Abstract
Background
The 11-item Facial Line Outcomes (FLO-11) questionnaire is content validated for measuring the negative psychological impacts of crow’s feet lines (CFL).
Objectives
The aims of this study were to determine psychological impacts of forehead lines (FHL) alone and upper facial lines (UFL: FHL + CFL + glabellar lines [GL]) and to assess adequacy of FLO-11 to measure these impacts.
Methods
Participants aged at least 18 years participated in concept elicitation and cognitive interviews to identify and define psychological impacts of UFL. They completed the FLO-11 questionnaire to assess its ability to measure psychological impacts of facial lines and its comprehensiveness in doing so.
Results
Forty interviews were completed by 29 participants. Twenty participants each provided interviews for FHL and for UFL. The most commonly reported psychological impacts for FHL and UFL, respectively, were feeling unattractive (85%, 80%), looking less attractive than desired (85%, 70%), feeling bothered (80%, 70%), feeling good/bad about appearance (80%, 70%), looking older than actual age (75%, 65%), and feeling stressed (70%, 70%). For FHL, 70% of participants also reported looking older than desired as a psychological impact. More than 50% of participants agreed that all 11 FLO-11 items measured a psychological impact for FHL. More than 50% reported that 9 of 11 items measured a psychological impact for UFL. The majority of participants (FHL, 65%; UFL, 60%) reported that the FLO-11 questionnaire is comprehensive in measuring psychological impacts of facial lines.
Conclusions
FHL and UFL have psychological impacts on patients, and FLO-11 is a content valid, comprehensive instrument for measuring them.
The functional role of human facial appearance and expression is complex and the subject of extensive research and review.1-4 As documented in social, psychological, and biological research, facial expression and appearance are fundamental to interpersonal communications. The appearance of the face is also a salient clue to perceived age and attractiveness. With aging, upper facial lines (UFL) appear and develop progressively.5,6 These lines include transverse forehead lines (FHL), lateral canthal lines, also called crow’s feet lines (CFL), and glabellar lines (GL). UFL, which result in part from repeated contracture of underlying musculature, are among the most visible signs of aging.6,7
Initially, UFL are apparent primarily during muscle contraction (dynamic lines). Over time, however, UFL also become apparent at rest (static lines).6,7 The presence of these static, or permanent, facial lines may result in substantial discord between an individual’s true emotions and their interpretation by others. Indeed, evidence suggests that increasing facial age diminishes the accuracy with which emotions are interpreted by an observer.8-10 Research conducted across a number of disciplines also has documented that the appearance of the face and signs of aging can substantially affect an individual’s mood, overall psychological well-being, feelings about self, and interpersonal communications.7,11-13 For example, erroneous signaling of emotions through facial expressions can result in anxiety as well as depression.12,13 For some individuals, the negative impact of their facial lines will drive them to seek treatment, frequently with minimally invasive procedures. Treatments with neuromodulator injections continue to be the most popular of these procedures.14,15
Historically, the evaluation of treatment effects in aesthetic medicine was based primarily on clinician-rated efficacy end points, such as the Facial Wrinkle Scale with photonumeric guide (FWS), sometimes supplemented with patient global assessments of improvement and satisfaction.16-21 As the field of aesthetic medicine has evolved, patient-reported outcomes (PROs), such as rating of severity using the FWS, identification of psychosocial impacts, perspective on treatment expectations, and evaluation of treatment satisfaction, are increasingly important. Incorporating content-validated PRO instruments into clinical trials may capture the benefits of treatment from the patients’ perspective and may provide support for product-labeling claims on patient-relevant outcomes.22 Recently, for instance, the prescribing information for onabotulinumtoxinA (Botox® Cosmetic; Allergan plc, Dublin, Ireland) was updated to include data on the Facial Line Satisfaction Questionnaire, a content-validated PRO instrument developed to evaluate treatment satisfaction in adults with FHL.23,24
Several aesthetic PRO instruments, including the Facial Line Outcomes (FLO) questionnaire, have been the subject of a number of literature reviews and studies.25–27 For example, different versions of the FLO questionnaire (eg, FLO-7 and FLO-11) in concert with other efficacy outcome measures have been used in a number of clinical research studies spanning more than a decade to assess PROs after treatment with onabotulinumtoxinA.27-36 The results of these studies consistently demonstrated that patients perceived onabotulinumtoxinA treatment to be beneficial for treating various areas of the upper face, alone or in combination, based on the various concepts measured by the FLO questionnaire.
The FLO instrument was created using an iterative development process that began with interviews of individuals who had received or were contemplating aesthetic treatment of lines of their upper face; it then underwent psychometric evaluation and content validation before the US Food and Drug Administration (FDA) issued its draft guidance on PRO instruments in 2009.22,37 According to the FDA Guidance for Industry on Patient-Reported Outcome Measures, a key feature of a PRO instrument is that it is based directly on information provided by the patient that is not subject to interpretation by other individuals, including clinicians.22 Evidence for an instrument’s content validity should be derived from subjects whose characteristics reflect relevant features of the target patient population.22 In accordance with this guidance, additional content validation studies on the 11-item Facial Line Outcomes (FLO-11) questionnaire were conducted, demonstrating it to be an appropriate and content-valid instrument for evaluating the impact of UFL as well as the psychological impacts of CFL from the patients’ perspective.22,38
This current FLO questionnaire comprises 11 concepts (Table 1) that may be used to assess individual or multiple areas of the upper face.38 As with the previous content validity research, the present study was designed in accordance with FDA guidance on PRO instruments.22,38 The primary objectives were to evaluate the psychological impacts of FHL alone and provide additional information on the impacts of FHL, CFL, and GL combined (UFL), and to assess whether the FLO-11 questionnaire is a content-valid instrument to measure these psychological impacts.
Table 1.
FLO-11 Questionnaire Concepts
| Item | Concept |
|---|---|
| 1 | Bothered by facial lines |
| 2 | Looking older than they want to look |
| 3 | Feeling unattractive |
| 4 | Looking older than their actual age |
| 5 | Looking less attractive than they want to look |
| 6 | Looking not well rested |
| 7 | Skin not looking smooth |
| 8 | Looking tired |
| 9 | Looking stressed |
| 10 | Looking angry |
| 11 | Feeling good about their facial appearance |
FLO-11, Facial Line Outcomes Questionnaire.
METHODS
Design
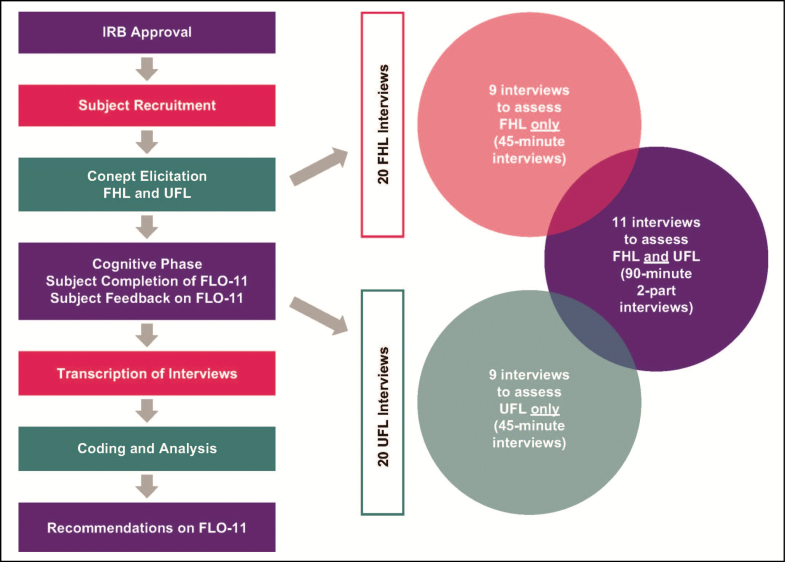
This qualitative research study (Figure 1), conducted between March 2013 and November 2013, comprised a concept elicitation phase and a cognitive interview phase, in which participants completed the FLO-11 questionnaire and participated in debriefing interviews with trained interviewers from the consulting firm Adelphi Values (Boston, MA). The qualitative interview phase was followed by interview transcription, data coding, and data analysis. All identifying information on participants was removed from transcripts, which were shared with the study sponsor; similarly, the identification of the study sponsor was not shared with the participants.
Figure 1.
Study design and interview number and distribution for forehead lines and upper facial lines. FHL, forehead lines; UFL, upper facial lines.
Participants
After Institutional Review Board approval was obtained from the Copernicus Group, participants were recruited directly through 2 US clinical study sites (Irvine, CA, and Chapel Hill, NC) identified by the study sponsor. Clinicians from these 2 sites identified potentially eligible participants from existing databases using patient medical records, guided by all eligibility criteria. Participants provided written informed consent, were told that the study was being conducted by Adelphi Values on behalf of an anonymous sponsor, and were compensated for their participation.
Inclusion Criteria
Participant inclusion criteria were: aged at least 18 years, with moderate or severe FHL or moderate or severe UFL (CFL + FHL + GL), all at maximum contraction as measured by the validated, investigator-rated FWS (0 = none, 1 = mild, 2 = moderate, and 3 = severe). All participants were required to be fluent in English and willing to participate in face-to-face 45-minute or 90-minute interviews.
Key Exclusion Criteria
Participants with severe resting UFL on the FWS were excluded. Participants were also excluded if they had received nonablative laser or light treatment, microdermabrasion, or superficial peels within 3 months of the enrollment day; any facial cosmetic procedure with medium-depth to deep facial chemical peels; midfacial or periorbital laser skin resurfacing or permanent makeup within 6 months of the enrollment day and/or midfacial, temple, forehead, glabellar, or periorbital treatment with nonpermanent soft tissue fillers, or treatment with oral retinoids, within 12 months of enrollment. Concurrent treatment or treatment within 6 months of enrollment with cosmetic botulinum toxin of any serotype was prohibited as was prior periorbital surgery, facial lift, brow lift, or any related surgical procedure. Participants could not have received midfacial or periorbital treatment with permanent soft tissue fillers, implantation of a synthetic material, such as polytetrafluorethylene, nor have undergone autologous fat transplantation. Participants were excluded if they had marked facial asymmetry, dermatochalasis, deep dermal scarring, brow or eyelid ptosis, infection or skin disorders at the relevant sites, a history of facial nerve palsy, excessively thick facial skin, or UFL that could not be lessened substantially by physically spreading them apart.
Conduct of the Study
All interviews were conducted by trained personnel using a semi-structured interview guide. Participants participated in either a 45-minute interview on just their FHL, a 45-minute interview on their 3 types of UFL, or a 90-minute interview that addressed FHL only during the first half and UFL during the second half. Interviews were recorded and/or videotaped, with each participant providing prior written informed consent.
Concept Elicitation Phase
During concept elicitation, interviews aimed to obtain information on the psychological impacts of FHL only, as well as impacts of the 3 areas of the upper face (CFL, FHL, and GL) combined. Concept elicitation interviews used both open-ended questions and targeted probes. Open-ended questions were used first with the intent of gathering spontaneously reported information on terminology and on the psychological impact of FHL and UFL. To facilitate elicitation of terminology, participants were shown a picture of a face and asked to describe the relevant anatomical areas, followed by the the terms they would use to describe wrinkles in these areas. After participants were given time to respond to open-ended questions, targeted probes could be used to obtain more information. For example, as an open-ended question, participants could be asked, “What do you call this part of your face?” [as the interviewer designates the forehead on the drawing of the face]. This could be followed by the open-ended question, “If there were wrinkles on this part of your face [as the interviewer points to the forehead], what would you call them?” To probe about terminology describing wrinkles in the forehead area, the interviewer could ask, “Have you heard of the term forehead lines?” Following the terminology elicitation portion of the interview, participants were questioned about how they felt about their various facial lines, to obtain information on psychological impacts.
FLO-11 Cognitive Debriefing Phase
For the cognitive interview phase, participants completed the FLO-11 questionnaire for the facial lines that were discussed during the concept elicitation phase. Each of the FLO-11 items is answered on an 11-point scale indicating the extent to which the individual “now” agrees with the statement. Item scores ranged from 0 (not at all) to 10 (very much).
Next, interviewers asked for feedback on the FLO-11 questionnaire, including whether it asks questions about the psychological impacts of the participant’s facial lines, whether there were any questions about the psychological impacts of facial lines that they felt were not asked, and whether the FLO-11 questionnaire comprehensively measures the psychological impacts of facial lines. At the completion of the interview, participants filled out demographic information forms.
Analysis
Researchers analyzed qualitative data collected during the interviews using a combination of grounded theory methods of data analysis involving constant comparison (ie, simultaneous and iterative data collection and analysis) and traditional content analysis, in which responses were tabulated based on questions in the semi-structured interview guide.39 In these methods of data collection and analysis, the meaning of a concept was determined through the words of participants from the ground up rather than on the basis of an a priori theory.40 Qualitative data were analyzed using ATLAS.ti version 7.0 (Atlas.ti GmbH, Berlin), a software package designed specifically for the analysis of qualitative data.41
Researchers developed a coding scheme to be applied to all transcripts. The preliminary coding scheme was based on the FLO-11 and the semi-structured interview guide. It was modified as coders analyzed the transcripts and added or modified codes. Independent coders read each transcript and coded relevant text regarding the FLO-11’s ability to measure psychological impacts. The data were aggregated around each item in the FLO-11 questionnaire. Any issues encountered in the FLO-11, as well as recommendations to address the issues, were documented.
RESULTS
Participants
A total of 29 participants who met all eligibility criteria participated in individual face-to-face interviews. This resulted in 9 FHL-only interviews (45 minutes), 9 UFL interviews (45 minutes), and 11 interviews that assessed both FHL alone and UFL (90 minutes) (Figure 1). Thus, a total of 20 FHL interviews and 20 UFL interviews provided data for analysis. Participants ranged in age from 24 to 72 years, and the majority were white and female (Table 2). Facial line severity at maximum contraction was moderate to severe for all areas. No participant had severe lines at rest.
Table 2.
Participant Demographics and Baseline Characteristics
| Characteristic | Interviews (n = 40) | |
|---|---|---|
| FHL cohort (n = 20) |
UFL cohort (n = 20) |
|
| Age, years, mean (SD) | 44.7 (15.6) | 50.4 (13.8) |
| Range, years | 24–72 | 24–72 |
| Female, n (%) | 14 (70.0) | 14 (70.0) |
| Race, n (%) | ||
| White | 17 (85.0) | 17 (85.0) |
| Black or African American | 2 (10.0) | 2 (10.0) |
| Asian | 1 (5.0) | 0 |
| Other | 0 | 1 (5.0) |
| FHL severity at maximum contraction, n (%) | ||
| Moderate | 8 (40.0) | 7 (35.0) |
| Severe | 12 (60.0) | 13 (65.0) |
| CFL severity at maximum contraction, n (%) | ||
| Moderate | NA | 11 (55.0) |
| Severe | NA | 9 (45.0) |
| GL severity at maximum frown, n (%) | ||
| Moderate | NA | 10 (50.0) |
| Severe | NA | 10 (50.0) |
| Education | ||
| College degree (2- or 4-year) | 10 (50.0) | 6 (30.0) |
| Some college or certificate program | 6 (30.0) | 8 (40.0) |
| Graduate degree | 2 (10.0) | 3 (15.0) |
| High school diploma/GED or less | 2 (10.0) | 3 (15.0) |
| Work status | ||
| Working full-time | 11 (55.0) | 10 (50.0) |
| Working part-time | 4 (20.0) | 4 (20.0) |
| Retired | 2 (10.0) | 3 (15.0) |
| Unemployed | 3 (15.0) | 2 (10.0) |
| Homemaker | 0 | 1 (5.0) |
| Other (eg, laid off) | 0 | 1 (5.0) |
| Annual household income, USD | ||
| $100,000 and over | 5 (25.0) | 7 (35.0) |
| $75,000 to $99,999 | 4 (20.0) | 5 (25.0) |
| $50,000 to $74,999 | 3 (15.0) | 3 (15.0) |
| $25,000 to $49,999 | 5 (25.0) | 3 (15.0) |
| < $25,000 | 3 (15.0) | 2 (10.0) |
| Relationship status | ||
| Married | 9 (45.0) | 9 (45.0) |
| Single | 6 (30.0) | 5 (25.0) |
| Divorced/separated | 1 (5.0) | 3 (15.0) |
| Widowed | 2 (10.0) | 2 (10.0) |
| Has a significant other | 2 (10.0) | 1 (5.0) |
| State of residence | ||
| Illinois | 7 (35.0) | 7 (35.0) |
| North Carolina | 6 (30.0) | 6 (30.0) |
| California | 3 (15.0) | 4 (20.0) |
| Florida | 4 (20.0) | 3 (15.0) |
| Subject-reported products or treatments previously used or currently usinga | ||
| Facial cream/moisturizer | 9 (45.0) | 7 (35.0) |
| None | 8 (40.0) | 7 (35.0) |
| Cosmetics | 5 (25.0) | 8 (40.0) |
| Botulinum toxin type A | 5 (25.0) | 6 (30.0) |
| Dermal filler | 1 (5.0) | 1 (5.0) |
| Facials | 1 (5.0) | 0 |
| Laser treatment | 1 (5.0) | 2 (10.0) |
| Intense pulsed light, facial | 0 | 1 (5.0) |
| Chemical peels | 0 | 1 (5.0) |
| Data missing | 1 (5.0) | 0 |
CFL, crow’s feet lines; FHL, forehead lines; GL, glabellar lines; NA, not applicable; SD, standard deviation.
aCounts are not mutually exclusive.
Concept Elicitation Phase
Forehead Lines
Of the 29 participants who were interviewed about FHL, all 29 referred to the forehead area as the “forehead.” FHL were termed “wrinkles” and/or “lines” by the majority (n = 25, 86%). Only 3 participants (10.3%) termed FHL “frown lines,” and 1 participant each (3.4%) used the terms “age lines” or “fine lines.” For this area, as well as for CFL and GL, participants may have used more than 1 descriptor so counts are not mutually exclusive.
Crow’s Feet Lines
A majority (n = 11; 55%) of the 20 participants interviewed about the CFL region, spontaneously referred to this area as the “sides of the eye,” “corner of the eye,” and/or “outside of the eyes.” Two participants (10%) used the term “crow’s feet.” Other terms used by 1 participant (5%) each were “end of eyelids,” “eye area,” “side of face,” “temple,” and “smile glands.” The term “crow’s feet lines” was used by 17 participants (85%) to describe their CFL. Other terms were “laugh lines,” used by 2 participants (10%), and “wrinkles” or “lines,” used by 1 participant each (5%).
Glabellar Lines
Terminology used to describe the glabellar area was more variable than for other areas. Of the 20 participants asked about their GL, 11 (55%) spontaneously referred to the area as “between the eyebrows” or “between the eyes.” Five (25%) participants referred to this area as the “bridge of the nose.” One participant (5%) each termed the glabellar area the “top of the nose” or the “unibrow spot.” None of the participants spontaneously used the term “glabellar lines” to refer to their GL, although 1 participant (5%) mentioned “grabella” lines, and 6 reported being familiar with the term “glabellar lines” when asked directly about it. Seven (35%) participants each used the term “wrinkles” and/or “frown lines” to describe GL. Four participants (20%) referred to GL as the “elevens,” and 1 (5%) referred to GL as “bridge wrinkles.”
Psychological Impact
Across both populations (N = 29), FHL and UFL participants defined the psychological impact of facial lines as a negative effect on one’s daily mental and/or emotional state, influencing one’s self-perception as well as his/her views toward the world. A majority of participants (n = 20, 69%) stated that psychological and emotional concepts were the same or similar, with the remaining participants considering them to be different. Distinction or lack of distinction between emotional and psychological impacts was variable; for example, a verbatim phrase indicating lack of distinction was: “I think that’s the same thing … well, emotions are psychological,” while a verbatim phrase indicating distinction was: “… psychological is dealing with your brain and what you’re thinking and emotions is what you feel, so I guess it’d be different.”
Impacts of FHL
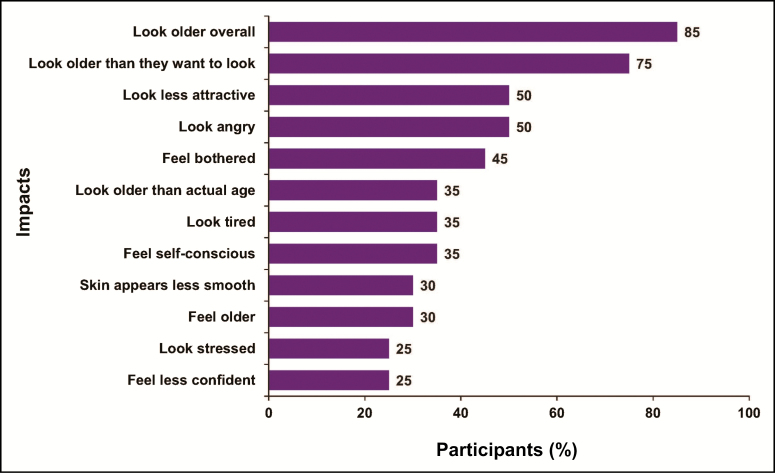
Before the cognitive interview phase with the FLO-11 questionnaire, at least 25% (n = 5) of participants reported impacts of FHL on appearance-related psychological impacts, including looking older overall, older than desired, less attractive, angry, tired, older than their actual age, and stressed. They also reported that their FHL made their skin appear less smooth (Figure 2). Other impacts of FHL reported by at least 25% of participants included feeling bothered, self-conscious, older, and less confident.
Figure 2.
Participant-reported impacts of forehead lines (concept elicitation phase), reported by at least 25% of participants.
Impacts of UFL
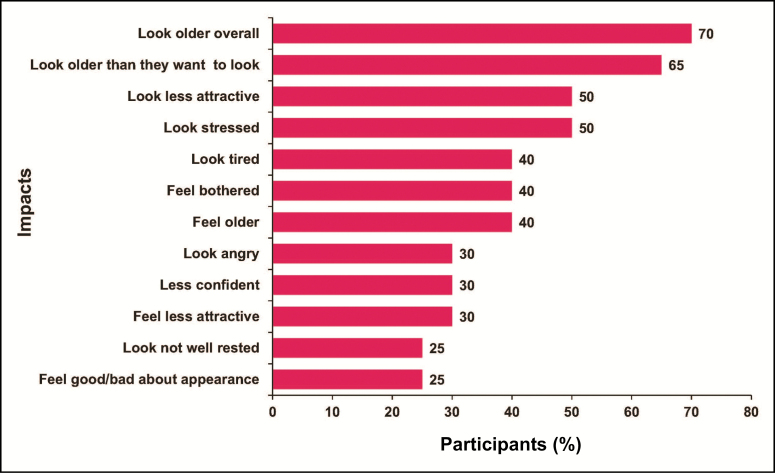
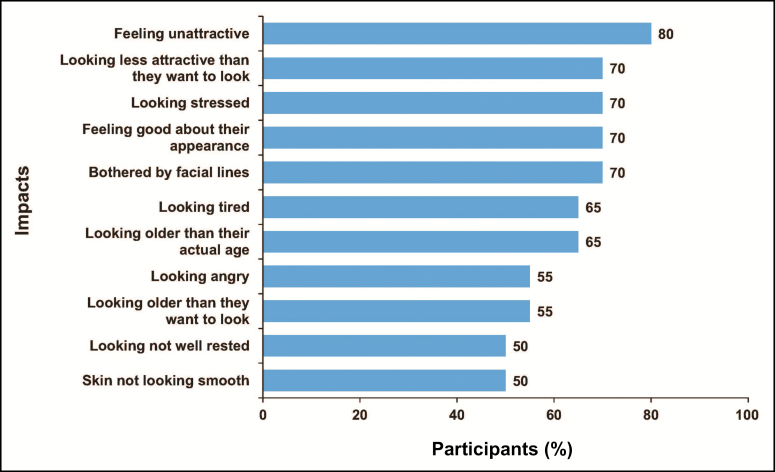
Participants reported a variety of appearance-related psychological impacts of their UFL (Figure 3). At least 25% of participants reported looking older overall as well as older than desired, less attractive, stressed, tired, angry, and not well rested. They also reported feeling older and bothered by their UFL, less confident, less attractive, and good or bad about their appearance.
Figure 3.
Participant-reported impacts of upper facial lines (concept elicitation phase),
reported by at least 25% of participants.
FLO-11 Questionnaire Cognitive Debriefing Phase
Forehead Lines
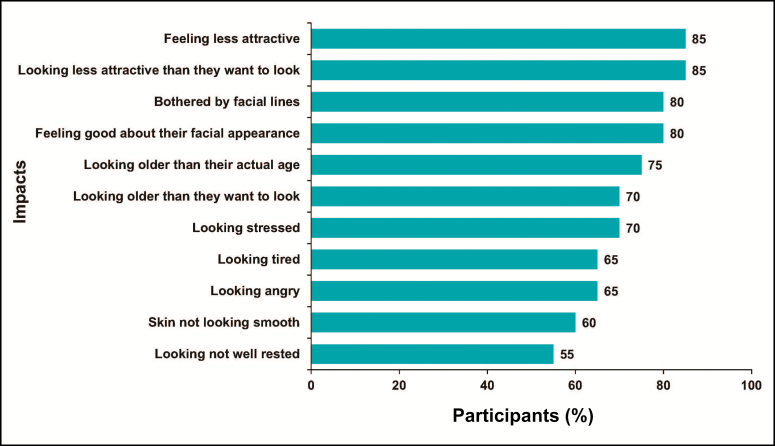
For FHL, more than 50% of participants agreed that each of the items on the FLO-11 measured a psychological impact (Figure 4). A majority (n = 13, 65%) of the participants interviewed about FHL reported it to be comprehensive in measuring psychological impacts of FHL. Five (25%) participants stated that the FLO-11 was not comprehensive for varied reasons, and data were missing for 2 participants.
Figure 4.
Forehead lines: participant-reported impacts of FLO-11 items (cognitive interview phase). Items identified by more than 50% of participants.
Upper Facial Lines
More than half of participants (range, 55%-80%) interviewed for UFL reported that 9 items of the FLO-11 measured psychological impacts of UFL (Figure 5). In addition, looking not well rested and having unsmooth skin were considered to measure psychological impact by 50% of participants. A majority (n = 12, 60%) reported the FLO-11 to be a comprehensive measure of the psychological impacts of UFL. Data were missing for 1 participant, and 7 did not consider the FLO-11 to be comprehensive for varying reasons.
Figure 5.
Upper facial lines: participant-reported impacts of FLO-11 items (cognitive interview phase). Items identified by more than 50% of participants.
Additional Psychological Impacts
Participants who underwent FHL and UFL interviews identified additional psychological impacts that were not included in the FLO-11. Each identified impact was reported by 2 or fewer participants for the FHL assessment and 4 or fewer for the UFL assessment. These impacts were “less confident,” “depression/sadness,” “comparing to others,” “angry,” “disappointed,” “feeling older,” “anxious,” “envious,” “frustrated,” “concerned with aging,” “aware of lines,” “self-conscious,” “irritable,” and “resigned.”
DISCUSSION
The present study was conducted to provide additional content validation of the FLO-11 questionnaire based on FDA recommendations that were published after the initial development of the FLO-11.22,37 Participants first identified the appearance-related psychological impacts of their FHL and UFL (concept elicitation) before completing the FLO-11 questionnaire. These participant-identified impacts were congruent with all of the FLO-11 key concepts; few participants (≤20%) identified concepts not already included in the FLO-11. Participants also defined psychological impacts of their facial lines as having a negative effect on their daily mental and/or emotional state. The most commonly reported psychological impacts were similar for both FHL and UFL, although frequencies varied somewhat. During the cognitive phase, after completing the FLO-11 questionnaire, the majority of participants with FHL and with UFL agreed that the individual FLO-11 items measured a psychological impact and that the questionnaire was comprehensive in measuring these impacts.
The results of the present study on FHL alone and on UFL are consistent with and extend the findings of a previous study that also followed FDA guidelines for developing PROs, including following the recommendation that study participants be representative of the target population for which treatment is intended.22,38 This previous study assessed the ability of the FLO-11 to capture the impacts of UFL comprehensively and accurately, determined whether the instrument was readily understandable for patients, and also specifically examined the psychological impact of CFL alone.38 Participants in both groups identified all key concepts of the FLO-11 during the concept elicitation phase of the studies, supporting its content validity. The most common spontaneously reported concepts were that facial lines affect how old one looks, including looking older than desired or looking older than actual age. In the CFL interviews, the majority of participants agreed that the FLO-11 assesses psychological impacts of CFL and reported it to be comprehensive in measuring these impacts.
The current study may be limited by some of the characteristics of the study population. Although the participants were representative of the majority of individuals who seek aesthetic treatment of UFL—ie, primarily white females—it remains to be determined how well the FLO-11 items reflect the concerns of other patient subgroups, including those of other races or ethnicities. Another potential limitation of this qualitative study may be researcher bias, specifically in how questions were asked and the way in which topics were probed with participants. In this study, however, the majority of concepts were reported spontaneously. Although these qualitative research studies do not directly assess treatment efficacy, the FLO questionnaire has been used in clinical trials for more than a decade, demonstrating subject-reported clinical efficacy consistent with other measures, such as the FWS.
CONCLUSIONS
This qualitative research study on the ability of the FLO-11 questionnaire to measure PROs demonstrated that participants with moderate-to-severe FHL and UFL experience multiple appearance-related psychological impacts from their lines and that these can be measured adequately by the FLO-11. Furthermore, participants agreed that the FLO-11 questionnaire is a comprehensive instrument for measuring these impacts. These findings, combined with the results of a prior PRO study,38 support the content validation of the FLO-11 questionnaire as a PRO instrument for assessing the psychological impacts of FHL and UFL. They also indicate that the FLO-11 may be helpful to both patients and clinicians in assessing the benefits of aesthetic facial treatment.
Disclosures
Dr. Dayan is an employee of DeNova Research, which received remuneration for this research from Allergan plc. Dr. Yoelin serves as an investigator and on a speakers’ bureau for Allergan plc. Dr. De Boulle serves as a consultant and investigator and on a speakers’ bureau for Allergan plc. Dr. Garcia is an employee of Allergan plc and may own stock/options in the company. The authors received no honoraria or other form of financial support related to the development of this article.
Funding
This study was funded by Allergan plc (Dublin, Ireland). Medical writing and editorial support for this article was provided by Paula Davis, PhD, of Peloton Advantage, LLC, an OPEN Health company (Parsippany, NJ, USA) and was funded by Allergan plc. Allergan employees were involved in the study design, the interpretation of data, the review of the manuscript, and the decision to submit the manuscript for publication.
Acknowledgments
The authors thank the study participants and the clinical personnel, and gratefully acknowledge Adelphi for conducting the participant interviews.
REFERENCES
- 1. Koblenzer CS. Psychologic aspects of aging and the skin. Clin Dermatol. 1996;14:171-177. [DOI] [PubMed] [Google Scholar]
- 2. Dimberg U. Facial reactions to fear-relevant and fear-irrelevant stimuli. Biol Psycshol. 1986;23:153-161. [DOI] [PubMed] [Google Scholar]
- 3. Ekman P. Facial expressions of emotion: an old controversy and new findings. Philos Trans R Soc Lond B Biol Sci. 1992;335:63-69. [DOI] [PubMed] [Google Scholar]
- 4. Adams RB Jr., Nelson AJ, Soto JA, Hess U, Kleck RE. Emotion in the neutral face: A mechanism for impression formation? Cogn Emot. 2012;26:431-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rossi A, Eviatar J, Green JB, et al. Signs of facial aging in men in a diverse, multinational study: Timing and preventive behaviors. Dermatol Surg. 2017;43(Suppl 2):S210-S220. [DOI] [PubMed] [Google Scholar]
- 6. Coleman SR, Grover R. The anatomy of the aging face: Volume loss and changes in 3-dimensional topography. Aesthet Surg J. 2006;26(1 Suppl):S4-S9. [DOI] [PubMed] [Google Scholar]
- 7. Finn CJ, Cox SE, Earl ML. Social implications of hyperfunctional facial lines. Dermatol Surg. 2003;29:450-455. [DOI] [PubMed] [Google Scholar]
- 8. Hess U, Adams RB Jr., Simard A, Stevenson MT, Kleck RE. Smiling and sad wrinkles: Age-related changes in the face and the perception of emotions and intentions. J Exp Soc Psychol. 2012;48:1377-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Folster M, Hess U, Werheid K. Facial age affects emotional expression decoding. Front Psychol. 2014;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freudenberg M, Adams RB Jr., Kleck RE, Hess U. Through a glass darkly: Facial wrinkles affect our processing of emotion in the elderly. Front Psychol. 2015;6:1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gupta MA, Gilchrest BA. Psychosocial aspects of aging skin. Dermatol Clin. 2005;23:643-648. [DOI] [PubMed] [Google Scholar]
- 12. Cox SE, Finn JC. Social implications of hyperdynamic facial lines and patient satisfaction outcomes. Int Ophthalmol Clin. 2005;45:13-24. [DOI] [PubMed] [Google Scholar]
- 13. VanSwearingen JM, Cohn JF, Bajaj-Luthra A. Specific impairment of smiling increases the severity of depressive symptoms in patients with facial neuromuscular disorders. Aesthetic Plast Surg. 1999;23:416-423. [DOI] [PubMed] [Google Scholar]
- 14. 2017 Cosmetic Plastic Surgery Statistics. American Society of Plastic Surgeons, 2018 https://www.plasticsurgery.org/documents/News/Statistics/2017/plastic-surgery-statistics-report-2017.pdf. Accessed April 18, 2019.
- 15. Cosmetic surgery national data bank statistics. Aesthet Surg J. 2017;37(Suppl 2):1-29. [DOI] [PubMed] [Google Scholar]
- 16. Carruthers JD, Lowe NJ, Menter MA, Gibson J, Eadie N. Double-blind, placebo-controlled study of the safety and efficacy of botulinum toxin type A for patients with glabellar lines. Plast Reconstr Surg. 2003;112:1089-1098. [DOI] [PubMed] [Google Scholar]
- 17. Carruthers A, Carruthers J, Cohen J. A prospective, double-blind, randomized, parallel-group, dose-ranging study of botulinum toxin type a in female subjects with horizontal forehead rhytides. Dermatol Surg. 2003;29:461-467. [DOI] [PubMed] [Google Scholar]
- 18. Harii K, Kawashima M. A double-blind, randomized, placebo-controlled, two-dose comparative study of botulinum toxin type A for treating glabellar lines in Japanese subjects. Aesthetic Plast Surg. 2008;32:724-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carruthers A, Carruthers J. Prospective, double-blind, randomized, parallel-group, dose-ranging study of botulinum toxin type A in men with glabellar rhytids. Dermatol Surg. 2005;31:1297-1303. [DOI] [PubMed] [Google Scholar]
- 20. Carruthers A, Carruthers J, Said S. Dose-ranging study of botulinum toxin type A in the treatment of glabellar rhytids in females. Dermatol Surg. 2005;31:414-422. [DOI] [PubMed] [Google Scholar]
- 21. De Boulle K. Patient satisfaction with different botulinum toxin type A formulations in the treatment of moderate to severe upper facial rhytids. J Cosmet Laser Ther. 2008;10:87-92. [DOI] [PubMed] [Google Scholar]
- 22. Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims U.S. Department of Health and Human Services, Food and Drug Administration, 2009. http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf. Accessed February 21, 2019. [Google Scholar]
- 23. Botox Cosmetic [package insert]. Dublin, Ireland: Allergan plc; 2017. [Google Scholar]
- 24. Pompilus F, Burgess S, Hudgens S, Banderas B, Daniels S. Development and validation of a novel patient-reported treatment satisfaction measure for hyperfunctional facial lines: Facial Line Satisfaction Questionnaire. J Cosmet Dermatol. 2015;14:274-285. [DOI] [PubMed] [Google Scholar]
- 25. Fagien S, Carruthers JD. A comprehensive review of patient-reported satisfaction with botulinum toxin type a for aesthetic procedures. Plast Reconstr Surg. 2008;122:1915-1925. [DOI] [PubMed] [Google Scholar]
- 26. Carruthers A, Carruthers J. Patient-reported outcomes with botulinum neurotoxin type A. J Cosmet Laser Ther. 2007;9(Suppl 1):32-37. [DOI] [PubMed] [Google Scholar]
- 27. Trindade de Almeida A, Carruthers J, Cox SE, Goldman MP, Wheeler S, Gallagher CJ. Patient satisfaction and safety with aesthetic onabotulinumtoxinA after at least 5 years: A retrospective cross-sectional analysis of 4,402 glabellar treatments. Dermatol Surg. 2015;41(Suppl 1):S19-S28. [DOI] [PubMed] [Google Scholar]
- 28. Beer KR, Boyd C, Patel RK, Bowen B, James SP, Brin MF. Rapid onset of response and patient-reported outcomes after onabotulinumtoxinA treatment of moderate-to-severe glabellar lines. J Drugs Dermatol. 2011;10:39-44. [PubMed] [Google Scholar]
- 29. Carruthers A, Bruce S, de Coninck A, et al. Efficacy and safety of onabotulinumtoxinA for the treatment of crow’s feet lines: A multicenter, randomized, controlled trial. Dermatol Surg. 2014;40:1181-1190. [DOI] [PubMed] [Google Scholar]
- 30. Carruthers J, Carruthers A. Botulinum toxin type A treatment of multiple upper facial sites: Patient-reported outcomes. Dermatol Surg. 2007;33:S10-S17. [DOI] [PubMed] [Google Scholar]
- 31. Carruthers J, Rivkin A, Donofrio L, et al. A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of repeated onabotulinumtoxinA treatments in subjects with crow’s feet lines and glabellar lines. Dermatol Surg. 2015;41:702-711. [DOI] [PubMed] [Google Scholar]
- 32. Dayan S, Coleman WP III, Dover JS, et al. Effects of onabotulinumtoxinA treatment for crow’s feet lines on patient-reported outcomes. Dermatol Surg. 2015;41(Suppl 1):S67-S74. [DOI] [PubMed] [Google Scholar]
- 33. Fagien S, Cox SE, Finn JC, Werschler WP, Kowalski JW. Patient-reported outcomes with botulinum toxin type A treatment of glabellar rhytids: A double-blind, randomized, placebo-controlled study. Dermatol Surg. 2007;33:S2-S9. [DOI] [PubMed] [Google Scholar]
- 34. Fagien S, Cohen JL, Coleman W, et al. Forehead line treatment with onabotulinumtoxinA in subjects with forehead and glabellar facial rhytids: A phase 3 study. Dermatol Surg. 2017;43:S274-S284. [DOI] [PubMed] [Google Scholar]
- 35. Moers-Carpi M, Carruthers J, Fagien S, et al. Efficacy and safety of onabotulinumtoxinA for treating crow’s feet lines alone or in combination with glabellar lines: A multicenter, randomized, controlled trial. Dermatol Surg. 2015;41:102-112. [DOI] [PubMed] [Google Scholar]
- 36. de Boulle KL, Werschler WP, Gold MH, et al. Phase 3 study of onabotulinumtoxinA distributed between frontalis, glabellar complex, and lateral canthal areas for treatment of upper facial lines. Dermatol Surg. 2018;44:1437-1448. [DOI] [PubMed] [Google Scholar]
- 37. Kowalski J, Kozma C, Reese PR, Slaton T, Lee J. Initial development of a patient-completed questionnaire to assess outcomes of aesthetic treatment for hyperfunctional facial lines of the upper face [poster]. Presented at the annual meeting of the American Academy of Dermatology, July 20-24, 2005, Chicago, IL. [Google Scholar]
- 38. Yaworsky A, Daniels S, Tully S, et al. The impact of upper facial lines and psychological impact of crow’s feet lines: Content validation of the Facial Line Outcomes (FLO-11) questionnaire. J Cosmet Dermatol. 2014;13:297-306. [DOI] [PubMed] [Google Scholar]
- 39. Charmaz K. Grounded theory. In: Smith JA, Harre R, Van Langenhove L, eds. Rethinking Methods in Psychology. London, United Kingdom: Sage Publications; 1995:27-49. [Google Scholar]
- 40. Glaser BG, Strauss A.. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago, IL: Aldine Transaction; 1967. [Google Scholar]
- 41. Weitzman EA, Miles MB.. Computer Programs for Qualitative Data Analysis. London, United Kingdom: Sage Publications; 1995. [Google Scholar]