Abstract
Context
Pharmacokinetic properties of cortisone acetate (CA) and hydrocortisone (HC) differ because CA needs to be converted into cortisol to become active.
Objective
This work analyzed the metabolic consequences of switching CA to an equivalent daily dose of HC in patients with secondary adrenal insufficiency (SAI).
Design
This was a post hoc analysis from a prospective study including individuals with hypopituitarism receiving growth hormone replacement. Data were collected before and after a switch from CA to an equivalent dose of HC (switch group). Two control groups were included: patients continuing CA replacement (CA control group) and adrenal-sufficient hypopituitary patients (AS control group).
Results
The analysis included 229 patients: 105, 31, and 93 in the switch, CA control, and AS control groups, respectively. After the change from CA to HC, increases in mean body weight (1.2 kg; P < .05), waist circumference (2.9 cm; P < .001), body fat measured by dual-energy x-ray absorptiometry (1.3 kg; P < .001), and glycated hemoglobin (0.3%; P < .05) were recorded in the switch group. The increase in mean waist circumference was greater than in the AS control group (0.9 cm; P < .05). Mean body fat increased in the switch group but not in the CA control group (–0.7 kg; P < .05).
Conclusions
A switch from CA to an equivalent dose of HC was associated with a worsened metabolic profile, suggesting that HC has a more powerful metabolic action than CA based on the assumption that 20 mg HC equals 25 mg CA.
Keywords: secondary adrenal insufficiency, cortisone acetate, hydrocortisone, metabolic outcome
Approximately 75% of European patients with adrenal insufficiency (AI) receive hydrocortisone (HC) for glucocorticoid (GC) replacement [1]. In some countries, however, cortisone acetate (CA) is more commonly used. The pharmacokinetic profiles of HC and CA differ. HC is the chemical equivalent of endogenous cortisol and, thus, is a biologically active GC, whereas CA requires conversion to cortisol by the enzyme 11-β-hydroxysteroid dehydrogenase type 1 to become active. Therefore, orally administered CA has a delayed peak of serum cortisol concentration compared to HC.
The dose of GC replacement has a strong impact on metabolic outcome in patients with secondary AI (SAI), irrespective of GC type [2, 3]. However, the type of GC may also influence metabolic outcome [2]. The conventional treatment for replacement therapy in patients with AI consists of HC or CA, divided into 2 or 3 oral doses per day, as recommended by current guidelines [4]. As the standard and most widely used conversion ratio for HC:CA is 4:5, the hydrocortisone equivalent (HCeq) dose of 20 mg HC is 25 mg of CA. This conversion ratio, however, is based on data from older in vitro studies comparing the anti-inflammatory potency of GCs [5-8]. There is limited information comparing the in vivo metabolic effects of various GCs during oral administration. In a previous study [2], the use of CA was associated with more favorable glucose metabolism, with glycated hemoglobin (HbA1c) levels being lower than in patients treated with HC. Thus, HC may have a more potent metabolic effect than the assumed equivalent dose of CA.
In Sweden, CA was the conventional GC used for replacement therapy in patients with AI until 2001, when it was withdrawn from the market by the manufacturer and HC was approved by the Swedish Medical Products Agency. We were therefore able, in an ongoing prospective study [9, 10], to follow patients with hypopituitarism through a period during which most patients were switched from CA to HC. The aim of this study was to assess the consequent metabolic effects of the change in GC replacement in patients with SAI.
1. Materials and Methods
A. Design
This was a post hoc analysis on data from an ongoing, prospective, open-label trial that started October 8, 1990, of replacement therapy with recombinant human growth hormone (GH) in adults with GH deficiency [11]. Anthropometric characteristics, body composition measurements, and blood samples were collected at baseline, when GH treatment was started, and at 6, 12, 24, 36, 60, 84, 120, and 144 months following inclusion.
B. Study Population
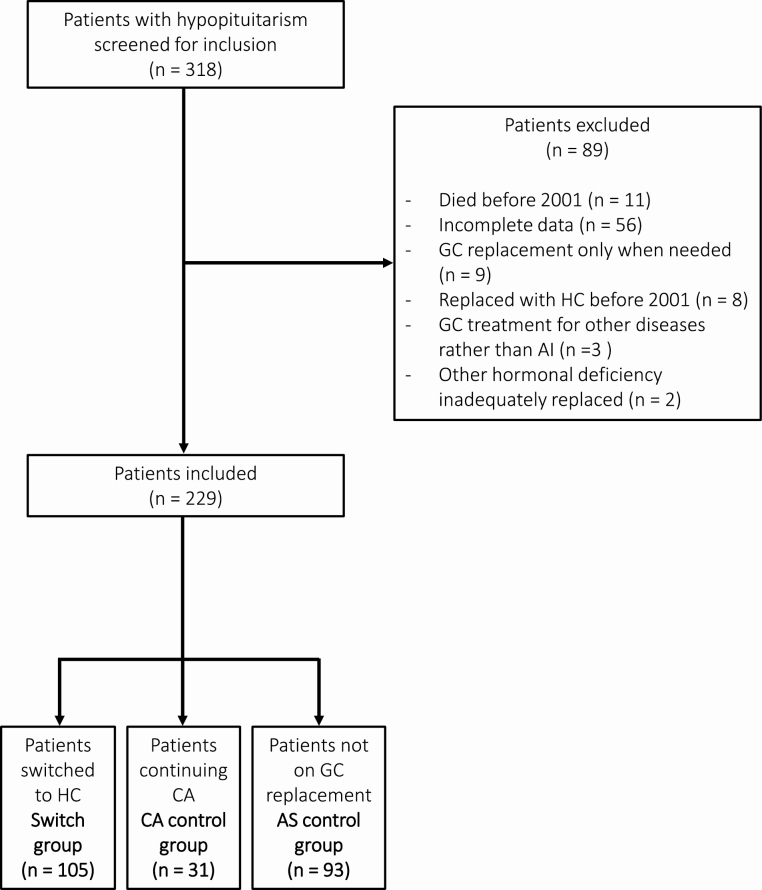
To be included in the analysis the patients had to be age 18 years or older, have a stable replacement dose with L-thyroxine, testosterone, estrogen, and GH for 3 months or more before study entry and during the rest of the study period, and have complete follow-up data. In addition, patients receiving GC replacement had to have a stable CA dose for 6 months or more before study inclusion and a stable HCeq dose of GCs during the rest of the study period. When the switch from CA to HC started (in 2001), 318 patients with hypopituitarism had entered the study (Fig. 1). Of these, 89 patients were excluded from the analysis: 56 because of incomplete data, 11 had died before the 2001, 9 had GC only when needed, 8 had already received HC as GC replacement before 2001, 3 had GC treatment for a disease other than AI, and 2 had other hormonal deficiencies that were not stable (see Fig. 1).
Figure 1.
Flow diagram of patients included in the study. AI, adrenal insufficiency; AS, adrenal sufficient; CA, cortisone acetate; HC, hydrocortisone, GC, glucocorticoid.
C. Data Collection
Data were collected for all patients from 2 visits: baseline (visit 0-32 months before the switch from CA to HC) and follow-up (visit 12-40 months following the switch). Similarly, for the control groups, data from 2 visits (baseline and follow-up) were collected, with comparable time intervals. HCeq-dose calculations were based on the standard conversion ratio of 4:5 for HC:CA [2].
D. Outcome Variables
Body height was measured to an accuracy of 0.5 cm and weight to an accuracy of one decimal point. Body mass index (BMI) was calculated as kilogram divided by meters squared (kg/m2). Waist circumference was measured between the iliac crest and the lowest level of the hip in the supine position. Resting blood pressure was measured in a sitting position.
Serum total cholesterol, high-density lipoprotein (HDL)-cholesterol, and triglycerides, fasting plasma glucose, and blood HbA1c were measured by standardized methods [10], and low-density lipoprotein cholesterol was calculated by the Friedewald formula as previously described [12].
Total body fat mass was measured by dual-energy x-ray absorptiometry (DEXA; Lunar DPX-L, Lunar Corporation; Software 1.1-8.70). Total body fat mass was also measured by bioelectrical impedance analysis (BIA) performed in the supine position on a BIA-101 device (RJL System) using a frequency of 50 kHz [13].
E. Statistical Analyses
Continuous variables are presented either as mean ± SD or median (range) and categorical variables as number (percentage; %). For comparison between groups, unpaired t test was used for normally distributed data and Mann-Whitney U test for nonnormally distributed data. For within-group comparisons, a paired t test was used for normally distributed data and a Wilcoxon signed rank tests for nonnormally distributed data. All tests were 2-tailed and P values less than .05 were considered statistically significant. The statistical analyses were performed using SPSS statistics, version 24.
F. Ethics
This study was approved by the Regional Ethical Committee of the University of Gothenburg, Sweden (permission No. 100-15). The study was conducted according to the Declaration of Helsinki.
2. Results
A. Patient Characteristics
A total of 229 patients with hypopituitarism (135 men, 94 women) were included in the study (see Fig. 1). The study population was divided into 3 groups: 1) a switch group (n = 105) that included SAI patients who changed GC replacement from CA to HC; 2) a CA control group (n = 31) that included SAI patients continuing CA therapy during the study period; and 3) an AS control group (n = 93) that included hypopituitary patients without adrenocorticotrophic hormone deficiency and thereby not requiring GC replacement (see Fig. 1). The most common causes of hypopituitarism were nonfunctioning pituitary adenoma and craniopharyngioma (Table 1). Clinical characteristics of the 3 groups are shown in Table 2. At baseline, mean (± SD) age was higher in the switch group than in the CA control group (57.1 ± 12.7 vs 50.7 ± 14.1 years, P < .05) (see Table 2). The mean daily dose of CA was 26.6 ± 9.1 mg and 21.6 ± 8.8 mg in the switch and CA control groups, respectively (P < .05 between groups). The mean GC dose remained stable in each group during the entire study period. Mean BMI at baseline was 27.6 ± 4.4 kg/m2 in the switch group, which was not different compared to the CA control (P = .8) or AS control groups (P = .3) (see Table 2). At baseline, no differences in mean body weight, waist circumference, total body fat (measured using DEXA), fasting plasma glucose, HbA1c, and diastolic blood pressure were recorded in the switch-group in comparison with both the CA and AS group (data not shown). Mean systolic blood pressure in the switch group (130.4 ± 17.0 mm Hg) was not different from in AS controls (131.3 ± 18.4 mm Hg) but higher than in CA controls (123.4 ± 15.4 mm Hg; P < .05). Mean observation time between baseline and follow-up visit was 31 ± 11 months in the switch group, 36 ± 9 months in the CA control group, and 37 ± 12 months in the AS control group (see Table 2).
Table 1.
Underlying causes of hypopituitarism in the study population
| Diagnosis | Switch group (n = 105) | CA control group(n = 31) | AS control group (n = 93) |
|---|---|---|---|
| Nonfunctioning pituitary adenoma | 44 (41.9) | 5 (16.1) | 35 (37.6) |
| Craniopharyngioma | 16 (15.2) | 6 (19.4) | 5 (5.4) |
| Idiopathic | 7 (6.7) | 2 (6.5) | 9 (9.7) |
| Cushing disease | 6 (5.7) | 4 (12.9) | 4 (4.3) |
| Prolactinoma | 6 (5.7) | 5 (16.1) | 10 (10.8) |
| Empty sella syndrome | 5 (4.8) | 0 (0.0) | 3 (3.2) |
| Acromegaly | 4 (3.8) | 0 (0.0) | 7 (7.5) |
| Other diagnosis | 17 (16.2) | 9 (29.0) | 20 (21.5) |
| Other diagnosis | (n = 17) | (n = 9) | (n = 20) |
| Congenital hypopituitarism | 2 | - | 1 |
| Cystic change | 1 | - | - |
| Dysgerminoma | 1 | - | - |
| Ependymoma | - | 1 | 1 |
| Granular cell tumor | - | - | 1 |
| Histiocytosis | - | 1 | - |
| Hypophysitis | 2 | 2 | 1 |
| Isolated idiopathic GHD | - | - | 5 |
| Leukemia | - | - | 2 |
| Medulloblastoma | - | - | 1 |
| Meningioma | 2 | 1 | 3 |
| Pituitary apoplexia | 1 | 1 | 1 |
| Rhabdomyosarcoma | - | - | 1 |
| Sarcoidosis | 1 | 1 | - |
| Septo-optic dysplasia | - | 1 | - |
| Sheehan syndrome | 2 | - | - |
| Trauma | 3 | - | 1 |
| Unknown | 2 | 1 | 2 |
Data are given as No. (%).
Abbreviations: AS, adrenal sufficient; CA, cortisone acetate; GHD, growth hormone deficiency.
Table 2.
Clinical characteristics of the study population
| Switch group (n = 105) | CA control group (n = 31) | AS control group (n = 93) | |
|---|---|---|---|
| Women, No. | 40 (38) | 16 (52) | 38 (40) |
| Men, No. | 65 (62) | 15 (48) | 56 (60) |
| Age, y | 57.1 ± 12.7 | 50.7 ± 14.1b | 51.6 ± 16.2b |
| GH replacement, No. | 105 (100%) | 31 (100%) | 93 (100%) |
| GC replacement, No. | 105 (100%) | 105 (100%) | 0 |
| Thyroid hormone replacement, No. | 84 (80%) | 24 (77%) | 35 (38%) |
| Sex steroid replacement, No. | 78 (74%) | 24 (77%) | 40 (43%) |
| GC replacement regimen | |||
| Once daily | 79 (75%) | 0 | – |
| Twice daily | 19 (18%) | 31 (100%) | – |
| Thrice daily | 7 (7%) | 0 | – |
| BMI, kg/m2 | 27.6 ± 4.4 | 27.4 ± 4.6 | 28.3 ± 5.3 |
| BSAa, sqm | 1.9 ± 0.2 | 1.9 ± 0.2 | 2 ± 0.3 |
| CA dose, mg | 26.6 ± 9.1 | 21.6 ± 8.8 | – |
| HCeq dose, mg | 21.3 ± 7.3c | 17.3 ± 7.0 | – |
| Observation time, mo | 31 ± 11 | 36 ± 9 | 37 ± 12 |
Data are given as No. (%) or mean ± SD.
Abbreviations: AS, adrenal sufficient; BMI, body mass index; BSA, body surface area; CA, cortisone acetate; GC, glucocorticoid; GH, growth hormone; HC, hydrocortisone; HCeq, hydrocortisone equivalent.
aBSA was calculated using the Dubois and DuBois formula: 0.007 184 × height (cm)0.725 × weight (kg)0.425.
b P less than .05 compared to the switch group.
c P less than .05 compared to the CA control group.
B. Body Weight and Body Composition
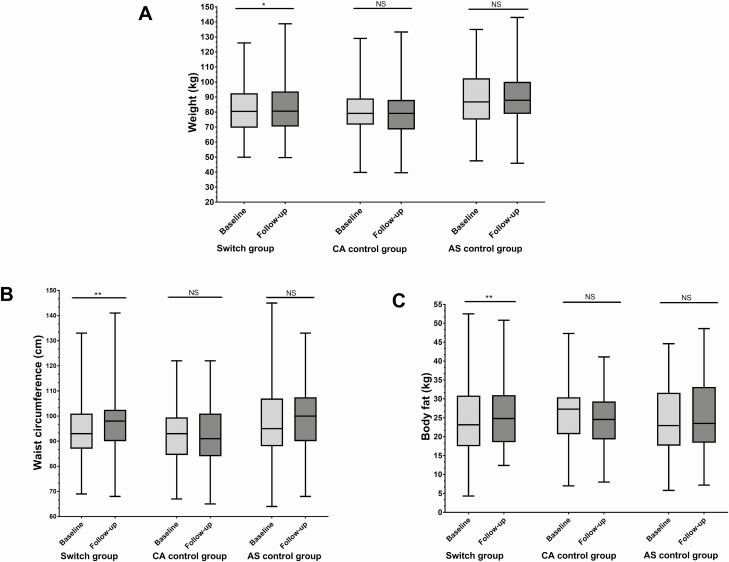
Body weight increased by 1.2 kg (95% CI, 0.3 to 2.0 kg) in the switch group (P < .05), whereas no significant changes were recorded in the control groups (Table 3; Fig. 2A). Waist circumference increased by 2.9 cm (95% CI, 1.8 to 4.1 cm) after the switch from CA to HC (P < .001), whereas waist circumference did not change in the control groups (see Table 3, Fig. 2B).
Table 3.
Body weight and body composition in the study population at baseline and follow-up
| Variable | Baseline | Follow-up | Within-group differences | P | Difference between switch and control groups |
|---|---|---|---|---|---|
| Weight, kg | |||||
| Switch group (n = 105) | 81.3 ± 16.1 | 82.4 ± 16.9 | 1.2 (0.3 to 2.0) | < .05 | |
| AS control group (n = 93) | 85.4 ± 20.0 | 85.5 ± 21.3 | 0.1 (–1.1 to 1.4) | .9 | –1.1, P = .2 |
| CA control group (n = 31) | 80.2 ± 16.9 | 79.7 ± 18.7 | –0.5 (–2.2 to 1.3) | .6 | –1.7, P = .07 |
| Waist, cm | |||||
| Switch group (n = 105) | 94.2 ± 10.8 | 97.1 ± 11.8 | 2.9 (1.8 to 4.1) | < .001 | |
| AS control group (n = 93) | 95.3 ± 12.4 | 96.2 ± 14.1 | 0.9 (–0.6 to 2.4) | .2 | –2.03, P < .05 |
| CA control group (n = 31) | 92.2 ± 11.7 | 93.0 ± 11.9 | 0.8 (–1.1 to 2.7) | .4 | –2.1, P = .08 |
| BIA total body fat mass, kg | |||||
| Switch group (n = 105) | 20.4 ± 7.8 | 22.5 ± 9.3 | 2.1 (1.1 to 3.2) | < .001 | |
| AS control group (n = 93) | 22.4 ± 9.5 | 24.1 ± 11.6 | 1.7 (0.4 to 3.0) | < .05 | –0.4, P = .6 |
| CA control group (n = 31) | 23.2 ± 15 | 22.5 ± 7.2 | –0.7 (–5.5 to –4.1) | .8 | –2.8, P = .08 |
| DEXA total body fat mass, kg | |||||
| Switch group (n = 105) | 24.6 ± 8.9 | 25.9 ± 9.1 | 1.3 (0.6 to 2.0) | < .001 | |
| AS control group (n = 93) | 26.5 ± 9.9 | 26.9 ± 9.8 | 0.4 (–0.6 to 1.3) | .4 | –1.1, P = .08 |
| CA control group (n = 31) | 25.2 ± 7.2 | 23.5 ± 7.7 | –0.7 (–1.9 to 0.5) | .2 | –2.0, P < .05 |
Data are given as mean ± SD or mean (95% CI).
Abbreviations: AS, adrenal sufficient; BIA, bioelectrical impedance analysis; CA, cortisone acetate; DEXA, dual-energy x-ray absorptiometry.
Figure 2.
Box (median and interquartile range) and whisker (range) plots of A, weight; B, waist circumference; and C, total body fat mass measured by dual-energy x-ray absorptiometry in the switch and control groups at baseline and follow-up. *P < .05. **P < .001. AS, adrenal sufficient; CA, cortisone acetate; NS, not significant.
Total body fat mass increased in the switch group by 1.3 kg (95% CI, 0.6 to 2.0 kg) when measured using DEXA (P < .001) (Fig. 2B) and 2.1 kg (95% CI, 1.1 to 3.2 kg) using BIA (P < .001), whereas there were no changes in the CA control group with either method (see Table 3).
C. Lipid and Glucose Metabolism and Blood Pressure Profile
Total cholesterol and triglycerides concentrations were not affected by the switch to HC (Table 4). However, an increase of mean HDL cholesterol by 0.2 mmol/L (95% CI, 0.1 to 0.2; P < .001 mmol/L) was recorded after the switch. A similar pattern was found in the AS control group, with higher mean (± SD) HDL cholesterol at follow-up compared to baseline (1.3 ± 0.4 vs 1.4 ± 0.5 mmol/L; P < .001) (see Table 4). The switch to HC led to increased diastolic blood pressure, but did not affect systolic blood pressure (see Table 4). In the control groups, both systolic and diastolic blood pressure remained stable throughout the study (see Table 4).
Table 4.
Metabolic characteristics in the study population at baseline and follow-up
| Variable | Baseline | Follow-up | Within-group differences | P | Difference between switch and control groups |
|---|---|---|---|---|---|
| Systolic BP, mm Hg | |||||
| Switch group (n = 105) | 130.4 ± 17.0 | 132.9 ± 20.2 | 2.5 (–1.3 to 6.3) | .2 | |
| AS control group (n = 93) | 131.3 ± 18.4 | 131.9 ± 18.7 | 0.5 (–3.0 to 4.0) | .8 | –2.0, P = .4 |
| CA control group (n = 31) | 123.4 ± 15.4 | 125.9 ± 17.5 | 2.4 (–2.4 to 7.3) | .3 | 0.0, P = 1.0 |
| Diastolic BP, mm Hg | |||||
| Switch group (n = 105) | 76.8 ± 8.5 | 79.3 ± 10.2 | 2.6 (0.2 to 4.9) | < .05 | |
| AS control group (n = 93) | 77.8 ± 9.7 | 78.6 ± 9.8 | 0.8 (–1.4 to 3.0) | .5 | –1.8, P = .5 |
| CA control group (n = 31) | 74.3 ± 8.6 | 73.6 ± 11.3 | –0.7 (–4.7 to 3.3) | .7 | –3.3, P = .2 |
| Serum cholesterol, mmol/L | |||||
| Switch group (n = 105) | 5.1 ± 1.0 | 5.2 ± 1.2 | 0.2 (0.0 to 0.4) | .1 | |
| AS control group (n = 93) | 5.3 ± 1.0 | 5.4 ± 1.2 | 0.1 (–1.7 to 0.3) | .6 | –0.1, P = .6 |
| CA control group (n = 31) | 5.4 ± 1.0 | 5.2 ± 1.2 | –0.2 (–0.4 to 0.1) | .1 | –0.4, P = .09 |
| Serum triglycerides, mmol/L | |||||
| Switch group (n = 105) | 1.5 (5.3–0.7) | 1.6 (0.3–9) | –0.1 (–1.7 to 7.2) | .7 | |
| AS control group (n = 93) | 1.5 (0.5–4.3) | 1.5 (0.1–6.7) | –0.1 (–1.8 to 4.2) | .3 | P = .6 |
| CA control group (n = 31) | 1.4 (0.6–6.7) | 1.6 (0.6–6.3) | –0.1 (–1.8 to 1.7) | .5 | P = .6 |
| Serum HDL-cholesterol, mmol/L | |||||
| Switch group (n = 105) | 1.3 ± 0.4 | 1.4 ± 0.5 | 0.2 (0.1 to 0.2) | < .001 | |
| AS control group (n = 93) | 1.3 ± 0.4 | 1.4 ± 0.5 | 0.1 (0.1 to 0.2) | < .001 | –0.1, P = .1 |
| CA control group (n = 31) | 1.2 ± 0.4 | 1.4 ± 0.5 | 0.1 (0.0 to 0.2) | .09 | –0.1, P = .3 |
| Fasting plasma glucose, mmol/L | |||||
| Switch group (n = 105) | 5.2 ± 1.5 | 5.4 ± 2.4 | 0.2 (–0.1 to 0.6) | .2 | |
| AS control group (n = 93) | 5.5 ± 1.5 | 5.4 ± 1.2 | –0.2 (–0.4 to 0.0) | .1 | –0.4 P = .07 |
| CA control group (n = 31) | 4.7 ± 0.6 | 4.7 ± 0.6 | –0.1 (–0.3 to 0.1) | .4 | –0.3 P = .4 |
| Blood HbA1c, % | |||||
| Switch group (n = 105) | 4.5 ± 0.7 | 4.8 ± 1.5 | 0.3 (0.0 to 0.5) | < .05 | |
| AS control group (n = 93) | 4.7 ± 0.9 | 4.7 ± 0.8 | 0.0 (–0.1 to 0.1) | .6 | –0.3 P < .05 |
| CA control group (n = 31) | 4.3 ± 0.4 | 4.2 ± 0.4 | –0.1 (–0.2 to 0.0) | .07 | –0.4, P = .07 |
Data are presented as mean ± SD or mean (95% CI), and for triglycerides as median (range).
Abbreviations: AS, adrenal sufficient; BP, blood pressure; CA, cortisone acetate; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein.
Mean (± SD) HbA1c increased following the switch to HC (4.5 ± 0.7 vs 4.8 ± 1.5%; P < .05), but did not change significantly in the control groups (see Table 4). In the CA control group, a trend toward a reduction of mean HbA1c was recorded (4.3 ± 0.4 vs 4.2 ± 0.4%; P = .07). There were no significant differences in plasma fasting glucose between the switch group and the control groups (see Table 4).
When body composition and metabolic outcome were analyzed in men and women separately, no sex differences were observed. However, body fat, measured using body fat percentage, increased in men but not in women (data not shown).
3. Discussion
In this study we evaluated whether the type of GC for replacement therapy has an impact on metabolic outcome in patients with SAI. We found that a switch from CA to an assumed equivalent dose of HC was associated with an increase in body weight, total fat mass, waist circumference, and HbA1c. These data suggest that the metabolic potency of HC in relation to CA is greater than previously thought when using the conversion ratio of 4:5 for HC:CA.
It is well recognized that overexposure to any type of GC leads to an adverse metabolic profile. In patients with SAI treated with different GCs, HCeq doses of more than 20 mg/day have been associated with larger waist circumference as well as higher BMI, total cholesterol, low-density lipoprotein cholesterol, and triglycerides [2]. Also, higher GC doses have been associated with an overall increased mortality in patients with SAI [14, 15]. In a randomized, prospective study [3], a higher HC dose was associated with higher BMI and blood pressure. On the other hand, a study that investigated the effects of a dose reduction from 30 to 15 mg HC/day in SAI was unable to show any beneficial effects on blood pressure [16].
There is sparse evidence on the metabolic properties of the different types of GCs in patients with AI. Long-acting GCs, such as prednisolone and dexamethasone, are more efficient in achieving successful adrenocorticotrophic hormone suppression in patients with congenital adrenal hyperplasia [17, 18]. However, long-acting GCs are more prone to cause adverse metabolic outcomes [2, 19]. Therefore, short-acting GCs, such as HC and CA, are usually recommended as replacement therapy in patients with AI [4, 20]. HC is often considered the most appropriate GC for replacement because it is chemically equivalent to endogenous cortisol. However, whether the slower and delayed cortisol exposure during CA administration is more favorable from a metabolic perspective is unknown since a formal comparison between HC and CA has not been performed.
In our prospective study, weight gain occurred after the switch from CA to HC. In addition, mean waist circumference increased by 3 cm in the switch group, whereas no significant change was found in patients continuing with CA and in individuals with sufficient GC production. These findings support the hypothesis that CA might be a more favorable choice than HC from a metabolic perspective. Filipsson et al [2] have previously analyzed the impact of glucocorticoid replacement regimens on metabolic outcome in 2424 hypopituitary patients, showing that patients treated with CA had a more favorable glucose metabolism, with HbA1c levels being lower than in patients treated with HC. A possible explanation could be that CA administration has a delayed cortisol peak and more prolonged exposure than HC [21, 22]. Another more likely explanation is that the assumption that 25 mg CA is equivalent to 20 mg HC is incorrect and that the equivalent dose of HC is actually lower. Older studies that have assessed serum cortisol profile after administration of oral CA and HC in the same individuals have shown that mean serum cortisol concentrations after CA are significantly lower than those obtained after HC administration [18, 21, 22]. Interestingly, in one study [18] analyzing serum cortisol profiles in patients with Addison disease reported that the equivalent dose of HC could be up to 40% lower than the dose of CA and not 20% as conventionally thought to achieve the same cortisol exposure.
To our knowledge, this is the first study to analyze total body fat mass in patients with SAI switching from CA to an equivalent dose of HC. Total body fat mass increased after the switch to HC, whereas it remained unchanged in patients continuing CA. At baseline, patients in the switch group were older than those in CA control group, which could possibly affect the comparison between these groups. However, at baseline, no differences in total body fat mass were found between these groups. The AS control group also showed an increase in total body fat mass measured using BIA, which could suggest that the increase in body fat is not an effect of switching the GC replacement regimen. Two commonly used methods for measurements of total body fat mass, DEXA and BIA, were used in this study. The 2 methods differ in their measurement of total body fat mass, as shown by the discrepancies found in this study (see Table 3); however, the 2 methods showed more consistent results when measuring changes during intervention, as demonstrated in a previous study of GH replacement therapy [13].
GCs have a major impact on the cardiovascular system [23, 24]. Hypovolemia and hypotension are common clinical signs in patients with AI [4, 25]. Conversely, overexposure to GCs is associated with high systolic and diastolic blood pressure [3]. In the present study, diastolic blood pressure increased at follow-up in the switch group but not in the control groups. Systolic blood pressure remained unchanged in all groups. A higher potency of HC, compared to CA, could be a plausible explanation for the increased diastolic blood pressure.
This study showed an adverse effect of HC on glucose metabolism, with HbA1c increasing in the switch group but not in the 2 control groups. Similar findings have been reported in a previous study in which higher HbA1c was observed in HC-treated patients compared to CA-treated patients [2]. The switch from CA to HC did not affect total cholesterol and triglycerides, although HDL cholesterol increased after the switch. This is in line with a previous study showing that increased cortisol exposure increases the activity of the cholesteryl ester transfer protein pathway, which in turn results in increased HDL cholesterol concentration and size [26]. The increase in HDL cholesterol is therefore another indirect indication that the switch from CA to HC, supposedly at equivalent doses, results in higher cortisol exposure.
The major strength of this study is the large group of patients with SAI and the inclusion of 2 control groups. There are, however, some limitations to be acknowledged. This was a post hoc analysis with data obtained from a prospective, open-label trial of replacement therapy with recombinant human GH in adults with GH deficiency. The control groups were chosen to have a comparison of metabolic characteristics over time but were not matched to the switch group. Moreover, we could not control for other factors influencing metabolism such as physical activity, food intake, and medications other than hormone replacement. The reasons why patients in the CA group did not change replacement but continued with CA were not available. Another source of error could be the different durations of observation in the different patient groups. Also, patients in the switch group had higher HCeq doses of CA compared to the CA control group at baseline, and this may have affected the comparison between these groups. Conversely, within-group comparisons were not affected by imbalances between groups. Indeed, within-group comparisons showed worse metabolic outcome in the switch group at 6 months’ follow-up. On the contrary, no differences were recorded in the CA and AS control groups between baseline and follow-up. This may suggest that the switch from CA to HC per se affects metabolic outcomes. Further studies, however, are needed to confirm this finding.
In conclusion, our findings suggest that the type of GC, and not only the dose, has affects the metabolic profile in patients with SAI. In this study, the switch from CA to an assumed equivalent dose of HC led to adverse metabolic effects, suggesting that the widely used conversion ratio of 4:5 for HC to CA may be imprecise and underestimate the metabolic potency of HC. We can also speculate that, in patients with SAI, replacement treatment with CA may have more favorable pharmacokinetic properties in terms of metabolic outcomes.
Acknowledgments
We thank Annika Reibring and Anna Cederberg-Olsson for their contribution of collecting data. We are indebted to all the research nurses at the Department of Endocrinology for their skillful support. We also would like to thank Peter Todd (Tajut Ltd, Kaiapoi, New Zealand) for third-party editorial assistance in drafting this manuscript, for which he received financial compensation from ALF Funding.
Financial Support: This work was supported by The Swedish Research Council (Project No. 2015-02561 and No. 2019-01112) and the Swedish federal government under the LUA/ALF agreement (Project No. ALFGBG-719531).
Glossary
Abbreviations
- AI
adrenal insufficiency
- AS
adrenal sufficient
- BIA
bioelectrical impedance analysis
- BMI
body mass index
- DEXA
dual-energy x-ray absorptiometry
- CA
cortisone acetate
- GC
glucocorticoid
- GH
growth hormone
- HbA1c
glycated hemoglobin
- HC
hydrocortisone
- HCeq
hydrocortisone equivalent
- HDL
high-density lipoprotein
- SAI
secondary adrenal insufficiency
Additional Information
Disclosure Summary: E.E. has nothing to disclose. D.E. has received lecture fees from Ipsen. O.R. has received lecture fees from Novo Nordisk, Ipsen, Sandoz, and Pfizer; an unrestricted research grant from HRA-Pharma; and consultancy fees from Novartis, Alnylam, and HRA-Pharma. J.I. has received lecture fees from Pfizer and Merck Serono. G.J. has served as a consultant for Novo Nordisk, Shire, and Astra Zeneca; and has received lecture fees from Eli Lilly, Ipsen, Novartis, Novo Nordisk, Merck Serono, Otsuka, and Pfizer.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Forss M, Batcheller G, Skrtic S, Johannsson G. Current practice of glucocorticoid replacement therapy and patient-perceived health outcomes in adrenal insufficiency—a worldwide patient survey. BMC Endocr Disord. 2012;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Filipsson H, Monson JP, Koltowska-Häggström M, Mattsson A, Johannsson G. The impact of glucocorticoid replacement regimens on metabolic outcome and comorbidity in hypopituitary patients. J Clin Endocrinol Metab. 2006;91(10):3954-3961. [DOI] [PubMed] [Google Scholar]
- 3. Werumeus Buning J, van Faassen M, Brummelman P, et al. Effects of hydrocortisone on the regulation of blood pressure: results from a randomized controlled trial. J Clin Endocrinol Metab. 2016;101(10):3691-3699. [DOI] [PubMed] [Google Scholar]
- 4. Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boland EW. Antirheumatic effects of hydrocortisone (free alcohol), hydrocortisone acetate, and cortisone (free alcohol) as compared with cortisone acetate; results from oral administration in patients with rheumatoid arthritis. Br Med J. 1952;1(4758):559-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boland EW. 16a-Methyl corticosteroids; a new series of anti-inflammatory compounds; clinical appraisal of their antirheumatic potencies. Calif Med. 1958;88(6):417-422. [PMC free article] [PubMed] [Google Scholar]
- 7. Liddle GW. Clinical pharmacology of the anti-inflammatory steroids. Clin Pharmacol Ther. 1961;2:615-635. [DOI] [PubMed] [Google Scholar]
- 8. Bunim JJ, Black RL, Bollet AJ, Pechet MM. Metabolic effects of metacortandralone and metacortandracin. Ann N Y Acad Sci. 1955;61(2):358-368. [DOI] [PubMed] [Google Scholar]
- 9. Svensson J, Sunnerhagen KS, Johannsson G. Five years of growth hormone replacement therapy in adults: age- and gender-related changes in isometric and isokinetic muscle strength. J Clin Endocrinol Metab. 2003;88(5):2061-2069. [DOI] [PubMed] [Google Scholar]
- 10. Götherström G, Svensson J, Koranyi J, et al. A prospective study of 5 years of GH replacement therapy in GH-deficient adults: sustained effects on body composition, bone mass, and metabolic indices. J Clin Endocrinol Metab. 2001;86(10):4657-4665. [DOI] [PubMed] [Google Scholar]
- 11. Johannsson G, Rosén T, Bosaeus I, Sjöström L, Bengtsson BA. Two years of growth hormone (GH) treatment increases bone mineral content and density in hypopituitary patients with adult-onset GH deficiency. J Clin Endocrinol Metab. 1996;81(8):2865-2873. [DOI] [PubMed] [Google Scholar]
- 12. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499-502. [PubMed] [Google Scholar]
- 13. Koranyi J, Bosaeus I, Alpsten M, Bengtsson BA, Johannsson G. Body composition during GH replacement in adults—methodological variations with respect to gender. Eur J Endocrinol. 2006;154(4):545-553. [DOI] [PubMed] [Google Scholar]
- 14. Zueger T, Kirchner P, Herren C, et al. Glucocorticoid replacement and mortality in patients with nonfunctioning pituitary adenoma. J Clin Endocrinol Metab. 2012;97(10):E1938-E1942. [DOI] [PubMed] [Google Scholar]
- 15. Hammarstrand C, Ragnarsson O, Hallén T, et al. Higher glucocorticoid replacement doses are associated with increased mortality in patients with pituitary adenoma. Eur J Endocrinol. 2017;177(3):251-256. [DOI] [PubMed] [Google Scholar]
- 16. Dunne FP, Elliot P, Gammage MD, et al. Cardiovascular function and glucocorticoid replacement in patients with hypopituitarism. Clin Endocrinol (Oxf). 1995;43(5):623-629. [DOI] [PubMed] [Google Scholar]
- 17. Caldato MCF, Fernandes VT, Kater CE. One-year clinical evaluation of single morning dose prednisolone therapy for 21-hydroxylase deficiency. Arq Bras Endocrinol Metabol. 2004;48(5):705-712. [DOI] [PubMed] [Google Scholar]
- 18. Khalid BA, Burke CW, Hurley DM, Funder JW, Stockigt JR. Steroid replacement in Addison’s disease and in subjects adrenalectomized for Cushing’s disease: comparison of various glucocorticoids. J Clin Endocrinol Metab. 1982;55(3): 551-559. [DOI] [PubMed] [Google Scholar]
- 19. Suliman AM, Freaney R, Smith TP, McBrinn Y, Murray B, McKenna TJ. The impact of different glucocorticoid replacement schedules on bone turnover and insulin sensitivity in patients with adrenal insufficiency. Clin Endocrinol (Oxf). 2003;59(3):380-387. [DOI] [PubMed] [Google Scholar]
- 20. Fleseriu M, Hashim IA, Karavitaki N, et al. Hormonal replacement in hypopituitarism in adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(11):3888-3921. [DOI] [PubMed] [Google Scholar]
- 21. Heazelwood VJ, Galligan JP, Cannell GR, Bochner F, Mortimer RH. Plasma cortisol delivery from oral cortisol and cortisone acetate: relative bioavailability. Br J Clin Pharmacol. 1984;17(1):55-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jenkins JS, Sampson PA. Conversion of cortisone to cortisol and prednisone to prednisolone. Br Med J. 1967;2(5546):205-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walker BR. Glucocorticoids and cardiovascular disease. Eur J Endocrinol. 2007;157(5):545-559. [DOI] [PubMed] [Google Scholar]
- 24. Johannsson G, Ragnarsson O. Cardiovascular and metabolic impact of glucocorticoid replacement therapy. Front Horm Res. 2014;43:33-44. [DOI] [PubMed] [Google Scholar]
- 25. Esposito D, Bobbio E, Di Fraia R, et al. Patients with adrenal insufficiency have cardiovascular features associated with hypovolemia. Endocrine. 2020;70(2):412-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Werumeus Buning J, Dimova LG, Perton FG, Tietge UJF, van Beek AP, Dullaart RPF. Downregulation of cholesteryl ester transfer protein by glucocorticoids: a randomised study on HDL. Eur J Clin Invest. 2017;47(7):494-503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.