Abstract
We previously identified a diazaborine series with potential for development as a new tuberculosis drug. This series has activity in vitro and in vivo and targets cell wall biosynthesis via inhibition of InhA. The overall aim of this study was to determine whether InhA inhibitors have activity against non-replicating Mycobacterium tuberculosis. We tested the ability of two molecules of the diazaborine series to kill non-replicating M. tuberculosis in the nutrient starvation model; both molecules were bactericidal, reducing viability by >3 logs in 21 days. Activity showed similar kill rates to other InhA inhibitors (isoniazid and NITD-916). We conclude that inhibition of InhA is bactericidal against nutrient-starved non-replicating M. tuberculosis.
Introduction
Mycobacterium tuberculosis, the causative agent of tuberculosis, remains a global health scourge with >1 million deaths annually [1, 2]. Drug treatment is lengthy, in part because anti-tubercular agents are more effective against rapidly growing bacteria, but much less effective against slowly replicating or non-replicating bacteria. There is a need for new agents that target non-replicating persisters [3]. We have previously identified a diazaborine series with potential for development as a new tuberculosis drug [4, 5]. This series has activity in vitro and in vivo and targets cell wall biosynthesis via direct inhibition of InhA [4]. Diazaborine activity does not require a cofactor or require activation by bacterial enzymes (unlike the frontline drug isoniazid which also targets InhA) and so the series has improved properties and a lower frequency of resistance than isoniazid [4, 5].
Materials and methods
Bacterial culture
M. tuberculosis H37Rv (London Pride: ATCC 25618) [6] was cultured in Middlebrook 7H9 medium containing 10% v/v oleic acid, albumen, dextrose, catalase (OADC) supplement and 0.05% w/v Tween 80
Generation of non-replicating M. tuberculosis
M. tuberculosis was grown to log phase in medium. Bacteria were harvested and resuspended in phosphate-buffered saline (PBS) pH 7.4 plus 0.05% w/v Tyloxapol for 2 weeks to generate nutrient-starved, non-replicating bacteria [7].
Kill kinetics against non-replicating M. tuberculosis
Compounds were added to nutrient-starved, non-replicating bacteria to a final DMSO concentration of 2% for 21 days. CFUs were determined by serial dilution and culture on Middlebrook 7H10 agar plates supplemented with 10% v/v OADC for 3–4 weeks.
Results and discussion
We had previously demonstrated that two representative molecules from the diazaborine series (AN12855 and AN12541) had bactericidal activity against replicating M. tuberculosis (Fig 1) [4]. It has long been suggested that drugs that target the cell wall would not be active against non-dividing bacteria and there is some evidence that the efficacy of isoniazid is reduced under these conditions [8]. However, we had previously noted that isoniazid was able to kill non-replicating M. tuberculosis in the nutrient starvation model with >3 logs kill in 21 days even at concentrations close to the minimum inhibitory concentration (MIC) [9]. We wanted to investigate whether the diazaborines could also kill non-replicating bacteria, which could be a good indicator of their ability to shorten treatment in a novel drug regimen [3].
Fig 1. Structure of molecules used in this study.
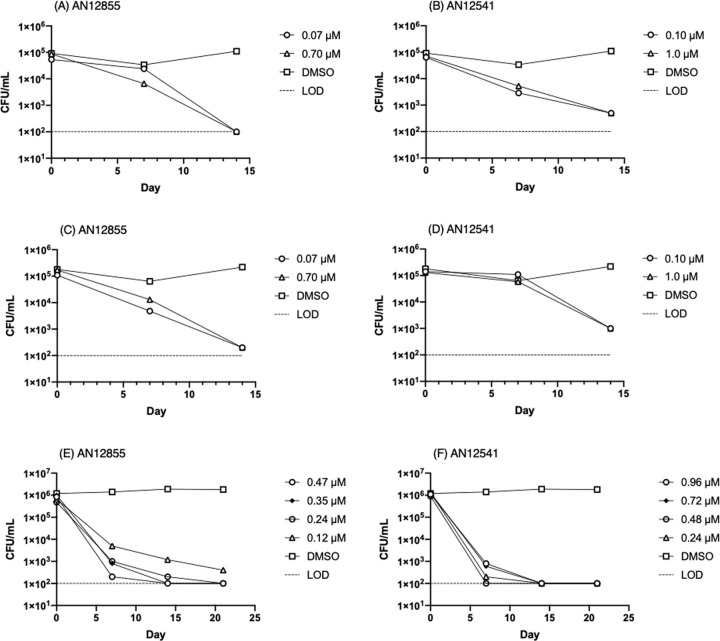
We tested the ability of two molecules of the diazaborine series in the nutrient starvation model [8]. M. tuberculosis H37Rv was grown to log phase in Middlebrook 7H9 medium containing 10% v/v oleic acid, albumen, dextrose, catalase (OADC) supplement and 0.05% w/v Tween 80. Bacteria were harvested and resuspended in phosphate-buffered saline (PBS) pH 7.4 plus 0.05% w/v Tyloxapol for 2 weeks to generate nutrient-starved, non-replicating bacteria [7]. Compounds were added and CFUs were determined over 21 days by serial dilution and culture on Middlebrook 7H10 agar. The experiment was carried out twice (independent cultures on different dates). AN12855 demonstrated time-dependent activity against non-replicating bacteria (Fig 2A and 2C) with ~3 log10 kill after 14 days at concentrations equivalent to the IC90 (0.090 ± 0.050 μM, n = 10 from [4]). AN12541 was similarly active against non-replicating bacteria (Fig 2B and 2D) but with a slightly slower kill rate, reaching ~2 log kill after 14 days (IC90 is 0.11 ± 0.21 μM, n = 2 from [4]). There was no outgrowth of resistant mutants, which is sometimes seen with isoniazid after 7 days [9].
Fig 2. Activity of diazaborines against non-replicating M. tuberculosis.
Kill kinetics of (A), (C) and (E) AN12855 and (B), (D) and (F) AN12541 against nutrient-starved, non-replicating bacteria. Limit of detection is marked by a dashed line.
Our initial kill kinetic experiments were run with a starting inoculum of 105 CFU/mL to ensure that we did not have any spontaneous resistant clones (which could mask bacterial kill during outgrowth) and over 14 days. We repeated the experiment at a higher inoculum of 106 CFU/mL to determine if there was any inoculum-dependent effect, which has been noted for other anti-tubercular agents with activity against non-replicating M. tuberculosis [9]. We also used an expanded range of concentrations and an extended period of time (21 days). For AN12855 we saw a similar kill profile over 14 days to our original experiment. Although there was a higher kill rate in the first 7 days and a slower kill rate over the second 7 days, the overall kill rate was similar at 14 days. For AN12541 we saw an accelerated kill rate using the higher inoculum, which was not expected. The difference in the initial kill rate is likely dependent on the physiological state of the bacteria during starvation which may be subtly different between cultures at different densities. Both compounds resulted in >3 log reduction in viable bacteria over 21 days confirming bactericidal activity (Fig 2E and 2F). There was no outgrowth of resistant mutants at this inoculum.
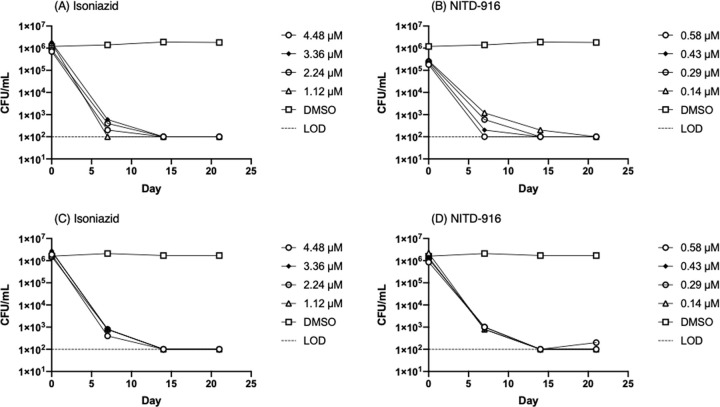
Isoniazid is inactive against non-replicating M. tuberculosis under oxygen limitation and in multi-stress models [10, 11], although we previously demonstrated it had good activity against nutrient-starved bacteria [9]. We tested isoniazid and another direct InhA inhibitor, NITD-916 [12], as a comparator for the diazaborine series (Fig 3). We ran two independent experiments (independent cultures on different dates) using the higher inoculum of 106 CFU/mL. Again, we noted that isoniazid had good activity against non-replicating bacteria, as did NITD-916. Both of these showed rapid kill with >3 logs reduction in viability over 21 days. Kill kinetics were similar between isoniazid and NITD-916. We did not see any outgrowth of resistant mutants for either compound. Thus, we conclude that inhibition of InhA is bactericidal against nutrient-starved non-replicating M. tuberculosis, and that rapid kill can be effected by InhA inhibitors regardless of the their binding mechanism.
Fig 3. Activity of InhA inhibitors against non-replicating M. tuberculosis.
Kill kinetics of (A) and (C) isoniazid and (B) and (D) NITD-916 against nutrient-starved, non-replicating bacteria. Limit of detection is marked by a dashed line.
Our work indicates that InhA is a viable target in non-replicating bacteria, at least those generated by starvation. Our data for isoniazid are consistent with those from Betts et al. [8], who demonstrated that isoniazid was able to reduce CFUs in starved bacteria by >1.5 log in 7 days at 10 μg/mL. They did not see activity at 1 μg/mL; however, their starting inoculum was >107 CFU/mL and at that high concentration it is likely that they would have outgrowth of resistant mutants, or that the longer period of starvation used in that study (6 weeks) could be a factor in generating isoniazid resistance.
Taken together with our previous data demonstrating activity against replicating and intracellular bacteria, as well as in vivo activity in mouse models of infection [4, 5], these data support the validity of both the target InhA and the diazaborine series for further exploration.
Acknowledgments
We thank Matthew McNeil and Dickon Alley for useful discussion.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This research was supported with funding from the Bill & Melinda Gates Foundation. Funding supported LF, AK and TP. https://www.gatesfoundation.org/ The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gordon SV, Parish T. Microbe profile: Mycobacterium tuberculosis: Humanity’s deadly microbial foe. Microbiol. 2018;164(4):437–9. 10.1099/mic.0.000601 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global tuberculosis report 2019. 2020.
- 3.Mandal S, Njikan S, Kumar A, Early JV, Parish T. The relevance of persisters in tuberculosis drug discovery. Microbiol. 2019;165(5):492–9. 10.1099/mic.0.000760 [DOI] [PubMed] [Google Scholar]
- 4.Xia Y, Zhou Y, Carter DS, McNeil MB, Choi W, Halladay J, et al. Discovery of a cofactor-independent inhibitor of Mycobacterium tuberculosis InhA. Life Sci Alliance. 2018;1(3):e201800025 10.26508/lsa.201800025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robertson GT, Ektnitphong VA, Scherman MS, McNeil MB, Dennison D, Korkegian A, et al. Efficacy and Improved resistance potential of a cofactor-independent InhA inhibitor of Mycobacterium tuberculosis in the C3HeB/FeJ Mouse Model. Antimicrob Agents Chemother. 2019. April;63(4). 10.1128/AAC.02071-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ioerger TR, Feng Y, Ganesula K, Chen X, Dobos KM, Fortune S, et al. Variation among genome sequences of H37Rv strains of Mycobacterium tuberculosis from multiple laboratories. J Bacteriol. 2010;192(14). 10.1128/JB.00166-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Early J, Alling T. Determination of compound kill kinetics against Mycobacterium tuberculosis. Methods Mol Biol. 2015;1285:269–79. 10.1007/978-1-4939-2450-9_16 [DOI] [PubMed] [Google Scholar]
- 8.Betts JC, Lukey PT, Robb LC, McAdam RA, Duncan K. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol Microbiol. 2002;43(3):717–31. 10.1046/j.1365-2958.2002.02779.x [DOI] [PubMed] [Google Scholar]
- 9.Bonnett SA, Dennison D, Files M, Bajpai A, Parish T. A class of hydrazones are active against nonreplicating Mycobacterium tuberculosis. PLoS One. 2018;13(10):e0198059 10.1371/journal.pone.0198059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gold B, Roberts J, Ling Y, Quezada LL, Glasheen J, Ballinger E, et al. Rapid, semiquantitative assay to discriminate among compounds with activity against replicating or nonreplicating Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2015;59(10):6521–38. 10.1128/AAC.00803-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wayne LG, Sramek HA. Metronidazole is bactericidal to dormant cells of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1994;38(9):2054–8. 10.1128/aac.38.9.2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manjunatha UH, Rao SPS, Kondreddi RR, Noble CG, Camacho LR, Tan BH, et al. Direct inhibitors of InhA are active against Mycobacterium tuberculosis. Sci Transl Med. 2015;7(269):269ra3. 10.1126/scitranslmed.3010597 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.