Abstract
Inflammatory bowel disease (IBD) increases the risk of colitis-associated cancer (CAC). Evidences suggest that Helicobacter pylori (H. pylori) infection is associated with a low risk of IBD and protects against experimental colitis in mouse models. However, the effect of H. pylori infection in CAC remains unclear. We previously reported that H. pylori infection increased M2 macrophages in dextran sodium sulfate (DSS)-induced chronic colitis. Tumor-associated macrophages (TAMs) play a pivotal role in colon cancer. Therefore, we established a H. pylori-infected CAC mouse model induced by azoxymethane and DSS to explore the effect of H. pylori infection on TAMs in CAC. Here, we demonstrated that H. pylori infection attenuated the development of CAC by decreasing tumor multiplicity, tumor size, tumor grade and colitis scores. Moreover, H. pylori infection reduced the infiltration of TAMs, particularly M2-like TAMs in CAC tumors, accompanied with the down-regulated pro-inflammatory and pro-tumorigenic factors TNF-α, IL-1β, IL-6 and IL-23 in tumors of CAC mice. Our study suggests that H. pylori infection can reduce TAMs infiltration and regulate cytokines expression in CAC.
Introduction
Colitis-associated cancer (CAC), associated with inflammatory bowel disease (IBD), only accounts for 1–2% of all colorectal cancer (CRC) cases in general population [1]. The risk of CAC increases with the duration and anatomic extent of inflammation [2–4]. Large epidemiological studies have demonstrated a low risk for IBD in patients with Helicobacter pylori (H. pylori) infection. Furthermore, animal studies have revealed the protective effect of H. pylori infection in acute and chronic colitis [5, 6]. However, some epidemiological studies and the latest meta-analysis have shown that CRC is associated with H. pylori infection [7, 8]. Therefore, the effect of H. pylori infection in CAC remains unclear.
Unlike the typical “adenoma-adenocarcinoma” process of sporadic CRC, CAC follows the sequence of “inflammation-dysplasia-adenocarcinoma” [4]. Moreover, in CAC tumorigenesis, the immunological mechanisms are more involved and immune cells interact with tumor cells [9]. We previously reported that H. pylori infection alleviated dextran sodium sulfate (DSS)-induced chronic colitis through balancing T helper cells 17 / regulatory T cells responses and increasing M2 macrophages [10]. However, the effect of H. pylori infection on macrophages in CAC is still unknown.
Macrophages have a critical role in inflammation and tumor [11, 12]. Usually, activated macrophages are divided into classical M1 and alternative M2 macrophages, based on their function. M1 macrophages express high levels of pro-inflammatory cytokines (e.g. TNF-α, IL-1β, IL-6 and IL-23) and are considered to exert tumoricidal effects. In contrast, M2 macrophages are involved in anti-inflammatory responses and tumor-promoting activities [13]. Tumor-associated macrophages (TAMs) refer to macrophages within tumor and closely resemble M2 macrophages, which are related to promote tumor growth and metastasis [14].
Considering that M2 macrophages have the different influences on inflammation and tumor, in this study, we established a H. pylori-infected CAC mouse model induced by azoxymethane (AOM)/DSS, aiming to explore the role of H. pylori infection on TAMs, especially on M2-like TAMs in CAC.
Materials and methods
H. pylori colonization and CAC induction
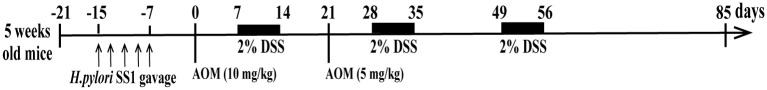
Male 5 weeks old C57BL/6 mice were purchased from Beijing Vital River Laboratory Animal Technology Company Limited, China. The mice were adapted to the environment for 1 week before entering the formal study. All mice were randomly divided into 4 groups: the control group (control), H. pylori-infected group (Hp), AOM/DSS-treated group (AOM/DSS), and AOM/DSS-treated with H. pylori infection group (Hp+AOM/DSS). In the Hp and Hp+AOM/DSS groups, 6-week-old mice fasted overnight were intragastrically inoculated 5 times with 200 μl brain-heart infusion containing 1×109 colony-forming units per ml H. pylori SS1 every other day. Mice in control and AOM/DSS groups were inoculated with brain-heart infusion without H. pylori SS1. One week later, CAC was induced as the protocol previously described [15]. Mice in AOM/DSS and Hp+AOM/DSS groups were intraperitoneally injected with AOM (10 mg/kg, Sigma-Aldrich, USA) on day 0. One week after AOM injection, mice were given 2% DSS (MP Biomedicals, USA) in drinking water for 7 days followed by substitution of regular water for 14 days. During this time, mice received a second intraperitoneal injection of AOM (5 mg/kg) on day 21. Then, mice were subjected to 2 more cycles of 2% DSS treatment (7 days/cycle). Mice were sacrificed on day 85 after AOM/DSS challenge (Fig 1). All animal experiments were reviewed and approved by the Animal Studies Committee of Peking University First Hospital (Protocol Number: J201823).
Fig 1. Schematic of the animal treatment.
Histopathological and immunohistochemical analyses
Histopathological appearances in colonic tissues were determined by hematoxylin and eosin (H&E) staining. The criteria of colitis score in non-tumor areas was described previously [16] as follows: severity scores for inflammation (0, normal; 1–3, mild-serve), scores for ulceration (0, normal; 1–3, mild-serve), scores for mucosal hyperplasia (0, normal; 1–3, mild-serve), and scores for extent of lesions (0, normal; 1–3, mild-serve). The score of each part was added to give a total histological score. For immunohistochemistry, the polyclonal rabbit anti-H. pylori antibody (1:250, Abcam, UK) was staining in gastric tissues to identify the colonization of H. pylori and the expression of F4/80, CD206 and CD31 in colon tissues was detected respectively using monoclonal rabbit anti-F4/80 antibody (1:300, Cell Signaling Technology, USA), polyclonal rabbit anti-CD206 (1:10000, Abcam, UK) and monoclonal rabbit anti-CD31 antibody (1:100, Cell Signaling Technology, USA). Each slide was randomly selected 5 high power fields in tumor sections (×400) and was calculated the average positive staining cells per filed.
Cell isolation and flow cytometry
Colonic Lamina propria mononuclear cells were isolated from fresh colonic tissues as described previously [17]. Colonic tissues were cut into small pieces and incubated twice in Hank’s Balanced Salt Solution (HBSS) containing 5 mM EDTA and 1 mM DTT for 20 min at 37 °C under slow rotation. After washing off the EDTA, the remaining tissues were cut into 1mm pieces and incubated twice in digestion solution containing 0.5 mg/ml DNase I and 0.5 mg/ml collagenase IV and 3 mg/ml dispase II (all from Sigma Aldrich, USA) for 20 min at 37 °C. The cell solution was filtered through a 70-μm cell strainer and then layered on a percoll density gradient to separate immunocytes. For macrophages surface staining, the mononuclear cell suspensions were incubated with antibodies on ice for 30 minutes, and analyzed on a BD Influx™ (BD Biosciences, USA). The following monoclonal rat anti-mouse antibodies were used: anti-CD45-APC/Cy7, anti-CD11b-PE/Cy7, anti-F4/80-PE, anti-CD206-APC (all from BioLegend, USA). In parallel, isotype antibodies were used as control. The results were analyzed using Flow Jo 7.6 software (Tree Star, USA).
Magnetic-activated cell sorting (MACS)
The lamina propria mononuclear cell were obtained from colon tissues as described above. TAMs were purified from these mononuclear cells using Anti-F4/80 MicroBeads UltraPure (Miltenyi Biotec, Germany) followed by magnetic separation.
Quantitative real-time PCR (qPCR)
Total RNA from colonic tissues or TAMs was extracted using Trizol reagent (Invitrogen, USA) and reverse-transcribed to cDNA using High-Capacity cDNA Reverse Transcription kits (Applied Biosystems, USA). Then, qPCR was performed with Power SYBR Green PCR Master MIX (Applied Biosystems, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control. The sequences of primers are listed in Table 1.
Table 1. Primers used in qPCR.
| Primers | Forward (5’-3’) | Reverse (5’-3’) |
|---|---|---|
| GAPDH | AGGTCGGTGTGAACGGATTTG | TGTAGACCATGTAGTTGAGGTCA |
| CD206 | CTCTGTTCAGCTATTGGACGC | CGGAATTTCTGGGATTCAGCTTC |
| Trem2 | CTGGAACCGTCACCATCACTC | CGAAACTCGATGACTCCTCGG |
| Ym1 | CAGGTCTGGCAATTCTTCTGAA | GTCTTGCTCATGTGTGTAAGTGA |
| Arginase1 | CTCCAAGCCAAAGTCCTTAGAG | AGGAGCTGTCATTAGGGACATC |
| MMP2 | ACCTGAACACTTTCTATGGCTG | CTTCCGCATGGTCTCGATG |
| IL-1β | GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGTCCGTCAACT |
| TNF-α | CAGGCGGTGCCTATGTCTC | CGATCACCCCGAAGTTCAGTAG |
| IL-6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| IL-23 | ATGCTGGATTGCAGAGCAGTA | ACGGGGCACATTATTTTTAGTCT |
Statistical analysis
All data were checked for normal distribution and were expressed as mean ± SEM. Differences were analyzed by Student’s t test, and a P value of less than 0.05 was considered statistically significant. Statistics were performed using GraphPad Prism 7.0 software.
Results
H. pylori infection attenuated the development of CAC
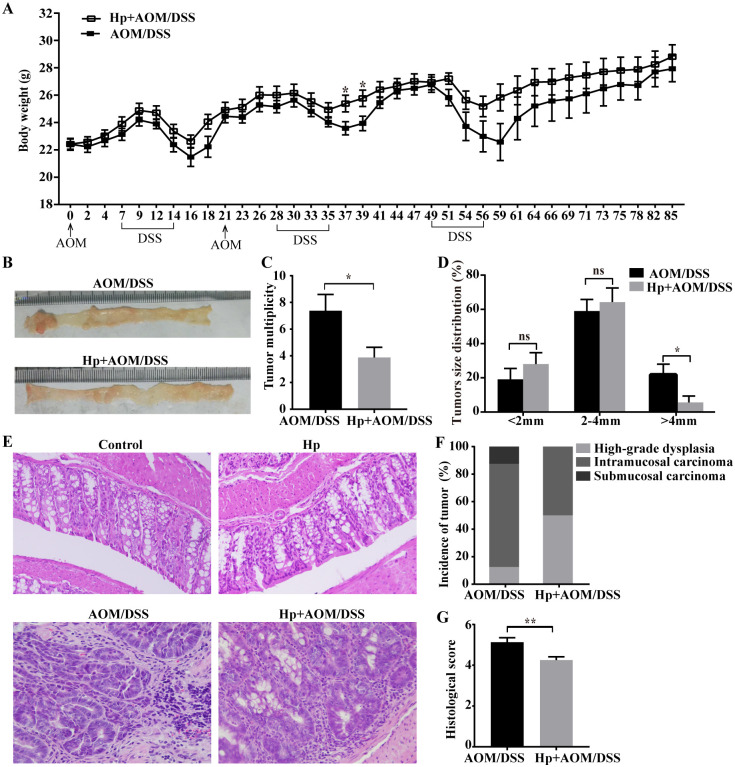
Mice’s body weight of each group over time was shown in Fig 2A. During each cycle of DSS administration, mice in AOM/DSS group lost more body weight. All mice developed colitis-associated tumor after 85-day AOM/DSS challenge (Fig 2B). Mice in Hp+AOM/DSS group displayed the significantly decreased tumor multiplicity and low frequency of tumor size (>4mm) compared with AOM/DSS group (Fig 2C and 2D). To further assess the colitis-associated tumor, H&E staining was performed. All tumors were adenomas with high-grade dysplasia or carcinomas (Fig 2E). As shown in Fig 2F, 12.5% (1/8), 75% (6/8) and 12.5% (1/8) of tumors in CAC mice were high-grade dysplasia, intramucosal and submucosal carcinoma respectively, whereas 50% (4/8) and 50% (4/8) of tumors in H. pylori-infected CAC mice were high-grade dysplasia and intramucosal carcinoma respectively. Moreover, scores of colitis were higher in the AOM/DSS group than in the Hp+AOM/DSS group (Fig 2G), and there were no histological changes in colonic tissues of control group and Hp group. Collectively, these findings suggested that H. pylori infection attenuated the progression of CAC. In addition, there was only one mouse in Hp+AOM/DSS group with negative anti-H. pylori staining in gastric tissues, and data from this mouse were discarded.
Fig 2. H. pylori infection attenuated the development of CAC.
CAC was induced in mice of AOM/DSS-treated group (AOM/DSS) and AOM/DSS-treated with H. pylori infection group (Hp+AOM/DSS). (A) Body weight change (n = 8/group). (B) Representative images of colon tumors. (C) Tumor multiplicity (n = 8/group). (D) Tumor size distribution (n = 8/group). (E) Representative images of colonic tissues in control and H. pylori infection (Hp) group and tumor tissues in AOM/DSS group and Hp+AOM/DSS group by H&E staining (×200). (F) The incidence of each tumor grading (n = 8/group). (G) Histological inflammation scores (n = 8/group). Data are represented as mean ± SEM. *P < 0.05, **P < 0.01, ns, not significant.
H. pylori infection decreased TAMs infiltration in CAC
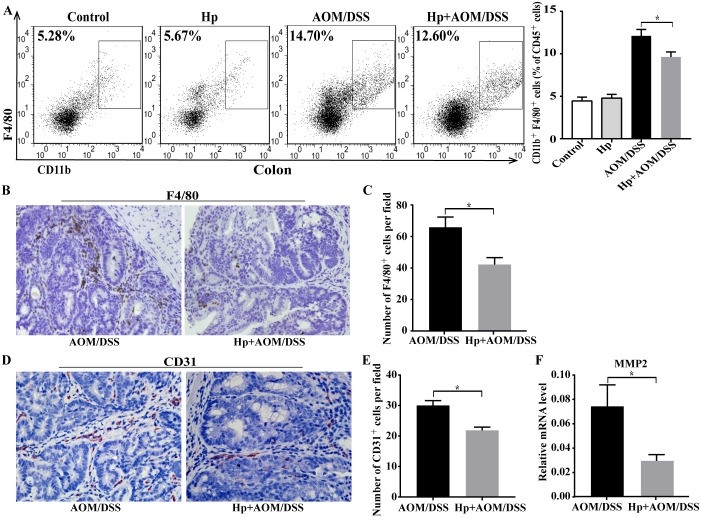
At the end of 85-day CAC challenge, immunocytes were isolated from fresh colonic tissues, and were characterized by flow cytometry. In CAC-bearing mice, the percentages of CD45+CD11b+F4/80+ TAMs were notably increased in colon, but there was a marked reduction of TAMs in Hp+AOM/DSS group when compared with AOM/DSS group (Fig 3A). Then, we further confirmed the results by F4/80 staining. Similar to the results of flow cytometry analysis, immunohistochemical analysis showed that H. pylori-infected CAC mice significantly decreased the number of F4/80+ macrophages in colonic tumors versus CAC mice (Fig 3B and 3C). These results supported that H. pylori infection diminished the infiltration of TAMs in CAC tumors.
Fig 3. H. pylori infection decreased TAMs infiltration in CAC.
(A) Flow cytometry analysis of CD45+CD11b+F4/80+ TAMs in colonic tissues from AOM/DSS-treated group (AOM/DSS) and AOM/DSS-treated with H. pylori infection group (Hp+AOM/DSS) (n = 6/group). (B) Representative immunohistochemical images of the TAM marker F4/80 staining in tumor tissues (×400) and (C) the numbers of F4/80+ cells per field (n = 6/group). (D) Representative immunohistochemical images of the CD31 staining in tumor tissues (×400) and (E) the numbers of CD31+ cells per field (n = 6/group). (F) Relative mRNA expression of MMP2 in colonic tumor tissues (n = 6/group). Data are represented as mean ± SEM. *P < 0.05.
TAMs can promote the tumor invasion and metastasis. Therefore, we assessed the vascular density in by CD31 immunostaining. As shown in Fig 3D and 3E, the expression of CD31 was clearly decreased in tumors of Hp+AOM/DSS group. TAMs could produce matrix metalloproteinases (MMPs) [18]. Thus, our study detected the MMP2 expression in colonic tumors by qPCR. As shown in Fig 3F, H. pylori infection obviously reduced the mRNA levels of MMP2 in mice after CAC challenge. Thereby H. pylori infection might limit the progression of CAC.
H. pylori infection reduced M2 macrophages proportion in CAC
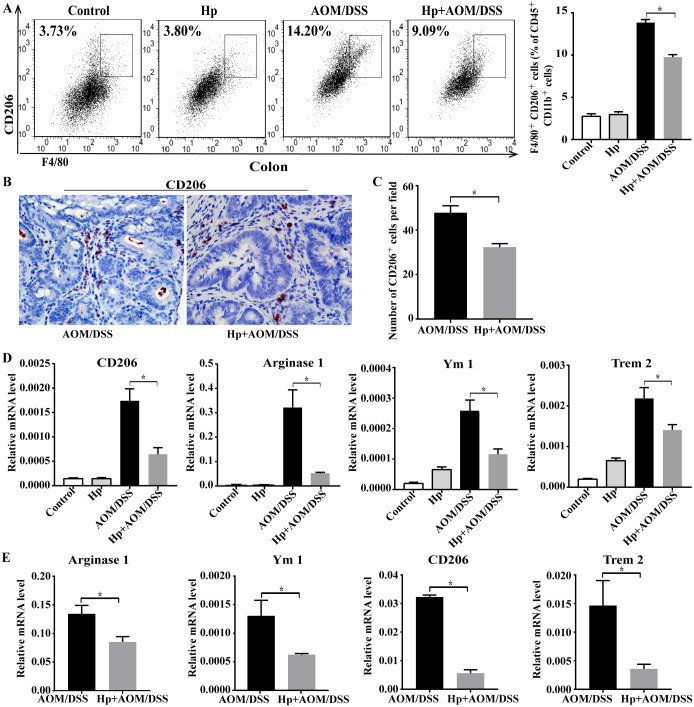
As M2 macrophages play a pro-tumor role in colon cancer, we explored the effect of H. pylori infection on them and used a common marker CD206 to identify M2 subtype from total macrophages. As shown in Fig 4A, H. pylori-infected CAC mice exhibited lower percentages of CD45+CD11b+F4/80+CD206+ M2 macrophages in colon than CAC mice. Consist with the decreased proportion of M2 macrophages, the CD206 expression in tumor by immunostaining and the mRNA levels of representative M2 genes (Arginase1, CD206, Trem2 and Ym1) by qPCR were also markedly down-regulated in colonic tumors of H. pylori-infected CAC mice (Fig 4B–4D).
Fig 4. H. pylori infection reduced M2 macrophages proportion in CAC.
(A) Flow cytometry analysis of CD45+CD11b+F4/80+CD206+ M2 macrophages in colonic tissues from AOM/DSS-treated group (AOM/DSS) and AOM/DSS-treated with H. pylori infection group (Hp+AOM/DSS) (n = 6/group). (B) Representative immunohistochemical images of the M2 marker CD206 staining in tumor tissues (×400) and (C) the numbers of CD206+ cells per field (n = 6/group). (D) Relative mRNA expression of M2-associated genes (Arginase1, CD206, Trem2 and Ym1) in colonic tumors (n = 5-6/group). (E) Arginase1, Ym1, CD206 and Trem2 mRNA expression in F4/80+ macrophages isolated from tumors of AOM/DSS group and Hp+AOM/DSS group (n = 5-6/group). Data are represented as mean ± SEM. *P < 0.05.
To further confirm the effect of H. pylori infection on the M2 phenotype of TAMs in CAC, we isolated F4/80+ TAMs from colonic tumor tissues and analyzed the M2-associated genes expression. As shown in Fig 4E, the mRNA levels of Arginase1, Ym1, CD206 and Trem2 were significantly decreased in TAMs from H. pylori-infected CAC mice compared with TAMs from CAC mice. Taken together, these findings indicated that H. pylori infection reduced the infiltration of M2-like TAMs in CAC tumor.
H. pylori infection regulated the pro-inflammatory and pro-tumorigenic cytokines expression in CAC
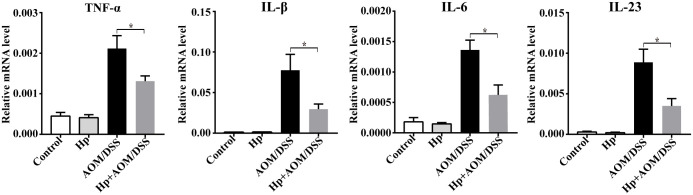
To further explore the effect of H. pylori infection on the function of TAMs in CAC, we investigated mRNA expression of pro-inflammatory and pro-tumorigenic cytokines, which are associated with TAMs. In accordance with the decreased TAMs proportion, mRNA levels of TNF-α, IL-1β, IL-6 and IL-23 were also obviously down-regulated in tumors of H. pylori-infected CAC mice. Thus, H. pylori infection appears to affect the TAMs in CAC through regulating the cytokines expression (Fig 5).
Fig 5. H. pylori infection regulated the pro-inflammatory and pro-tumorigenic cytokines expression in CAC mice.
Relative mRNA expression of pro-inflammatory and pro-tumorigenic cytokines (TNF-α, IL-1β, IL-6 and IL-23) in colonic tumors (n = 5-6/group). Data are represented as mean ± SEM. *P < 0.05.
Discussion
In this study, we have revealed that H. pylori infection attenuated the development of CAC by decreasing tumor multiplicity and tumor size. Histologically, H. pylori infection also slowed down the colitis-associated tumor progression, supported by tumor grading and colitis scores. Moreover, H. pylori infection reduced infiltration of TAMs, particularly M2-like TAMs in CAC tumors. This effect might be associated with the down-regulated pro-inflammatory and pro-tumorigenic cytokines in colonic tumors of H. pylori-infected CAC mice.
Accumulations of TAMs is common in tumors, including colon cancer [19, 20]. TAMs also include two subtypes: M1 and M2. At the early stage of tumor development, TAMs exhibit M1-like phenotype, which promote immune responses that kill tumor cells. During the late stage of tumor progression, TAMs generally switch to M2-like phenotype, which exert tumor-promoting activities [13, 21]. In inflammatory and tumor microenvironment, TAMs can produce cytokines such as IL-6/IL-17/IL-23 to induce the tumor initiation and progression via the NF-κB or STAT3 signaling pathway. TAMs-derived TNF-α can transport to destination organs, which promote tumor cells recruitment in metastatic foci [22]. Evidences also suggest that TAMs promote tumor metastasis through producing matrix metallopeptidase (MMP), such as MMP2, MMP7, and MMP9 [18]. Previous study has showed that VEGF, TNF-α, IL-1β and MMPs derived from TAMs involve in tumor angiogenesis [23].
In an AOM/DSS-induced CAC mouse model, the percentages of CD68+ macrophages and CD206+ M2 macrophages increased in tumors, accompanied with the up-regulated expression of TNF-α, IL-1β, IL-6 [24]. Studies revealed that anti-tumor drugs inhibited the M2-associated genes, such as CD206, Arginase 1, CD204 and MMP2 in AOM/DSS mouse models [25, 26]. The inflammatory cytokines, including TNF-α, IL-1β and IL-6, were also suppressed and the percentages of macrophages were decreased, after using a kind of anti-ulcerative colitis medicine in CAC [27].
Evidences have demonstrated that H. pylori infection decreased the pro-inflammatory cytokines (TNF-α, IFN-γ, IL-1β, IL- 6, IL-17A and IL23) in colitis mouse models [6, 10], and we also have reported that IL-6 and IL23 expression were down-regulated in H. pylori-infected chronic colitis mice. In present study, we have found that H. pylori infection significantly reduced the proportion of TAMs and the expression of pro-inflammatory and pro-tumorigenic cytokines (TNF-α, IL-1β, IL-6 and IL-23) in a CAC model, suggesting that H. pylori infection might affect TAMs by regulating the cytokine milieu in CAC mice. H. pylori infection also decreased the MMP2 expression related to TAMs, and thereby H. pylori infection can limit colonic tumor metastasis. Moreover, we also found the infiltration of M2 TAMs were diminished in H. pylori infected CAC, suggesting that H. pylori infection appears to attenuate CAC through regulating differentiation of TAMs. However, the specific mechanism about how H. pylori infection regulates the TAMs in CAC remains unknown.
In Conclusion, our study suggests that H. pylori infection reduces TAMs infiltration especially M2-like TAMs and regulates the cytokines expression in CAC.
Acknowledgments
We would like to thank Yi Ding for technical assistance with BD Influx.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This work was supported by the Natural Science Foundation of Beijing Municipality (7182165). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lin KD, Chiu GF, Waljee AK, Owyang SY, El-Zaatari M, Bishu S, et al. Effects of Anti-Helicobacter pylori Therapy on Incidence of Autoimmune Diseases, Including Inflammatory Bowel Diseases. Clin Gastroenterol Hepatol. 2019;17(10):1991–9. 10.1016/j.cgh.2018.12.014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48(4):526–35. 10.1136/gut.48.4.526 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment Pharmacol Ther. 2006;23(8):1097–104. 10.1111/j.1365-2036.2006.02854.x . [DOI] [PubMed] [Google Scholar]
- 4.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140(6):1807–16. 10.1053/j.gastro.2011.01.057 . [DOI] [PubMed] [Google Scholar]
- 5.Li X, Tan J, Zhang F, Xue Q, Wang N, Cong X, et al. H. pylori Infection Alleviates Acute and Chronic Colitis with the Expansion of Regulatory B Cells in Mice. Inflammation. 2019;42(5):1611–21. 10.1007/s10753-019-01022-0 . [DOI] [PubMed] [Google Scholar]
- 6.Wu YZ, Tan G, Wu F, Zhi FC. H. pylori attenuates TNBS-induced colitis via increasing mucosal Th2 cells in mice. Oncotarget. 2017;8(43):73810–6. 10.18632/oncotarget.17962 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi DS, Seo SI, Shin WG, Park CH. Risk for Colorectal Neoplasia in Patients With Helicobacter pylori Infection: A Systematic Review and Meta-analysis. Clin Transl Gastroenterol. 2020;11(2):e00127 10.14309/ctg.0000000000000127 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Hoffmeister M, Weck MN, Chang-Claude J, Brenner H. Helicobacter pylori infection and colorectal cancer risk: evidence from a large population-based case-control study in Germany. Am J Epidemiol. 2012;175(5):441–50. 10.1093/aje/kwr331 . [DOI] [PubMed] [Google Scholar]
- 9.Luo C, Zhang H. The Role of Proinflammatory Pathways in the Pathogenesis of Colitis-Associated Colorectal Cancer. Mediators Inflamm. 2017;2017:5126048 10.1155/2017/5126048 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Dai Y, Liu Y, Wu T, Li J, Wang X, et al. Helicobacter pylori Colonization Protects Against Chronic Experimental Colitis by Regulating Th17/Treg Balance. Inflamm Bowel Dis. 2018;24(7):1481–92. 10.1093/ibd/izy107 . [DOI] [PubMed] [Google Scholar]
- 11.Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 2015;36(4):229–39. 10.1016/j.it.2015.02.004 . [DOI] [PubMed] [Google Scholar]
- 12.Dehne N, Mora J, Namgaladze D, Weigert A, Brune B. Cancer cell and macrophage cross-talk in the tumor microenvironment. Curr Opin Pharmacol. 2017;35:12–9. 10.1016/j.coph.2017.04.007 . [DOI] [PubMed] [Google Scholar]
- 13.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel). 2014;6(3):1670–90. 10.3390/cancers6031670 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090 10.1016/j.ejphar.2020.173090 . [DOI] [PubMed] [Google Scholar]
- 15.Dai Y, Jiao H, Teng G, Wang W, Zhang R, Wang Y, et al. Embelin reduces colitis-associated tumorigenesis through limiting IL-6/STAT3 signaling. Molecular cancer therapeutics. 2014;13(5):1206–16. 10.1158/1535-7163.MCT-13-0378 . [DOI] [PubMed] [Google Scholar]
- 16.Zaki MH, Vogel P, Malireddi RK, Body-Malapel M, Anand PK, Bertin J, et al. The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell. 2011;20(5):649–60. 10.1016/j.ccr.2011.10.022 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath MF. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat Protoc. 2007;2(10):2307–11. 10.1038/nprot.2007.315 . [DOI] [PubMed] [Google Scholar]
- 18.Deryugina EI, Quigley JP. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol. 2015;44–46:94–112. 10.1016/j.matbio.2015.04.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. 10.1038/nrclinonc.2016.217 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szebeni GJ, Vizler C, Kitajka K, Puskas LG. Inflammation and Cancer: Extra- and Intracellular Determinants of Tumor-Associated Macrophages as Tumor Promoters. Mediators Inflamm. 2017;2017:9294018 10.1155/2017/9294018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ngambenjawong C, Gustafson HH, Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev. 2017;114:206–21. 10.1016/j.addr.2017.04.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. 2017;10(1):58 10.1186/s13045-017-0430-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. 10.1016/j.cell.2010.03.014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Li X, Zheng D, Zhang D, Peng X, Zhang X, et al. Dynamic changes and functions of macrophages and M1/M2 subpopulations during ulcerative colitis-associated carcinogenesis in an AOM/DSS mouse model. Mol Med Rep. 2015;11(4):2397–406. 10.3892/mmr.2014.3018 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Li L, Mei H, Pan G, Wang X, Huang X, et al. Antitumor properties of triptolide: phenotype regulation of macrophage differentiation. Cancer Biol Ther. 2020;21(2):178–88. 10.1080/15384047.2019.1679555 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu T, Dai Y, Wang W, Teng G, Jiao H, Shuai X, et al. Macrophage targeting contributes to the inhibitory effects of embelin on colitis-associated cancer. Oncotarget. 2016;7(15):19548–58. 10.18632/oncotarget.6969 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin X, Yi Z, Diao J, Shao M, Zhao L, Cai H, et al. ShaoYao decoction ameliorates colitis-associated colorectal cancer by downregulating proinflammatory cytokines and promoting epithelial-mesenchymal transition. J Transl Med. 2014;12:105 10.1186/1479-5876-12-105 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.