Abstract
Background and aim
Most patients who present with a fourth ventricle tumor have concurrent hydrocephalus, and some demonstrate persistent hydrocephalus after tumor resection. There is still no consensus on the management of hydrocephalus in patients with fourth ventricle tumor after surgery. The purpose of this study was to identify the factors that predispose to postoperative hydrocephalus and the need for a postoperative cerebrospinal fluid (CSF) diversion procedure.
Materials and methods
We performed a retrospective analysis of patients who underwent surgery of the fourth ventricle tumor between January 2013 and December 2018 at the Department of Neurosurgery in West China Hospital of Sichuan University. The characteristics of patients and the tumor location, tumor size, tumor histology, and preventive external ventricular drainage (EVD) that were potentially correlated with CSF circulation were evaluated in univariate and multivariate analysis.
Results
A total of 121 patients were enrolled in our study; 16 (12.9%) patients underwent postoperative CSF drainage. Univariate analysis revealed that superior extension (p = 0.004), preoperative hydrocephalus (p<0.001), and subtotal resection (p<0.001) were significantly associated with postoperative hydrocephalus. Multivariate analysis revealed that superior extension (p = 0.013; OR = 44.761; 95% CI 2.235–896.310) and subtotal resection (p = 0.005; OR = 0.087; 95% CI 0.016–0.473) were independent risk factors for postoperative hydrocephalus after resection of fourth ventricle tumor.
Conclusion
Superior tumor extension (into the aqueduct) and failed total resection of tumor were identified as independent risk factors for postoperative hydrocephalus in patients with fourth ventricle tumor.
Introduction
Posterior fossa tumors represent 7.9% of intracranial lesions and approximately 20–90% of patients with posterior fossa tumors present with hydrocephalus before tumor surgery.[1–6] Although in posterior fossa tumor, tumor removal can restore CSF circulation, 10–30% of patients tend to experience persistent hydrocephalus following posterior fossa resection.[4, 5, 7–9] The patients may present with signs and symptoms of increased intracranial pressure from hydrocephalus (headache, nausea/vomiting, vertigo, unsteady gait, diplopia, papilledema, etc.), which affect the patient's life quality and may result in a prolonged hospital stay.[2, 10] Fourth ventricle tumors, in particular, present a high rate of postoperative hydrocephalus because of the compression of tumor to the cerebrospinal fluid (CSF) pathways.[11, 12]
Surgery for fourth ventricular tumors is plagued by potential pitfalls caused by proximity to deep eloquent structures and the risk of injury to perforating arteries supplying subcortical regions and lesions of the fourth ventricle, which make up only a fraction of this subset.[13, 14] The lack of cases limits clinical experience data on the spectrum of pathologies in this region.[2–9, 15–18] We aimed to sought factors, which might be correlated with the development of persistent hydrocephalus following resection of fourth ventricle tumors to evaluate the indication for postoperative CSF drainage.
Materials and methods
Study population and data collection
This retrospective descriptive cohort study investigated the incidence of postoperative hydrocephalus and its causative factors in a consecutive group of patients who underwent surgery of fourth ventricle tumors between January 2013 and December 2018 at the Department of Neurosurgery in West China Hospital of Sichuan University.
The inclusion criteria were: 1) single intracranial neoplasm detected by preoperative magnetic resonance imaging; 2) surgical resection of the lesion; 3) a tumor confirmed by pathologic diagnosis. Patients were excluded if 1) there was evidence of multiple tumors or repeated surgery for tumor; 2) the patients underwent biopsy rather than resection.
The West China Hospital Ethics Committee approved this study. Individual patient identification data were not collected when the database was developed, and this is the reason why patient consent was not required for this study.
After selecting the patient population, the data recorded during the course of routine clinical practice (statistical data, case histories, surgery, and procedure reports) were taken from the electronic health record of the hospital by the first author. The following clinical and statistical data were included in the analysis: basic patient characteristics, tumor histology, EVD placement, surgical treatment, and shunt placement. An independent neuroradiologist reviewed preoperative MRI scans for the location of the tumor, tumor size, and preoperative hydrocephalus. Assessment of hydrocephalus was made by an Evans index (the maximum width between the frontal horns divided by the maximal width of the inner table) larger than 0.3 with or without clinical symptoms and signs (headache, nausea, vomiting, lethargy, papilledema, etc.) [16, 19]. Location of the tumor was classified as follows: superiorly (into the aqueduct of Sylvius), caudally (into the foramen magnum), laterally (into the foramen of Luschka or cerebellopontine angle), or anteriorly (invading or distorting the brainstem) (Fig 1). The extent of resection was evaluated by reviewing the postoperative MRI images obtained within 72 hours after surgical treatment, and the gross-total resection was defined as complete tumor resection with no evidence of residual tumor on postoperative MRI [20]. The number and duration of the external ventricular drain (EVD) and ventriculoperitoneal (VP) shunts were noted after surgery. The follow-up was done on postoperative days 30 and 90 and at 6 months after surgery.
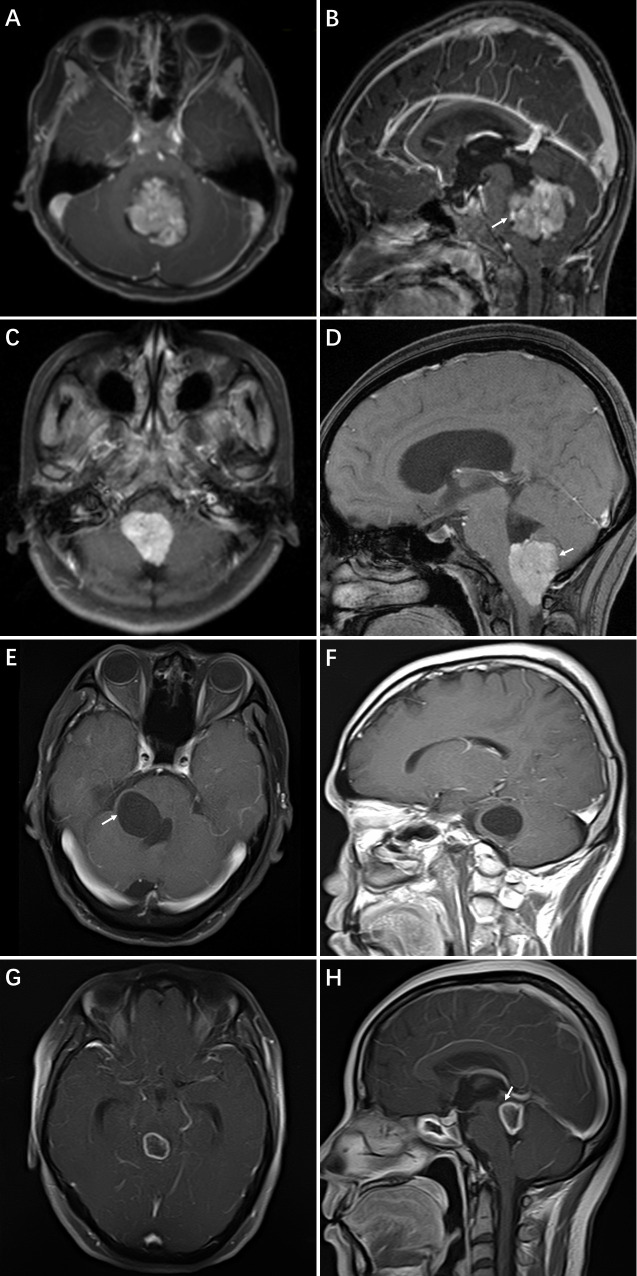
Fig 1. Cases of extension of fourth ventricle tumor.
(A and B) The axial and sagittal contrast-enhanced T1-weighted image shows the lesion invading into the brainstem. (C and D) The axial and sagittal contrast-enhanced T1-weighted image shows the lesion invading into the foramen magnum. (E and F) The axial and sagittal contrast-enhanced T1-weighted image shows the lesion invading into the left foramen of Luschka. (G and H) The axial and sagittal contrast-enhanced T1-weighted image shows the lesion invading into the aqueduct of Sylvius.
Clinical management and surgical procedures
The surgical procedures were all performed by three senior neurosurgeons (Yuekang Z, Xuhui H, and Yan J). Resection of the tumor was performed with the patient in a prone or a park bench/side position. The lesions were approached through a standard midline suboccipital approach. The indication of prophylactic EVD placement included one of the following two types: 1) symptomatic hydrocephalus and 2) asymptomatic ventriculomegaly. Prophylactic EVD placement was performed immediately after preoperative hydrocephalus had been confirmed by imaging radiological examination. Gross-total removal or subtotal resection was achieved in all patients. During the operation, electrophysiological monitoring was performed. Postoperatively, all patients were monitored in the ICU for at least 1 day. In patients with EVD, CSF was drained via the EVD for 3–5 days. The EVD was removed when no further symptomatic elevated ICP appeared over a period of turning off EVD for 24 hours. In patients without EVD placement, an EVD was rapidly placed if symptomatic high ICP was observed.
Outcomes
The indication for VP shunt placement was a failed reduction of CSF drainage due to constantly elevated ICP with clinical symptoms (headache, vomiting, lethargy, papilledema, and upward gaze paresis) lasting for more than 1–2 weeks. The postoperative hydrocephalus was defined as symptomatic hydrocephalus requiring CSF drainage following tumor removal.
Statistical analysis
Data analysis was performed using the SPSS software version 23.0 (IBM Corp., Armonk, New York, USA). Categorical variables were reported as counts (%) and continuous variables were described by median (interquartile range [IQR]). The chi-square test and Fisher exact test were employed to complete the univariate analysis of patients with and without postoperative hydrocephalus. Differences in continuous variables between patients with and without postoperative hydrocephalus were compared by the Wilcoxon-Mann-Whitney test. The significant factors in the univariate analysis were used as covariates in the multivariate analysis, which was performed using logistic regression. All statistical tests were 2-sided; if P <0.05, the data were considered statistically significant.
Results
Patient characteristic
In total, 121 patients (60 males and 61 females) were included in our study. The median patient age was 24 years (IQR, 9–41 years). Histologically, the most common tumor was ependymoma (30.6%), followed by medulloblastoma (24.2%) and pilocytic astrocytoma (16.5%). Rare lesions included cholesteatoma, hemangioblastoma, vascular malformation, choroid plexus papilloma, and metastatic lesions. Most tumors extended beyond the boundaries of the fourth ventricle. The anterior extension was the most frequent and was observed in 81.0% of cases, while the caudal extension (57.0%) was the least frequent. The lateral extension was apparent in 28.1% of cases. The superior extension was found in 11.6% of cases. In terms of surgery, gross-total resection was achieved in 91 patients (73.4%), and there was no perioperative mortality. Details are shown in Table 1.
Table 1. Patients’ characteristics and details of tumor.
| Variables | Value |
|---|---|
| Sex | |
| Female | 61 (50.4%) |
| Male | 60 (49.6%) |
| Tumor size (mm) | 37 (30–44) |
| Age (years) | 24 (9–41) |
| <3 | 11 (9.1%) |
| 3–5 | 10 (8.3%) |
| 5–18 | 35 (28.9%) |
| >18 | 65 (53.7%) |
| Tumor pathology | |
| Ependymoma | 37 (30.6%) |
| Medulloblastoma | 29 (24.0%) |
| Astrocytoma | 20 (16.5%) |
| Hemangioblastoma | 7 (5.8%) |
| Cholesteatoma | 7 (5.8%) |
| Choroid plexus papilloma | 7 (5.8%) |
| Metastatic | 4 (3.3%) |
| Non-Hodgkin lymphoma | 1 (0.8%) |
| Other | 9 (7.5%) |
| Tumor growth characteristics a | |
| Lateral extension | 34 (28.1%) |
| Anterior extension | 98 (81.0%) |
| Caudal extension | 69 (57.0%) |
| Superior extension | 14 (11.6%) |
| No extension beyond the fourth ventricle | 8 (6.6%) |
Values are number of patients (%) or median (interquartile range).
a Percentages do not add up to 100 because some patients had more than 1 growth characteristic.
Predictors for postoperative hydrocephalus which need CSF diversion
Overall, there were 56 patients with prophylactic EVD placement and 65 patients without EVD insert before tumor resection. Fifteen patients underwent postoperative CSF diversion, which included 10 VP shunts and 5 EVDs. Of the 10 VP shunts, 9 cases had a prophylactic EVD placement, and only one underwent postoperative EVD before VP shunt placement. This accounted for 17.9% (10/56) of the patients with prophylactic inserted EVDs. In the univariate analysis, the need for postoperative CSF drainage in patients was significantly correlated with sex (p = 0.012), superior extension (p = 0.015), preoperative hydrocephalus (p<0.001), and subtotal resection (p<0.001) (Table 2). Meanwhile, the need for postoperative VP in patients was significantly correlated with superior extension (p = 0.016), preoperative hydrocephalus (p<0.005), prophylactic EVD (0.006), and subtotal resection (p<0.001) (Table 3). In the multivariate analysis, superior extension (p = 0.013; OR = 44.761; 95% CI 2.235–896.310) and subtotal resection (p = 0.005; OR = 0.087; 95% CI 0.016–0.473) were confirmed as independent influencing factors for CSF drainage requirement (Table 4). However, only prophylactic EVD (p = 0.042; OR = 10.431; 95% CI 1.093–99.514) were confirmed as independent influence factors for postoperative VP. When considering the subgroup of patients who had prophylactic EVD placement prior to surgery, superior extension (p = 0.003), and subtotal resection (p<0.001) were significantly correlated with the need for postoperative CSF drainage (Table 5). Multivariate analysis identified superior extension (p = 0.012; OR = 23.400; 95% CI 1.982–276.230) and subtotal resection (p = 0.004; OR = 0.036; 95% CI 0.004–0.344) as the independent influencing factors (Table 6).
Table 2. Univariate analysis of the association between each factor and postoperative hydrocephalus.
| Variables | Postoperative CSF diversion | P-value | |
|---|---|---|---|
| Yes (15) | No (106) | ||
| Sex | 0.012a | ||
| Female | 3 (4.9%) | 51 (95.1%) | |
| Male | 12 (20%) | 48 (80%) | |
| Tumor size (mm) | 40 (34–49) | 36 (30–43) | 0.100 |
| Age (years) | 18 (3–38) | 24 (9–41) | 0.301 |
| Tumor pathology | |||
| Ependymoma | 3 (8.1%) | 34 (91.9%) | 0.226b c |
| Medulloblastoma | 5 (17.2%) | 24 (82.8%) | 1.0b c |
| Astrocytoma | 4 (20.0%) | 16 (80.0%) | |
| Lateral extension | 0.553b | ||
| Yes | 3 (8.8%) | 31 (91.2%) | |
| No | 12 (13.8%) | 75 (86.2%) | |
| Anterior extension | 0.159b | ||
| Yes | 88 (89.8%) | 10 (10.2%) | |
| No | 5 (21.7%) | 18 (78.3%) | |
| Caudal extension | 0.420a | ||
| Yes | 10 (14.5%) | 59 (85.5%) | |
| No | 5 (9.6%) | 47 (90.4%) | |
| Superior extension | 0.015b | ||
| Yes | 5 (35.7%) | 9 (64.3%) | |
| No | 10 (9.3%) | 97 (90.7%) | |
| Extent of resection | <0.001b | ||
| GTR | 3 (3.3%) | 87(96.7%) | |
| STR | 12 (38.7%) | 19(61.3%) | |
| Preoperative hydrocephalus | <0.001a | ||
| Yes | 15 (21.4%) | 55 (78.6%) | |
| No | 0 (0%) | 51 (100%) | |
| Prophylactic EVD | 0.091a | ||
| Yes | 10 (17.9%) | 46 (82.1%) | |
| No | 5 (7.7%) | 60 (92.3%) | |
CSF, cerebrospinal fluid; GTR, gross total resection; STR, subtotal resection; EVD, external ventricular drainage.
a Chi-square test.
b Fisher exact test.
c p value compared with astrocytoma.
Table 3. Univariate analysis of the association between each factor and postoperative VP.
| Variables | Postoperative VP | P-value | |
|---|---|---|---|
| Yes (10) | No (110) | ||
| Sex | 0.054b | ||
| Female | 2 (3.3%) | 59 (96.7%) | |
| Male | 8 (13.3%) | 52 (86.7%) | |
| Tumor size (mm) | 44 (33–53) | 36 (30–43) | 0.032 |
| Age (years) | 16 (1–45) | 24 (9–41) | 0.533 |
| Tumor pathology | |||
| Ependymoma | 2 (5.4%) | 35 (94.6%) | 0.170b c |
| Medulloblastoma | 3 (10.3%) | 26 (89.7%) | 0.422b c |
| Astrocytoma | 4 (20.0%) | 16 (80.0%) | |
| Lateral extension | 0.724b | ||
| Yes | 2 (5.9%) | 32 (94.1%) | |
| No | 8 (9.2%) | 79 (90.8%) | |
| Anterior extension | 0.339b | ||
| Yes | 7 (7.1%) | 91 (92.9%) | |
| No | 3 (13%) | 20 (87%) | |
| Caudal extension | 1.000b | ||
| Yes | 6 (8.7%) | 63 (91.3%) | |
| No | 4 (7.7%) | 48 (92.3%) | |
| Superior extension | 0.016b | ||
| Yes | 4 (28.6%) | 10 (71.4%) | |
| No | 6 (5.6%) | 101 (94.4%) | |
| Extent of resection | <0.001b | ||
| GTR | 1 (1.1%) | 89(98.9%) | |
| STR | 9 (29%) | 22(71%) | |
| Preoperative hydrocephalus | <0.005b | ||
| Yes | 10 (14.3%) | 60 (85.7%) | |
| No | 0 (0%) | 51 (100%) | |
| Prophylactic EVD | 0.006b | ||
| Yes | 9 (16.1%) | 47 (83.9%) | |
| No | 1 (1.5%) | 64 (98.5%) | |
a Chi-square test.
b Fisher exact test.
c p value compared with astrocytoma.
Table 4. Multivariate analysis of factors associated with postoperative hydrocephalus.
| Variables | Odds ratio (95% CI) | p-value |
|---|---|---|
| Superior extension | 44.761(2.235–896.310) | 0.013 |
| Gross total resection | 0.087(0.016–0.473) | 0.005 |
Table 5. Univariate analysis of the association between each factor and postoperative hydrocephalus in a subgroup of perioperative EVD placement.
| Variables | Postoperative CSF diversion | p-value | |
|---|---|---|---|
| Yes (10) | No (46) | ||
| Sex | 0.162b | ||
| Female | 2(8.3%) | 22(91.7%) | |
| Male | 8(25.0%) | 24(75.0%) | |
| Tumor size (mm) | 41(33–50) | 36(31–41) | 0.174 |
| Age (years) | 22.5(1–45) | 25.5(11–41) | 0.528 |
| Tumor pathology | |||
| Ependymoma | 3(20.0%) | 12(80.0%) | 0.239b c |
| Medulloblastoma | 2(15.4%) | 11(84.6%) | 0.357b c |
| Astrocytoma | 4(36.4%) | 7(63.6%) | |
| Lateral extension | 0.713b | ||
| Yes | 2(13.3%) | 13(86.7%) | |
| No | 8(19.5%) | 33(80.5%) | |
| Anterior extension | 0.361b | ||
| Yes | 7(15.2%) | 39(84.8%) | |
| No | 3(30.0%) | 7(70.0%) | |
| Caudal extension | 0.727b | ||
| Yes | 7(20.0%) | 28(80.0%) | |
| No | 3(14.3%) | 18(85.7%) | |
| Superior extension | 0.003b | ||
| Yes | 5(62.5%) | 3(37.5%) | |
| No | 5(10.4%) | 43(89.6%) | |
| Extent of resection | <0.001b | ||
| GTR | 2(5.0%) | 38(95.0%) | |
| STR | 8(50.0%) | 8(50.0%) | |
a Chi-square test.
b Fisher exact test.
c p value compared with astrocytoma.
Table 6. Multivariate analysis of factors associated with postoperative hydrocephalus in a subgroup of perioperative EVD placement.
| Variables | Odds ratio (95% CI) | p-value |
|---|---|---|
| Superior extension | 23.400(1.982–276.230) | 0.012 |
| Gross total resection | 0.036(0.004–0.344) | 0.004 |
Discussion
There is still no consensus for management of hydrocephalus before and after fourth ventricle lesions surgery. In the present study, we retrospectively analyzed clinical parameters, including sex, age, preoperative hydrocephalus, tumor type, size and location, the extent of resection, and prophylactic EVD to identify whether these factors were correlated with persistent postoperative hydrocephalus. Superior tumor extension and failed total resection of tumors were identified as significant risk factors for the development of postoperative hydrocephalus. To the best of our knowledge, this is the first study confirming that superior tumor extension (into the aqueduct) increases the incidence of postoperative hydrocephalus. Our findings may be used to preoperatively identify patients at high risk of postoperative hydrocephalus after resection of the fourth ventricle tumor.
Previous studies have shown that younger patients with posterior fossa are at higher risk for the development of persistent hydrocephalus.[3, 4, 15, 21, 22] Bognar et al.[3] and Kumar et al. [21] have demonstrated that age< 3 years at tumor surgery is a significant predictor of postoperative CSF diversion. This might be explained by the finding that the younger patient with fourth ventricle tumor related to the higher incidence of congenital and malignant tumors, like medulloblastoma, frequently accompanied by leptomeningeal metastases which may develop impaired CSF absorption at the subarachnoid level because of the more aggressive nature of tumors [22–24]. However, we did not find age to be significantly associated with postoperative CSF drainage, which might be explained by a larger number of enrolled adults in the current compared to previous studies and a small group of medulloblastoma. The relationship between age and postoperative hydrocephalus in patients with fourth ventricle tumor needs to be further explored.
There are conflicting findings on the association between preoperative hydrocephalus and postresection hydrocephalus. Our results did not confirm preoperative hydrocephalus to be correlated with the need for a postoperative CSF drainage. However, Gopalakrishnan et al. [7] and Morelli et al. [18] found that patients with severe hydrocephalus on preoperative MRI were at a higher rate of postoperative CSF division procedure, which might be because severe hydrocephalus leads to higher venous and CSF pressures and possibly a longer time to resolution of the pressure. This inconsistency with previous reports may be due to evaluating preoperative hydrocephalus by a dichotomized variable rather than calculating the extent of hydrocephalus into 3 groups (mild, moderate, and marked).
In previous studies, tumors in posterior fossa predominantly located in the midline were associated with a higher incidence of postoperative CSF diversion procedure compared with those tumors situated in the cerebellar hemispheres [12, 15, 25]. In our study, we classified the location of tumors by displayed extension: superior extension (into the aqueduct), caudal extension (into the foramen magnum), lateral extension (into the foramen of Luschka or cerebellopontine angle), and anterior extension (invading or distorting the brainstem). Notably, we find superior extension was a significant predictor for needing the postoperative CSF division, while other extensions were not associated with a postoperative shunt. The inflation after resection of tumor could result in stenosis of the CSF pathway in patients with tumor extension (aqueduct, foramina of Luschka, and foramen of Magendie). For the management of hydrocephalus in patients with fourth ventricle tumor, endoscopic third ventriculostomy (ETV) is a safe and durable means of controlling obstruction hydrocephalus and the risk of ETV failure may be lower than the risk of shunt failure surgery when ETV Success Score (ETVSS) ≥ 80 [26–30]. However, fourth ventricle tumor resection frequently developed adhesive arachnoiditis and secondary hydrocephalus when some cases with communication hydrocephalus after tumor resection could benefit from shunt rather than ETV in the study. In cases where the CSF flow from the aqueduct was well seen, there was a trend for shunt insertion based on the assumption that there was impaired CSF absorption at the subarachnoid level. Factors like the presence of postoperative cerebellar edema, especially in cases with triventricular hydrocephalus appeared on imaging, favored ETV rather than shunt insertion.
In the present study, the size of the tumor was not correlated with the need for a postoperative CSF drainage. Similarly, in their study, Sherise et al. [16] found no association between the size of the tumor and persistent postoperative hydrocephalus. The study by Kumar et al. [21] demonstrated that incidence of a CSF diversion procedure was highest after the surgical removal of medulloblastoma and ependymoma, and lowest among patients with astrocytoma, which may be explained by the observed higher incidence of astrocytoma in a lateral rather than a midline location and the higher number of such patients in the series. Meanwhile, Won et al. [23] found medulloblastoma was a predictor for the development of hydrocephalus, including noncommunicating hydrocephalus due to occlusion of the fourth ventricle or its outlets and communicating hydrocephalus secondary to leptomeningeal metastases due to the more aggressive nature of medulloblastoma. Yet, no significant correlation was found in this study. Inconsistency between our results and those reported by previous studies may be explained by the small group of medulloblastoma. The small group of each kind of tumor in this study might make us fail to find the association between pathology and the incidence of postoperative hydrocephalus.
In the present series, gross total resection of the tumor was statistically associated with a lower incidence of the need for postoperative CSF diversion procedure, by the way, there is no residual tumor near the aqueduct and the residual tumors are located in the brain stem and the bottom of the fourth ventricle in failed-total resection cases on postoperative MRI. This association was also confirmed by Culley et al. [15], Kumar et al. [21], and Gnanalingham et al. [31], which may be due to residual tumor obstructing CSF flow. Conversely, more recent studies did not find a relationship between the extent of tumor resection and postoperative hydrocephalus [2, 5, 7, 32], which could be due to the less volume of the residual tumor with sophisticated surgical techniques and the continuous development of neurosurgical instruments. However, compared with GTR, whether near-total resection (< 1.5 cm3 residual) could lead to a higher incidence of postoperative hydrocephalus remained unknown because the volume of residual tumor was not accurately measured in these studies. Therefore, further studies are necessary to determine the association between the volume of residual tumor and postoperative hydrocephalus.
Our results showed no statistically significant association between the prophylactic EVD placement and postoperative CSF drainage. In contrast to our study, Culley et al. [15] demonstrated that a more extended EVD placement might be significantly correlated with persistent hydrocephalus. A possible explanation for this may be a different indication of prophylactic EVD placement in our study. Nevertheless, it is important to note that prolonged placement of an EVD might be a sign of difficult weaning instead of a physiological predictor of persistent hydrocephalus.
The present study has some limitations. First, as a single-center retrospective study, admission bias may be present in our sample. Second, details of the treatment of posterior fossa tumor, including surgical techniques and perioperative management, may vary among hospitals. Thus, our findings should be confirmed by other multi-center prospective studies with a larger sample.
Conclusion
Superior tumor extension (into the aqueduct) and failed total resection of tumor resulted as significant risk factors for postoperative hydrocephalus in patients with fourth ventricle tumor. These findings may help to identify the patients who are at high risk of postoperative hydrocephalus and require medical interventions.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Program of Chengdu Science and Technology Bureau in the form of a grant awarded to YZ (2019-YF05-00392-SN). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-oncology. 2019;21(Suppl 5). 10.1093/neuonc/noz150 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Won SY, Gessler F, Dubinski D, Eibach M, Behmanesh B, Herrmann E, et al. A novel grading system for the prediction of the need for cerebrospinal fluid drainage following posterior fossa tumor surgery. J Neurosurg. 2019;132(1):296–305. Epub 2019/01/06. 10.3171/2018.8.JNS181005 . [DOI] [PubMed] [Google Scholar]
- 3.Bognár L, Borgulya G, Benke P, Madarassy G. Analysis of CSF shunting procedure requirement in children with posterior fossa tumors. Child's nervous system: ChNS: official journal of the International Society for Pediatric Neurosurgery. 2003;19(5–6):332–6. 10.1007/s00381-003-0745-x . [DOI] [PubMed] [Google Scholar]
- 4.Sainte-Rose C, Cinalli G, Roux FE, Maixner R, Chumas PD, Mansour M, et al. Management of hydrocephalus in pediatric patients with posterior fossa tumors: the role of endoscopic third ventriculostomy. Journal of neurosurgery. 2001;95(5):791–7. 10.3171/jns.2001.95.5.0791 . [DOI] [PubMed] [Google Scholar]
- 5.Marx S, Reinfelder M, Matthes M, Schroeder HWS, Baldauf J. Frequency and treatment of hydrocephalus prior to and after posterior fossa tumor surgery in adult patients. Acta Neurochir (Wien). 2018;160(5):1063–71. Epub 2018/02/20. 10.1007/s00701-018-3496-x . [DOI] [PubMed] [Google Scholar]
- 6.Bhatia R, Tahir M, Chandler CL. The management of hydrocephalus in children with posterior fossa tumours: the role of pre-resectional endoscopic third ventriculostomy. Pediatric neurosurgery. 2009;45(3):186–91. 10.1159/000222668 . [DOI] [PubMed] [Google Scholar]
- 7.Gopalakrishnan CV, Dhakoji A, Menon G, Nair S. Factors predicting the need for cerebrospinal fluid diversion following posterior fossa tumor surgery in children. Pediatr Neurosurg. 2012;48(2):93–101. Epub 2012/10/06. 10.1159/000343009 . [DOI] [PubMed] [Google Scholar]
- 8.Hosainey SA, Lassen B, Helseth E, Meling TR. Cerebrospinal fluid disturbances after 381 consecutive craniotomies for intracranial tumors in pediatric patients. J Neurosurg Pediatr. 2014;14(6):604–14. Epub 2014/10/18. 10.3171/2014.8.PEDS13585 . [DOI] [PubMed] [Google Scholar]
- 9.Lin CT, Riva-Cambrin JK. Management of posterior fossa tumors and hydrocephalus in children: a review. Childs Nerv Syst. 2015;31(10):1781–9. Epub 2015/09/10. 10.1007/s00381-015-2781-8 . [DOI] [PubMed] [Google Scholar]
- 10.Raimondi AJ, Tomita T. Hydrocephalus and infratentorial tumors. Incidence, clinical picture, and treatment. Journal of neurosurgery. 1981;55(2):174–82. 10.3171/jns.1981.55.2.0174 . [DOI] [PubMed] [Google Scholar]
- 11.Taylor WA, Todd NV, Leighton SE. CSF drainage in patients with posterior fossa tumours. Acta neurochirurgica. 1992;117(1–2):1–6. 10.1007/BF01400627 . [DOI] [PubMed] [Google Scholar]
- 12.Papo I, Caruselli G, Luongo A. External ventricular drainage in the management of posterior fossa tumors in children and adolescents. Neurosurgery. 1982;10(1):13–5. . [PubMed] [Google Scholar]
- 13.Mussi AC, Rhoton AL. Telovelar approach to the fourth ventricle: microsurgical anatomy. Journal of neurosurgery. 2000;92(5):812–23. 10.3171/jns.2000.92.5.0812 . [DOI] [PubMed] [Google Scholar]
- 14.Rhoton AL. Cerebellum and fourth ventricle. Neurosurgery. 2000;47(3 Suppl):S7–27. 10.1097/00006123-200009001-00007 . [DOI] [PubMed] [Google Scholar]
- 15.Culley DJ, Berger MS, Shaw D, Geyer R. An analysis of factors determining the need for ventriculoperitoneal shunts after posterior fossa tumor surgery in children. Neurosurgery. 1994;34(3). 10.1227/00006123-199403000-00003 . [DOI] [PubMed] [Google Scholar]
- 16.Ferguson SD, Levine NB, Suki D, Tsung AJ, Lang FF, Sawaya R, et al. The surgical treatment of tumors of the fourth ventricle: a single-institution experience. J Neurosurg. 2018;128(2):339–51. Epub 2017/04/15. 10.3171/2016.11.JNS161167 . [DOI] [PubMed] [Google Scholar]
- 17.Le Fournier L, Delion M, Esvan M, De Carli E, Chappé C, Mercier P, et al. Management of hydrocephalus in pediatric metastatic tumors of the posterior fossa at presentation. Childs Nerv Syst. 2017;33(9):1473–80. Epub 2017/05/13. 10.1007/s00381-017-3447-5 . [DOI] [PubMed] [Google Scholar]
- 18.Morelli D, Pirotte B, Lubansu A, Detemmerman D, Aeby A, Fricx C, et al. Persistent hydrocephalus after early surgical management of posterior fossa tumors in children: is routine preoperative endoscopic third ventriculostomy justified? Journal of neurosurgery. 2005;103(3 Suppl):247–52. 10.3171/ped.2005.103.3.0247 . [DOI] [PubMed] [Google Scholar]
- 19.Schmid UD, Seiler RW. Management of obstructive hydrocephalus secondary to posterior fossa tumors by steroids and subcutaneous ventricular catheter reservoir. Journal of neurosurgery. 1986;65(5):649–53. 10.3171/jns.1986.65.5.0649 . [DOI] [PubMed] [Google Scholar]
- 20.Lescher S, Schniewindt S, Jurcoane A, Senft C, Hattingen E. Time window for postoperative reactive enhancement after resection of brain tumors: less than 72 hours. Neurosurgical focus. 2014;37(6):E3 10.3171/2014.9.FOCUS14479 . [DOI] [PubMed] [Google Scholar]
- 21.Kumar V, Phipps K, Harkness W, Hayward RD. Ventriculo-peritoneal shunt requirement in children with posterior fossa tumours: an 11-year audit. British journal of neurosurgery. 1996;10(5):467–70. 10.1080/02688699647096 . [DOI] [PubMed] [Google Scholar]
- 22.Bateman GA, Fiorentino M. Childhood hydrocephalus secondary to posterior fossa tumor is both an intra- and extraaxial process. J Neurosurg Pediatr. 2016;18(1):21–8. Epub 2016/04/02. 10.3171/2016.1.PEDS15676 . [DOI] [PubMed] [Google Scholar]
- 23.Won SY, Dubinski D, Behmanesh B, Bernstock JD, Seifert V, Konczalla J, et al. Management of hydrocephalus after resection of posterior fossa lesions in pediatric and adult patients-predictors for development of hydrocephalus. Neurosurg Rev. 2020;43(4):1143–50. Epub 2019/07/10. 10.1007/s10143-019-01139-8 . [DOI] [PubMed] [Google Scholar]
- 24.Kim HS, Park JB, Gwak HS, Kwon JW, Shin SH, Yoo H. Clinical outcome of cerebrospinal fluid shunts in patients with leptomeningeal carcinomatosis. World J Surg Oncol. 2019;17(1):59 Epub 2019/03/29. 10.1186/s12957-019-1595-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLaurin RL. Disadvantages of the preoperative shunt in posterior fossa tumors. Clin Neurosurg. 1983;30:286–92. 10.1093/neurosurgery/30.cn_suppl_1.286 . [DOI] [PubMed] [Google Scholar]
- 26.Di Vincenzo J, Keiner D, Gaab MR, Schroeder HW, Oertel JM. Endoscopic third ventriculostomy: preoperative considerations and intraoperative strategy based on 300 procedures. J Neurol Surg A Cent Eur Neurosurg. 2014;75(1):20–30. Epub 2013/06/05. 10.1055/s-0032-1328953 . [DOI] [PubMed] [Google Scholar]
- 27.Tamburrini G, Pettorini BL, Massimi L, Caldarelli M, Di Rocco C. Endoscopic third ventriculostomy: the best option in the treatment of persistent hydrocephalus after posterior cranial fossa tumour removal? Child's Nervous System. 2008;24(12):1405–12. 10.1007/s00381-008-0699-0 [DOI] [PubMed] [Google Scholar]
- 28.Yanamadala V, Walcott BP, Nahed BV, Barker FG 2nd. Retrograde third ventriculocisternostomy from the posterior fossa. Neurosurgery. 2013;72(1 Suppl Operative):9–13; discussion -4. Epub 2012/10/06. 10.1227/NEU.0b013e3182744b67 . [DOI] [PubMed] [Google Scholar]
- 29.Kulkarni AV, Drake JM, Kestle JR, Mallucci CL, Sgouros S, Constantini S. Predicting who will benefit from endoscopic third ventriculostomy compared with shunt insertion in childhood hydrocephalus using the ETV Success Score. J Neurosurg Pediatr. 2010;6(4):310–5. Epub 2010/10/05. 10.3171/2010.8.PEDS103 . [DOI] [PubMed] [Google Scholar]
- 30.Kulkarni AV, Drake JM, Mallucci CL, Sgouros S, Roth J, Constantini S. Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. The Journal of pediatrics. 2009;155(2):254–9.e1. Epub 2009/05/19. 10.1016/j.jpeds.2009.02.048 . [DOI] [PubMed] [Google Scholar]
- 31.Gnanalingham KK, Lafuente J, Thompson D, Harkness W, Hayward R. The natural history of ventriculomegaly and tonsillar herniation in children with posterior fossa tumours—an MRI study. Pediatr Neurosurg. 2003;39(5):246–53. Epub 2003/09/27. 10.1159/000072869 . [DOI] [PubMed] [Google Scholar]
- 32.El-Gaidi MA, El-Nasr AH, Eissa EM. Infratentorial complications following preresection CSF diversion in children with posterior fossa tumors. J Neurosurg Pediatr. 2015;15(1):4–11. Epub 2014/11/08. 10.3171/2014.8.PEDS14146 . [DOI] [PubMed] [Google Scholar]