Abstract
Androgens can affect the reproductive axis of both sexes. In healthy women, as in men, elevated exogenous androgens decrease gonad function and lower gonadotropin levels; such circumstances occur with anabolic steroid abuse or in transgender men (genetic XX individuals) taking androgen supplements. The neuroendocrine mechanisms by which endogenous or exogenous androgens regulate gonadotropin release, including aspects of pulsatile luteinizing hormone (LH) secretion, remain unknown. Because animal models are valuable for interrogating neural and pituitary mechanisms, we studied effects of androgens in the normal male physiological range on in vivo LH secretion parameters in female mice and in vitro LH secretion patterns from isolated female pituitaries. We also assessed androgen effects on hypothalamic and gonadotrope gene expression in female mice, which may contribute to altered LH secretion profiles. We used a nonaromatizable androgen, dihydrotestosterone (DHT), to isolate effects occurring specifically via androgen receptor (AR) signaling. Compared with control females, DHT-treated females exhibited markedly reduced in vivo LH pulsatility, with decreases in pulse frequency, amplitude, peak, and basal LH levels. Correlating with reduced LH pulsatility, DHT-treated females also exhibited suppressed arcuate nucleus Kiss1 and Tac2 expression. Separate from these neural effects, we determined in vitro that the female pituitary is directly inhibited by AR signaling, resulting in lower basal LH levels and reduced LH secretory responses to gonadotropin-releasing hormone pulses, along with lower gonadotropin gene expression. Thus, in normal adult females, male levels of androgen acting via AR can strongly inhibit the reproductive axis at both the neural and pituitary levels.
Keywords: GnRH, kisspeptin, Kiss1, neurokinin B, Tac2, androgen, DHT, androgen receptor, pituitary, gonadotrope
A fundamental tenet of hypothalamic-pituitary-gonadal (HPG) axis regulation is sex steroid negative feedback, including actions by androgens. Although first proposed in 1932 (1), the actual mechanisms through which endogenous or exogenous androgens act to regulate gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH) secretion still remain unknown. Although androgens strongly affect male fertility and are often thought of as male hormones, androgens can also have important actions on the female reproductive axis. Indeed, in rodents, global androgen receptor (AR) deficiency impacts female reproduction, with altered cycles, premature ovarian failure, and subfertility, and some of these impairments occur at the neuroendocrine level (2-5). Although endogenous androgen levels are normally low in otherwise healthy females, those findings implicate endogenous androgens as important modulators of the female HPG axis. Separately, elevated androgens, from exogenous or endogenous sources, can negatively impact the reproductive axis in both sexes. In healthy women, as in men, elevated exogenous androgen exposures can inhibit the reproductive axis, decreasing gonad size and lowering gonadotropin levels, along with suppressed menstrual cycles; such circumstances occur with anabolic steroid abuse or in transgender men (XX individuals assigned female at birth who identify as male) taking androgen supplements to masculinize physical characteristics (6-14). Indeed, the global prevalence of transgender individuals is rising, with >1 million people in the United States alone (15-17). Yet, the neuroendocrine effects of such exogenous androgens in women and transgender men have not been well-studied, with most androgen-related clinical studies focusing on women with polycystic ovary syndrome (PCOS), a complex hyperandrogenemia disorder that may not recapitulate normal HPG axis functioning in healthy females. Moreover, the circulating androgen levels in most transgender men taking supplements are higher than the slightly elevated androgens in PCOS women, making functional comparisons between them difficult. It is therefore imperative to understand the fundamental neuroendocrine mechanisms of androgen action not just in males or female PCOS patients, but also in healthy females who are exposed to high androgens through steroid abuse or androgen supplementation for transgender transitioning. In this regard, the use of genetically tractable animal models, such as mice, to tease apart neural and pituitary mechanisms is invaluable; however, few studies have studied adulthood androgen effects on the reproductive endocrinology of female mice, and those focused primarily on gonadal measures rather than neural effects on LH secretion patterns (18-21).
At the level of the brain, at least in males, androgens provide negative feedback by decreasing hypothalamic GnRH secretion and Gnrh mRNA levels, thereby lowering LH secretion (22-25). Because GnRH neurons do not express AR, negative feedback effects on GnRH, either by endogenous or exogenous androgens, occur indirectly via “upstream” afferent neurons that express AR. Indeed, androgen treatment into the medial basal hypothalamus region is able to alter GnRH levels in males, but similar implants into the preoptic area (where GnRH cell bodies residue) do not (26), supporting the idea that androgen does not act on GnRH soma. Prime candidates to mediate neural androgen effects on GnRH/LH secretion are AR-expressing kisspeptin neurons. Kisspeptin, encoded by Kiss1, stimulates GnRH neurons and is essential for proper reproductive axis function in both males and females (27-30). In the rodent hypothalamus of both sexes, kisspeptin neurons are detected primarily in 2 regions, the anteroventral periventricular nucleus (AVPV) and the arcuate nucleus (ARC) (31, 32). The ARC region is implicated in sex steroid negative feedback on GnRH pulse secretion in both sexes (33, 34), and ARC Kiss1 neurons may participate in this negative feedback process (35). Indeed, evidence suggests that ARC Kiss1 neurons, which also co-express the neuropeptides neurokinin B (NKB) and dynorphin, are key components of the GnRH pulse generator mechanism (36-39). In contrast, Kiss1 neurons in the AVPV likely mediate estrogen positive feedback induction of the preovulatory GnRH/LH surge in females only (40, 41).
Both ARC and AVPV Kiss1 populations express estrogen receptor alpha (ERα) and AR, but the 2 Kiss1 populations are regulated oppositely by sex steroids: estradiol (E2) or testosterone (T) inhibit Kiss1 gene expression in the ARC but stimulate Kiss1 in the AVPV (32). In male mice, the nonaromatizable androgen dihydrotestosterone (DHT) lowers Kiss1 mRNA levels in the ARC, as does T or E2, but DHT has no effect on AVPV Kiss1 levels (42, 43). This indicates that either AR or ERα signaling can inhibit ARC Kiss1 expression, whereas AVPV Kiss1 is exclusively regulated by ERα, at least in males (42, 43). Similar DHT effects on ARC Kiss1 levels in adult female mice, or on ARC Tac2 levels, have not yet been reported.
Besides the brain, AR is also expressed in the pituitary, along with ER and progesterone receptor (PR) (44), predominantly in gonadotropes, lactotropes, and thyrotropes (45). Because gonadotropes are technically difficult to isolate from other pituitary cells, most studies have measured in vivo androgen effects in whole pituitary or in vitro in immortalized cell lines that represent late embryonic gonadotropes (LβT2 cells). Those studies focused mostly on changes in gene expression and found that androgen regulates mRNA levels of gonadotropins (Lhβ and Fshβ) and GnRH receptor (46-49). However, direct effects of androgens on pituitary LH pulse secretion, separate from gene expression effects, have not been well-studied in females and are necessary to understand how androgens might functionally alter pituitary hormone release patterns.
In the present study, we studied neuroendocrine effects of androgen in normal females on both in vivo LH secretion profiles and in vitro gonadotrope LH secretion patterns. Assessment of LH pulse profiles allowed us to assess androgen effects on multiple secretion parameters, including LH pulse frequency, pulse amplitude, basal levels, and pulse peak. This determined if (and how) androgen signaling alters one or a combination of these various pulse parameters. In addition, we determined whether expression of neural reproductive genes, such as Kiss1 and Tac2 in the ARC, and gonadotrope genes are altered by elevated androgen treatment in adult female mice, perhaps contributing to their altered LH secretion profiles. For all studies, we implemented physiological male doses of a nonaromatizable androgen, DHT, to specifically isolate androgen effects occurring via AR signaling pathways, thereby avoiding confounding effects of aromatization of androgen to E2.
Materials and Methods
Animals and surgeries
C57BL/6 mice of both sexes were weaned at 3 weeks of age and housed on a 12:12 light-dark cycle, with food and water available ad libitum. Mice were housed 2 or 3 per cage. All experiments were approved by the University of California San Diego Institutional Animal Care and Use Committee. In Experiment 1, some males were bilaterally gonadectomized (GDX) to remove endogenous testicular hormone negative feedback on GnRH/LH secretion. Similarly, in Experiments 2 and 3, females were bilaterally ovariectomized (OVX) to remove all ovarian hormone negative feedback and induce elevated GnRH/LH pulsatility. For both castrations and ovariectomies, animals were briefly anesthetized with isoflurane during the surgical procedure and a subcutaneous (s.c.) injection of buprenorphine analgesic was given at the time of surgery. Figure 1 shows a schematic overview of the experimental paradigms for each of the 4 experiments.
Figure 1.
Schematic of experimental paradigms for testing androgen effects on LH levels in young adult male mice (Experiment 1) or LH pulse secretion and gene expression in young adult female mice (Experiments 2-4).
Experiment 1: DHT dose-response study in male mice
This experiment sought to determine in vivo doses of DHT in the male physiological range of androgens that effectively suppress endogenous LH secretion (i.e., mimic androgen negative feedback) in young adult male mice. Male mice that were 5 weeks old were used to mimic similar body weights (BW) of young adult females aged 7 weeks (studied in Experiments 2-4). At 5 weeks of age, males were either left gonad-intact (controls) or GDX and given an s.c. DHT implant made of Silastic tubing (inner diameter 1.47 mm, outer diameter 1.96 mm) containing 1 of 3 different DHT doses, achieved by varying the length of DHT powder packed within the tubing (4 mm, 10 mm, or 16 mm of DHT, corresponding to ~4 mg, 10 mg, and 16 mg DHT powder, respectively) (n = 9-11 per group). Each end of the implant was sealed with ~3 mm silicone adhesive. DHT implants were soaked for ~6 hours in sterile physiological saline prior to implantation. A separate cohort of GDX control males underwent a sham s.c. surgery with no implant (n = 9). Two weeks after DHT implantation, all males were sacrificed, and blood collected for LH measurement (see Fig. 1 for experimental timeline). Seminal vesicle (SV) weights, an androgen-dependent trait, were also measured in a subset of males upon sacrifice (n = 4-5/group). Blood was left to clot at room temperature for 90 minutes and then centrifuged to collect the serum, which was stored at −20 °C. Serum samples were assayed for LH at the University of Virginia Ligand Assay Core using a well-validated sensitive sandwich radioimmunoassay for mouse LH using antibodies against bovine LH and human LH-beta (50, 51) (sensitivity: 0.04 ng/mL; reportable range: 0.08-75.0 ng/mL). Values are reported as ng/mL of serum.
Experiment 2: effects of DHT on in vivo LH pulses in adult female mice
This experiment tested whether exposure to elevated male levels of androgens affects in vivo LH pulsatility in young adult females (see Fig. 1). At 5 weeks of age, after puberty, females were OVX to remove ovarian hormone negative feedback and given 1-week recovery. Beginning at 6 weeks of age, all females underwent daily handling for 3 weeks to become habituated to the tail-tip bleeding procedure used for collecting serial blood samples. OVX were performed 1 week before initiation of the daily handling, and 4 weeks before serial bleeding, to avoid daily handling of mice right after recovery from the surgery and to prevent any unintended short-term stress effects of the OVX surgery on in vivo LH pulse patterns. At 7 weeks of age, females were implanted s.c. with a Silastic implant containing 1 of 2 doses of DHT, 4 mm or 10 mm, as in Experiment 1, or given a sham implantation surgery without an implant (OVX control). At 9 weeks of age, 2 weeks after DHT implantation, all mice underwent serial blood sampling, conducted between 10:00 and 12:00 hours. The 2-week duration of DHT exposure was chosen to ensure the implant was not yet depleted on the day of LH sampling. For the serial bleeding, the tail-tip of each animal was first cut and then ~15 minutes later serial blood samples were collected every 6 minutes for a total duration of 2 hours (n = 5-7/group), as in previous studies (52, 53). For each sample, 3 μL of whole blood was pipetted from the tail and mixed with 57 μL of assay buffer, and then placed on ice until storage at −20 °C. Mice were awake the entire time and able to freely roam their home cage with access to food and water in between sampling.
Serial tail-tip blood samples were assayed for LH levels by the UVA Ligand Assay Core using an ultra-sensitive murine LH ELISA that uses capture monoclonal antibody (anti-bovine LH-beta subunit, 518B7) and detection polyclonal antibody (rabbit LH antiserum, AFP240580Rb) (50, 54). Functional sensitivity of the assay was 0.320 ng/mL. Values are reported as ng/mL of whole blood.
Serial blood LH measures were analyzed for endogenous LH pulses. An LH value was determined to be a pulse using a similar criteria as in our previous reports for mouse LH pulsatility (52, 53): the value must show a >20% increase from 1 of the 2 previous points followed by a decrease by >10% in 1 of the next 2 subsequent points. In addition, to qualify as a pulse, the pulse amplitude must exceed the detection sensitivity of the assay (≥0.32 ng/mL), as in previous reports (53, 55). For identified LH pulses, the following parameters were calculated: (i) pulse frequency (# pulses/60 min); (ii) interpulse interval, defined as the time between 2 pulses; (iii) pulse amplitude, defined as the difference between a pulse peak value and a preceding nadir, the lowest value of the 3 preceding values; (iv) pulse peak, defined as the zenith LH value of each identified pulse; (v) basal LH level, defined as the lowest of the 3 preceding values for each pulse event. In addition to these pulse measures, overall mean LH, the average of all the LH values for an animal for the entire 2-hour sampling period, was also calculated.
Experiment 3: DHT effects on hypothalamic gene expression in females
Females were OVX at 5 weeks old and given either DHT implants (4 mm or 10 mm) or no DHT (OVX control) (n = 6-7/group) at 7 weeks of age, similar to Experiment 2. At 9 weeks of age, 2 weeks after DHT implantation, females were sacrificed between 10:30 and 12:00 hours and their brains collected, immediately frozen on dry ice, and stored at −80 °C. Frozen brains were sectioned on a cryostat into 5 sets of 20 uM coronal sections and thaw mounted on Superfrost-plus slides that were stored at −80 °C until assaying.
Single-label in situ hybridization (ISH) for ARC Kiss1 or Tac2 (NKB) gene expression was performed, in each case, on 1 set of brain sections spanning the entire rostral-to-caudal ARC region. All ISH assays were performed using published protocols and validated riboprobes (56, 57). Briefly, slides with brain sections were fixed in 4% paraformaldehyde, treated with acetic anhydride, rinsed in saline sodium citrate (SSC), delipidated in chloroform, and dehydrated in graded ethanol. Slides were air-dried before the hybridization step, where P33 radiolabeled riboprobe (Kiss1 or Tac2; 0.04 pmol/mL) was combined with 1/20 volume yeast tRNA in 0.1 M Tris/0.01M EDTA (pH 8) to produce the probe mix. The probe mix was heat-denatured, iced for 5 minutes, and added to prewarmed hybridization buffer (60% deionized formamide, 5× hybridization salts, 0.1× Denhardt’s buffer, 0.2% SDS). 100 μL of this final hybridization mix was added to each slide. Slides were coverslipped and placed in a humidity chamber at 55 °C for 16 hours. Following hybridization, coverslips were removed and slides were washed with 4× SSC at room temperature. Slides were then placed in RNase A (10 mg/mL, Roche Biochemicals) in 0.15 M sodium chloride, 10mM Tris, 1mM EDTA (pH 8) for 30 minutes at 37 °C followed by buffer without RNase for another 30 minutes at 37 °C. Slides were then washed in 2× SSC for 30 minutes and 0.1× SSC at 62 °C for 60 minutes. Slides were then washed for 3 minutes in 0.1× SSC at room temperature and dehydrated with graded ethanol. After drying, slides were dipped in Kodak NTB emulsion, air-dried, and stored at 4 °C for 5 to 7 days (depending on the specific assay) until they were developed and coverslipped.
ISH slides were analyzed using previously published techniques with a computer-automated image processing system and custom counting software (GRAINS; Dr. Don Clifton, University of Washington) by a person unaware of the treatment group of each slide (58). The software counted, bilaterally in every section spanning the entire rostral-to-caudal ARC, the number of silver grain clusters representing Kiss1 or Tac2 cells, as well as the number of silver grains in each individual cell cluster (a semiquantitative measure of Kiss1 or Tac2 mRNA expressed per cell) (56, 58, 59). Cells were considered Kiss1 or Tac2 positive when the number of silver grains in a cluster exceeded that of background by 3-fold, as in previous studies (57, 60).
Experiment 4: Effects of DHT on in vitro LH pulses and gene expression from isolated female pituitaries
AR is expressed in both the brain and pituitary, and therefore androgens might theoretically act in either target to alter reproductive hormone levels. This experiment tested whether elevated androgens can directly act at the level of the pituitary to affect LH pulse secretion in females. To isolate possible DHT effects on pituitary from those that might occur in the brain, pituitaries were dissected from 8-week-old C57Bl/6 female mice (see Fig. 1 for experimental paradigm). Isolated pituitaries were pooled, dispersed, and the cellular equivalent of 5 pituitaries per treatment group (in vitro DHT or vehicle [VEH]) were cultured on 1-mL bed volume of Cytodex 3 microcarrier beads (GE Healthcare) for 48 hours, as previously described (61, 62). Cell suspensions cultured in 3 mL 10% FBS Dulbecco’s Modified Eagle Medium (DMEM) were changed to serum-free DMEM with antibiotics for 16 hours, to adjust media to 1% serum. Cultures were then loaded into perifusion columns and equilibrated for 40 minutes in serum-free DMEM with 0.1% BSA alone (VEH) or supplemented with male physiological concentrations of DHT (1 nM or 10 nM; normal androgen serum range in female mice is 0.25 to 0.5 nM and up to 12 nM in male mice (63)) at a flow rate of 200 μL/min. Subsequently, cells were pulsed with 10 nM peak concentration of GnRH for 2 minutes. GnRH pulses were repeated every 30 minutes for 4 hours. Perifusion culture and pulse methodology were described previously (64). Fractions of ~1 mL were collected every 5 minutes and LH concentrations were measured in sampled fractions during the baseline period, after the first GnRH pulse, and after the fourth GnRH pulse with a well-validated mouse LH RIA (as in Experiment 1) by the University of Virginia Ligand Assay Core. To normalize for cell content differences between columns, secreted LH levels were normalized to Gapdh expression levels measured by real-time quantitative polymerase chain reaction (RT-qPCR) in RNA harvested from the same pituitary cells post-perifusion, as described below. LH values in the initial baseline period before any GnRH treatment (“basal LH”), after the first GnRH pulse, and after the fourth GnRH pulse were averaged from 3 independent experiments and compared between VEH and DHT treatments.
To assess direct pituitary effects of androgen signaling on female gonadotrope gene expression, separate from possible effects on LH secretion, RT-qPCR analysis was performed on RNA isolated from the same female mouse pituitary cells used in the pulsed with GnRH under perifusion. At the conclusion of the 4-hour pulsing with GnRH, total RNA was isolated from pituitary samples from VEH and DHT treatments using TRIzol (Thermo Fisher Scientific). The cDNA was synthesized using qScript cDNA SuperMix per the manufacturer’s instructions (Quanta Biosciences). For quantitative RT-PCR, real-time PCR was carried out using the CFX Connect Real-Time PCR Detection System (Bio-Rad Laboratories) with KAPA SYBR Green PCR kit (KAPA Biosystems) supplemented with 200 nM of transcript-specific primers and cycled as recommended by the manufacturer. A melt curve was performed after each PCR run to ensure that a single product was amplified. The relative transcript levels were determined with Gapdh as an endogenous control. Primer sequences were designed against murine mRNA sequences, as follows: Lhb forward (F): 5′- TGTCCTAGCATGGTCCGAGT-3′, Lhb reverse (R): 5′- CCCCCACAGTCAGAGCTACT-3′; Fshb F: 5′-GCCGTTTCTGCATAAGC-3′, Fshb R: 5′-CAATC TTACGGTCTCGTATACC-3′; Egr1 F: 5′-ATTTTTCCTG AGCCCCAAAGC-3′, Egr1 R: 5′-ATGGGAACCTGGAAA CCACC-3′; Gapdh F: 5′-TGCACCACCACCTGCTTAG-3′, Gapdh R: 5′-GGATGCAGGGATGATGTTC-3′.
Statistical analysis
All data were analyzed in GraphPad Prism 6 (La Jolla, CA). Mean data were analyzed as a 1-way ANOVA (group), followed by post hoc Bonferroni’s multiple comparison test or Fishers PLSD post hoc test. All data are expressed as mean ± standard error of the mean. A value of P < 0.05 was considered statistically significant.
Results
Experiment 1: DHT dose-dependently lowers LH in male mice
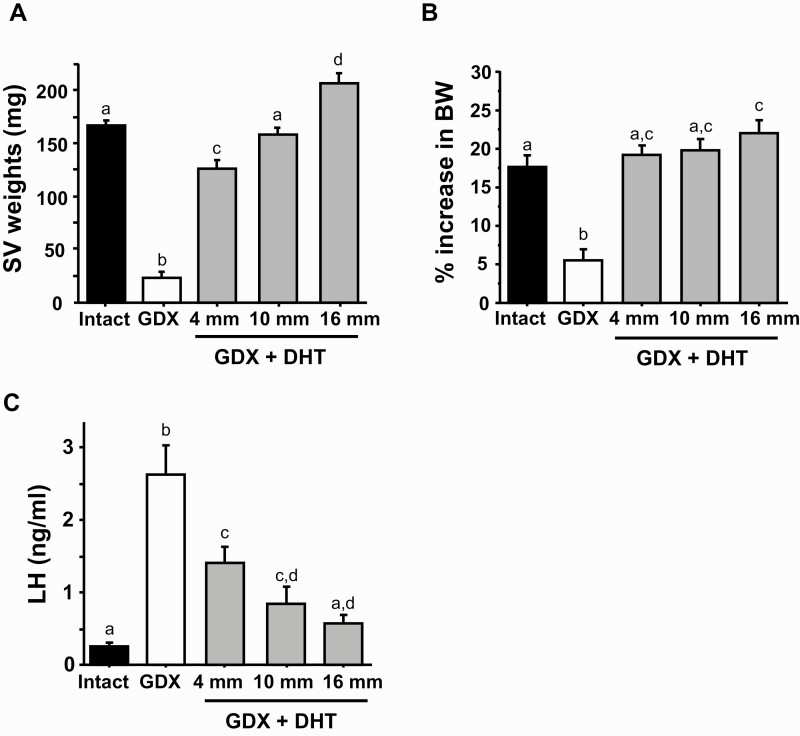
This experiment tested whether different doses of exogenous DHT effectively suppress in vivo LH levels (i.e., mimic androgen negative feedback) in young adult male mice. SV weights and BW gain were used to assess androgen doses that were functionally in the normal male physiological range. All 3 DHT doses increased SV weights relative to GDX males lacking DHT (P < 0.05 in each case; Fig. 2A). SV weights of the middle (10 mm) DHT dose were not significantly different from those of gonad-intact controls, whereas the lowest and highest DHT doses stimulated SV weights that were smaller and greater, respectively, than intact controls (P < 0.05, Fig. 2A). Likewise, all 3 DHT doses increased BW gain over the 2-week treatment period relative to GDX controls lacking DHT (P < 0.05; Fig. 2B); the lowest and middle DHT doses resulted in similar BW gain as gonad-intact controls, whereas BW gain with the highest DHT dose was slightly higher than intact controls (P < 0.05 vs intact; Fig. 2B). Taken together, these SV and BW data indicate that the middle (10 mm) DHT dose is functionally in the normal male range, whereas the lowest dose (4 mm) is functionally at or just below the low end of the male range. By contrast, the highest dose (16 mm) is somewhat supraphysiological based on slightly higher SV weights and BW gain.
Figure 2.
Dose-response study comparing effects of different DHT implant doses, given for 2 weeks, on LH levels and BW in young adult male mice. A) Mean seminal vesicle (SV) weights of males after 2 weeks of DHT treatment. B) Mean % increase in BW of males during the 2-week DHT treatment period between 5 and 7 weeks of age. C) LH levels in gonad-intact males compared to GDX males with or without 2-week DHT treatment. Bars with different letters are significantly different from each other (P < 0.05).
DHT treatment dose-dependently lowered LH levels in GDX male mice (Fig. 2C). GDX control males (no DHT treatment) exhibited highly elevated LH levels compared with gonad-intact control males, as expected due to lack of steroid feedback (Fig. 2C). All 3 DHT doses significantly lowered LH from the high GDX control levels (P < 0.05 for each dose relative to GDX control); only the highest DHT dose (16 mm) resulted in LH levels that were not significantly different from gonad-intact controls (Fig. 2C). The lowest (4 mm) and middle (10 mm) doses of DHT caused large reductions in LH levels compared with GDX controls, by approximately 50% and 70%, respectively (P < 0.05), but these LH levels were still slightly elevated relative to gonad-intact controls (P < 0.05; Fig. 2C).
Experiment 2: DHT inhibits endogenous LH pulses in female mice
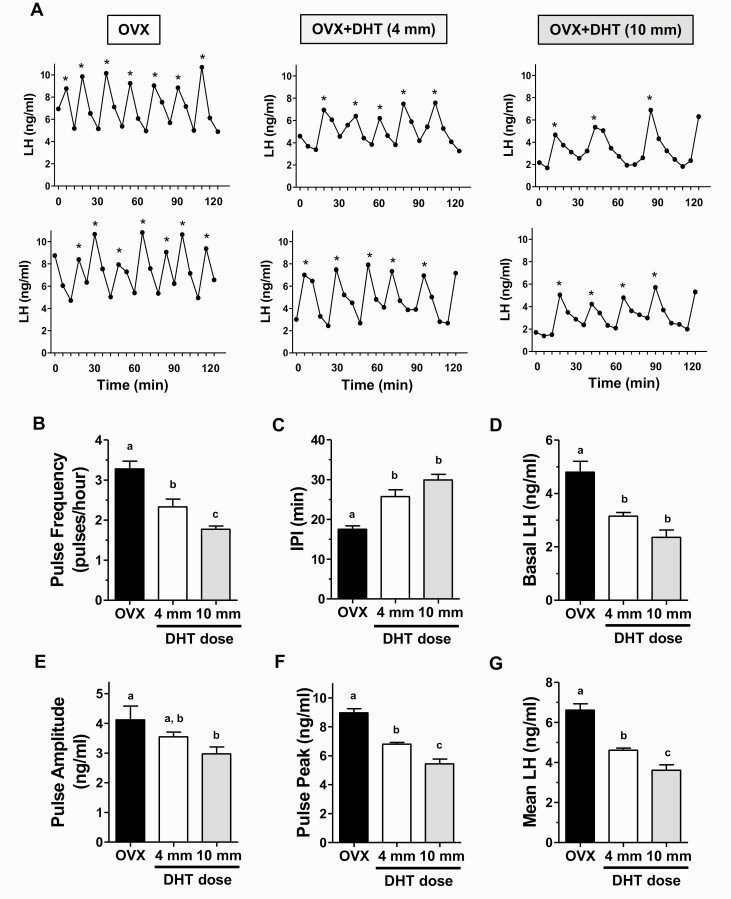
This experiment tested whether constant elevated androgens, in the low to normal male physiological range, impact endogenous LH pulse secretion in young adult females. We used DHT, rather than T, to isolate androgen actions occurring via AR and to avoid possible activation of ERα or ERβ if T were aromatized to E2. Based on Experiment 1, we studied effects of the 10 mm DHT dose in females, as this dose in males strongly lowered LH versus GDX controls and also induced normal SV weights and BW gain, unlike the highest DHT dose which was supraphysiological. For comparison, we also included a cohort of females given the lowest DHT dose (4 mm), which also yielded significant inhibition on LH in GDX males but with slightly lower stimulation of SV weights than intact controls. Figure 3 shows representative in vivo LH secretion profiles during serial blood collection for control OVX females and OVX+DHT females for each dose. OVX control females exhibited rapid, high-amplitude pulsatile LH secretion pattern, along with a high basal level and high LH pulse peaks (Fig. 3A). By contrast, females given DHT had slower and lower level pulses (Fig. 3A).
Figure 3.
DHT treatment reduces endogenous LH pulse secretion in young adult OVX females. (A) Representative profiles of in vivo LH secretion in DHT-treated OVX female mice (middle and right columns), and control OVX littermates (left column). LH was measured in serial tail-tip bleeds from awake, unrestrained females every 6 minutes for 2 hours. Identified pulses are indicated by *. Mean LH pulse frequency (pulses/hour) (B), interpulse interval (IPI; the number of minutes between pulses) (C), basal LH level (D), pulse amplitude (E), pulse peak (zenith value of a pulse) (F), and mean LH across the entire sampling period (G) were all significantly different in OVX+DHT vs control OVX females. Different letters above bars indicate significantly different (P < 0.05) from each other.
Mean analysis of individual LH pulse parameters in females with androgen exposure indicated significant reductions in virtually all pulse parameters (Fig. 3B-3G). Specifically, the mean number of detectable pulses (pulse frequency) was significantly decreased by 30% to 45% in DHT mice versus OVX controls (P < 0.01; Fig. 3B) while mean interpulse interval was correspondingly increased by 45% to 70% in DHT females (P < 0.01; Fig. 3C). There were also large decreases in mean basal levels (by 35%-50%), mean pulse amplitude (by ~30%), and mean pulse peak levels (i.e., the zenith of a pulse; by 25%-40%) in DHT mice (P < 0.01 for each parameter compared to OVX control females; Fig. 3D-3F). Overall mean LH for the entire sampling period was significantly lower, by 30% to 45%, in OVX+DHT females versus OVX controls (P < 0.01; Fig. 3G). As with mean LH (Fig. 3G), pulse frequency and pulse peak which were both significantly lower in the 10 mm DHT group relative to the 4 mm DHT group (P < 0.05; Fig. 3B and 3F), whereas other individual pulse parameters did not differ significantly between the 2 DHT doses.
Experiment 3: DHT inhibits hypothalamic Kiss1 and Tac2 gene expression in females
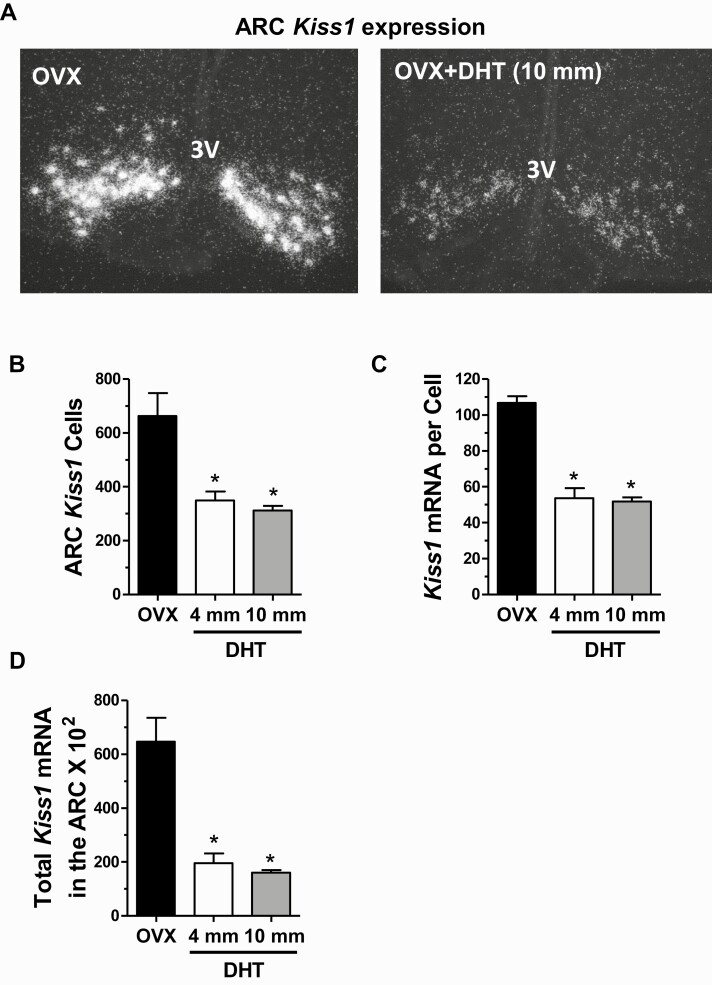
Experiment 2 determined that elevated androgen exposure is sufficient to provide inhibition on endogenous LH pulses in females, mimicking androgen-mediated negative feedback in males. Experiment 3 tested whether androgens might reduce female LH pulse secretion by inhibiting ARC kisspeptin neurons, which are critical components of the GnRH pulse generator and therefore involved in stimulating downstream LH secretion (36, 39, 65). Indeed, we found that Kiss1 expression in the ARC, measured via radiolabeled ISH, was strongly decreased in female mice given DHT compared with control littermates without DHT (Fig. 4A). Both doses of DHT resulted in lower ARC Kiss1 mRNA levels compared to non-DHT controls (Fig. 4B-4D). Total Kiss1 mRNA levels in the ARC region were >70% lower in DHT than control females (P < 0.01 for each dose relative to controls; Fig. 4D), with both the number of Kiss1 cells (Fig. 4B) and levels of Kiss1 mRNA per cell (Fig. 4C) being significantly decreased (P < 0.01 for both doses, for each measure). There was no significant difference between the 2 DHT doses in any Kiss1 mRNA measure.
Figure 4.
Androgens suppress Kiss1 levels in the arcuate nucleus of young adult female mice. A) Representative microscope images of Kiss1 mRNA expression in the ARC nucleus, determined with in situ hybridization, in OVX females with and without 2-week DHT treatment. Mean Kiss1 cell number (B), Kiss1 mRNA per cell (C), and total Kiss1 mRNA levels (D) in the ARC of females with and without 2-week DHT treatment. *, significantly different from OVX control group (P < 0.05).
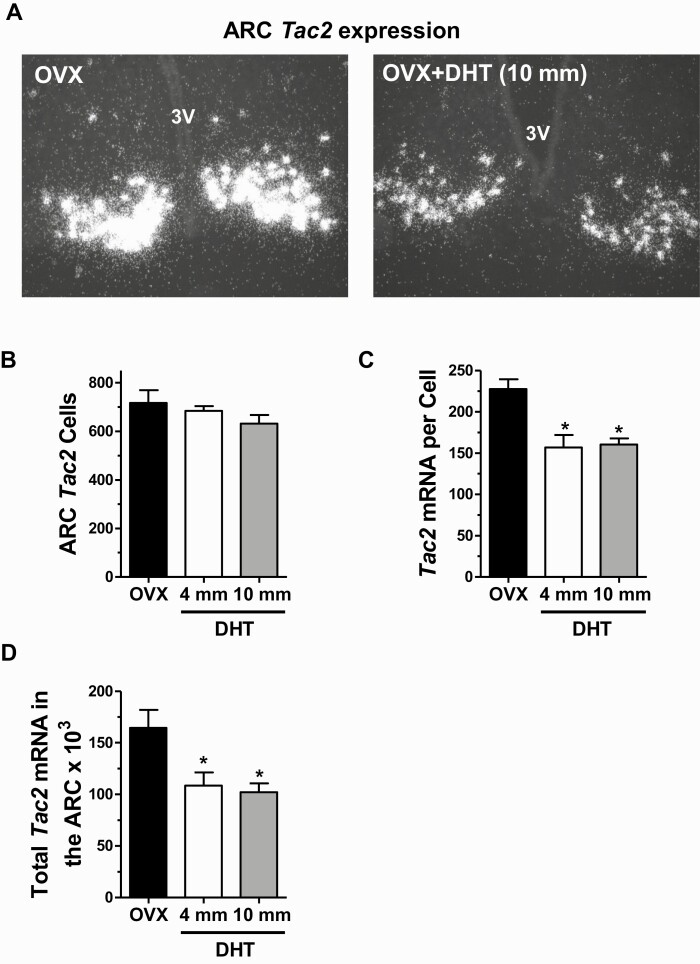
Similar to Kiss1, expression levels of Tac2 in the ARC of females were significantly decreased by DHT exposure (Fig 5A). The number of detected ARC Tac2 cells was not significantly different between either DHT group and control females (Fig. 5B). However, the relative amount of Tac2 mRNA per cell was significantly lower, by ~30%, in females with DHT of either dose versus control females (P < 0.01; Fig. 5C), indicating a marked reduction in the amount of NKB synthesized by these cells. Correspondingly, the overall total levels of Tac2 in the ARC region was significantly lower in both DHT groups versus controls (Fig. 5D), reaching ~40% reduction in the 10 mm DHT dose group (P < 0.01).
Figure 5.
Androgens suppress Tac2 levels in the arcuate nucleus of young adult female mice. A) Representative microscope images of Tac2 mRNA expression (encoding NKB) in the ARC nucleus, determined with in situ hybridization, in OVX females with and without 2-week DHT treatment. Mean Tac2 cell number (B), Tac2 mRNA per cell (C), and total Tac2 mRNA levels (D) in the ARC of females with and without 2-week DHT treatment. *, significantly different from OVX control group (P < 0.05).
Experiment 4: DHT inhibits LH pulse responses to GnRH in female pituitaries in vitro
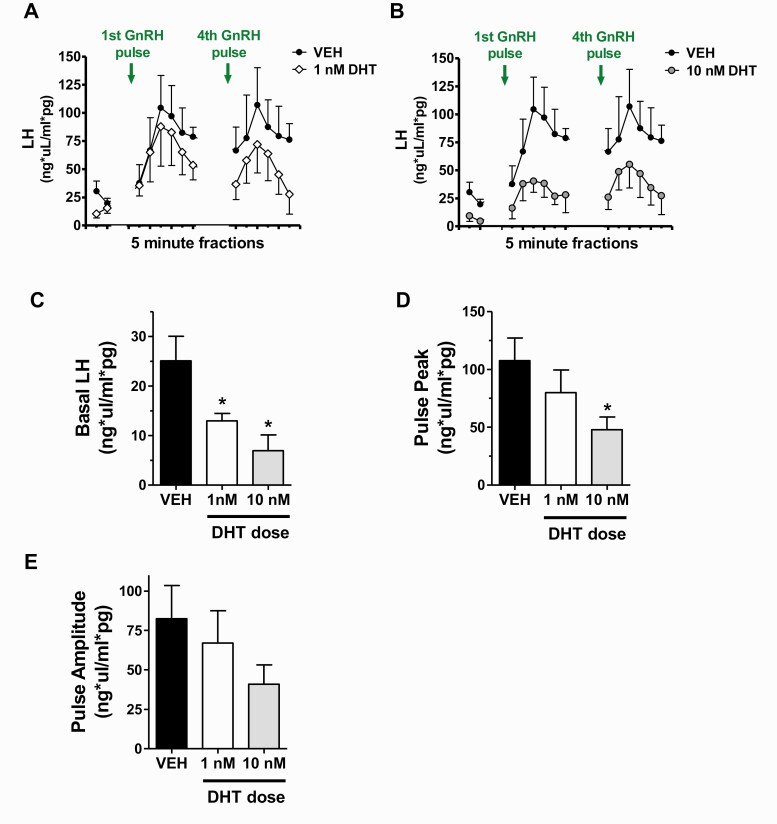
This experiment tested whether androgens might impact LH pulse secretion directly at the level of the pituitary, separate from any possible androgen actions in the brain. To elucidate possible pituitary-specific androgen effects on LH secretion, we used isolated pituitaries from adult female mice and treated them in culture with or without DHT prior to pulsing them with periodic GnRH treatment. Figures 6A and 6B show mean in vivo LH secretion profiles during serial 5-minute fraction collections for control VEH pituitaries and 2 doses of DHT-treated pituitaries. DHT dose-dependently lowered the overall LH secretory response to an incoming GnRH pulse, with more pronounced effects at the 10 nM (Fig. 6B) than 1 nM dose (Fig 6A). To analyze these secretion patterns, we compared several pulse secretion parameters between VEH and DHT groups. Mean basal LH levels, measured in the baseline period before the first GnRH pulse, were significantly lower in each DHT group, reaching a 75% decrease in the 10 nM DHT group (P < 0.05 vs VEH for each DHT dose). Upon pulse treatments with GnRH, LH pulse secretion responses were significantly dampened at the 10 nM DHT dose, with pulse peak being 55% lower versus VEH controls (P < 0.05; Fig. 6D). Pulse amplitude had a lower mean level for the 10 nM DHT group but was not significantly different from VEH controls (Fig. 6E), likely due to low statistical power (only n = 3/group). By contrast, the 1 nM DHT group did not show significant decreases in GnRH-induced LH secretion versus the VEH group (Figs. 6D and 6E).
Figure 6.
Effects of androgen on in vitro LH secretion from isolated female pituitaries in response to GnRH pulse input. A, B) Mean LH secretion measured in 5-minute fractions from DHT-treated pituitaries (1 nM or 10 nM DHT) before and after brief (2-minute) GnRH pulses. The first 2 fractions show basal LH levels prior to any GnRH input, and subsequent fractions show mean LH secretion immediately following GnRH pulses. For better viewing, 1 nM (A) and 10 nM (B) DHT groups are graphed separately with vehicle (VEH) controls. Analyses of various pulse parameters are shown in subsequent panels, including mean basal LH (C), mean pulse peak (D), and mean pulse amplitude (E). *, significantly different from VEH control group (P < 0.05).
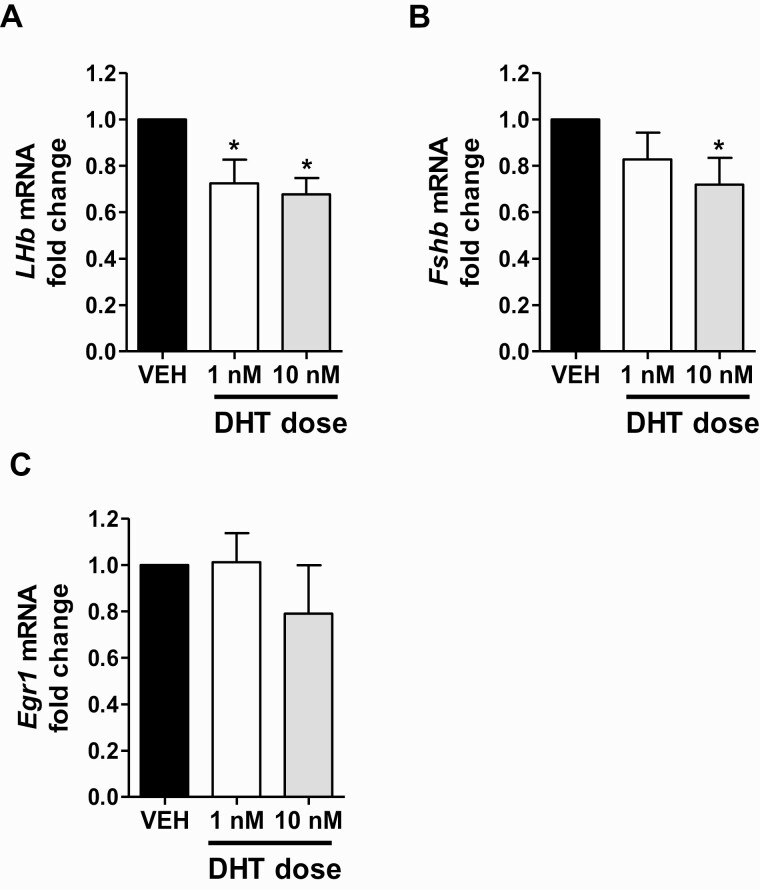
To assess direct pituitary effects of androgen on female gonadotrope gene expression, separate from androgen effects on LH secretion, we performed qPCR analysis on RNA isolated from DHT-treated pituitary cells after 4 hours of GnRH pulse treatment. Lhβ mRNA levels were significantly lower, by ~30%, in both DHT groups relative to the VEH control group (P < 0.05 for each DHT dose versus VEH; Fig. 7A). Fshβ expression levels were also significantly decreased, by ~30%, with 10 nM DHT treatment (P < 0.05) but not by the lower 1 nM DHT dose (Fig. 7B). In contrast to the gonadotropin genes, pituitary Egr1 expression levels were not significantly altered by either DHT dose (Fig. 7C).
Figure 7.
Androgen lowers gonadotropin gene expression levels in isolated pituitaries of adult female mice. DHT treatment significantly lowers mean Lhβ mRNA (A) and Fshβ (B) levels, but not Egr1 (C) levels in GnRH-pulsed female pituitaries, as measured by qPCR and normalized to Gapdh expression. *, significantly different from VEH control group (P < 0.05).
Discussion
In the present study, we studied AR-specific effects of elevated androgens in adult females on both in vivo LH secretion profiles and in vitro on gonadotrope LH secretion patterns. Assessment of LH pulse profiles allowed us to determine how AR signaling affects different secretion parameters, including LH pulse frequency, pulse amplitude, basal levels, and pulse peak. The high-amplitude, high-frequency LH pulses (occurring approximately every 18 minutes) observed in vivo in our control GDX females matched that of previous reports (53, 66). In clear contrast to these controls’ rapid, high-amplitude pulse events, GDX+DHT females demonstrated markedly suppressed LH pulses that included significant decreases in pulse frequency, pulse amplitude, pulse peak levels, and basal LH levels. Several of the in vivo LH pulse parameters showed significant dose-dependent reductions between the 2 tested androgen doses, the higher of which (10 mm) is functionally in the physiological range of young adult males, as evidenced by our SV and BW data from Experiment 1. This androgen dose was chosen to mimic the human situation of transgender men (TGM) exposure to elevated androgens, which are often in the male range, as well as high androgen levels in women abusing anabolic steroids. We demonstrate that such male level androgens can inhibit virtually all parameters of endogenous LH pulsatile secretion in otherwise healthy females; in fact, even lower androgen doses (the 4 mm dose) that are near the low end of the male range can also provide suppression, to a lower degree, on female LH pulses.
As demonstrated in our in vivo LH pulse experiment, androgens acting through AR can suppress LH secretion in females, but it is not fully understood how this occurs. We studied the brains of DHT-treated females to gain insight into possible underlying neural causes of the altered LH secretion. We hypothesized that ARC kisspeptin neurons, implicated as essential factors in the GnRH pulse generation mechanism, would show signs of inhibition in DHT-treated females. Indeed, both Kiss1 and Tac2 gene expression in the ARC were strongly downregulated by elevated androgen exposure, suggestive of a reduced capacity for these ARC cells to make kisspeptin and NKB. Since both kisspeptin and NKB are stimulatory to GnRH pulse output, lower levels of these factors may contribute to decreased basal LH levels as well as decreased LH amplitude, pulse peak, and/or pulse frequency. Given the stimulatory role of NKB in producing GnRH pulses, the reduced Tac2 levels in DHT-treated females may result in diminished stimulatory input for initiating ARC kisspeptin/NKB neuron “pulse firing,” leading to less frequent GnRH pulses and possibly also smaller magnitude (amplitude, pulse peak) GnRH pulses. Such reductions in GnRH pulse frequency and amplitude would be expected to reduce generation of downstream LH pulses from the pituitary.
The effects of diminished kisspeptin/NKB levels on GnRH pulses suggested here may act in concert with our observed DHT-induced reductions in in vitro gonadotrope secretory responses to each GnRH pulse signal, combining to attenuate basal LH or pulse peak LH levels. Current consensus is that the speed of LH pulses (i.e., pulse frequency) is determined solely in the brain, by the GnRH pulse generator mechanism. Conversely, the pituitary is not considered to play a critical role in governing the timing of LH pulse events. However, the concentration of secreted LH, either basal LH or peak LH levels, can be regulated by changes in both the brain and pituitary, owing to, for example, the amounts of kisspeptin, GnRH, or LH synthesized/released. Thus, androgen actions on neural circuits, including ARC Kiss1/NKB cells, likely contributed to the observed decreases in both pulse frequency and magnitude (basal and peak LH levels) while androgen actions at the pituitary likely contributed to decreases in just the latter. Unfortunately, the sample size of 3 in our in vitro experiment was too small to detect significant DHT effects on amplitude; future studies with greater sample size and statistical power are needed to determine if amplitude is altered by DHT.
The present study intentionally isolated androgen effects that occur via AR signaling pathways. In addition to binding AR, some androgens, including T, may be converted by aromatase to E2, allowing for subsequent activation of ERs. In such cases, experimental use of T or other aromatizable androgens would not identify which receptor—AR or ER (or both)—is responsible for any observed experimental effects. By using DHT in the present study we were able to focus on the possible contribution of just AR in female HPG axis regulation. Our findings clearly demonstrate a robust effect of AR signaling that is inhibitory to the female neuroendocrine reproductive axis, at least under the conditions studied, similar to classic androgen negative feedback in males. Relatedly, it has been reported that adult female ERα knockout (KO) mice, lacking ERα, show inhibited mean LH levels (measured as one-off samples) after either T or DHT treatment (18), supporting the concept that AR signaling is capable of inhibiting the female HPG axis, independent from any ERα regulation. This AR effect certainly does not rule out possible ERα signaling effects also modulating the female reproductive axis during treatment with aromatizable androgens, like T. However, whether exogenous T actually gets aromatized sufficiently to have significant consequences on ARC kisspeptin/NKB in females is unknown. Future studies addressing these issues need to use physiological doses of DHT, E2, and T, to best compare between steroids; our current study identified DHT doses that are in the male physiological range and similar dose-response studies taking into account steroid-sensitive measures as controls are needed for other steroids.
AR is expressed in multiple hypothalamic populations, including kisspeptin neurons, and several pituitary cell types, including gonadotropes. Our present findings show an unambiguous inhibitory effect on both LH and Kiss1/Tac2 mRNA levels, but whether this DHT effect is directly in Kiss1 neurons (which express AR) or indirect via other AR-expressing neurons remains to be determined. Likewise, while the most likely mechanism underlying DHT suppression of in vitro pituitary LH secretory responses to GnRH is via AR action in gonadotropes specifically, our pulse experiment cannot rule out AR actions in non-gonadotrope pituitary cells that somehow indirectly affect LH secretion. Future pituitary studies confirming AR action directly—and perhaps exclusively—in gonadotropes are required to confirm this likelihood and are being initiated in our lab.
Among transgender individuals, between one-third and one-half are TGM and report male gender identity after female sex assignment at birth. Concerns for reproductive health and fertility are significant in this population, and as many as 62% of TGM report current or previous desire for childbearing (67). However, this is challenging with concurrent androgen supplementation, a mainstay of gender-affirming medical care in TGM, with utilization rates of 86% to 100% (67, 68). Indeed, a majority of TGM treated with androgen become amenorrheic and unable to conceive. Secondary amenorrhea is also common in androgen-treated individuals (11-13), but the mechanism of menstrual suppression by androgens is still unknown. Indeed, few clinical studies have assessed effects of androgens on GnRH or gonadotropin secretion in healthy females as a potential “upstream” mechanism for menstrual suppression. Although some studies were unable to detect significant changes in serum gonadotropin secretion among treated TGM, there are concerns with low statistical power in those reports, and other clinical studies do in fact report reduced levels of LH, follicle-stimulating hormone, or both (10, 69, 70) during androgen treatment. Additional clinical studies utilizing more rigorous experimental designs and greater participant numbers (for statistical power) are needed on this important, emerging issue. Regardless, whether such gonadotropin suppression is the result of androgen signaling in the brain and/or pituitary is not known and technically difficult to assess in humans; our present results in female mice suggest the possibility that androgens provide HPG inhibition by acting through AR on both hypothalamic brain circuits (including ARC kisspeptin neurons) and the pituitary.
Current concepts of the mechanisms by which androgens alter HPG function in women are extremely limited and stem largely from studies evaluating the relationship between reproductive dysfunction and androgen overproduction in clinical disorders, such as PCOS. In this typically anovulatory condition, women have both elevated androgens and rapid, elevated LH pulses (71-73). Though the pathophysiologic basis for PCOS is undetermined, some studies have postulated that androgen overproduction may contribute organizationally and/or activationally to promoting the abnormally increased LH secretion (74, 75). This proposed mechanism for androgen action in PCOS, which still requires additional supporting evidence, does not match the pathophysiology of amenorrhea in androgen-treated TGM with otherwise normal ovulatory function. Indeed, in PCOS, LH levels increase proportionally to androgen levels and remain elevated in the face of hyperandrogenemia (76), differing from androgen’s typical inhibitory effects on LH in otherwise healthy individuals. This difference may reflect developmental pathophysiology or dysfunction in the neuroendocrine mechanisms controlling GnRH pulses in PCOS. If so, androgen “stimulatory” effects on LH pulses in women with PCOS may not occur in a normal brain/pituitary system and may not generalize to androgen “inhibitory” effects in non-PCOS females.
In summary, we show that compared with control females, young adult DHT-treated female mice exhibit markedly slower and lower-magnitude in vivo LH pulsatility, with decreases in pulse frequency, pulse amplitude, pulse peak, and basal levels. DHT-treated females also exhibit highly suppressed ARC Kiss1 and Tac2 expression, correlating with their reduced LH pulsatility. Moreover, separate from any AR effects in the female hypothalamus, we show that the female pituitary can also be inhibited directly by AR signaling, resulting in lower basal LH levels and reduced LH secretory response to individual GnRH pulses, along with lower gonadotropin gene expression. Thus, androgen signaling via AR can provide inhibition of the female reproductive axis at both the neural and pituitary levels. Our findings of substantial androgen suppression of LH in females under non-PCOS conditions suggests that such androgen inhibition may be disabled or altered in the PCOS state to allow for hyperactive LH pulse secretion in the face of elevated circulating androgens.
Acknowledgments
We thank Paige Steffen, Danmei Li, and Danielle Schafer for technical support.
Financial Support: This research was supported by National Institutes of Health (NIH) grants P50 HD012303, R01 HD090161, R01 HD082567, and NIH R24 HD102061.
Glossary
Abbreviations
- AR
androgen receptor
- ARC
arcuate nucleus
- AVPV
anteroventral periventricular nucleus
- BW
body weight
- DHT
dihydrotestosterone
- GDX
gonadectomized
- E2
estradiol
- ERα
estrogen receptor alpha
- GnRH
gonadotropin-releasing hormone
- HPG
hypothalamic-pituitary-gonadal
- ISH
in situ hybridization
- LH
luteinizing hormone
- NKB
neurokinin B
- OVX
ovariectomized
- PCOS
polycystic ovary syndrome
- PR
progesterone receptor
- RT-qPCR
real-time quantitative polymerase chain reaction
- s.c.
subcutaneous
- SSC
saline sodium citrate
- SV
seminal vesicle
- T
testosterone
- TGM
transgender men
- VEH
vehicle
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
Some or all datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Moore CR, Price D. Gonad hormone functions, and the reciprocal influence between gonads and hypophysis with its bearing on the problem of sex hormone antagonism. Am J Anatomy. 1932;50:13-71. [Google Scholar]
- 2. Yeh S, Tsai MY, Xu Q, et al. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci U S A. 2002;99(21):13498-13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu YC, Wang PH, Yeh S, et al. Subfertility and defective folliculogenesis in female mice lacking androgen receptor. Proc Natl Acad Sci U S A. 2004;101(31):11209-11214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shiina H, Matsumoto T, Sato T, et al. Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci U S A. 2006;103(1):224-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walters KA, McTavish KJ, Seneviratne MG, et al. Subfertile female androgen receptor knockout mice exhibit defects in neuroendocrine signaling, intraovarian function, and uterine development but not uterine function. Endocrinology. 2009;150(7):3274-3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duca Y, Aversa A, Condorelli RA, Calogero AE, La Vignera S. Substance abuse and male hypogonadism. J Clin Med. 2019;8(5):732. doi:10.3390/jcm8050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Christou MA, Tigas S. Recovery of reproductive function following androgen abuse. Curr Opin Endocrinol Diabetes Obes. 2018;25(3):195-200. [DOI] [PubMed] [Google Scholar]
- 8. Shankara-Narayana N, Yu C, Savkovic S, et al. Rate and extent of recovery from reproductive and cardiac dysfunction due to androgen abuse in men. J Clin Endocrinol Metab. 2020;105(6):1827-1839 [DOI] [PubMed] [Google Scholar]
- 9. Horwitz H, Andersen JT, Dalhoff KP. Health consequences of androgenic anabolic steroid use. J Intern Med. 2019;285(3):333-340. [DOI] [PubMed] [Google Scholar]
- 10. Spinder T, Spijkstra JJ, van den Tweel JG, et al. The effects of long term testosterone administration on pulsatile luteinizing hormone secretion and on ovarian histology in eugonadal female to male transsexual subjects. J Clin Endocrinol Metab. 1989;69(1):151-157. [DOI] [PubMed] [Google Scholar]
- 11. McFarland J, Craig W, Clarke NJ, Spratt DI. Serum testosterone concentrations remain stable between injections in patients receiving subcutaneous testosterone. J Endocr Soc. 2017;1(8):1095-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakamura A, Watanabe M, Sugimoto M, et al. Dose-response analysis of testosterone replacement therapy in patients with female to male gender identity disorder. Endocr J. 2013;60(3):275-281. [DOI] [PubMed] [Google Scholar]
- 13. Deutsch MB, Bhakri V, Kubicek K. Effects of cross-sex hormone treatment on transgender women and men. Obstet Gynecol. 2015;125(3):605-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nieschlag E, Vorona E. MECHANISMS IN ENDOCRINOLOGY: Medical consequences of doping with anabolic androgenic steroids: effects on reproductive functions. Eur J Endocrinol. 2015;173(2):R47-R58. [DOI] [PubMed] [Google Scholar]
- 15. Conron KJ, Scott G, Stowell GS, Landers SJ. Transgender health in Massachusetts: results from a household probability sample of adults. Am J Public Health. 2012;102(1):118-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Caenegem E, Wierckx K, Elaut E, et al. Prevalence of gender nonconformity in Flanders, Belgium. Arch Sex Behav. 2015;44(5):1281-1287. [DOI] [PubMed] [Google Scholar]
- 17. Meerwijk EL, Sevelius JM. Transgender population size in the United States: a meta-regression of population-based probability samples. Am J Public Health. 2017;107(2):e1-e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wersinger SR, Haisenleder DJ, Lubahn DB, Rissman EF. Steroid feedback on gonadotropin release and pituitary gonadotropin subunit mRNA in mice lacking a functional estrogen receptor alpha. Endocrine. 1999;11(2):137-143. [DOI] [PubMed] [Google Scholar]
- 19. Nandedkar TD, Munshi SR. Effect of dihydrotestosterone on follicular development, ovulation and reproductive capacity of mice. J Reprod Fertil. 1981;62(1):21-24. [DOI] [PubMed] [Google Scholar]
- 20. Kinnear HM, Constance ES, David A, et al. A mouse model to investigate the impact of testosterone therapy on reproduction in transgender men. Hum Reprod. 2019;34(10):2009-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andrisse S, Childress S, Ma Y, et al. Low-dose dihydrotestosterone drives metabolic dysfunction via cytosolic and nuclear hepatic androgen receptor mechanisms. Endocrinology. 2017;158(3):531-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Toranzo D, Dupont E, Simard J, et al. Regulation of pro-gonadotropin-releasing hormone gene expression by sex steroids in the brain of male and female rats. Mol Endocrinol. 1989;3(11):1748-1756. [DOI] [PubMed] [Google Scholar]
- 23. Selmanoff M, Shu C, Petersen SL, Barraclough CA, Zoeller RT. Single cell levels of hypothalamic messenger ribonucleic acid encoding luteinizing hormone-releasing hormone in intact, castrated, and hyperprolactinemic male rats. Endocrinology. 1991;128(1):459-466. [DOI] [PubMed] [Google Scholar]
- 24. Gross DS. Effect of castration and steroid replacement on immunoreactive gonadotropin-releasing hormone in hypothalamus and preoptic area. Endocrinology. 1980;106(5):1442-1450. [DOI] [PubMed] [Google Scholar]
- 25. Roselli CE, Kelly MJ, Ronnekleiv OK. Testosterone regulates progonadotropin-releasing hormone levels in the preoptic area and basal hypothalamus of the male rat. Endocrinology. 1990;126(2):1080-1086. [DOI] [PubMed] [Google Scholar]
- 26. Kalra PS, Kalra SP. Modulation of hypothalamic luteinizing hormone-releasing hormone levels by intracranial and subcutaneous implants of gonadal steroids in castrated rats: effects of androgen and estrogen antagonists. Endocrinology. 1980;106(1):390-397. [DOI] [PubMed] [Google Scholar]
- 27. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614-1627. [DOI] [PubMed] [Google Scholar]
- 28. Lapatto R, Pallais JC, Zhang D, et al. Kiss1-/- mice exhibit more variable hypogonadism than Gpr54-/- mice. Endocrinology. 2007;148(10):4927-4936. [DOI] [PubMed] [Google Scholar]
- 29. Messager S, Chatzidaki EE, Ma D, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102(5):1761-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Han SK, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349-11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kauffman AS, Gottsch ML, Roa J, et al. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148(4):1774-1783. [DOI] [PubMed] [Google Scholar]
- 32. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686-3692. [DOI] [PubMed] [Google Scholar]
- 33. Scott CJ, Kuehl DE, Ferreira SA, Jackson GL. Hypothalamic sites of action for testosterone, dihydrotestosterone, and estrogen in the regulation of luteinizing hormone secretion in male sheep. Endocrinology. 1997;138(9):3686-3694. [DOI] [PubMed] [Google Scholar]
- 34. Smith ER, Davidson JM. Location of feedback receptors: effects of intracranially implanted steroids on plasma LH and LRF response. Endocrinology. 1974;95(6):1566-1573. [DOI] [PubMed] [Google Scholar]
- 35. Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin- GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 2007;30(10):504-511. [DOI] [PubMed] [Google Scholar]
- 36. Herbison AE. The Gonadotropin-Releasing Hormone Pulse Generator. Endocrinology. 2018;159(11):3723-3736. [DOI] [PubMed] [Google Scholar]
- 37. Plant TM. The neurobiological mechanism underlying hypothalamic GnRH pulse generation: the role of kisspeptin neurons in the arcuate nucleus. F1000Res. 2019;8:F1000 Faculty Rev-982. doi:10.12688/f1000research.18356.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wakabayashi Y, Yamamura T, Sakamoto K, Mori Y, Okamura H. Electrophysiological and morphological evidence for synchronized GnRH pulse generator activity among Kisspeptin/neurokinin B/dynorphin A (KNDy) neurons in goats. J Reprod Dev. 2013;59(1):40-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qiu J, Nestor CC, Zhang C, et al. High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. eLife. 2016;5:e16246. doi:10.7554/eLife.16246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26(25):6687-6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology. 2009;150(8):3664-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S, Kauffman AS. Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology. 2011;152(5):2020-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smith JT, Dungan HM, Stoll EA, et al. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146(7):2976-2984. [DOI] [PubMed] [Google Scholar]
- 44. Stefaneanu L. Pituitary sex steroid receptors: localization and function. Endocr Pathol. 1997;8(2):91-108. [DOI] [PubMed] [Google Scholar]
- 45. Okada Y, Fujii Y, Moore JP Jr, Winters SJ. Androgen receptors in gonadotrophs in pituitary cultures from adult male monkeys and rats. Endocrinology. 2003;144(1):267-273. [DOI] [PubMed] [Google Scholar]
- 46. Shupnik MA. Gonadotropin gene modulation by steroids and gonadotropin-releasing hormone. Biol Reprod. 1996;54(2):279-286. [DOI] [PubMed] [Google Scholar]
- 47. Winters SJ, Ishizaka K, Kitahara S, Troen P, Attardi B. Effects of testosterone on gonadotropin subunit messenger ribonucleic acids in the presence or absence of gonadotropin-releasing hormone. Endocrinology. 1992;130(2):726-734. [DOI] [PubMed] [Google Scholar]
- 48. Jorgensen JS, Nilson JH. AR suppresses transcription of the alpha glycoprotein hormone subunit gene through protein-protein interactions with cJun and activation transcription factor 2. Mol Endocrinol. 2001;15(9):1496-1504. [DOI] [PubMed] [Google Scholar]
- 49. Curtin D, Jenkins S, Farmer N, et al. Androgen suppression of GnRH-stimulated rat LHbeta gene transcription occurs through Sp1 sites in the distal GnRH-responsive promoter region. Mol Endocrinol. 2001;15(11):1906-1917. [DOI] [PubMed] [Google Scholar]
- 50. RRID: AB_2665514, https://antibodyregistry.org/AB_2665514.
- 51. RRID: AB_2665513, https://antibodyregistry.org/AB_2665513.
- 52. Yang JA, Hughes JK, Parra RA, Volk KM, Kauffman AS. Stress rapidly suppresses in vivo LH pulses and increases activation of RFRP-3 neurons in male mice. J Endocrinol. 2018;239(3):339-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Esparza LA, Schafer D, Ho BS, Thackray VG, Kauffman AS. Hyperactive LH pulses and elevated kisspeptin and NKB gene expression in the arcuate nucleus of a PCOS mouse model. Endocrinology. 2020;161(4):bqaa018. doi:10.1210/endocr/bqaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. RRID: AB_2665533, https://antibodyregistry.org/AB_2665533.
- 55. Kreisman MJ, McCosh RB, Tian K, Song CI, Breen KM. Estradiol enables chronic corticosterone to inhibit pulsatile luteinizing hormone secretion and suppress Kiss1 neuronal activation in female mice. Neuroendocrinology. 2020;110(6):501-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Semaan SJ, Kauffman AS. Daily successive changes in reproductive gene expression and neuronal activation in the brains of pubertal female mice. Mol Cell Endocrinol. 2015;401:84-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab. 2009;297(5):E1212-E1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chowen JA, Clifton DK. Semiquantitative analysis of cellular somatostatin mRNA levels by in situ hybridization histochemistry. Method Neurosci. 1991;5:137-158. [Google Scholar]
- 59. Chowen JA, Argente J, Vician L, Clifton DK, Steiner RA. Pro-opiomelanocortin messenger RNA in hypothalamic neurons is increased by testosterone through aromatization to estradiol. Neuroendocrinology. 1990;52(6):581-588. [DOI] [PubMed] [Google Scholar]
- 60. Poling MC, Luo EY, Kauffman AS. Sex differences in steroid receptor coexpression and circadian-timed activation of Kisspeptin and RFRP-3 Neurons may contribute to the sexually dimorphic basis of the LH surge. Endocrinology. 2017;158(10):3565-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Do MH, Santos SJ, Lawson MA. GNRH induces the unfolded protein response in the LbetaT2 pituitary gonadotrope cell line. Mol Endocrinol. 2009;23(1):100-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim T, Lawson MA. GnRH regulates gonadotropin gene expression through NADPH/dual oxidase-derived reactive oxygen species. Endocrinology. 2015;156(6):2185-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Desai R, Harwood DT, Handelsman DJ. Simultaneous measurement of 18 steroids in human and mouse serum by liquid chromatography–mass spectrometry without derivatization to profile the classical and alternate pathways of androgen synthesis and metabolism. Clin Mass Spectrom. 2019;11:42-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lawson MA, Tsutsumi R, Zhang H, et al. Pulse sensitivity of the luteinizing hormone beta promoter is determined by a negative feedback loop Involving early growth response-1 and Ngfi-A binding protein 1 and 2. Mol Endocrinol. 2007;21(5):1175-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lehman MN, Hileman SM, Goodman RL. Neuroanatomy of the kisspeptin signaling system in mammals: comparative and developmental aspects. Adv Exp Med Biol. 2013;784:27-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yang JA, Song CI, Hughes JK, et al. Acute psychosocial stress inhibits LH pulsatility and Kiss1 neuronal activation in female mice. Endocrinology. 2017;158(11):3716-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wierckx K, Van Caenegem E, Pennings G, et al. Reproductive wish in transsexual men. Hum Reprod. 2012;27(2):483-487. [DOI] [PubMed] [Google Scholar]
- 68. Light A, Wang LF, Zeymo A, Gomez-Lobo V. Family planning and contraception use in transgender men. Contraception. 2018;98(4):266-269. [DOI] [PubMed] [Google Scholar]
- 69. Pelusi C, Costantino A, Martelli V, et al. Effects of three different testosterone formulations in female-to-male transsexual persons. J Sex Med. 2014;11(12):3002-3011. [DOI] [PubMed] [Google Scholar]
- 70. Pache TD, Hop WC, de Jong FH, et al. 17 beta-Oestradiol, androstenedione and inhibin levels in fluid from individual follicles of normal and polycystic ovaries, and in ovaries from androgen treated female to male transsexuals. Clin Endocrinol (Oxf). 1992;36(6):565-571. [DOI] [PubMed] [Google Scholar]
- 71. Rebar R, Judd HL, Yen SS, Rakoff J, Vandenberg G, Naftolin F. Characterization of the inappropriate gonadotropin secretion in polycystic ovary syndrome. J Clin Invest. 1976;57(5):1320-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Baird DT, Corker CS, Davidson DW, Hunter WM, Michie EA, Van Look PF. Pituitary-ovarian relationships in polycystic ovary syndrome. J Clin Endocrinol Metab. 1977;45(4):798-801. [DOI] [PubMed] [Google Scholar]
- 73. Coutinho EA, Kauffman AS. The role of the brain in the pathogenesis and physiology of polycystic ovary syndrome (PCOS). Med Sci. 2019;7(8):84. doi:10.3390/medsci7080084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McCartney CR, Eagleson CA, Marshall JC. Regulation of gonadotropin secretion: implications for polycystic ovary syndrome. Semin Reprod Med. 2002;20(4):317-326. [DOI] [PubMed] [Google Scholar]
- 75. Eagleson CA, Gingrich MB, Pastor CL, et al. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85(11):4047-4052. [DOI] [PubMed] [Google Scholar]
- 76. Lawson MA, Jain S, Sun S, Patel K, Malcolm PJ, Chang RJ. Evidence for insulin suppression of baseline luteinizing hormone in women with polycystic ovarian syndrome and normal women. J Clin Endocrinol Metab. 2008;93(6):2089-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.