experiments that isolate the effects of different components that contribute to placebo responses. The standard placebo manipulation depicted in Fig. 1 (and employed in many of the experiments in the present meta-analysis) combines many features, only some of which have been directly investigated with neuroimaging tools. Participants generally receive both verbal suggestions and conditioning/paired association. A number of laboratory experiments have sought to separate the contributions of verbal suggestions and conditioning (Benedetti et al. 2003; Montgomery and Kirsch 1996, 1997), but such paradigms have yet to be extended to the neuroimaging domain. While one study examined the neural correlates of the conditioning phase as well as accompanying placebo analgesia during test (Lui et al. 2010), conditioning-based placebo was not directly compared to a placebo condition based on suggestion alone, nor was conditioning examined in the absence of explicit verbal instructions. Another key component of the placebo effect involves the psychosocial aspects of the patient–doctor relationship (Kaptchuk 2002). While few studies have formally investigated this interaction, some evidence comes from studies showing that patient responses are influenced by doctors’ expectations (Gracely et al. 1985; Levine and Gordon 1984). One recent experiment attempted to identify neural mechanisms contributing to the patient–doctor relationship by focusing on brain responses in physicians as they administered treatment (Jensen et al. 2013). Future studies can elaborate on this work and directly address the interactive nature of this relationship by adapting designs from social neuroscience optimized to study interpersonal interactions.
Finally, placebo effects might induce changes in attention and/or induce positive affective shifts (Atlas and Wager 2013), and, as mentioned above, changes in regions like insula and dACC might reflect these nonspecific processes. Experiments that relate placebo effects, brain responses, and performance on well-validated attention and emotion experiments can evaluate the extent to which this is true. For example, one study (Scott et al. 2007) related placebo-induced changes in striatal dopamine binding with performance on a reward task (Knutson et al. 2001), suggesting that this region might play a role in the rewarding aspects of receiving treatment. We have interwoven attention probes with placebo administration (Atlas et al. 2014) and stimulus expectancy cues (Johnston et al. 2012) to determine whether expectancy-based pain modulation depends on changes in attention. Finally, studies have tested whether placebo involves changes in emotion processing by relating responses during placebo analgesia with placebo effects on responses to aversive images (Petrovic et al. 2010; Zhang and Luo 2009) and by testing whether responses to emotional stimuli are altered during placebo analgesia (Atlas et al. 2014).
In sum, we envision an iterative process that will ultimately lead to precision and specificity with regard to the contributions of the individual regions and networks identified here. The regions we have identified can serve as regions of interest for future studies designed to isolate specific elements of placebo analgesia and other forms of expectancy-based pain modulation. As studies on a specific subprocess (e.g., positive affect/reward processing) accumulate, future meta-analyses will determine which regions are reliably activated as a function of that process. This iterative science will ultimately provide us with a detailed picture of how distinct psychological and neural processes combine to influence pain under placebo, which can then be extended to develop targeted interventions at a clinical level.
Acknowledgments
This work was supported in part by the Intramural Research program of the NIH’s National Center for Complementary and Alternative Medicine.
Contributor Information
Lauren Y. Atlas, National Center for Complementary and Alternative Medicine, National Institutes of Health, 10 Center Drive, Rm 4-1741, Bethesda, MD 20892
Tor D. Wager, Departments of Psychology and Neuroscience, University of Colorado, Boulder, CO, USA
References
- Amanzio M, Benedetti F, Porro CA, Palermo S, Cauda F (2011) Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Hum Brain Mapp 34:738–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede R-D, Zubieta J-K (2005) Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain (Lond) 9:463–484 [DOI] [PubMed] [Google Scholar]
- Atlas LY, Wager TD (2012) How expectations shape pain. Neurosci Lett 520:140–148 [DOI] [PubMed] [Google Scholar]
- Atlas LY, Wager TD (2013) Expectancies and beliefs: insights from cognitive neuroscience In: Ochsner KN, Kosslyn SM (eds) Oxford handbook of cognitive neuroscience. Oxford University Press, Oxford, NY, pp 359–381 [Google Scholar]
- Atlas LY, Bolger N, Lindquist MA, Wager TD (2010) Brain mediators of predictive cue effects on perceived pain. J Neurosci 30:12964–12977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Whittington RA, Lindquist MA, Wielgosz J, Sonty N, Wager TD (2012) Dissociable influences of opiates and expectations on pain. J Neurosci 32:8053–8064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Wielgosz J, Whittington RA, Wager TD (2014) Specifying the non-specific factors underlying opioid analgesia: expectancy, attention, and affect. Psychopharmacology 231:813–82324096537 [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV (2009) Parsing pain perception between nociceptive representation and magnitude estimation. J Neurophysiol 101:875–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett B, Muller D, Rakel D, Rabago D, Marchand L, Scheder JC (2006) Placebo, meaning, and health. Perspect Biol Med 49:178–198 [DOI] [PubMed] [Google Scholar]
- Baumgartner U, Iannetti GD, Zambreanu L, Stoeter P, Treede R-D, Tracey I (2010) Multiple somatotopic representations of heat and mechanical pain in the operculo-insular cortex: a high-resolution fMRI study. J Neurophysiol 104:2863–2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecher HK (1955) The powerful placebo. J Am Med Assoc 159:1602–1606 [DOI] [PubMed] [Google Scholar]
- Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I (2003) Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci 23:4315–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JF, Huang GF, Besson JM (1992) Nucleus centralis of the amygdala and the globus pallidus ventralis: electrophysiological evidence for an involvement in pain processes. J Neurophysiol 68:551–569 [DOI] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Glauche V, Knab R, Glascher J, Weiller C, Buchel C (2004) Somatotopic organization of human somatosensory cortices for pain: a single trial fMRI study. NeuroImage 23:224–232 [DOI] [PubMed] [Google Scholar]
- Bingel U, Lorenz J, Schoell E, Weiller C, Buchel C (2006) Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain 120:8–15 [DOI] [PubMed] [Google Scholar]
- Bingel U, Wanigasekera V, Wiech K, Mhuircheartaigh RN, Lee MC, Ploner M, Tracey I (2011) The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med 3:70ra14. [DOI] [PubMed] [Google Scholar]
- Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C (2002) Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain 125:1326–1336 [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS (2004) Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 8:539–546 [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Ceko M, Low LA (2013) Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 14:502–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WC (1969) Sensory-decision theory analysis of the placebo effect on the criterion for pain and thermal sensitivity. J Abnorm Psychol 74:363–371 [DOI] [PubMed] [Google Scholar]
- Craggs J, Price D, Perlstein W, Nicholas Verne G, Robinson M (2008) The dynamic mechanisms of placebo induced analgesia: evidence of sustained and transient regional involvement. Pain 139:660–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2002) How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 3:655–666 [DOI] [PubMed] [Google Scholar]
- Craig ADB (2009) How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 10:12. [DOI] [PubMed] [Google Scholar]
- Craig ADB, Chen K, Bandy D, Reiman EM (2000) Thermosensory activation of insular cortex. Nat Neurosci 3:184–190 [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ (2004) Neural systems supporting interoceptive awareness. Nat Neurosci 7:189–195 [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ (2001) The amygdala: vigilance and emotion. Mol Psychiatry 6:13–34 [DOI] [PubMed] [Google Scholar]
- de Craen AJ, Tijssen JG, de Gans J, Kleijnen J (2000) Placebo effect in the acute treatment of migraine: subcutaneous placebos are better than oral placebos. J Neurol 247:183–188 [DOI] [PubMed] [Google Scholar]
- De Pascalis V, Chiaradia C, Carotenuto E (2002) The contribution of suggestibility and expectation to placebo analgesia phenomenon in an experimental setting. Pain 96:393–402 [DOI] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE (2006) A core system for the implementation of task sets. Neuron 50:799–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden EG, Albanese M-C (2011) Localization of pain-related brain activation: a meta-analysis of neuroimaging data. Hum Brain Mapp 34:109–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Levinthal DJ, Strick PL (2009) The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. J Neurosci 29:14223–14235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT (2009) Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30:2907–2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C (2009a) Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 63:533–543 [DOI] [PubMed] [Google Scholar]
- Eippert F, Finsterbusch J, Bingel U, Buchel C (2009b) Direct evidence for spinal cord involvement in placebo analgesia. Science 326:404. [DOI] [PubMed] [Google Scholar]
- Fields HL (2000) Pain modulation: expectation, opioid analgesia and virtual pain. Prog Brain Res 122:245–253 [DOI] [PubMed] [Google Scholar]
- Fields HL (2004) State-dependent opioid control of pain. Nat Rev Neurosci 5:565–575 [DOI] [PubMed] [Google Scholar]
- Friebel U, Eickhoff S, Lotze M (2011) Coordinate-based meta-analysis of experimentally induced and chronic persistent neuropathic pain. NeuroImage 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuter S, Buchel C (2013) Facilitation of pain in the human spinal cord by nocebo treatment. J Neurosci 33:13784–13790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuter S, Eippert F, Attar CH, Büchel C (2012) Cortical and subcortical responses to high and low effective placebo treatments. Neuroimage:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffaux P, Redmond WJ, Rainville P, Marchand S (2007) Descending analgesia–when the spine echoes what the brain expects. Pain 130:137–143 [DOI] [PubMed] [Google Scholar]
- Gracely RH, Dubner R, Deeter WR, Wolskee PJ (1985) Clinicians’ expectations influence placebo analgesia. Lancet 1:43. [DOI] [PubMed] [Google Scholar]
- Harris RE, Zubieta J-K, Scott DJ, Napadow V, Gracely RH, Clauw DJ (2009) Traditional Chinese acupuncture and placebo (sham) acupuncture are differentiated by their effects on mu-opioid receptors (MORs). NeuroImage 47:1077–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ (1992) The amygdala is essential for the expression of conditional hypoalgesia. Behav Neurosci 106:518–528 [DOI] [PubMed] [Google Scholar]
- Jensen KB, Petrovic P, Kerr CE, Kirsch I, Raicek J, Cheetham A, Spaeth R, Cook A, Gollub RL, Kong J, Kaptchuk TJ (2013) Sharing pain and relief: neural correlates of physicians during treatment of patients. Mol Psychiatry 19:392–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston NE, Atlas LY, Wager TD (2012) Opposing effects of expectancy and somatic focus on pain. PLoS ONE 7:e38854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptchuk TJ (2002) The placebo effect in alternative medicine: can the performance of a healing ritual have clinical significance? Ann Intern Med 136:817–825 [DOI] [PubMed] [Google Scholar]
- Kaptchuk TJ, Goldman P, Stone DA, Stason WB (2000) Do medical devices have enhanced placebo effects? J Clin Epidemiol 53:786–792 [DOI] [PubMed] [Google Scholar]
- Keltner J, Furst A, Fan C, Redfern R, Inglis B et al. (2006) Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. J Neurosci 26:4437–4443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I (1985) Response expectancy as a determinant of experience and behavior. Am Psychol 40:1189–1202 [Google Scholar]
- Kirsch I (1997) Response expectancy theory and application: a decennial review. Appl Prev Psychol 6:69–79 [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D (2001) Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21:RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koban L, Ruzic L, Wager TD (2013) Brain predictors of individual differences in placebo responding In: Colloca L, Flaten MA, Meissner K(eds) Placebo and pain (89–101). Elsevier/Academic Press, Burlington, MA [Google Scholar]
- Kober H, Wager TD (2010) Meta-analysis of neuroimaging data. WIREs Cogn Sci 1:293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, Kaptachuk TJ (2006) Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci 26:381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Kaptachuk T, Polich G, Kirsch I, Angel M, Zyloney C, Rosen B, Gollub R (2009a) An fMRI study on the interaction and dissociation between expectation of pain relief and acupuncture treatment. NeuroImage 47:1066–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Kaptachuk TJ, Polich G, Kirsch I, Vangel MG, Zyloney C, Rosen BR, Gollub RL (2009b) Expectancy and treatment interactions: a dissociation between acupuncture analgesia and expectancy evoked placebo analgesia. NeuroImage 45:940–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Jensen K, Loiotile R, Cheetham A, Wey H-Y, Tan Y, Rosen B, Smoller JW, Kaptchuk TJ, Gollub RL (2013) Functional connectivity of the frontoparietal network predicts cognitive modulation of pain. Pain 154:459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T, McHaffie JG, Laurienti P, Coghill RC (2005) The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci USA 102:12950–12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Berman MG, Mischel W, Smith EE, Wager TD (2011) Social rejection shares somatosensory representations with physical pain. Proc Natl Acad Sci USA 108:6270–6275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni B, Bentley DE, Elliott R, Youell P, Watson A, Derbyshire SW, Frackowiak RS, Friston KJ, Jones AK (2005) Attention to pain localization and unpleasantness discriminates the functions of the medial and lateral pain systems. Eur J Neurosci 21:3133–3142 [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA (1998) Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron 20:937–945 [DOI] [PubMed] [Google Scholar]
- LeDoux JE (1995) Emotion: clues from the brain. Annu Rev Psychol 46:209–235 [DOI] [PubMed] [Google Scholar]
- Lee H-F, Hsieh J-C, Lu C-L, Yeh T-C, Tu C-H, Cheng C-M, Niddam DM, Lin H-C, Lee F-Y, Chang F-Y (2012) Enhanced affect/cognition-related brain responses during visceral placebo analgesia in irritable bowel syndrome patients. Pain 153:1301–1310 [DOI] [PubMed] [Google Scholar]
- Levine JD, Gordon NC (1984) Influence of the method of drug administration on analgesic response. Nature 312:755–756 [DOI] [PubMed] [Google Scholar]
- Levine JD, Gordon NC, Fields HL (1978) The mechanism of placebo analgesia. Lancet 2:654–657 [DOI] [PubMed] [Google Scholar]
- Liberman R (1964) An experimental study of the placebo response under three different situations of pain. J Psychiatr Res 33:233–246 [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Jarcho JM, Berman S, Naliboff BD, Suyenobu B, Mandelkern M, Mayer EA (2004) The neural correlates of placebo effects: a disruption account. NeuroImage 22:447–455 [DOI] [PubMed] [Google Scholar]
- Liljeholm M, O’Doherty JP (2012) Contributions of the striatum to learning, motivation, and performance: an associative account. Trends Cogn Sci 16:467–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H-C, Hsieh J-C, Lu C-L, Niddam DM, Wu Y-T, Yeh T-C, Cheng C-M, Chang F-Y, Lee S-D (2010) Neuronal correlates in the modulation of placebo analgesia in experimentally-induced esophageal pain: A 3 T-fMRI study. Pain 148:75–83 [DOI] [PubMed] [Google Scholar]
- Lui F, Colloca L, Duzzi D, Anchisi D, Benedetti F, Porro CA (2010) Neural bases of conditioned placebo analgesia. Pain 151:816–824 [DOI] [PubMed] [Google Scholar]
- Maihöfner C, Herzner B, Otto Handwerker H (2006) Secondary somatosensory cortex is important for the sensory-discriminative dimension of pain: a functional MRI study. Eur J Neurosci 23:1377–1383 [DOI] [PubMed] [Google Scholar]
- Matre D, Casey KL, Knardahl S (2006) Placebo-induced changes in spinal cord pain processing. J Neurosci 26:559–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzola L, Isnard J, Peyron R, Mauguiere F (2011) Stimulation of the human cortex and the experience of pain: Wilder Penfield’s observations revisited. Brain 135:631–640 [DOI] [PubMed] [Google Scholar]
- Meissner K, Bingel U, Colloca L, Wager TD, Watson A, Flaten MA (2011) The placebo effect: advances from different methodological approaches. J Neurosci 31:16117–16124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK (2000) The prefrontal cortex and cognitive control. Nat Rev Neurosci 1:59–65 [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001) An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202 [DOI] [PubMed] [Google Scholar]
- Moerman DE, Jonas WB (2002) Deconstructing the placebo effect and finding the meaning response. Ann Intern Med 136:471–476 [DOI] [PubMed] [Google Scholar]
- Montgomery G, Kirsch I (1996) Mechanisms of placebo pain reduction: an empirical investigation. Psychol Sci 7:174–176 [Google Scholar]
- Montgomery GH, Kirsch I (1997) Classical conditioning and the placebo effect. Pain 72:107–113 [DOI] [PubMed] [Google Scholar]
- Murray EA, O’Doherty JP, Schoenbaum G (2007) What we know and do not know about the functions of the orbitofrontal cortex after 20 years of cross-species studies. J Neurosci 27:8166–8169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M (2002) Placebo and opioid analgesia– imaging a shared neuronal network. Science 295:1737–1740 [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Andersson J, Fransson P, Ingvar M (2010) A prefrontal non-opioid mechanism in placebo analgesia. Pain 150:59–65 [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L (2000) Functional imaging of brain responses to pain. A review and meta-analysis. Clin Neurophysiol 30:263–288 [DOI] [PubMed] [Google Scholar]
- Phelps EA (2006) Emotion and cognition: insights from studies of the human amygdala. Annu Rev Psychol 57:27–53 [DOI] [PubMed] [Google Scholar]
- Price DD, Milling L, Kirsch I, Duff A (1999) An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain 83:147–156 [DOI] [PubMed] [Google Scholar]
- Price DD, Craggs J, Verne G, Perlstein W, Robinson ME (2007) Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain 127:63–72 [DOI] [PubMed] [Google Scholar]
- Rainville P (2002) Brain mechanisms of pain affect and pain modulation. Curr Opin Neurobiol 12:195–204 [DOI] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B et al. (1997) Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277:968–971 [DOI] [PubMed] [Google Scholar]
- Rainville P, Carrier B, Hofbauer RK, Bushnell MC, Duncan GH (1999) Dissociation of sensory and affective dimensions of pain using hypnotic modulation. Pain 82:159–171 [DOI] [PubMed] [Google Scholar]
- Rogan MT, Stäubli UV, LeDoux JE (1997) Fear conditioning induces associative long-term potentiation in the amygdala. Nature 390:604–607 [DOI] [PubMed] [Google Scholar]
- Rosen JB, Donley MP (2006) Animal studies of amygdala function in fear and uncertainty: relevance to human research. Biol Psychol 73:49–60 [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager T (2012) Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci 16:147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Roesch MR, Stalnaker TA, Takahashi YK (2009) A new perspective on the role of the orbitofrontal cortex in adaptive behaviour. Nat Rev Neurosci 10:885–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk C, Wang H et al. (2007) Individual differences in reward responding explain placebo-induced expectations and effects. Neuron 55:325–336 [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler C, Egnatuk C, Wang H, Koeppe R, Zubieta JK (2008) Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry 65:220–231 [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour B, O’Doherty JP, Dayan P, Koltzenburg M et al. (2004) Temporal difference models describe higher-order learning in humans. Nature 429:664–667 [DOI] [PubMed] [Google Scholar]
- Seymour B, O’Doherty JP, Koltzenburg M, Wiech K et al. (2005) Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nat Neurosci 8:1234–1240 [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ (2011) The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 12:154–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski SM, Pinsk MA, Douglas MM, Kastner S, Saalmann YB (2013) Functional and structural architecture of the human dorsal frontoparietal attention network. Proc Natl Acad Sci USA 110:15806–15811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tölle TR, Kaufmann T, Siessmeier T, Lautenbacher S, Berthele A, Munz F, Zieglgänsberger W, Willoch F, Schwaiger M, Conrad B, Bartenstein P (1999) Region-specific encoding of sensory and affective components of pain in the human brain: a positron emission tomography correlation analysis. Ann Neurol 45:40–47 [DOI] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Lindquist MA, Roy M, Choong-Wan W, Kross E (2013) An fMRI-based neurologic signature of physical pain. N Engl J Med 368:1388–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Fields H (2013) Placebo analgesia In: McMahon S, Koltzenburg M, Tracey I, and Turk DC (eds) Wall and Melzack’s Textbook of pain, pp 362–373. Philadelphia: Elsevier/ Saunders [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD (2004) Placebo-induced changes in FMRI in the anticipation and experience of pain. Science 303:1162–1167 [DOI] [PubMed] [Google Scholar]
- Wager T, Lindquist M, Kaplan L (2007a) Meta-analysis of functional neuroimaging data: current and future directions. Soc Cogn Affect Neurosci 2:150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, Zubieta J-K (2007b) Placebo effects on human μ-opioid activity during pain. Proc Natl Acad Sci USA 104:11056–11061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Lindquist MA, Nichols TE, Kober H, Snellenberg JXV (2009) Evaluating the consistency and specificity of neuroimaging data using meta-analysis. NeuroImage 45:S210–S221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Leotti LA, Rilling JK (2011) Predicting individual differences in placebo analgesia: contributions of brain activity during anticipation and pain experience. J Neurosci 31:439–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A, El-Deredy W, Iannetti G, Lloyd D, Tracey I, Vogt B, Nadeau V, Jones A (2009) Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain 145:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ (2007) The uncertainty of it all. Trends Cogn Sci 11:499–500 [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA (1998) Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neurosci 18:411–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Kagan J, Cook RG, Davis FC, Kim H, Polis S, McLaren DG, Somerville LH, McLean AA, Maxwell JS, Johnstone T (2004) Human amygdala responsivity to masked fearful eye whites. Science 306:2061. [DOI] [PubMed] [Google Scholar]
- Wiech K, C-s L, Brodersen KH, Bingel U, Ploner M, Tracey I(2010) Anterior insula integrates information about salience into perceptual decisions about pain. J Neurosci 30:16324–16331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD, Westlund KN (1997) Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol 14:2–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD (2011) Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 8:665–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Luo J (2009) The transferable placebo effect from pain to emotion: changes in behavior and EEG activity. Psychophysiology 46:626–634 [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS (2001) Regional μ opioid receptor regulation of sensory and affective dimensions of pain. Science 293:311–315 [DOI] [PubMed] [Google Scholar]
- Zubieta J-K, Bueller JA, Jackson L, Scott DJ et al. (2005) Placebo effects mediated by endogenous opioid activity on μ-opioid receptors. J Neurosci 25:7754–7762 [DOI] [PMC free article] [PubMed] [Google Scholar]