Abstract
Background and purpose
The objective of our systematic review is to identify prognostic factors that may be used in decision-making related to the care of patients infected with COVID-19.
Data sources
We conducted highly sensitive searches in PubMed/MEDLINE, the Cochrane Central Register of Controlled Trials (CENTRAL) and Embase. The searches covered the period from the inception date of each database until April 28, 2020. No study design, publication status or language restriction were applied.
Study selection and data extraction
We included studies that assessed patients with confirmed or suspected SARS-CoV-2 infectious disease and examined one or more prognostic factors for mortality or disease severity.
Reviewers working in pairs independently screened studies for eligibility, extracted data and assessed the risk of bias. We performed meta-analyses and used GRADE to assess the certainty of the evidence for each prognostic factor and outcome.
Results
We included 207 studies and found high or moderate certainty that the following 49 variables provide valuable prognostic information on mortality and/or severe disease in patients with COVID-19 infectious disease: Demographic factors (age, male sex, smoking), patient history factors (comorbidities, cerebrovascular disease, chronic obstructive pulmonary disease, chronic kidney disease, cardiovascular disease, cardiac arrhythmia, arterial hypertension, diabetes, dementia, cancer and dyslipidemia), physical examination factors (respiratory failure, low blood pressure, hypoxemia, tachycardia, dyspnea, anorexia, tachypnea, haemoptysis, abdominal pain, fatigue, fever and myalgia or arthralgia), laboratory factors (high blood procalcitonin, myocardial injury markers, high blood White Blood Cell count (WBC), high blood lactate, low blood platelet count, plasma creatinine increase, high blood D-dimer, high blood lactate dehydrogenase (LDH), high blood C-reactive protein (CRP), decrease in lymphocyte count, high blood aspartate aminotransferase (AST), decrease in blood albumin, high blood interleukin-6 (IL-6), high blood neutrophil count, high blood B-type natriuretic peptide (BNP), high blood urea nitrogen (BUN), high blood creatine kinase (CK), high blood bilirubin and high erythrocyte sedimentation rate (ESR)), radiological factors (consolidative infiltrate and pleural effusion) and high SOFA score (sequential organ failure assessment score).
Conclusion
Identified prognostic factors can help clinicians and policy makers in tailoring management strategies for patients with COVID-19 infectious disease while researchers can utilise our findings to develop multivariable prognostic models that could eventually facilitate decision-making and improve patient important outcomes.
Systematic review registration
Prospero registration number: CRD42020178802. Protocol available at: https://www.medrxiv.org/content/10.1101/2020.04.08.20056598v1.
Introduction
COVID-19 is an infectious disease caused by the SARS-CoV-2 coronavirus [1]. It was first identified in Wuhan, China, on December 31, 2019 [2]; five months later, more than six million cases had been identified across 215 countries [3]. On March 11, 2020, WHO characterised the COVID-19 outbreak as a pandemic [1]. While the majority of cases present with mild symptoms, a minority progress to acute respiratory illness and hypoxia requiring hospitalization, and a subset develop acute respiratory distress syndrome, multi-organ failure or have fatal outcomes [4]. The case fatality rate reported across countries, settings and age groups is highly variable, ranging from about 0.5% to 10% [5]. In hospitalised patients it has been reported to be higher than 20% [6].
Prognostic factors (stand-alone or combined in risk assessment models) may guide the stratification of patients with SARS-CoV-2 infectious disease based on their risk of severe disease or death. This risk stratification may subsequently guide optimised management and resource utilisation strategies in the care of these patients.
Although multiple prognostic factors have been proposed and some have been accepted as “established” by the scientific community (i.e age), the predictive value of most of these potential prognostic factors has not been robustly evaluated and remains uncertain. As pointed out by Wynants et.al: “unreliable predictors could cause more harm than benefit in guiding clinical decisions” [7]. For example, aggressive and risky interventions might be attempted if the risk of poor outcomes are inaccurately defined as high based on unreliable predictors.
Using innovative and agile processes supported by advanced evidence synthesis tools and collaborative efforts across several international research groups, this systematic review aims to provide a rigorous summary of the evidence available on prognostic factors that may be used in decision-making related to the care of patients infected with COVID-19.
Methods
Protocol registration
We published [8] and registered the protocol for this systematic review with PROSPERO (CRD42020178802).
Search strategy
We conducted highly sensitive searches in PubMed/MEDLINE, the Cochrane Central Register of Controlled Trials (CENTRAL) and Embase. The searches covered the period from the inception date of each database until April 28, 2020. No study design, publication status or language restriction were applied.
Detailed strategies for each database are reported in the S1 Text.
In order to identify articles that might have been missed in the electronic searches, we reviewed the reference list of each included study and performed cross citation in Google Scholar using each included study as the index reference.
Study selection
Four reviewers working independently and in duplicate, performed study selection, including screening of titles and abstracts and of potentially eligible full-text articles. Reviewers resolved disagreements by discussion.
We included studies examining individual prognostic factors or risk assessment models based on the typologies of prognosis proposed by Iorio and colleagues [9] and the PROGnosis RESearch Strategy (PROGRESS) Group framework [10] without applying any restrictions based on analytical methods (i.e performing multivariable analysis).
Specifically, we included studies that evaluated patients with confirmed SARS-CoV-2 infectious disease regardless of the healthcare setting (i.e ambulatory or inpatients) and of baseline disease severity. We investigated all candidate prognostic factors reported by individual studies and compared patients exposed (the factor was present) with patients unexposed (the factor was absent) to each one of those factors.We considered studies that assessed mortality or severe COVID-19 disease as outcomes and accepted the author's definitions of the latter. When severe COVID-19 disease was reported as a multi-categorical scale, we used the most severe category. Additionally, when severe COVID-19 disease was not reported as an outcome, we considered ICU requirement, invasive mechanical ventilation (IVM) and acute respiratory distress syndrome (ARDS) as surrogate outcomes.
Data extraction
For each eligible study, five pairs of reviewers, independently, abstracted the following information on study characteristics (year of publication, country, medical center and time period in which the study was conducted); population characteristics (sample size, context in which the study was conducted and other population characteristics); description of prognostic factors and outcomes and their definitions; and study results (measures of association or crude event rates for every candidate prognostic factor and outcome reported).
Risk of bias assessment
Two reviewers assessed the risk of bias of individual included studies independently and in duplicate. Discrepancies were resolved by consensus. We used the Quality in Prognosis Studies (QUIPS) tool for prognostic factor studies [11] which considers population characteristics, attrition, prognostic factor and outcome measurement and potential residual confounding. For “study confounding summary” and “statistical analysis and presentation domains”, in order to assess adequacy of the multivariable models, we considered appropriate model adjustment as based on inclusion of age, one comorbidity (e.g diabetes) and one parameter of disease severity (e.g. respiratory rate) at minimum.
Data synthesis and analysis
We presented the results of individual prognostic factors in both tabular and narrative formats. We standardized the units of measurement for each prognostic factor, unifying the direction of the predictors and adjusting the weights of the studies, and calculated crude effect estimates when not provided [12]. When possible, we meta-analysed all prognostic factors whose association with the selected outcomes of interest was explored and were reported by more than one study. We used the generic inverse variance-based method to produce an overall measure of association and random-effect models based on the DerSimonian-Laird method provided by the metafor package for R software [13].
For every candidate prognostic factor, we presented the measure of association as odds ratios (ORs) and their corresponding 95% confidence intervals (CI). In studies that reported the measure of association as a hazard ratio (HR) or risk ratio (RR), we converted them to ORs using the baseline risk (death rate or incidence of severe COVID-19 out of the total sample) reported in the studies [14,15]. For dichotomous variables, we used the crude event rate to calculate ORs when no measures of association were provided. We excluded information on continuous variables for which no measures of association were available. We also calculated absolute risk differences (RDs) that can be attributed to every individual candidate prognostic factor by applying the ORs to estimated baseline risks (see below “Baseline risks”). When the same candidate prognostic factor was assessed in alternative ways (e.g. dichotomic and continuous) we used the one for which we found better certainty of evidence.
For every explored candidate variable, we performed sensitivity analysis excluding high risk of bias studies and studies that did not report adjusted estimates. In cases where the effect estimates provided by the primary analysis and the sensitivity analysis significantly differed, we either presented the moderate/low risk of bias–adjusted estimates or the primary analysis estimates but rated down certainty of the evidence because for risk of bias (see below). In addition, when we observed inconsistent results for disease severity outcome, we performed subgroup analyses accounting for outcome definition (i.e severity scale vs. IVM vs. ARDS) as a potential source of heterogeneity.
Assessment of certainty of the evidence
We assessed certainty of the evidence for each candidate prognostic factor, by outcome, based on the GRADE approach [16]. The approach considers the following domains: risk of bias, indirectness, inconsistency, imprecision, and publication bias. We produced summary of findings tables and rated the certainty of the evidence as high, moderate, low or very low depending on the grading of the individual domains [16]. See S1 Text for a detailed description of the certainty of the evidence assessment.
Result interpretation
To define which candidate variables provide valuable prognostic information we adopted a minimally contextualized approach [17]. To this end, we arbitrarily set thresholds to define important incremental increase in the risk of our outcomes. In setting those thresholds we aimed to define the minimal incremental increase in the risk of mortality or severe COVID-19 disease that could be interpreted as valuable prognostic information without considering the potential consequences of using that information in healthcare decision-making. These thresholds represent the line that separates a risk increase that is trivial from a small but important risk increase. We set those thresholds in 0.5% increase in mortality and 1% increase insevere COVID-19 disease. We performed a sensitivity analysis in which we adopted a purely non-contextualized approach [17] to assess mortality outcome. In doing so we only considered the relative measures of association and used an OR of 1 as the threshold for minimal important risk increment.
Baseline risks
To define baseline risks we selected clinical scenarios based in the severity categories proposed by WHO [18]. To assess the prognostic value on mortality, we used the clinical scenario of a patient infected with COVID-19 with severe but not critical disease (i.e patients with respiratory failure but not invasive mechanical ventilation and/or hemodynamic support requirement). We identified one study informing prognosis in this specific subgroup with a mortality risk of 9% [19]. However, as we identified significant variability in mortality risks reported for similar clinical scenarios (i.e in the RECOVERY trial [20] mortality risk in hospitalized patients assigned to the control arm, with no baseline oxygen requirement was 14%), we performed a sensitivity analysis using a baseline risk of 26% as reported by a large cohort of non-ICU inpatients treated in 255 sites across 36 countries [6].
To assess the prognostic value on severe COVID-19 disease, we used the clinical scenario of a patient infected with COVID-19 with non-severe disease. We identified 7 studies informing prognosis in this specific subgroup with a median risk of progression to severe or critical state of 13% [21–27]. We calculated baseline risks (risks in patients not exposed to the prognostic factor) by also considering the prevalence of every prognostic factor and the estimates of association [28]. When prevalence of prognostic factors was not available we used described baseline risks (9% for mortality and 13% for severe COVID-19 disease).
Update of this systematic review
An artificial intelligence algorithm deployed in the Coronavirus/COVID-19 topic of the L.OVE platform (https://app.iloveevidence.com/loves/5e6fdb9669c00e4ac072701d) will provide instant notification of articles with a high likelihood of eligibility. These will be screened by paired reviewers iteratively and will conduct data extraction and iterative updates of estimates for selected prognostic factors accordingly. We will consider resubmission to a journal if there is a substantial modification on the measure of association or the certainty of the evidence for a given prognostic factor such that it is clinically significant, at the discretion of the reviewer team. This review is part of a larger project established to produce multiple parallel systematic reviews relevant to COVID-19 [29].
Results
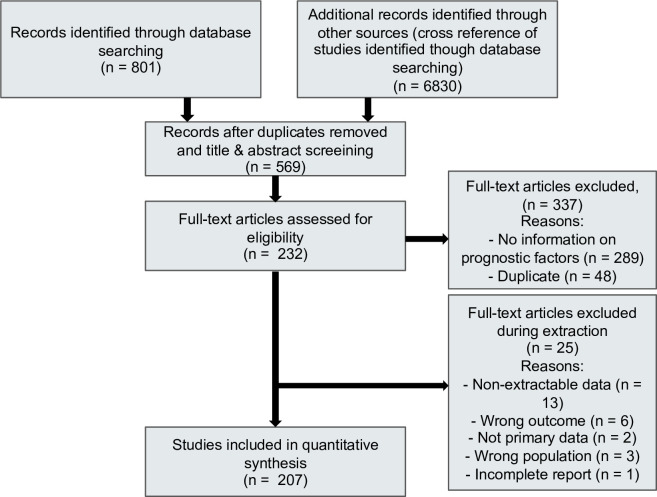
(Fig 1) illustrates the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. Our search identified 7631 citations of which we included 569 studies for full text assessment. 207 studies fulfilled the inclusion criteria [21,23–25,27,30–231]. These 207 studies, with sample sizes from 10 to 8910, enrolled a total of 75607 patients and were conducted in 12 different countries (China, USA, Canada, Spain, France, Turkey, Korea, Japan, Italy, Germany, India and Singapore).
Fig 1. PRISMA (preferred reporting items for systematic reviews and meta-analyses) flowchart of study inclusions and exclusions.
Description of included studies
S1 Table describes the characteristics of the included studies reporting on mortality and/or severe COVID-19 disease. Regarding candidate prognostic factors, of the 207 included studies, 184 (88.9%) reported socio-demographic variables, 180 (86.9%) comorbidities, 178 (86%) clinical findings, 176 (85%) laboratory findings and 106 (51.3%) imaging findings. The outcomes reported were mortality in 116 (56%) and progression to severe/critical status in 131 (63.3%). In 78 (37.7%) of the included studies a multivariable analysis was performed. In the 150 studies in which the severity of included patients was described the mean proportion of patients in each category was: non-severe disease 63.8%, severe disease 22.6%, critical disease 13.6%.
Risk of bias
Risk of bias was high across most identified studies. Among the 207 included studies only 7 were judged as low risk of bias [21,24,56,57,59,130,142] as the remaining presented important limitations in at least one domain or item. Most frequent were retrospective design, which may have introduced classification bias, and lack or inappropriate adjusted analysis. S2 Table provides detailed judgements for each of the risk of bias domains criteria.
Prognostic factors for mortality
We investigated 96 candidate prognostic factors for mortality from 116 studies including 57044 patients. S3 Table provides a summary of findings for all the candidate prognostic factors and S1 Appendix includes the corresponding forest plots.
We found high or moderate certainty that the following 35 variables provide valuable prognostic information on mortality outcome (Table 1).
Table 1. Prognostic factors for mortality and/or severe COVID-19 disease.
| Prognostic factor | Mortality | Severe COVID-19 disease | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients (studies) | Odds ratio (95%CI) | Risk without prognostic factor | Risk with prognostic factor | Certainty of the evidence | Number of patients (studies) | Odds ratio (95%CI) | Risk without prognostic factor | Risk with prognostic factor | Certainty of the evidence | |
| Socio-demographic characteristics | ||||||||||
| Age Definition: 10 years increase | 11962 (19) | 1.80 (1.54–2.10) | 9% | 15.1% | ⨁⨁⨁⨁ HIGH | 14456 (53) | 1.63 (1.47–1.80) | 13% | 19.6% | ⨁⨁⨁⨁ HIGH |
| 6.1% increase in mortality. Between 4.2% more and 8.2% more | 6.6% increase in severe COVID-19 disease. Between 5% more and 8.2% more | |||||||||
| Sex Definition: Male | 31948 (58) | 1.72 (1.5–1.98) | 8% | 13% | ⨁⨁⨁◯ MODERATEd | 25032 (122) | 1.53 (1.4–1.67) | 10.8% | 15.5% | ⨁⨁⨁⨁ HIGH |
| 5% increase in mortality. Between 4% more and 7% more | 4.7% increase in severe COVID-19 disease. Between 3.7% more and 5.6% more | |||||||||
| Smoking Definition: Active, present smoker | 12025 (16) | 1.57 (1.19–2.07) | 8.7% | 13% | ⨁⨁⨁⨁ HIGH | 9147 (45) | 1.65 (1.25–2.17) | 12.1% | 18.4% | ⨁⨁⨁◯ MODERATE d |
| 4.3% increase in mortality. Between 1.5% more and 7.5% more | 6.3% increase in severe COVID-19 disease. Between 2.7% more and 10.2% more | |||||||||
| Medical illness and patient history | ||||||||||
| Any chronic condition or comorbidities | 4406 (16) | 3.3 (2.18–5) | 5.9% | 16.2% | ⨁⨁⨁◯ MODERATE a | 6640 (40) | 3.16 (2.71–3.68) | 8.2% | 20.1% | ⨁⨁⨁⨁ HIGH |
| 10.3% increase in mortality. Between 6.8% more and 13.4% more | 12% increase in severe COVID-19 disease. Between 10.6% more and 13.2% more | |||||||||
| Cerebrovascular disease Definition: History of stroke or CNS disease | 15294 (26) | 2.85 (2.02–4.01) | 8.7% | 21.3% | ⨁⨁⨁⨁ HIGH | 11050 (42) | 2.67 (1.84–3.87) | 12.7% | 27.8% | ⨁⨁⨁◯ MODERATE d |
| 12.6% increase in mortality. Between 7.5% more and 18.5% more | 15.1% increase in severe COVID-19 disease. Between 8.4% more and 22.8% more | |||||||||
| COPD | 34759 (41) | 2.43 (1.88–3.14) | 8.5% | 18.4% | ⨁⨁⨁⨁ HIGH | 15468 (65) | 2.7 (2.14–3.4) | 12.6% | 27.9% | ⨁⨁⨁⨁ HIGH |
| 9.8% increase in mortality. Between 6.4% more and 13.6% more | 15.3% increase in severe COVID-19 disease. Between 11% more and 20% more | |||||||||
| Chronic kidney disease Definition: KDIGO definition of CKD | 23448 (28) | 2.27 (1.69–3.05) | 8.5% | 17.2% | ⨁⨁⨁⨁ HIGH | 12056 (42) | 2.21 (1.51–3.24) | 12.8% | 24.5% | ⨁⨁◯◯ LOW a,d |
| 8.8% increase in mortality. Between 5.1% more and 12.9% more | 11.7% increase in severe COVID-19 disease. Between 5.4% more and 19.2% more | |||||||||
| Cardiovascular disease Definition: Coronary heart disease or congestive heart failure | 37156 (51) | 2.12 (1.77–2.56) | 8.1% | 15.5% | ⨁⨁⨁◯ MODERATE d | 16679 (73) | 3.34 (2.71–4.1) | 12.2% | 31.3% | ⨁⨁⨁◯ MODERATE d |
| 7.5% increase in mortality. Between 5.4% more and 9.7% more | 19.1% increase in severe COVID-19 disease. Between 15.1% more and 23.1% more | |||||||||
| Cardiac arrhythmia | 12729 (6) | 2.13 (1.72–2.65) | 7% | 13.6% | ⨁⨁⨁⨁ HIGH | 747 (4) | 16.51 (6.69–40.77) | 6.5% | 35.5% | ⨁⨁◯◯ LOW a,c,e |
| 6.5% increase in mortality. Between 4.7% more and 8.4% more | 29% increase in severe COVID-19 disease.Between 22.6% more and 32.3% more | |||||||||
| Arterial hypertension | 31341 (52) | 2.02 (1.71–2.38) | 7% | 13% | ⨁⨁⨁⨁ HIGH | 20817 (94) | 2.5 (2.21–2.92) | 11.1% | 23.3% | ⨁⨁⨁◯ MODERATEd |
| 6% increase in mortality. Between 4.5% more and 7.3% more | 12.1% increase in severe COVID-19 disease. Between 10.4% more and 14.4% more | |||||||||
| Diabetes | 30303 (52) | 1.84 (1.61–2.1) | 7.9% | 13.6% | ⨁⨁⨁⨁ HIGH | 21381 (97) | 2.51 (2.2–2.87) | 12% | 25.2% | ⨁⨁⨁⨁ HIGH |
| 5.6% increase in mortality. Between 4.3% more and 7% more | 13.2% increase in severe COVID-19 disease. Between 11% more and 15.5% more | |||||||||
| Dementia | 8922 (3) | 1.54 (1.31–1.81) | 9% | 13.2% | ⨁⨁⨁⨁ HIGH | 0 (0) | NA | NA | NA | NA |
| 4.2% increase in mortality. Between 2.5% more and 6.2% more | NA | |||||||||
| Obesity: BMI > 25–30 | 9127 (3) | 1.41 (1.15–1.74) | 8.5% | 11.5% | ⨁⨁⨁⨁ HIGH | 1140 (8) | 3.74 (2.37–5.89) | 10.2% | 35% | ⨁⨁⨁⨁ HIGH |
| 3.1% increase in mortality. Between 1.2% more and 5.1% more | 16.7% increase in severe COVID-19 disease. Between 9.6% more and 24.7% more | |||||||||
| Cancer Definition: Solid or active haematologic cancer | 22734 (25) | 1.35 (1.17–1.55) | 8.9% | 11.6% | ⨁⨁⨁⨁ HIGH | 15156 (58) | 2.06 (1.64–2.58) | 12.8% | 23.2% | ⨁⨁⨁◯ MODERATEd |
| 2.7% increase in mortality. Between 1.4% more and 4.2% more | 10.4% increase in severe COVID-19 disease. Between 6.6% more and 14.5% more | |||||||||
| Dyslipidemia | 11273 (4) | 1.26 (1.06–1.5) | 8.9% | 11% | ⨁⨁⨁◯ MODERATEb | 559 (4) | 0.63 (0.22–1.83) | 13.1% | 8.7% | ⨁⨁◯◯ LOW a,b |
| 2.1% increase in mortality. Between 0.5% more and 3.9% more | 4.4% decrease in severe COVID-19 disease. Between 10% less and 8.3% more | |||||||||
| Symptoms, vital signs and physical examination | ||||||||||
| Respiratory failure Definition: increased respiratory rate, abnormal blood gases (hypoxemia, hypercapnia, or both), and evidence of increased work of breathing | 1887 (8) | 21.17 (4.9–91.3) | 3.1% | 23.4% | ⨁⨁⨁◯ MODERATEa | 1156 (7) | 23.21 (12.07–44.62) | NA | NA | NA |
| 20.3% increase in mortality. Between 13.4% more and 22.4% more | NA | |||||||||
| Low blood pressure Definition SBP less than 90–100 mmHg | 1269 (2) | 6.7 (3.14–14.33) | 9% | 39.9% | ⨁⨁⨁◯ MODERATEa | 480 (2) | 1.29 (0.72–2.29) | NA | NA | NA |
| 30.9% increase in mortality. Between 14.7% more and 49.6% more | NA | |||||||||
| Hypoxemia Definition: Low digital saturation (below 90–93%) | 1047 (5) | 5.46 (2.05–14.53) | 2.3% | 9.1% | ⨁⨁⨁◯ MODERATEa | 1331 (5) | 4.69 (1.56–14.09) | NA | NA | NA |
| 6.7% increase in mortality. Between 4.2% more and 7.7% more | NA | |||||||||
| Tachycardia Definition: More than 90–100 bpm | 1269 (2) | 2.61 (1.62–4.22) | 9% | 20.5% | ⨁⨁⨁◯ MODERATEa | 78 (1) | 1.54 (0.31–7.58) | 13% | 18.7% | ⨁◯◯◯ VERY LOWa,f |
| 11.5% increase in mortality. Between 4.8% more and 20.4% more | 5.7% increase in severe COVID-19 disease. Between 8.6% less and 40.1% more | |||||||||
| Dyspnea Definition: Dyspnea or shortness of breath | 6613 (28) | 3.45 (2.72–4.38) | 4.9% | 13.8% | ⨁⨁⨁⨁ HIGH | 16803 (78) | 4.23 (3.32–5.38) | 9.3% | 27.8% | ⨁⨁◯◯ LOWa,d |
| 8.9% increase in mortality. Between 7.5% more and 10.2% more | 18.5% increase in severe COVID-19 disease. Between 15.4% more and 21.3% more | |||||||||
| Anorexia | 1483 (8) | 2.16 (1.14–4.12) | 7.3% | 14.4% | ⨁⨁⨁◯ MODERATEc | 5495 (26) | 2.86 (2.16–3.84) | 10.4% | 24% | ⨁⨁⨁◯ MODERATEa |
| 7% increase in mortality. Between 1.1% more and 13.1% more | 13.6% increase in severe COVID-19 disease. Between 9.8% more and 17.5% more | |||||||||
| Tachypnea Definition: More than 20–24 bpm | 202 (1) | 1.21 (1.12–1.31) | 7.6% | 9% | ⨁⨁⨁◯ MODERATEa | 518 (7) | 7.51 (1.66–33.91) | 13% | 52.9% | ⨁⨁⨁◯ MODERATEd |
| 1.4% increase in mortality. Between 0.9% more and 1.9% more | 39.9% increase in severe COVID-19 disease. Between 6.9% more and 70.5% more | |||||||||
| Haemoptysis | 781 (5) | 2.91 (0.74–11.4) | 8.3% | 20.6% | ⨁⨁◯◯ LOWa,b | 3317 (14) | 4.39 (2.18–8.81) | 12.7% | 38.6% | ⨁⨁⨁◯ MODERATEa |
| 12.3% increase in mortality. Between 2.2% less and 34.6% more | 25.9% increase in severe COVID-19 disease. Between 11.4% more and 42.1% more | |||||||||
| Abdominal pain | 1127 (5) | 1.06 (0.53–2.11) | 9% | 9.5% | ⨁⨁◯◯ LOWa,b | 4896 (22) | 1.95 (1.36–2.79) | 12.7% | 22% | ⨁⨁⨁◯ MODERATEa |
| 5% increase in mortality. Between 4% less and 8% more | 9.4% increase in severe COVID-19 disease. Between 4% more and 15.8% more | |||||||||
| Fatigue | 3725 (21) | 1.66 (1.27–2.17) | 7.1% | 11.1% | ⨁⨁◯◯ LOWa,d | 13262 (71) | 1.41 (1.19–1.68) | 11.6% | 15.5% | ⨁⨁⨁◯ MODERATEd |
| 4% increase in mortality. Between 1.9% more and 5.9% more. | 3.9% increase in severe COVID-19 disease. Between 2% more and 5.9% more. | |||||||||
| Fever. Definition: More than 37.5°C | 6154 (31) | 1.04 (0.77–1.4) | 8.7% | 9.1% | ⨁⨁◯◯ LOWa,b | 20026 (102) | 1.84 (1.54–2.21) | 9.3% | 15.4% | ⨁⨁⨁◯ MODERATEd |
| 0.3% increase in mortality. Between 2.3% less and 2.5% more | 6.1% increase in severe COVID-19 disease. Between 4.5% more and 7.6% more | |||||||||
| Myalgia/arthralgia Definition: myalgia or arthralgias | 3436 (18) | 0.96 (0.77–1.23) | 9.1% | 8.7% | ⨁⨁◯◯ LOWa,b | 13814 (61) | 1.29 (1.03–1.61) | 12.5% | 15.6% | ⨁⨁⨁◯ MODERATEd |
| 0.3% decrease in mortality. Between 2% less and 1.8% more | 3% increase in severe COVID-19 disease. Between 0.3% more and 5.9% more | |||||||||
| Laboratory measures (blood or plasma) | ||||||||||
| High procalcitonin Definition: More than 0.01–05 ng/ml | 4735 (10) | 12.42 (7.18–21.5) | 6.3% | 38.5% | ⨁⨁⨁◯ MODERATEc | 7923 (28) | 5.13 (3.16–8.35) | 10.6% | 35.4% | ⨁⨁◯◯ LOWa,d |
| 32.3% increase in mortality. Between 25% more and 38.1% more | 24.8% increase in severe COVID-19 disease. Between 16.7% more and 32.3% more | |||||||||
| Myocardial injury Definition: Reported as myocardial injury or as increase in blood troponins | 3855 (21) | 10.89 (5.39–22.04) | 3.5% | 20.4% | ⨁⨁⨁◯ MODERATEd | 3627 (20) | 10 (6.84–14.62) | 11.1% | 51.3% | ⨁⨁⨁⨁ HIGH |
| 16.9% increase in mortality. Between 13.4% more and 19% more | 40.2% increase in severe COVID-19 disease. Between 33.1% more and 46.4% more | |||||||||
| High WBC Definition: greater than 10.0 x 109/L | 2870 (10) | 4.06 (2.7–6.12) | 7.8% | 24.7% | ⨁⨁⨁◯ MODERATEd | 9331 (32) | 4.67 (3.17–6.88) | 11.2% | 35.6% | ⨁⨁⨁⨁ HIGH |
| 16.9% increase in mortality. Between 11% more and 23.3% more | 24.3% increase in severe COVID-19 disease. Between 17.3% more and 31.2% more | |||||||||
| High lactate: Definition More than 1.5–2.2 mmol/L | 1078 (1) | 3.66 (2.26–5.94) | 7.3% | 21.7% | ⨁⨁⨁◯ MODERATEa | 812 (3) | 3.74 (0.69–20.16) | 12.1% | 33.3% | ⨁◯◯◯ VERY LOW a,b,d |
| 14.3% increase in mortality. Between 8.3% more and 20.6% more | 21.2% increase in severe COVID-19 disease. Between 3.7% less and 51.4% more | |||||||||
| Low platelet count Definition: Less than 100–150 x 109/L | 3676 (10) | 5.43 (2.55–11.56) | 5% | 19.3% | ⨁⨁⨁⨁ HIGH | 8081 (32) | 1.93 (1.52–2.46) | 11.1% | 19.2% | ⨁⨁◯◯ LOWa,d |
| 14.3% increase in mortality. Between 8.3% more and 18.6% more | 8% increase in severe COVID-19 disease. Between 5% more and 11.1% more | |||||||||
| High D-dimer Definition: More than 500–1000 ng/ml | 4361 (17) | 4.81 (3.15–7.34) | 4.3% | 15.6% | ⨁⨁⨁◯ MODERATEd | 6356 (24) | 3.27 (2.46–4.36) | 8.2% | 20.7% | ⨁⨁⨁◯ MODERATEd |
| 11.2% increase in mortality. Between 8.8% more and 13.1% more | 12.5% increase in severe COVID-19 disease. Between 9.8% more and 14.8% more | |||||||||
| High LDH Definition: More than 240–250 U/L | 1440 (6) | 4.09 (1.18–14.17) | 4.7% | 15.2% | ⨁⨁⨁◯ MODERATEd | 7955 (26) | 4.48 (3.21–6.25) | 7.8% | 23.9% | ⨁⨁⨁◯ MODERATEd |
| 10.4% increase in mortality. Between 1.4% more and 15.3% more | 16.2% increase in severe COVID-19 disease. Between 13.1% more and 18.8% more | |||||||||
| High CRP Definition: More than 1–100 mg/l | 2107 (8) | 6.6 (3.36–12.99) | 2.3% | 10.3% | ⨁⨁⨁◯ MODERATEd | 9094 (37) | 4.5 (3.1–6.23) | 6.3% | 19.5% | ⨁⨁⨁⨁ HIGH |
| 7.9% increase in mortality. Between 6.4% more and 8.7% more | 13.2% increase in severe COVID-19 disease. Between 10.8% more and 14.9% more | |||||||||
| Decrease in Lymphocyte count Definition: per 1 x 109 U/L decrease | 544(3) | 3.57 (2–6.67) | 9% | 26.1% | ⨁⨁⨁◯ MODERATEd | 1909 (7) | 2.28 (1.21–4.30) | 13% | 25.4% | ⨁⨁⨁◯ MODERATEd |
| 17.1% increase in mortality. Between 7.5% more and 30.7% more | 12.4% increase in severe COVID-19 disease. Between 2.3% more and 26.1% more | |||||||||
| High AST level Definition: More than 32–40 U/l | 2969 (7) | 3.5 (1.59–7.71) | 6% | 17.1% | ⨁⨁⨁◯ MODERATEd | 9179 (32) | 3.41 (2.7–4.3) | 9.9% | 25.8% | ⨁⨁⨁◯ MODERATEa |
| 11.1% increase in mortality. Between 4% more and 16.8% more | 15.8% increase in severe COVID-19 disease. Between 12.7% more and 18.8% more | |||||||||
| Decrease in albumin: Definition: 20 g/L decrease | 336 (3) | 1.53 (1.32–1.78) | 9% | 13.2% | ⨁⨁⨁◯ MODERATEc | 1266 (5) | 1.11 (1.01–1.21) | 13% | 14.2% | ⨁⨁⨁◯ MODERATEb |
| 4.2% increase in mortality. Between 2.5% more and 6% more | 1.2% increase in severe COVID-19 disease. Between 0.1% more and 2.3% more | |||||||||
| Increase in creatinine Definition: per 1 mg/dL increase | 1508 (9) | 1.14 (1.02–1.28) | 9% | 10.1% | ⨁⨁⨁◯ MODERATEb | 1116 (4) | 1.89 (0.87–4.10) | 13% | 22% | ⨁⨁⨁◯ MODERATEb |
| 1.1% increase in mortality. Between 0.2% more and 2.3% more | 9% increase in severe COVID-19 disease. Between 1.5% less and 25% more | |||||||||
| High Neutrophil count Definition: greater than 6.3 x 109/L | 727 (2) | 6.78 (3.07–14.97) | 5.2% | 23% | ⨁⨁◯◯ LOWa,c | 4945 (16) | 5.66 (3.71–8.63) | 9% | 31% | ⨁⨁⨁◯ MODERATEa |
| 17.8% increase in mortality. Between 10% more and 23% more | 22% increase in severe COVID-19 disease. Between 17% more and 27% more | |||||||||
| High BNP: More than 500–900 pg/mL | 1283 (6) | 3.27 (1.24–8.63) | 7% | 19% | ⨁⨁◯◯ LOWa,d | 1086 (1) | 4.99 (3.2–7.77) | 9.4% | 30.9% | ⨁⨁⨁◯ MODERATEa |
| 12% increase in mortality. Between 1.9% more and 21.9% more | 21.5% increase in severe COVID-19 disease. Between 15.5% more and 26.7% more | |||||||||
| High BUN Definition: mmol/L, > 5.2–9.5 | 1258 (2) | 10.56 (6.76–16.48) | 5.2% | 29.6% | ⨁⨁◯◯ LOWa,c,e | 3890 (10) | 3.66 (2.82–4.74) | 11.1% | 30.2% | ⨁⨁⨁◯ MODERATEa |
| 24.4% increase in mortality. Between 20.2% more and 27.7% more | 19.1% increase in severe COVID-19 disease. Between 14.8% more and 23.4% more | |||||||||
| High CPK Definition: More than 185–200 U/L | 407 (3) | 1.35 (0.58–3.14) | 8.8% | 11.5% | ⨁⨁◯◯ LOWa,b | 3292 (13) | 3.1 (2.32–4.16) | 11.5% | 28.1% | ⨁⨁⨁◯ MODERATEa |
| 2.7% increase in mortality. Between 3.7% less and 13% more | 16.5% increase in severe COVID-19 disease. Between 11.7% more and 21.6% more | |||||||||
| High total bilirubin Definition: More than 17–21 pg/ml | 2715 (3) | 3.03 (1.87–4.92) | 8.1% | 20.7% | ⨁⨁◯◯ LOWa,c | 5098 (14) | 2.94 (2.18–3.97) | 12.5% | 29.3% | ⨁⨁⨁◯ MODERATEa |
| 12.6% increase in mortality. Between 6.3% more and 19.9% more | 16.8% increase in severe COVID-19 disease. Between 11.3% more and 22.9% more | |||||||||
| High interleukin-6 Definition: More than 5–20 pg/ml | 436 (4) | 1.31 (0.14–12.27) | 8.1% | 10.3% | ⨁⨁◯◯ LOWa,b | 1211 (7) | 7.36 (2.97–18.27) | 6.5% | 26.2% | ⨁⨁⨁◯ MODERATEa |
| 2.2% increase in mortality. Between 11.6% less and 15% more | 19.7% increase severe COVID-19 disease. Between 12.2% more and 23.9% more | |||||||||
| High Definition More than 10–20 mm/H | 628 (3) | 0.89 (0.54–1.45) | 9.7% | 8.7% | ⨁⨁◯◯ LOWa,b | 2557 (12) | 3.08 (2.04–4.65) | 6.6% | 15.6% | ⨁⨁⨁◯ MODERATEa |
| 1% decrease in mortality. Between 5.6% less and 2.8% more | 9.4% increase in severe COVID-19 disease. Between 6.7% more and 11.3% more | |||||||||
| Radiological signs | ||||||||||
| Pleural effusion Definition: X ray or CT assessment | 820 (5) | 1.38 (0.63–3.06) | 8.8% | 11.7% | ⨁⨁◯◯ LOWa,b | 5289 (23) | 3.31 (2.03–5.38) | 12.5% | 32% | ⨁⨁⨁◯ MODERATEd |
| 3% increase in mortality. Between 3% less and 13% more | 19% increase in severe COVID-19 disease. Between 10% more and 30% more | |||||||||
| Consolidation pattern Definition: X ray or CT assessment | 795 (4) | 1.93 (1.31–2.84) | 7.5% | 13% | ⨁⨁◯◯ LOWa,c | 6133 (27) | 2.46 (1.54–3.93) | 11.2% | 23.2% | ⨁⨁⨁◯ MODERATEd |
| 5.8% increase in mortality. Between 2.3% more and 9.4% more | 12% increase in severe COVID-19 disease. Between 5.4% more and 18.8% more | |||||||||
| Others | ||||||||||
| High SOFA score Definition: More than 2 | 585 (3) | 1.97 (1.22–3.2) | 9% | 16.3% | ⨁⨁⨁◯ MODERATEd | 92 (2) | 21.31 (6.26–72.6) | 13% | 76.1% | ⨁⨁◯◯ LOWa,c |
| 7.3% increase in mortality. Between 1.8% more and 15% more | 63% increase in severe COVID-19 disease. Between 35.3% more and 78.6% more | |||||||||
Glossary
FDP: Fibrin Degradation Product.
PT: Prothrombin time.
APTT: Activated partial thromboplastin time.
APACHE: Acute Physiology And Chronic Health Evaluation II.
SOFA: The sequential organ failure assessment score.
qSOFA: Quick sepsis related organ failure assessment.
AST: Aspartate aminotransferase.
ALT: Alanine aminotransferase.
BUN: Blood urea nitrogen.
NA: Not applicable, either because there is no information or because the addressed variable does not represent a potential prognostic factor in that clinical scenario.
Explanations
a. Risk of bias due to study limitations (unadjusted estimates, inappropriate prognostic factor or outcome assessment, inappropriate population inclusion criteria, study attrition).
b. Imprecision: Confidence interval includes significant and non-significant risk increase.
c. Imprecision due to fragility: Less than 200 events.
d. Inconsistency: Unexplained visual heterogeneity.
e. Risk of selective reporting: Most of the pooled estimate weight from studies that performed multivariable analysis but did not report adjusted estimates.
f. Very serious imprecision: Very wide confidence interval.
Demographic factors
Age per 10 years increase (OR 1.8, 95% CI 1.54 to 2.1; RD 6.1%, 95% CI 4.2 to 8.2%), male sex (OR 1.72, 95% CI 1.5 to 1.98; RD 5%, 95% CI 4 to 7%) and active smoker (OR 1.57, 95% CI 1.19 to 2.07; RD 4.3%, 95%CI 1.5 to 7.5%).
Medical illness and patient history factors
Any chronic condition or comorbidity (OR 3.3, 95% CI 2.18 to 5; RD 10.3%, 95% CI 6.8 to 13.4%), cerebrovascular disease (OR 2.85, 95% CI 2.02 to 4.01; RD 12.6%, 95% CI 7.5 to 18.5%), chronic obstructive pulmonary disease (COPD) (OR 2.43, 95% CI 1.88 to 3.14; RD 9.8%, 95% CI 6.4 to 13.6%), chronic kidney disease (CKD), (OR 2.27, 95% CI 1.69 to 3.05; RD 8.8%, 95% CI 5.1 to 12.9%), cardiovascular disease (defined as coronary heart disease and/or cardiac failure) (OR 2.12, 95% CI 1.77 to 2.56; RD 7.5%, 95% CI 5.4 to 9.7%), cardiac arrhythmia (OR 2.13, 95% CI 1.72 to 2.65; RD 6.5%, 95% CI 4.7 to 8.4%), arterial hypertension (OR, 2.02, 95% CI 1.71 to 2.38; RD 6%, 95% CI 4.5 to 7.33), diabetes (OR 1.84, 95% CI 1.61 to 2.1; RD 5.6%, 95% CI 4.3 to 7%), dementia (OR 1.54, 95% CI 1.31 to 1.81; RD 4.2%, 95% CI 2.5 to 6.2%), obesity (OR 1.41, 95% CI 1.15 to 1.74; RD 3.1%, 95% CI 1.2 to 5.1%), cancer (OR 1.35, 95% CI 1.17 to 1.55; RD 2.7%, 95% CI 1.4 to 4.2) and dyslipidemia (OR 1.26, 95%CI 1.06–1.5; RD 2.1%, 95%CI 0.5% to 3.9%).
Symptoms, vital signs and physical examination factors
Respiratory failure (OR 21.17, 95% CI 4.9 to 91.3; RD 20.3%, 95% CI 13.4% to 22.4%), low blood pressure (OR 6.7, 95% CI 3.14 to 14.33; RD 30.9%, 95% CI 14.7% to 49.6%), hypoxemia (OR 5.46, 95% CI 2.05 to 14.53; RD 6.7%, 95% CI 4.2% to 7.7%), tachycardia (OR 2.61, 95% CI 1.62 to 4.22; RD 11.5%, 95% CI 4.8% to 20.4%), dyspnea (OR 3.45, 95% CI 2.72 to 4.38; RD 8.9%, 95% CI 7.5% to 10.2%), anorexia (OR 2.16, 95% CI 1.14 to 4.12; RD 7%, 95% CI 1.1% to 13.1%) and tachypnea (OR 1.21, 95%CI 1.12 to 1.31; RD 1.4%, 95% CI 0.9% to 1.9%).
Laboratory factors (measured in blood or plasma)
High procalcitonin (OR 12.42, 95% CI 7.18 to 21.5; RD 32.3%, 95% CI 25% to 38.1%), myocardial injury markers (OR 10.89, 95% CI 5.39 to 22.04; RD 16.9%, 95% CI 13.4% to 19%), high white cell count (WBC) (OR 4.06, 95% CI 2.7 to 6.12; RD 16.9%, 95% CI 11% to 23.3%), high lactate (OR 3.66, 95% CI 2.26 to 5.94; RD 14.3%, 95% CI 8.3% to 20.6%), low platelet count (OR 5.43, 95% CI 2.55 to 11.56; RD 14.3%, 95% CI 8.3% to 18.6%), high D-dimer (OR 4.81, 95% CI 3.15 to 7.34; RD 11.2%, 95% CI 8.8% to 13.1%), high lactate dehydrogenase (LDH) (OR 4.09, 95% CI 1.18 to 14.17; RD 10.4%, 95% CI 1.4% to 15.3%), high c-reactive protein (CRP) (OR 6.6, 95% CI 3.36 to 12.99; RD 7.9%, 95% CI 6.4% to 8.7%), decrease in lymphocyte count (OR 3.57, 95% CI 2 to 6.67; RD 17.1%, 95% CI 7.5% to 30.7%), high aspartate aminotransferase (AST) (OR 3.5, 95% CI 1.59–7.71; RD 11.1%, 95% CI 4% to 16.8%), albumin increase (OR 1.53, 95% CI 1.32 to 1.78; RD 4.2%, 95% CI 2.5% to 6%) and creatinine increase (OR 1.14, 95%CI 1.02 to 1.28; RD 1.1%, 95% CI 0.2% to 2.3%).
Others
SOFA score> 2 (OR 1.97, 95% CI 1.22 to 3.2; RD 7.3%, 95% CI 1.8% to 15%).
Prognostic factors for severe COVID-19 disease
We investigated 96 candidate prognostic factors for severe COVID-19 disease from 131 studies including 28538 patients. S3 Table provides a summary of findings for all the candidate prognostic factors and S2 Appendix includes the corresponding forest plots.
In addition to identified prognostic factors for mortality, we found high or moderate certainty that the following 14 variables provide valuable prognostic information on severe COVID-19 disease outcome (Table 1).
Symptoms, vital signs and physical examination factors
Haemoptysis (OR 4.39, 95% CI 2.18 to 8.81; RD 25.9%, 95% CI 11.4% to 42.1%), abdominal pain (OR 1.95, 95% CI 1.36 to 1.79; RD 9.4%, 95% CI 4% to 15.8%), fatigue (OR 1.41, 95% CI 1.19 to 1.68; RD 3.9%, 95% CI 2% to 5.9%), fever (OR 1.84, 95% CI 1.54 to 2.21; RD 6.1%, 95% CI 4.5% to 7.6%) and myalgia or arthralgia (OR 1.29, 95% CI 1.03 to 1.61; RD 3%, 95%CI 0.3% to 5.9%).
Laboratory factors (measured in blood or plasma)
High neutrophil count (OR 5.66, 95% CI 3.71 to 8.63; RD 22%, 95% CI 17% to 27%), high B-type natriuretic peptide (BNP) (OR 4.99, 95% CI 3.2 to 7.77; RD 21.5%, 95% CI 15.5% to 26.7%), High urea nitrogen (BUN) (OR 3.66, 95% CI 2.82 to 4.74; RD 19.1%, 95% CI 14.8% to 23.4%), high creatine kinase (CK) (OR 3.1, 95% CI 2.32 to 4.16; RD 16.5%, 95% CI 11.7% to 21.6%), high bilirubin (OR 2.94, 95% CI 2.18 to 3.97; RD 16.8%, 95% CI 11.3% to 22.9%), high interleukin-6 (IL-6) (OR 7.36, 95% CI 2.97 to 18.27; RD 13.3%, 95% CI 8.5% to 15.9%), high erythrocyte sedimentation rate (ESR) (OR 3.08, 95% CI 2.04 to 4.65; RD 9.4%, 95% CI 6.7% to 11.3%).
Radiological factors
Consolidative infiltrate (OR 2.46, 95% CI 1.54 to 3.93; RD 12%, 95% CI 5.4% to 18.8%) and pleural effusion (OR 3.31, 95% CI 2.03 to 5.38; RD 19%, 95% CI 10% to 30%).
Other analysed variables
The remaining variables analysed were: asthma, tuberculosis, HIV infection, immunocompromise, autoimmune disease, malnutrition, chronic liver disease, thyroid disease, chronic gastric disease, chest pain, high fever, cough, rhinorrhea, odynophagia, conjunctivitis, sputum production, enlarged lymph nodes, rash, headache, vomits, diarrhea, anemia, low WBC, low neutrophil count, glomerular filtration rate, blood urea, cystatin C, prothrombin time, APTT time, ferritin, cholinesterase, alanine aminotransferase (ALT), fibrinogen degradation products, globulin, prealbumin, blood glucose, alfa-HBDH, low density lipoprotein (LDL), triglycerides, any abnormal radiologic finding, radiological interstitial pattern, ground glass opacity, crazy paving pattern, radiological evidence of enlarged lymph nodes, bilateral radiological compromise, APACHE (Acute Physiology And Chronic Health Evaluation II), qSOFA (quick sepsis related organ failure assessment).
For all of these variables we found low or very low certainty evidence both for mortality and severe COVID-19 disease. Hence, it is uncertain if these variables provide prognostic value in the context of COVID-19 infected patients.
Additional analysis
We performed a sensitivity analysis on mortality outcome using a non-contextualized approach and assuming adjusted estimates as at low risk of being biased (less demanding risk of bias approximation in comparison with the primary analysis) (see methods, risk of bias assessment in S1 Text). The results were similar to the primary analysis. However our certainty increased to moderate or high for some prognostic factors for which we had very low or low certainty: Chest pain, cough, sputum production, anemia, high ferritin, high ALT, increase in blood glucose and high APACHE score.
A second sensitivity analysis in which we set a significantly higher baseline mortality risk (26%) for patients with severe but non-critical COVID-19 disease did not show differences with the primary analysis.
When we found inconsistent results for severe COVID-19 disease outcome (n = 43), we performed subgroup analyses accounting on outcome definition. Observed heterogeneity could not be explained by this analysis for any of the candidate prognostic factors explored (see S3 Appendix).
Discussion
In this systematic review we evaluated prognostic factors for poor outcome in patients with covid-19 infectious disease. We found 49 variables that provide valuable prognostic information for mortality and/or severe COVID-19 disease. Identified prognostic factors include socio-demographic characteristics (age, male sex and smoking) medical illness and patients history information (comorbidities including chronic respiratory, cardiac and endocrinologic conditions), physical examination findings (respiratory failure related symptoms as well as general clinical condition deterioration), laboratory (multiple biomarkers and alterations in basic laboratory tests) and radiological findings (consolidation pattern and pleural effusion) (Table 1). Overall the risk of severe COVID-19 disease or death resulted higher in older patients and those with previous medical conditions including COPD and cardiovascular disease as some of the most relevant predictors. Additionally, those patients that presented with clinical signs and symptoms suggesting respiratory failure or laboratory biomarkers showing inflammation or organ damage were also at increased risk of severe COVID-19 disease or death. Radiological features did not show good predictive value.
Strengths and limitations of the study
Our systematic review has a number of strengths. First, it provides the most comprehensive and trustworthy body of evidence up to date as it includes a significant number of studies not included in prior reviews. Secondly, we followed the GRADE approach to summarize and rate the certainty on the evidence. And thirdly, we presented our results both as relative estimates of association as well as absolute risk differences and used the latter to interpret and analyse our results. We consider that the absolute risk modification that can be attributed to a prognostic factor is a critical piece of information for those aiming to make decisions using prognostic information.
Regarding limitations, most of the studies included in this review were not published in peer review journals (only as preprint) at the time we performed the search. We identified most of those studies by cross reference search in google scholar, but it is possible that some may have not been detected by our search strategy. Additionally, given the high publication speed of COVID-19 studies it is probable that new relevant information not included in our review is available at the time our review is published. We aim to address this issue by updating our results in the short term. Although we made efforts to identify data duplication, in many instances it was not clear if studies reported, totally or partially, on the same cohorts of patients hence we assume there is a considerable chance of some degree of data overlap between included publications.
Significant variability in study design, study type, patient eligibility criteria, prognostic factor definition was observed, however, given the huge amount of information analysed, it was not feasible to explore subgroup effects accounting for those differences.
In analysing our results we implemented a minimally contextualized approach for which we arbitrarily set thresholds to define the minimal important risk difference necessary to assume valuable prognostic information. As the degree of contextualization was minimal, we set very low thresholds (near the point of no effect). We acknowledge that readers might find those thresholds inappropriate, hence we also provided relative estimates of association which can be used with alternative thresholds or analytical approximations (e.g partially contextualized approach). In addition, for some candidate prognostic factors, baseline risk could not be adjusted for prevalence which might have resulted in an overestimation or underestimation of the risk difference estimates. Finally, for risk of bias assessment, we defined a set of requirements for dealing with potential confounders that were not previously validated.
Relation to prior work
We identified multiple systematic reviews addressing prognostic factors in patients with COVID-19 infectious disease [232–251]. All analysed certain prognostic factors or groups or prognostic factors that we included in the present review, and measured mortality and/or disease severity as outcomes. Most of the reported results are in consonance with our findings with only a few exceptions. Kumar et.al [246] reported diarrhea, productive cough and high ALT as prognostic factors however we found low certainty evidence on those variables in our primary analysis. Wang et.al [249] reported no association between chronic kidney disease and malignancy with poor outcomes in COVID-19 patients however we found that both conditions are associated with an increased risk of mortality and severe COVID-19 disease. Other significant differences of our review in relation to these prior reviews include multiple characteristics that were previously not identified as prognostic factors. In contrast to previous reviews, here we provide both relative and absolute estimates of risk and provide our certainty in those estimates.
Significant information has been published since our search was finalized. An update of the ISARIC registry [252] including 15194 hospitalised patients discharged or dead, the openSAFELY registry [253] included 17425445 adults potentially exposed to COVID-19 infection and a Chinese registry [254] that included 44672 patients with COVID-19.
These studies identified the following variables as prognostic factors for COVID-19 related mortality: Age, sex (male), obesity, cardiovascular disease, diabetes, arterial hypertension, dyslipidemia, COPD, smoking, malignancy, cerebrovascular disease, dementia and chronic kidney disease. All these variables were captured by our analysis as predictors of COVID-19 related mortality or severe COVID-19 disease for which moderate or high certainty evidence exists. In addition, other prognostic factors were identified by these studies: race (not white) [253] and deprivation [253], two variables we did not explore, and immunocompromise [253], asthma [253], autoimmune diseases [253], and chronic liver disease [252,253], four variables for which we found low certainty evidence.
Implications of study
Our approach considered two clinical scenarios in which we assumed that the prognostic information of each predictor for each outcome could potentially impact decision-making. In patients presenting with mild disease, predicting the risk of progression to severe status could support decisions on the level of healthcare required and more extensive follow up strategies. In the same way, in patients presenting with severe disease, predicting mortality risk could support the use of certain, more aggressive, therapeutic interventions. Clinicians or decision-makers can use our results to tailor management strategies for patients with COVID-19. For example, they could select a set of prognostic factors for which there is high certainty in a significant risk incremental increase (e.g Age, gender, comorbidities, respiratory failure and myocardial injury) and use them to define hospitalization rules for patients consulting to the emergency department. However, to what extent accounting for these prognostic factors will improve clinically important outcomes is a question that cannot be addressed with our results. Furthermore using information on multiple individual prognostic factors for outcome prediction is challenging. Multivariable models provide a solution to this limitation, however, considering the high demand for accurate risk prediction models for patients with COVID-19 [7], our work can also provide solid grounds for development of these prognostic tools.
Conclusions
We have identified a set of variables that provide valuable prognostic information in patients with COVID-19 infectious disease. Clinicians and policy makers can use our results to tailor management strategies for patients with this condition while researchers can utilise our findings to develop multivariable prognostic models that could eventually facilitate decision-making and improve patient important outcomes.
Supporting information
This table presents detailed information on individual included studies.
(PDF)
This table contains a detailed RoB assessment of included studies.
(PDF)
This table presents the complete results including all assessed candidate variables.
(DOCX)
This file contains additional details on methods.
(DOCX)
This file contains the references of the pages according to PRISMA Statement.
(DOCX)
This file contains the Forest plots for all assessed candidate variables.
(PDF)
This file contains the Forest plots for all assessed candidate variables.
(PDF)
This file contains the Forest plots for all performed subgroup analyses.
(PDF)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work
References
- 1.World Health Organization. Director-General's remarks at the media briefing on 2019-nCoV on 11 February 2020. World Health Organization 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020.
- 2.Hui DS, Azhar Ei, Madani TA, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis 2020; 91: 264–266. 10.1016/j.ijid.2020.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Worldometer. COVID-19 coronavirus pandemic. 2020. https://www.worldometers.info/coronavirus/ (accessed June 2, 2020).
- 4.Hu Y, Sun J, Dai Z, et al. Prevalence and Severity of Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis. J Clin Virol 2020; 127: 104371 10.1016/j.jcv.2020.104371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centre for Evidence-Based Medicine. Global Covid-19 Case Fatality Rates. https://www.cebm.net/covid-19/global-covid-19-case-fatality-rates/ (accessed April 6, 2020).
- 6.International Severe Acute Respiratory and Emerging Infections Consortium (ISARIC). COVID-19 Report: 19 May 2020. https://media.tghn.org/medialibrary/2020/05/ISARIC_Data_Platform_COVID-19_Report_19MAY20.pdf. (accessed June 2, 2020).
- 7.Wynants L, Van Calster B, Bonten MMJ, et al. Prediction models for diagnosis and prognosis of Covid-19 infection: Systematic review and critical appraisal. BMJ-Brit Med J 2020; 369:m1328 10.1136/bmj.m1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Izcovich A, Ragusa M, Sanguine V, Tortosa F, Espinosa F, Agnoletti C, et al. Prognostic factors for severity and mortality in patients infected with COVID-19: A living systematic review protocol. medRxiv 2020; published online April 11. 10.1101/2020.04.08.20056598 [preprint]. [DOI] [Google Scholar]
- 9.Iorio A, Spencer FA, Falavigna M, et al. Use of GRADE for assessment of evidence about prognosis: Rating confidence in estimates of event rates in broad categories of patients. BMJ-Brit Med J 2015; 350:h870 10.1136/bmj.h870 [DOI] [PubMed] [Google Scholar]
- 10.Hemingway H, Croft P, Perel P, et al. Prognosis research strategy (PROGRESS) 1: A framework for researching clinical outcomes. BMJ-Brit Med J 2013; 346: e5595 10.1136/bmj.e5595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayden JA, Van Der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med 2013; 158(4): 280–286. 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- 12.Ahn S, Fessler JA. Standard errors of mean, variance, and standard deviation estimators. 2003. Available from: 10.1037/e572902006-006. [DOI]
- 13.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw 2010; 36 (3). [Google Scholar]
- 14.Grant RL. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ-Brit Med J 2014; 348: f7450 10.1136/bmj.f7450 [DOI] [PubMed] [Google Scholar]
- 15.Wang Z. Converting odds ratio to relative risk in cohort studies with partial data information. J Stat Softw 2013; 55 (5). [Google Scholar]
- 16.Farid F, Guyatt G, Zuk V, et al. GRADE Guidelines 28: Use of GRADE for the assessment of evidence about prognostic factors: Rating certainty in identification of groups of patients with different absolute risks. J Clin Epidemiol 2020; 121: 60–70. [DOI] [PubMed] [Google Scholar]
- 17.Hultcrantz M, Rind D, Akl EA, T, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017; 87: 4–13. 10.1016/j.jclinepi.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO. Clinical management of COVID-19 (interim guidance). https://www.who.int/publications-detail/clinical-management-of-covid-19. (accessed on June 2, 2020).
- 19.Hu L, Chen S, Fu Y, et al. Risk factors associated with clinical outcomes in 323 COVID-19 patients in Wuhan, China. medRxiv 2020; published online March 30. 10.1093/cid/ciaa539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19—Preliminary Report. N Engl J Med. 2020 Jul 17;NEJMoa2021436.
- 21.Bai X, Fang C, Zhou Y, et al. Predicting COVID-19 malignant progression with AI techniques. medRxiv 2020; published online March 27. 10.1101/2020.03.20.20037325 [preprint]. [DOI] [Google Scholar]
- 22.Yan L, Zhang H-T, Goncalves J, et al. A machine learning-based model for survival prediction in patients with severe COVID-19 infection. medRxiv 2020; published online March 17. 10.1101/2020.02.27.20028027 [preprint]. [DOI] [Google Scholar]
- 23.Dong J, Zhang D, Xu J, et al. prediction for progression risk in patients with COVID-19 pneumonia: The CALL score. Clin Infect Dis 2020; published online April 09. 10.1093/cid/ciaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bi Q, Hong C, Meng J, et al. Characterization of clinical progression of COVID-19 patients in Shenzhen, China. medRxiv 2020; published online May 13. 10.1101/2020.04.22.20076190 [preprint]. [DOI] [Google Scholar]
- 25.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol 2020; S0091-6749(20): 30495–4. 10.1016/j.jaci.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Fang J, Zhu Y, et al. Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang hospital. Clin Microbiol Infect 2020; published online April 03. 10.1016/j.cmi.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong J, Ou J, Qiu X, et al. A tool to early predict severe 2019-novel coronavirus pneumonia (COVID-19): A multicenter study using the risk nomogram in Wuhan and Guangdong, China. medRxiv 2020; published online April 22. 10.1101/2020.03.17.20037515 [preprint]. [DOI] [Google Scholar]
- 28.Farid F, Iorio A, Thabane L, Guyatt G. Calculation of absolute risk for important outcomes in patients with and without a prognostic factor of interest. J Clin Epidemiol 2020; 117: 46–51. 10.1016/j.jclinepi.2019.08.012 [DOI] [PubMed] [Google Scholar]
- 29.Rada G, Verdugo-Paiva F, Ávila C, et al. ; COVID-19 L.OVE Working Group. Evidence synthesis relevant to COVID-19: A protocol for multiple systematic reviews and overviews of systematic reviews. Medwave 2020; 20(3): e7867. [DOI] [PubMed] [Google Scholar]
- 30.Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 Patients with COVID-19 in New York: Retrospective case series. medRxiv 2020; published online May 07. 10.1101/2020.04.20.20072116 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Auld S, Caridi-Scheible M, Blum JM, et al. ICU and ventilator mortality among critically ill adults with COVID-19. medRxiv 2020; published online April 26. 10.1101/2020.04.23.20076737 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bai T, Tu S, Wei Y, et al. Clinical and laboratory factors predicting the prognosis of patients with COVID-19: An analysis of 127 patients in Wuhan, China. SSRN 2020; published online March 5. 10.2139/ssrn.3546118 [preprint]. [DOI] [Google Scholar]
- 33.Benelli G, Buscarini E, Canetta C, et al. SARS-COV-2 comorbidity network and outcome in hospitalized patients in Crema, Italy. medRxiv 2020; published online April 30. 10.1101/2020.04.14.20053090 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai Q, Huang D, Ou P, et al. COVID-19 in a designated infectious diseases hospital outside Hubei province, China. Allergy 2020; 00: 1–11. [DOI] [PubMed] [Google Scholar]
- 35.Cao M, Zhang D, Wang Y, et al. Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China. medRxiv 2020; published online March 06. 10.1101/2020.03.04.20030395 [preprint]. [DOI] [Google Scholar]
- 36.Cao W. Clinical features and laboratory inspection of novel coronavirus pneumonia (COVID-19) in Xiangyang, Hubei. medRxiv 2020; published online February 25. 10.1101/2020.02.23.20026963 [preprint]. [DOI] [Google Scholar]
- 37.Chao C, Meiping C, Yiting L, et al. Clinical features and predictors for patients with severe SARS-CoV-2 pneumonia: A retrospective multicenter cohort study. SSRN 2020; published online March 31. 10.2139/ssrn.3556663 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen G, Wu D, Guo W, et al. Clinical and immunologic features in severe and moderate forms of coronavirus disease 2019. medRxiv 2020; published online February 19. 10.1101/2020.02.16.20023903 [preprint]. [DOI] [Google Scholar]
- 39.Chen J, Pan H, Wang Y, et al. Clinical characteristics of critically ill patients with SARS-CoV-2 infected pneumonia (COVID-19) in Wenzhou, China: A retrospective, single-center, descriptive study. SSRN 2020; published online April 20. 10.2139/ssrn.3571553 [preprint]. [DOI] [Google Scholar]
- 40.Chen J, Qi T, Liu L, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect 2020; 80: e1–e6. 10.1016/j.jinf.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen L, Liu HG, Liu W, et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi (Chinese journal of tuberculosis and respiratory diseases) 2020;43(0):E005 10.3760/cma.j.issn.1001-0939.2020.0005. [DOI] [PubMed] [Google Scholar]
- 42.Chen M, Tu C, Tan C, et al. Key to successful treatment of COVID-19: Accurate identification of severe risks and early intervention of disease progression. medRxiv 2020; published online April 11. 10.1101/2020.04.06.20054890 [preprint]. [DOI] [Google Scholar]
- 43.Chen M, Yongzhen F, Xiaoyan W, et al. Clinical characteristics and risk factors for fatal outcome in patients with 2019-coronavirus infected disease (COVID-19) in Wuhan, China. SSRN 2020; published online March 03. 10.2139/ssrn.3546069 [preprint]. [DOI] [Google Scholar]
- 44.Chen R, Liang W, Jiang M, et al. Risk factors of fatal outcome in hospitalized subjects with coronavirus disease 2019 from a nationwide analysis in China. Chest 2020; published online April 15. 10.1016/j.chest.2020.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen TL, Dai Z, Mo P, et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China (2019): A single-centered, retrospective study. J Gerontol 2020; published online April 11. 10.1093/gerona/glaa089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen W, Chen C, Huang L, et al. Clinical characteristics of 85 patients infected by SARS-CoV-2 in Guangxi, China. SSRN 2020; published online April 01. 10.2139/ssrn.3559599 [preprint]. [DOI] [Google Scholar]
- 47.Chen X, Liu Z. Early prediction of mortality risk among severe COVID-19 patients using machine learning. medRxiv 2020; published online April 19. 10.1101/2020.04.13.20064329 [preprint]. [DOI] [Google Scholar]
- 48.Chen X, Zhang Y, Zhu B, et al. Associations of clinical characteristics and antiviral drugs with viral RNA clearance in patients with COVID-19 in Guangzhou, China. SSRN 2020; published online April 14. 10.2139/ssrn.3566234 [preprint]. [DOI] [Google Scholar]
- 49.Chen X, Zhao B, Qu Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated Interleukin 6 (IL-6) level in critically ill COVID-19 patients. medRxiv 2020; published online March 03. 10.1101/2020.02.29.20029520 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X, Zheng F, Qing Y, et al. Epidemiological and clinical features of 291 cases with coronavirus disease 2019 in areas adjacent to Hubei, China: A double-center observational study. medRxiv 2020; published online March 06. 10.1101/2020.03.03.20030353 [preprint]. [DOI] [Google Scholar]
- 51.Chen Y, Zhang K, Zhu G, et al. Clinical characteristics and current treatment of critically ill patients with COVID-19 outside Wuhan, China: A multicenter, retrospective, observational study.SSRN 2020; published online March 23. 10.2139/ssrn.3551426 [preprint]. [DOI] [Google Scholar]
- 52.Cheng Y, Luo R, Wang K, et al. Kidney impairment is associated with in-hospital death of COVID-19 patients. medRxiv 2020; published online February 20. 10.1101/2020.02.18.20023242 [preprint]. [DOI] [Google Scholar]
- 53.Chu J, Yang N, Wei Y, et al. Clinical characteristics of 54 medical staff with COVID-19: A retrospective study in a single center in Wuhan, China. J Med Virol 2020; 92(7): 807–813. 10.1002/jmv.25793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Colaneri M, Sacchi P, Zuccaro V, et al. Clinical characteristics of coronavirus disease (COVID-19) early findings from a teaching hospital in Pavia, North Italy, 21 to 28 February 2020. Euro Surveill 2020; 25(16). 10.2807/1560-7917.ES.2020.25.16.2000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Colombi D, Bodini FC, Petrini M, et al. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology 2020; published online April 17. 10.1148/radiol.2020201433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cumming MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. medRxiv 2020; published online April 20. 10.1101/2020.04.15.20067157 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol. medRxiv 2020; published online April 28. 10.1101/2020.04.23.20076042 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dreher M, Kersten A, Bickenbach J, et al. The characteristics of 50 hospitalized COVID-19 patients with and without ARDS. Dtsch Arztebl Int 2020; 117: 271–8. 10.3238/arztebl.2020.0271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du R-H, Liang L-R, Yang C-Q, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: A prospective cohort study. Eur Respir J 2020; 55: 2000524 10.1183/13993003.00524-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duan Q, Guo G, Ren Y, et al. Treatment outcomes, influence factors of 116 hospitalized COVID-19 patients with longer/prolonged treatment course in Wuhan, China. SSRN 2020; published online March 13. 10.2139/ssrn.3550017 [preprint]. [DOI] [Google Scholar]
- 61.Fan J, Hui W, Guangming Y, et al. Low-density lipoprotein is a potential predictor of poor prognosis in patients with coronavirus disease 2019. Metabolism 2020; 107:154–243. 10.1016/j.metabol.2020.154243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fan T, Hao B, Yang S, et al. Clinical characteristics of COVID-19 and establishment of a disease risk prediction model. SSRN 2020; published online March 31. 10.2139/ssrn.3556660 [preprint]. [DOI] [Google Scholar]
- 63.Fang L, Lin L, MengDa X, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol 2020; 127:104370 10.1016/j.jcv.2020.104370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fei J, Lin F, Ying L, et al. Reduction of lymphocyte at early stage elevates severity and death risk of COVID-19 patients: A hospital-based case-cohort study. medRxiv 2020; published online April 06. 10.1101/2020.04.02.20050955 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feng Y, Yun L, Tao B, et al. COVID-19 with different severity: A multi-center study of clinical features. Am J Respir Crit Care Med 2020; 201(11): 1380–1388. 10.1164/rccm.202002-0445OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feng Z, Yu Q, Yao S, et al. Early prediction of disease progression in 2019 novel coronavirus pneumonia patients outside Wuhan with CT and clinical characteristics. medRxiv 2020; published online February 23. 10.1101/2020.02.19.20025296 [preprint]. [DOI] [Google Scholar]
- 67.Fu L, Fei J, Xiang HX, et al. Analysis of death risk factors among 200 COVID-19 patients in Wuhan, China: A hospital-based case-cohort study. SSRN 2020; published online March 23. 10.2139/ssrn.3551430 [preprint]. [DOI] [Google Scholar]
- 68.Fu L, Fei J, Xu S, et al. Acute liver injury and its association with death risk of patients with COVID-19: A hospital-based prospective case-cohort study.medRxiv 2020; published online April 06. 10.1101/2020.04.02.20050997 [preprint]. [DOI] [Google Scholar]
- 69.Gao L, Jiang D, Wen XS, et al. Prognostic value of NT-ProBNP in patients with severe COVID-19. Respir Res 2020; 21: 83 10.1186/s12931-020-01352-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gao Y, Li T, Han M, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol 2020; 92(7): 791–796. 10.1002/jmv.25770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020; 323(16):1574–1581. 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gu T, Chu Q, Yu Z, et al. History of coronary heart disease increases the mortality rate of coronavirus disease 2019 (COVID-19) patients: A nested case-control study based on publicly reported confirmed cases in Mainland China. medRxiv 2020; published online April 03. 10.1101/2020.03.23.20041848 [preprint]. [DOI] [Google Scholar]
- 73.Guan W, Liang W, Zhao Y, et al. Comorbidity and its impact on 1590 Patients with Covid-19 in China: A nationwide analysis. Eur Respir J 2020; 55: 2000547 10.1183/13993003.00547-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tao Guo, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020; published online March 27. 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guo TM, Tong Y, Chen J, Huang L, Cheng B, Zhoue J. Clinical features predicting mortality risk in older patients with COVID-19. SSRN 2020; published online April 14. 10.2139/ssrn.3569846 [preprint] [DOI] [PubMed] [Google Scholar]
- 77.Guo W, Li M, Dong Y, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev 2020. 10.1002/dmrr.3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Han H, Xie L, Liu R et al. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J M Virol 2020; 92(7): 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Han M, Zhang Y, Liu Z, et al. Assessing SARS-CoV-2 RNA levels and lymphocyte/T cell counts in COVID-19 patients revealed initial immune status of patients as a major determinant of disease severity. SSRN 2020; published online March 31. 10.1007/s00430-020-00693-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Han Y, Zhang H, Mu S, et al. Lactate dehydrogenase, a risk factor of severe COVID-19 patients. medRxiv 2020; published online March 27. 10.18632/aging.103372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He W, Chen L, Chen L, et al. Clinical characteristics and outcome of 31 cases with Covid-19 diagnosed in haematology units in Wuhan, China: A retrospective cohort study. SSRN 2020; published online March 24. 10.2139/ssrn.3558004 [preprint]. [DOI] [Google Scholar]
- 82.He XW, Lai JS, Cheng J, et al. Impact of complicated myocardial injury on the clinical outcome of severe or critically ill COVID-19 Patients. Zhonghua Xin Xue Guan Bing Za Zhi (Chinese journal of cardiovascular diseases) 2020; 48(0):E011 Published online March 14. 10.3760/cma.j.cn112148-20200228-00137. [DOI] [PubMed] [Google Scholar]
- 83.Herold T, Jurinovic V, Arnreich C, et al. Level of IL-6 predicts respiratory failure in hospitalized symptomatic COVID-19 patients.medRxiv 2020; published online April 10. 10.1101/2020.04.01.20047381 [preprint]. [DOI] [Google Scholar]
- 84.Hu B, Wang D, Hu C, et al. Clinical features of critically ill patients with COVID-19 infection in China. Research Square 2020; published online March 08. 10.21203/rs.3.rs-16250/v1 [preprint]. [DOI] [Google Scholar]
- 85.Hu L, Chen S, Fu Y, et al. Risk factors associated with clinical outcomes in 323 COVID-19 patients in Wuhan, China. medRxiv 2020; published online March 30. 10.1093/cid/ciaa539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hu X, Zeng W, Zhang Y, et al. CT imaging features of different clinical types of COVID-19: a chinese multicenter study. SSRN 2020; published online March 13. 10.2139/ssrn.3550043 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu Z, Gan J, Li J, et al. Leucocyte subsets effectively predict the clinical outcome of patients with COVID-19 pneumonia: A retrospective case-control study. SSRN 2020; published online April 1. 10.2139/ssrn.3559566 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang H, Cai H, Li Y, et al. Prognostic factors for COVID-19 pneumonia progression to severe symptom based on the earlier clinical features: A retrospective analysis. medRxiv 2020; published online March 30. 10.1101/2020.03.28.20045989 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang M, Yang Y, Shang F, et al. Early and critical care in severe patients with COVID-19 in Jiangsu Province, China: A descriptive study. SSRN 2020; published online March 3. 10.2139/ssrn.3546056 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang X, Tao J, Wu H, et al. Clinical features and management of severe COVID-19: A retrospective study in Wuxi, Jiangsu Province, China. medRxiv 2020; published online April 14. 10.1101/2020.04.10.20060335 [preprint]. [DOI] [Google Scholar]
- 92.Jin JM, Bai P, He W, et al. Gender differences in patients with COVID-19: Focus on severity and mortality. medRxiv 2020; published online March 05. 10.3389/fpubh.2020.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jin X, Lian JS, Hu JH, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 2020; 69: 1002–1009. 10.1136/gutjnl-2020-320926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kalligeros M, Shehadeh F, Mylona EK, et al. Association of obesity with disease severity among patients with COVID‐19. Obesity 2020; published online April 30. 10.1002/oby.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kuang Y, He S, Lin S, et al. Clinical characteristics and CT manifestations of 143 hospitalized patients with 2019 novel coronavirus disease (COVID-19) outside Wuhan: A multi-center study in Taizhou City, Zhejiang, China. SSRN 2020; published online March 31. 10.2139/ssrn.3556667 [preprint]. [DOI] [Google Scholar]
- 96.Lee HY, Ahn J, Kang CK, et al. Association of angiotensin II receptor blockers and angiotensin-converting enzyme inhibitors on COVID-19-related outcome. SSRN 2020; published online April 14. 10.2139/ssrn.3569837 [preprint]. [DOI] [Google Scholar]
- 97.Lei L, Gao JY. Clinical characteristics of 51 patients discharged from hospital with COVID-19 in Chongqing, China. medRxiv 2020; published online February 23. 10.1101/2020.02.20.20025536 [preprint]. [DOI] [Google Scholar]
- 98.Lei S, Jiang F, Su W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine 2020; 21: 100331 10.1016/j.eclinm.2020.100331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li H, Xiang X, Ren H, et al. Serum amyloid A is a biomarker of severe coronavirus disease and poor prognosis. J Infect 202; 80: 646–655. 10.1016/j.jinf.2020.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li J, Long X, Luo H, et al. Clinical characteristics of deceased patients infected with SARS-CoV-2 in Wuhan, China. SSRN 2020; published online March 3. 10.2139/ssrn.3546043 [preprint]. [DOI] [Google Scholar]
- 101.Li J, Li M, Zheng S, et al. Leukopenia predicts risk for death in critically ill patients with COVID-19 in Wuhan, China: A single-centered, retrospective study. SSRN 2020; published online. 10.2139/ssrn.3555248. [DOI] [Google Scholar]
- 102.Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol 2020; published online April 23. 10.1001/jamacardio.2020.1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol 2020; 55(6): 327–331. 10.1097/RLI.0000000000000672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li Y, Wang Z, Yan Hui, et al. Clinical characteristics of 77 novel coronavirus 2019 infected patients with respiratory failure in the terminal stage in Wuhan. SSRN 2020; published online March 17. 10.2139/ssrn.3551325 [preprint]. [DOI] [Google Scholar]
- 105.Li Y-K, Peng S, Li L-Q, et al. Clinical and transmission characteristics of Covid-19—A retrospective study of 25 cases from a single thoracic surgery department. Curr Med Sci 2020; 40(2): 295–300. 10.1007/s11596-020-2176-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lian J, Jin X, Hao S, et al. Analysis of epidemiological and clinical features in older patients with coronavirus disease 2019 (COVID-19) out of Wuhan. Clin Infect Dis 2020; published online March 25. 10.1093/cid/ciaa242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol 2020; 21: 335–337. 10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liao X, Chen H, Wang B, et al. Critical care for severe COVID-19: A population-based study from a province with low case-fatality rate in China. medRxiv 2020; published online April 21. 10.1101/2020.03.22.20041277 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu F, Xu A, Zhang Y, et al. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis 2020; 95: 183–191. 10.1016/j.ijid.2020.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu J, Tu C, Zhu M, et al. Exploring the law of development and prognostic factors of common and severe COVID-19: A retrospective case-control study in 122 patients with complete course of disease. SSRN 2020; published online March 24. 10.2139/ssrn.3555209 [preprint]. [DOI] [Google Scholar]
- 111.Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. medRxiv 2020; published online February 22. 10.1016/j.ebiom.2020.102763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu J, Liu Y, Xiang P, et al. Neutrophil-to-Lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. medRxiv 2020; published online February 12. 10.1186/s12967-020-02374-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu K-C, Xu P, Lv W-F, et al. CT Manifestations of coronavirus disease-2019: A retrospective analysis of 73 cases by disease severity. Eur J Radiol 2020; 126: 108941 10.1016/j.ejrad.2020.108941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu R, Ming X, Xu O, et al. Association of cardiovascular manifestations with in-hospital outcomes in patients with COVID-19: A hospital staff data. medRxiv 2020; published online March 12. 10.1101/2020.02.29.20029348 [preprint]. [DOI] [Google Scholar]
- 115.Liu T, Zhang J, Yang Y, et al. The potential role of IL-6 in monitoring severe case of coronavirus disease 2019. medRxiv 2020; published online March 10. 10.1101/2020.03.01.20029769 [preprint]. [DOI] [Google Scholar]
- 116.Liu W, Tao ZW, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J 2020; 133(9):1032–1038. 10.1097/CM9.0000000000000775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu Y, Mao B, Liang S, et al. Association between age and clinical characteristics and outcomes of coronavirus disease 2019. SSRN 2020; published online March 31. 10.2139/ssrn.3556689 [preprint]. [DOI] [Google Scholar]
- 118.Liu Y, Bi L, Chen Y, et al. Active or latent tuberculosis increases susceptibility to COVID-19 and disease severity. medRxiv 2020; published online March 16. 10.1101/2020.03.10.20033795 [preprint]. [DOI] [Google Scholar]
- 119.Liu Y, Du X, Chen J, et al. Neutrophil-to-Lymphocyte Ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect 2020; 81: e6–e12. 10.1016/j.jinf.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu Y, Sun W, Guo Y, et al. Association between platelet parameters and mortality in coronavirus disease 2019: Retrospective cohort study. Platelets 2020; 31: 490–496. 10.1080/09537104.2020.1754383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu Y, Sun W, Li J, et al. Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019. medRxiv 2020; published online Febraury 27. 10.11817/j.issn.1672-7347.2020.200384 [DOI] [PubMed] [Google Scholar]
- 122.Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019-NCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 2020; 63(3): 364–374. 10.1007/s11427-020-1643-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lu J, Hu S, Fan R, et al. ACP Risk Grade: A simple mortality index for patients with confirmed or suspected severe acute respiratory syndrome coronavirus 2 disease (COVID-19) during the early stage of outbreak in Wuhan, China. medRxiv 2020; published online Febraury 23. 10.1101/2020.02.20.20025510 [preprint]. [DOI] [Google Scholar]
- 124.Luo XM, Zhou W, Yan X, et al. Prognostic value of C-Reactive Protein in patients with COVID-19. medRxiv 2020; published online March 23. 10.1101/2020.03.21.20040360 [preprint]. [DOI] [Google Scholar]
- 125.Luo XM, Zhou W, Xia H, et al. Characteristics of SARS-CoV-2 infected patients with clinical outcome during epidemic ongoing outbreak in Wuhan, China. SSRN 2020; published online March 24. 10.2139/ssrn.3552812 [preprint]. [DOI] [Google Scholar]
- 126.Lv X, Song C, Luo T, et al. Clinical characteristics between COVID-19 patients in and outside Wuhan: A multiple-centered retrospective study. SSRN 2020; published online April 23. 10.2139/ssrn.3572907 [preprint]. [DOI] [Google Scholar]
- 127.Ma J, Yin J, Qian Y, and Wu Yuan. Clinical characteristics and prognosis in cancer patients with COVID-19: A single center’s retrospective study. J Infec 2020; published online April 14. 10.1016/j.jinf.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ma KL, Liu ZH, Cao C, et al. COVID-19 myocarditis and severity factors: an adult cohort study. medRxiv 2020; published online March 23. 10.1101/2020.03.19.20034124 [preprint]. [DOI] [Google Scholar]
- 129.Ma Y, Shi N, Fan Y, et al. Predictive value of the neutrophil-to-lymphocyte ratio(NLR) for diagnosis and worse clinical course of the COVID-19: Findings from ten provinces in China. SSRN 2020; published online April 14. 10.2139/ssrn.3569838 [preprint]. [DOI] [Google Scholar]
- 130.Mehra MR, Desai SS, Kuy SR, Henry TD, Patel AN. Cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med 2020; published online May 01. 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 131.Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis 2020, published online March 16. 10.1093/cid/ciaa270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Niu S, Tian S, Lou J, et al. Clinical characteristics of older patients infected with COVID-19: A descriptive study. Arch Gerontol Geriatr 2020; 89: 104058 10.1016/j.archger.2020.104058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pan L, Mu M, Yang P, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: A descriptive, cross-sectional, multicenter study. Am J Gastroenterol 2020, 115(5): 766–773. 10.14309/ajg.0000000000000620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Paranjpe I, Russak A, De Freitas JK, et al. Clinical characteristics of hospitalized Covid-19 patients in New York City. medRxiv 2020; published online April 26. 10.1101/2020.04.19.20062117 [preprint]. [DOI] [Google Scholar]
- 135.Peng YD, Meng K., Guan HQ, et al. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-NCoV. Zhonghua Xin Xue Guan Bing Za Zhi (Chinese journal of cardiovascular diseases) 2020; 48: E004 10.3760/cma.j.cn112148-20200220-00105 [DOI] [PubMed] [Google Scholar]
- 136.Qi D, Yan X, Tang X, et al. Epidemiological and clinical features of 2019-NCoV acute respiratory disease cases in Chongqing Municipality, China: A retrospective, descriptive, multiple-center study. medRxiv 2020; published online March 20. 10.1101/2020.03.01.20029397 [preprint]. [DOI] [Google Scholar]
- 137.Qi X, Jiang Z, YU Q, et al. Machine learning-based CT radiomics model for predicting hospital stay in patients with pneumonia associated with SARS-CoV-2 infection: a multicenter study. medRxiv 2020; published online March 03. 10.21037/atm-20-3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Qin X, Qiu S, Yuan Y, et al. Clinical characteristics and treatment of patients infected with COVID-19 in Shishou, China. SSRN 2020; published online February 20. 10.2139/ssrn.3541147 [preprint]. [DOI] [Google Scholar]
- 139.Qu R, Ling Y, Zhang YH, et al. Platelet-to-lymphocyte ratio is associated with prognosis in patients with corona virus disease-19. J Med Virol 2020; 2020: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ran J, Dai B, Wang D, and Li XM. Clinical predictors of severity based on an analysis of data of COVID-19 pneumonia. SSRN 2020; published online April 01. 10.2139/ssrn.3559525 [preprint]. [DOI] [Google Scholar]
- 141.Rentsch CT, Kidwai-Khan F, Tate JP, et al. Covid-19 testing, hospital admission, and intensive care among 2,026,227 United States veterans aged 54–75 years. medRxiv 2020; published online April 14. 10.1101/2020.04.09.20059964 [preprint]. [DOI] [Google Scholar]
- 142.Rossi PG, Marino M, Formisano D, et al. Characteristics and outcomes of a cohort of SARS-CoV-2 patients in the province of Reggio Emilia, Italy. medRxiv 2020, published online April 16. 10.1101/2020.04.13.20063545 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020; 46(5): 846–848. 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sarkar J, and Chakrabarti P. A machine learning model reveals older age and delayed hospitalization as predictors of mortality in patients with COVID-19. medRxiv 2020; published online March 30. 10.1101/2020.03.25.20043331 [preprint]. [DOI] [Google Scholar]
- 145.Shi H, He L, Sun W, et al. Clinical characteristics and prognostic factors of 148 COVID-19 cases in a secondary epidemic area. SSRN 2020; published online April 23. 10.2139/ssrn.3572911 [preprint]. [DOI] [Google Scholar]
- 146.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020; published online March 25. 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shi W, Peng X, Liu T, et al. Deep learning-based quantitative computed tomography model in predicting the severity of COVID-19: A retrospective study in 196 patients. SSRN 2020; published online March 03. 10.2139/ssrn.3546089 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shi Y, Yu X, Hong Zhao, Hao Wang, Zhao R, Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: Findings of 487 cases outside Wuhan. Crit Care 2020; 24: 108 10.1186/s13054-020-2833-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Song CY, Xu J, He JQ, Lu YQ. COVID-19 early warning score: A multi-parameter screening tool to identify highly suspected patients. medRxiv 2020, published online March 08. 10.1101/2020.03.05.20031906 [preprint]. [DOI] [Google Scholar]
- 150.Sun F, Kou S, Wang S, et al. Medication patterns and disease progression among 165 patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: A single-centered, retrospective, observational study. SSRN 2020; published online March 17. 10.2139/ssrn.3551323 [preprint]. [DOI] [Google Scholar]
- 151.Sun X, Wang J, Liu Z, et al. Characteristics of patients with COVID-19 pneumonia admitted to the intensive care unit and predictors of mortality in Wuhan, China: A single-centered retrospective cohort study. SSRN 2020; published online April 06. 10.2139/ssrn.3559629 [preprint]. [DOI] [Google Scholar]
- 152.Sun Y, Dong Y, Wang Y, et al. Characteristics and prognostic factors of disease severity in patients with COVID-19: the Beijing experience. J Autoimmun 2020; published online April 24. 10.1016/j.jaut.2020.102473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tabata S, Imai K, Kawano S, et al. Non-severe vs severe symptomatic COVID-19: 104 cases from the outbreak on the cruise ship ‘Diamond Princess’ in Japan. SSRN 2020; published online April 07. 10.1101/2020.03.18.20038125 [preprint]. [DOI] [Google Scholar]
- 154.Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduct Target Ther 2020; 5: 33 10.1038/s41392-020-0148-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020; 18(4): 844–847. 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tian S, Hu N, Lou J, et al. Characteristics of COVID-19 infection in Beijing. J Infect 2020; 80(4): 401–6. 10.1016/j.jinf.2020.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tu WJ, Cao J, Yu L, Hu X, Liu Q. Clinicolaboratory study of 25 fatal cases of COVID-19 in Wuhan. Intensive Care Med 2020; published online April 06. 10.1007/s00134-020-06023-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wan S, Yi Q, Fan S, et al. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID‐19) infected patients. Br J Haematol 2020; 189(3):428–437. 10.1111/bjh.16659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323(11): 1061–1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang D, Yin Y, Hu C, et al. Clinical course and outcome of 107 patients infected with the novel coronavirus, SARS-CoV-2, discharged from two hospitals in Wuhan, China. Crit Care 2020; 24: 188 10.1186/s13054-020-02895-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang G, Wu C, Zhang Q, et al. Epidemiological and clinical features of coronavirus cisease 2019 (COVID-19) in Changsha, China. SSRN 2020; published online March 10. 10.2139/ssrn.3548770 [preprint]. [DOI] [Google Scholar]
- 162.Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect 2020; 80: 639–645. 10.1016/j.jinf.2020.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wang L, He WB, Yu XM, Liu HF, Zhou WJ, Jiang H. Prognostic value of myocardial injury in patients with COVID-19. Zhonghua Yan Ke Za Zhi (Chinese journal of ophthalmology) 2020; 56. [DOI] [PubMed] [Google Scholar]
- 164.Wang L, Li X, Chen H, et al. Coronavirus disease 19 infection does not result in acute kidney injury: An analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol 2020; 51: 343–348. 10.1159/000507471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang L, Zou A, Wang J, et al. Matrix analysis of clinical characteristics and dynamic observation of immunological features in 90 cases of COVID-19. SSRN 2020, published online April 02. 10.2139/ssrn.3556688 [preprint]. [DOI] [Google Scholar]
- 166.Wang R, Pan M, Zhang X, et al. Epidemiological and clinical features of 125 hospitalized patients with COVID-19 in Fuyang, Anhui, China. Int J Infect Dis 2020; 95: 421–428. 10.1016/j.ijid.2020.03.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang X, Fang J, Zhu Y, et al. Clinical characteristics of non-critically ill patients with novel coronavirus infection (COVID-19) in a Fangcang hospital. Clin Microbiol Infect 2020; S1198-743X(20): 30177–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang Y, Luo H, Liu S, et al. Respiratory failure among patients with COVID-19 in Jiangsu province, China: A multicentre retrospective cohort study. SSRN 2020; published online March 24. 10.2139/ssrn.3552836 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang Y, Lu X, Chen H, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med 2020; 201(11): 1430–34. 10.1164/rccm.202003-0736LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang Y, Zhou Y, Yang Z, Xia D, Geng S. Clinical characteristics of patients with severe pneumonia caused by the 2019 novel coronavirus in Wuhan, China. medRxiv 2020; published online March 03. 10.1101/2020.03.02.20029306 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 2020; published online March 16. 10.1093/cid/ciaa272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wen C, Yali Q, Zirui G, et al. Prevalence of acute kidney injury in severe and critical COVID-19 patients in Wuhan, China. SSRN 2020; published online March 24. 10.2139/ssrn.3555223 [preprint]. [DOI] [Google Scholar]
- 173.Wen Y, Wei L, Li Y, et al. Epidemiological and clinical characteristics of COVID-19 in Shenzhen, the largest migrant city of China. medRxiv 2020; published online March 23. 10.1101/2020.03.22.20035246 [preprint]. [DOI] [Google Scholar]
- 174.Wang S, Chen Z, Lin Y, et al. Epidemical and clinical characteristics of 165 patients infected with SARS-CoV-2 in Fujian province, China. SSRN 2020; published online March 23. 10.2139/ssrn.3551402 [preprint]. [DOI] [Google Scholar]
- 175.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020; published online March 13. 10.1001/jamainternmed.2020.0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wu J, Li W, Shi X, et al. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID‐19). J Intern Med 2020; published online March 27. 10.1111/joim.13063. [DOI] [PubMed] [Google Scholar]
- 177.Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int 2020; 40: 1321–1326. 10.1111/liv.14449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xie J, Covassin N, Fan Z, et al. Association between hypoxemia and mortality in patients with COVID-1. Mayo Clin Proc 2020; 95(6): 1138–1147. 10.1016/j.mayocp.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Xie J, Hungerford D, Chen H, et al. Development and external validation of a prognostic multivariable model on admission for hospitalized patients with COVID-19. medRxiv 2020; published online April 07. 10.1101/2020.03.28.20045997 [preprint]. [DOI] [Google Scholar]
- 180.Xu S, Fu L, Fei J, et al. Acute kidney injury at early stage as a negative prognostic indicator of patients with COVID-19: A hospital-based retrospective analysis. medRxiv 2020; published online March 26. 10.1101/2020.03.24.20042408 [preprint]. [DOI] [Google Scholar]
- 181.Xu W, Qu S, Xing M, et al. Epidemiologic features and clinical findings of COVID-19-infected patients in Suzhou. SSRN 2020; published online March 18. 10.2139/ssrn.3551352 [preprint]. [DOI] [Google Scholar]
- 182.Xu Y, Xu Z, Liu X, et al. Clinical findings in critical ill patients infected with SARS-Cov-2 in Guangdong province, China: A multi-center, retrospective, observational study. medRxiv 2020; published online March 06. 10.1101/2020.03.03.20030668 [preprint]. [DOI] [Google Scholar]
- 183.Xu YH, Dong JH, An WM, et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-CoV-2. J Infect. 2020; 80(4): 394–400. 10.1016/j.jinf.2020.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yan S, Song X, Lin F, et al. Clinical characteristics of coronavirus disease 2019 in Hainan, China. medRxiv 2020; published online March 23. 10.1101/2020.03.19.20038539 [preprint]. [DOI] [Google Scholar]
- 185.Yan X, Wang C, Peng D, et al. Clinical features, treatment and outcomes of 218 patients with COVID-19: A retrospective, multicenter study based on clinical classification. SSRN 2020; published online April 06. 10.2139/ssrn.3559594 [preprint]. [DOI] [Google Scholar]
- 186.Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol 2020; 84: 106504 10.1016/j.intimp.2020.106504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yang G, Tan Z, Zhou L, et al. Angiotensin II receptor blockers and angiotensin-converting enzyme inhibitors usage is associated with improved inflammatory status and clinical outcomes in COVID-19 patients with hypertension. medRxiv 2020; published online April 04. 10.1101/2020.03.31.20038935 [preprint]. [DOI] [Google Scholar]
- 188.Yang JK, Jin JM, Liu S, et al. Blood glucose is a representative of the clustered indicators of multi-organ injury for predicting mortality of COVID-19 in Wuhan, China. SSRN 2020; published online April 14. 10.2139/ssrn.3569852 [preprint]. [DOI] [Google Scholar]
- 189.Yang L, Lei Y, Zhang R, et al. Epidemiological and clinical features of 200 hospitalized patients with corona virus disease 2019 in Yichang, China: A descriptive study. J Clin Virol 2020; 129: 104475 10.1016/j.jcv.2020.104475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID‐19. J Thromb Haemost, 2020; 00: 1–4. 10.1111/jth.14848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med 2020; 8(5): 475‐481. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yin Y, Zhou S, Zhang X, et al. Critically ill patients with COVID-19 in China: A multicenter retrospective observational study. SSRN 2020; published online April 07. 10.1016/j.clnu.2020.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Young BE, Ong SWX., Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020; 323(15): 1488‐1494. 10.1001/jama.2020.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yu C, Lei Q, Li W, Wang X, Liu W, Li W. Epidemiological and clinical characteristics of 1663 hospitalized patients infected with COVID-19 in Wuhan, China. SSRN 2020; published online April 08. 10.1016/j.jiph.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yu M, Xu D, Lan L, et al. Thin-section chest CT imaging of coronavirus disease 2019 pneumonia: Comparison between patients with mild and severe disease. Radiology 2020; 2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yu T, Cai S, Zheng Z, et al. Association between clinical manifestations and prognosis in patients with COVID-19. Clin Ther 2020; published online April 27. 10.1016/j.clinthera.2020.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Yuan M, Yin W, Tao Z, Tan W, Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS One 2020; 15(3): e0230548 10.1371/journal.pone.0230548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang L, Yan X, Fan Q, et al. D‐dimer levels on admission to predict in‐hospital mortality in patients with Covid‐19. J Thromb Haemost 2020; 18: 1324–1329. 10.1111/jth.14859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zeng L, Li J, Liao M, et al. Risk assessment of progression to severe conditions for patients with COVID-19 pneumonia: A single-center retrospective study. medRxiv 2020; published online March 30. 10.1101/2020.03.25.20043166 [preprint]. [DOI] [Google Scholar]
- 200.Zeng Z, Sha T, Zhang Y, et al. Hypertension in patients hospitalized with COVID-19 in Wuhan, China: A single-center retrospective observational study. medRxiv 2020; published online April 11. 10.1101/2020.04.06.20054825 [preprint]. [DOI] [Google Scholar]
- 201.Zhang F, Yang D, Li J, et al. Myocardial injury is associated with in-hospital mortality of confirmed or suspected COVID-19 in Wuhan, China: A single center retrospective cohort study. medRxiv 2020; published online March 24. 10.1101/2020.03.21.20040121 [preprint]. [DOI] [Google Scholar]
- 202.Zhang G, Chang H, Linjie L, et al. Clinical features and outcomes of 221 patients with COVID-19 in Wuhan, China. medRxiv 2020; published online March 06. 10.1016/j.jcv.2020.104364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang G, Zhang J, Wang B, et al. Analysis of clinical characteristics and laboratory findings of 95 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A retrospective analysis. Respir Res 2020; 21: 74 10.1186/s12931-020-01338-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhang H, Xiaoying W, Zongqiang F, et al. Potential factors for prediction of disease severity of COVID-19 patients. medRxiv 2020; published online March 23. 10.1101/2020.03.20.20039818 [preprint]. [DOI] [Google Scholar]
- 205.Zhang H-Y, Wang L-W, Chen Y-Y, et al. A Multicentre Study of 2019 Novel Coronavirus Disease Outcomes of Cancer Patients in Wuhan, China. medRxiv 2020; published online April 15. 10.1101/2020.03.21.20037127 [preprint]. [DOI] [Google Scholar]
- 206.Zhang J, Peng L, Morong W, et al. The clinical data from 19 critically ill patients with coronavirus disease 2019: A single-centered, retrospective, observational study. J Publ Health 2020; published online April 21. 10.1007/s10389-020-01291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhang JJ, Dong X, Cao Y, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Eur J Allergy Clin Immunol 2020; 00: 1–12. 10.1111/all.14238 [DOI] [PubMed] [Google Scholar]
- 208.Zhang L, Tao B, Yu J, et al. Digestive involvement of the coronavirus disease 2019: Need attention on the “gut-type” in the novel coronaviral infection. SSRN 2020; published online April 06. 10.2139/ssrn.3559590 [preprint]. [DOI] [Google Scholar]
- 209.Zhang L, Tao B, Yu J, et al. Diarrhea in patients with coronavirus disease 2019: Clinical characteristics, outcomes and implications. SSRN 2020; published online April 09. 10.2139/ssrn.3566201 [preprint]. [DOI] [Google Scholar]
- 210.Zhang L, Wenwu S, Liangkai C, et al. Clinical features and a simple model for predicting the mortality of coronavirus disease 2019 patients on admission. SSRN 2020; published online March 23. 10.2139/ssrn.3551386 [preprint]. [DOI] [Google Scholar]
- 211.Zhang L, Zhu F, Xie L, et al. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann Oncol 2020; published online March 25. 10.1016/j.annonc.2020.03.296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang P, Zhu L, Cai J, et al. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension. Circ Res AHA 2020; 126 (12):1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhang R, Huangqing O, Lingli F, et al. CT features of SARS-CoV-2 pneumonia according to clinical presentation: A retrospective analysis of 120 consecutive patients from Wuhan City. Eur Radiol 2020; published online April 11. 10.1007/s00330-020-06854-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhang S, Guo M, Duan L, et al. Short term outcomes and risk factors for mortality in patients with COVID-19 in Wuhan, China: A retrospective study. SSRN 2020; published online March 23. 10.2139/ssrn.3551390 [preprint]. [DOI] [Google Scholar]
- 215.Zhang S, Zhao J, Wu Z, et al. Potential predictors for disease progression and medication evaluation of 2019 novel coronavirus-infected pneumonia in Wuhan, China. SSRN 2020; published online March 23. 10.2139/ssrn.3551399 [preprint]. [DOI] [Google Scholar]
- 216.Zhang X, Guo W, Hua J, et al. The incidence, risk factors and clinical outcomes of acute kidney injury in critically ill patients with COVID-19: A multicenter study. SSRN 2020; published online April 23. 10.2139/ssrn.3572908 [preprint]. [DOI] [Google Scholar]
- 217.Zhang X, Huan C, Jianhua H, et al. Epidemiological, clinical characteristics of cases of SARS-CoV-2 infection with abnormal imaging findings. Int J Infect Dis 2020; 94: 81–87. 10.1016/j.ijid.2020.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhang Y, Yanhui C, Minxue S, et al. Comorbid diabetes mellitus was associated with poorer prognosis in patients with COVID-19: A retrospective cohort study. medRxiv 2020; published online March 26. 10.1101/2020.03.24.20042358 [preprint]. [DOI] [Google Scholar]
- 219.Zhao W, Zheng Z, Qizhi Y, Xie X, Liu J. Clinical outcomes of patients with 2019-NCoV: A preliminary summary. SSRN 2020; published online February 25. 10.2139/ssrn.3542167 [preprint]. [DOI] [Google Scholar]
- 220.Zhao W, Shikai Y, Xiangyi Z, et al. Clinical characteristics and durations of hospitalized patients with COVID-19 in Beijing: A retrospective cohort study. medRxiv 2020; published online March 30. 10.1101/2020.03.13.20035436 [preprint]. [DOI] [Google Scholar]
- 221.Zhen L, Ming W, Jiwei Y, et al. Caution on kidney dysfunctions of COVID-19 patients. medRxiv 2020; published online March 27. 10.1101/2020.02.08.20021212 [preprint]. [DOI] [Google Scholar]
- 222.Zheng F, Tang W, Li H, Huang YX, Xie YL, Zhou ZG. Clinical characteristics of 161 cases of corona virus disease 2019 (COVID-19) in Changsha. Eur Rev Med Pharmacol Sci 2020; 24(6): 3404–10. 10.26355/eurrev_202003_20711 [DOI] [PubMed] [Google Scholar]
- 223.Zheng X, Jiehua C, Lisi D, et al. Clinical features and risk factors for the severity of inpatients with COVID-19: A retrospective cohort study. SSRN 2020; published online April 6. 10.2139/ssrn.3562460 [preprint]. [DOI] [Google Scholar]
- 224.Zhou B, She J, Wang Y, Ma X. The clinical characteristics of myocardial injury in severe and very severe patients with 2019 novel coronavirus disease. SSRN 2020; published online February 19. 10.1016/j.jinf.2020.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020; 395: 1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhou H, Zhang Z, Fan H, et al. Urinalysis, but not blood biochemistry, detects the early renal-impairment in patients with COVID-19. SSRN 2020; published online April 16. 10.2139/ssrn.3569863 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhou H, Wanxin C, Ziping L, et al. Delayed-phase thrombocytopenia in patients of coronavirus disease 2019 (COVID-19). medRxiv 2020; published online April 15. 10.1101/2020.04.11.20059170 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Zhou M, Xu J, Hao X, et al. A 'Burning Point' is found before the composite end point event happened in critically ill patients with COVID-19: A multicenter retrospective study. SSRN 2020; published online April 20. https://ssrn.com/abstract=3572864 [preprint]. [Google Scholar]
- 229.Zhou Y, Zhen Y, Yanan G, et al. A new predictor of disease severity in patients with COVID-19 in Wuhan, China. Respir Med 2020; published online March 27. 10.1101/2020.03.24.20042119 [preprint]. [DOI] [Google Scholar]
- 230.Zhu B, Chunguo J, Xiaokai F, et al. Correlation between fasting blood glucose level at admission and mortality in COVID-19 patients: A retrospective study. BMC Infect Dis 2020; published online March 24. 10.21203/rs.3.rs-18484/v1 [preprint]. [DOI] [Google Scholar]
- 231.Zhu Q, Zhao S, Lai X, et al. Dose-response association between risk factors and incidence of COVID-19 in 325 hospitalized patients: A multicenter retrospective cohort study. SSRN 2020; published online April 08. 10.2139/ssrn.3562478 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Li B, Yang J, Zhao F,et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol 2020; 109: 531–538. 10.1007/s00392-020-01626-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog Cardiovasc Dis 2020; published online March 10. 10.1016/j.pcad.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. Clin Chim Acta 2020; 505: 190–191. 10.1016/j.cca.2020.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ji W, Zhang J, Bishnu G, et al. Comparison of severe and non-severe COVID-19 pneumonia: Review and meta-analysis. medRxiv 2020; published online March 09. 10.1101/2020.03.04.20030965 [preprint]. [DOI] [Google Scholar]
- 236.Jain V, and Yuan J-M. Systematic review and meta-analysis of predictive symptoms and comorbidities for severe COVID-19 infection. medRxiv 2020; published online March 16. 10.1101/2020.03.15.20035360 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lippi G, and Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med 2020; 75: 107–108. 10.1016/j.ejim.2020.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lippi G, Henry BM. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19). Respir Med 2020; 167: 105941 10.1016/j.rmed.2020.105941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lippi G, Plebani M, and Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta 2020; 506:145–148. 10.1016/j.cca.2020.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhao X, Zhang B, Li P, et al. Incidence, clinical characteristics and prognostic factor of patients with COVID-19: A systematic review and meta-analysis. medRxiv 2020; published online March 20. 10.1101/2020.03.17.20037572 [preprint]. [DOI] [Google Scholar]
- 241.Ebrahimi M, Saki A, and Rahim F. Laboratory findings, signs and symptoms, clinical outcomes of patients with COVID-19 infection: An updated systematic reeview and meta-analysis. medRxiv 2020; published online March 30. 10.1101/2020.03.25.20043703 [preprint]. [DOI] [Google Scholar]
- 242.Henry BM, Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol 2020; 52:1193–1194. 10.1007/s11255-020-02451-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wang B, Li B, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: Evidence from meta-analysis. Aging 2020; 12(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Xiong M, Liang X, Wei YD. Changes in blood coagulation in patients with severe coronavirus disease 2019 (COVID‐19): A meta‐analysis. Br J Haematol 2020; published online May 14. 10.1111/bjh.16725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Santoso A, Pranata R, Wibowo A, et al. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: A meta-analysis. Am J Emerg Med 2020; published online April 19. 10.1016/j.ajem.2020.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kumar A, Arora A, Sharma P, et al. Clinical features of COVID-19 and factors associated with severe clinical course: A systematic review and meta-analysis. SSRN 2020; published online April 21. 10.2139/ssrn.3566166 [preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ali A, Mohamed S, Elkhidir I, et al. The association of lymphocyte count and levels of CRP, D-Dimer, and LDH with severe coronavirus disease 2019 (COVID-19): A meta-analysis. medRxiv 2020; published online April 24. 10.1101/2020.04.20.20072801 [preprint]. [DOI] [Google Scholar]
- 248.Aggarwal G, Lippi G, Michael Henry B. Cerebrovascular disease is associated with an increased disease severity in patients with coronavirus disease 2019 (COVID-19): A pooled analysis of published literature. Int J Stroke 2020; 15: 4 10.1177/1747493020921664 [DOI] [PubMed] [Google Scholar]
- 249.Wang X, Fang X, Cai Z, et al. Comorbid chronic diseases and acute organ injuries are strongly correlated with disease severity and mortality among COVID-19 patients: A systematic review and meta-analysis. Research 2020; 2020:2402961 10.34133/2020/2402961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Han P, Diao K, Pang T, Huang S, Yang Z. Comparison of clinical features between critically and non-critically Ill patients in SARS and COVID-19: A systematic review and meta-analysis. SSRN 2020; published online April 09. 10.2139/ssrn.3566133 [preprint]. [DOI] [Google Scholar]
- 251.Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect 2020; 15: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ-Brit Med J; 369: m1985 10.1136/bmj.m1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.The OpenSAFELY Collaborative, Williamson E, Walker AJ, Bhaskaran KJ, et al. OpenSAFELY: Factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv 2020; published online May 07. 10.1101/2020.05.06.20092999 [preprint]. [DOI] [Google Scholar]
- 254.Deng G, Yin M, Chen X, Zeng F. Clinical determinants for fatality of 44,672 patients with COVID-19. Crit Care. 2020; 24(1): 179 10.1186/s13054-020-02902-w [DOI] [PMC free article] [PubMed] [Google Scholar]