Abstract
Aim:
To investigate the therapeutic effect of FZHY on hepatic fibrosis in mice and to determine the mechanism of its action.
Methods:
Wild type mice were subjected to toxic (carbon tetrachloride, CCl4) or cholestatic (bile duct ligation, BDL). Upon induction of liver fibrosis, mice were treated with FZHY (4.0g/kg, 2w, oral gavage) or vehicle (PBS). Livers were analyzed by Sirius Red staining, immunostaining and RT-PCR for profibrogenic and pro-inflammatory genes. The effect of FZHY on hepatocytes, inflammatory responses, activation of fibrogenic myofibroblasts, and ROS production was assessed.
Results:
FZHY strongly inhibited the development of CCl4- and BDL-induced liver fibrosis in mice. Liver fibrosis was significantly improved in FZHY-treated mice, as demonstrated by reduced content of hepatic hydroxyproline and Sirius Red positive area. Moreover, the number of SMA +and Desmin+ myofibroblasts was significantly reduced in the livers of FZHY-treated mice, and correlated with downregulation of the mRNA levels of α-SMA, collagen-α1(I), tissue inhibitor of matrix metalloproteinase-1 (TIMP-1), TGF-β1 and its receptor TGF-βRI, and platelet-derived growth factor-β (PDGF-β), suggesting that FZHY inhibits activation of fibrogenic myofibroblasts. Furthermore, administration of FZHY markedly decreased recruitment of F4/80+ inflammatory macrophages to the livers of CCl4- and BDL-injured mice, and this effect was associated with downregulation of monocyte chemoattractant protein-1(MCP-1) and macrophage inflammatory protein-1 (MIP-1) mRNA. In addition, the lipid peroxidation products 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA) were reduced, demonstrating that treatment with FZHY can effectively block ROS production in livers of CCl4- and BDL-injured mice.
Conclusions:
Traditional Chinese Medicine FZHY has a variety of anti-fibrotic effects, including strong anti-oxidant, anti-inflammatory and anti-fibrotic effects on myeloid cells and hepatocytes. Although FZHY compound does not seem to directly affect HSCs, it regulates HSC activation via inhibition of macrophage recruitment to fibrotic liver.
Keywords: Traditional Chinese medicine, Fuzheng Huayu, Liver fibrosis, Reactive oxygen species, Toxic and cholestatic liver injury
1. Introduction
Liver fibrosis results from many chronic liver diseases, such as hepatitis B and C, alcoholic liver disease, non-alcoholic steatohepatitis (NASH), autoimmune hepatitis (AIH) and hepatotoxic drugs [1]. In western countries, hepatitis C virus (HCV) infection, alcohol abuse and NASH are the major causes of liver fibrosis, while in China, chronic hepatitis B virus (HBV) infection is still the most important cause of liver fibrosis, whereas the incidence of NASH is now increasing drastically [2]. Liver fibrosis is characterized by extensive deposition of extracellular matrix (ECM) proteins, which distorts the hepatic architecture by forming fibrous scar, and consequently develops into cirrhosis. The pathogenesis of liver fibrosis reflects a common wound-healing response to chronic injury [3]. Many cell types contribute to the pathogenesis of liver fibrosis, including hepatocytes, inflammatory cells, Kupffer cells and hepatic stellate cells (HSCs, the predominant source of myofibroblasts) [4]. Activation of HSCs into myofibroblasts, which are not present in the normal liver, is triggered by release of pro-fibrogenic cytokines such as TGF-β1, tumor necrosis factor-α (TNF-α), and induction of reactive oxygen species (ROS) in the damaged liver [5].
The data obtained from patient material and experimental animal models of liver fibrosis suggest that hepatic fibrosis and even cirrhosis can regress [6, 7]. Resolution of liver fibrosis is associated with resorption of fibrous scar and disappearance of fibrogenic myofibroblasts. Since activated HSCs/myofibroblasts are the major source of ECM in fibrotic liver, they serve as a primary target for anti-fibrotic therapy. Several important mechanisms facilitating regression of liver fibrosis has been identified: a) inhibition of HSC proliferation in injured liver; b) inhibition of Collagen Type I production by activated (a) HSCs; c) induction of aHSC apoptosis; and d) inactivation of aHSCs into a quiescent-like phenotype. Several potential anti-fibrotic therapies have been recently developed. Inhibitors of TGF-β1 (the major pro-fibrogenic cytokine), are effective in animal models of liver fibrosis, but may not be suitable for long term treatments in patients due to side the effects of broad inhibition of TGFβ1 involved in homeostasis, modulation of immune response, oncogenesis, and tissue repair [8, 9]. The renin angiotensin system (RAS) is another attractive target for anti-fibrotic therapy since angiotensin II induces ROS and accelerates activation of HSCs into myofibroblasts [10, 11]. Anti-inflammatory drugs have been assessed as anti-fibrotic therapy, since inflammation strongly promotes progression of liver fibrosis [12]. Antioxidants such as vitamin E, phosphatidylcholine, silymarin, and S-adenosyl-L-methionine can inhibit HSC activation, provide hepatoprotection, and attenuate liver fibrosis in mouse models of liver fibrosis [1].
Traditional Chinese medicines have been used in China for thousands of years to treat patients with chronic liver diseases. Fuzheng Huayu (FZHY) is a Chinese herbal product developed 20 years ago by the physicians at the Shanghai University of Traditional Chinese Medicine to treat liver fibrosis [13, 14]. FZHY is a compound that consists of six Chinese medicinal herbs, namely Radix Salvia Miltiorrhizae, Cordyceps (Chongcao), Semen Persicae, Gynostemma Pentaphyllammak (Jiaogulan), Pollen Pini (Songhuafen), Fructus Schisandrae Chinensis (Wuweizi) (see Table 1). Previous clinical and experimental studies have suggested that FZHY might exhibit anti-fibrotic properties [6, 15, 16]. However, little is known about the mechanism of FZHY action. In this study, we systematically investigated the effect of FZHY on liver fibrosis in mice of different etiologies, toxic (carbon tetrachloride, CCl4), and cholestatic (caused by ligation of common bile duct, BDL), and characterized the mechanism by which FZHY mediates its anti-fibrotic effects.
Table 1:
Composition of FZHY recipe.
| Herbal components | Botanic family | Medicinal part | g |
|---|---|---|---|
| Radix Salviae Miltiorrhizae | Lamiaceae | Root and rhizome | 8.0 |
| Fermentation Mycelium Powder | Clavicipitaceae | Stroma | 4.0 |
| Fructus Schisandrae Chinensis | Schisandraceae | Ripe fruit | 2.0 |
| Semen Persicae | Rosaceae | Seed | 2.0 |
| Pollen Pini | Pinaceae | Pollen | 2.0 |
| Gynostemmaa Pentaphyllammak | Cucurbitaceae | Rhizome | 6.0 |
Amount in 4.8 g of FZHY recipe powder
2. Materials and Methods
2.1. Preparation of FZHY recipe extract powder
FZHY compound consists of a mixture of six Chinese herbs (Table 1). FZHY compound was purified by Shanghai Sundise Medicine Technology Development Co. Ltd, China (SFDA approval No. Z20050564), which also determined the major bioactive ingredients and quality controls (Table 2). FZHY powder was resuspended in PBS, and administered to mice at concentration of 4.0 g/kg body weight (0.5 g/ml, twice a week, by oral gavage), or filtered and used for in vitro treatment of isolated HSCs or hepatocytes at concentration of 0.08 μg/ml or 1.25 μg/ml.
Table 2:
Quality control standard for FZHY recipe.
| Compounds (Marker) | Quality criterion |
|---|---|
| Salvianolic acid B | Referred to Radix Salvia Miltiorrhizae, should not be less than 3.15mg in 1 g of extract of TCM 319 recipe powder |
| Sodiumc danshensu | Referred to Radix Salvia Miltiorrhizae, should not be less than 2.75mg in 1 g of extract of TCM 319 recipe powder |
| Adenosine | Referred to Mycelium powder, should not be less than 1mg in 1 g of extract of TCM 319 recipe powder |
| Schisandrin B | Referred to Fructus Schisandrae Chinensis, should not be less than 0.475mg in 1 g of extract of TCM 319 recipe powder |
2.2. Experimental design
Wt C57BL/6 mice (10 week old males, 24–26 g weight) were purchased from Charles River Laboratories and were housed under standard conditions with ad libitum access to food and water at all times during the study. Hepatic fibrosis was induced either by CCl4 (200 μl, 1:4 dilution in corn oil, by oral gavage, twice a week, for 6 weeks), or BDL (3 weeks). Control mice received corn oil by oral gavage, or were sham operated. Total 47 mice were used for this study.
To test the effect of FZHY on liver homeostasis and fibrogenic liver injury, all the mice were randomly divided into seven groups: Group 1: control animals without any treatment (n=5); Group 2: control animals that receive vehicle (corn oil) and FZHY for 6 weeks (n=5); Group 3: CCl4-treated mice that received vehicle (corn oil) for 6 weeks (n=10); Group 4: CCl4-treated mice treated with FZHY for 6 weeks (n=10); Group 5: sham-operated mice that received vehicle (PBS) for 3 weeks (n=3); Group 6: BDL-operated mice that received vehicle (PBS) for 3 weeks (n=7); Group 7: BDL-operated mice treated with FZHY for 3 weeks (n=7). All animal studies were approved by The University of California, San Diego Institutional Animal Care and Use Committee (protocol number S07088).
2.3. Serum alanine aminotransferase (ALT) level assay
Sham- and BDL-operated mice were sacrificed after 3 weeks, while corn oil and CCl4-treated mice were sacrificed 72 hours after the last oral gavage. Whole blood was collected, serum was separated, and ALT levels were measured by conventional colorimetric assays.
2.4. Immunofluorescence and immunohistochemistry
Formalin-fixed frozen livers were stained with Sirius Red and anti-α-SMA Ab (Abcam). Immunohistochemistry was performed using DAB staining (Vector), and counterstaining with Hematoxilin. The following antibodies were used for immunostaining: rabbit anti-mouse α-SMA (Abcam, Cambridge, MA), rabbit anti-mouse Desmin (Thermo Fisher Scientific, Fremont, CA), rat anti-mouse F4/80 (eBioscience, San Diego, CA), or rabbit anti-mouse 4-HNE (Alpha Diagnostic Intl Inc., San Antonio, TX) antibodies, following incubation with Alexa Fluor ® secondary antibody. The images were taken using Nikon microscope, and analyzed by Image J.
2.5. Measurement of hepatic collagen content
Hepatic hydroxyproline content was measured as described [17]. Hepatic collagen content was also assessed by Sirius Red staining. Sirius Red positive areas were analyzed in six random fields (magnification×4) on each slide and quantified using NIH imaging software.
2.6. Quantitative real-time polymerase chain reaction (qPCR)
Total RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA), and RNeasy kit (Qiagen, Valencia, CA). RNA was reverse-transcribed with a high-capacity complementary DNA reverse-transcription kit (Applied Biosystems, Foster City, CA). Quantitative real-time PCR was performed with an ABIPrism 7000 sequence detector (Applied Biosystems) [18]. Gene expression levels were calculated after normalization to the standard housekeeping gene 18S using the ΔΔ CT method as described by the manufacturer (Invitrogen), and expressed as relative mRNA levels compared with control. The results are represented as mean ± SEM, p<0.0001.
2.7. Hepatocytes and HSC isolation, cell culture and treatment
Hepatocytes and HSCs were isolated as previously described [19] using collagenase/pronase-perfusion and gradient centrifugation method. Isolated hepatocytes were cultured on collagen-coated plates in medium supplemented with 10% FBS+antibiotics, in the presence or absence of CCl4 (5 mmol/L) ± FZHY (0.08 μg/ml or 1.25 μg/ml) for 6 hours. Cells were then stained with Hoechst and PI, and the number of living and apoptotic cells was counted. Alternatively, hepatocytes were harvested and expression of anti-apoptotic genes was analyzed by qPCR. The culture supernatant was collected and analyzed for ALT levels.
HSCs (1 × 105 cell/well) were isolated from a collagen-α1(I)-GFP reporter mice (Col-GFP) mice [20], which upregulates GFP upon cellular activation. The purity of isolated HSCs was estimated by the presence of retinoids (autofluorescent signal detected by fluorescent microscope at 405 wave length) at > 93% of isolated cells. HSCs were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco; Life Technologies Inc.) supplemented with 10% FBS + antibiotics in the presence or absence of FZHY recipe solution (60 μg/ml), and activation of HSCs was evaluated by upregulation of GFP, qPCR for expression of collagen α1(I), α-SMA, TGF-β1, and TGFRI mRNA.
2.8. Assessment of lipid peroxidation
Hepatic lipid peroxidation was evaluated by measurement the level of thiobarbituric acid reactive substances (OxiSelect ™ TBARS Assay Kit, Cell Biolabs, Inc. San Diego, CA) as previously described [21]. Livers were homogenized, and protein concentrations were determined by a modified Lowry assay using the Biorad Dc protein assay (Bio-Rad Laboratories). Sodium dodecyl sulphate and TBARS diluent were added to samples and incubated at 95°C for 1h, and absorbance was measured at 530 nm. The Malondialdehyde (MDA) concentration in liver samples was determined by comparison with the predetermined MDA standard curve and was normalized to total protein concentration.
2.9. Statistical Analysis
All data were expressed as mean ± standard error of mean. Multiple groups were compared using one-way ANOVA with post-hoc multiple comparisons (SPSS 11.5 for Windows Software). Two groups were compared using an unpaired Student’s t test (two tailed). p values less than 0.05 were considered statistically significant.
2.10. Animal Care and Use Statement
The animal protocol was designed to minimize pain or discomfort to the animals. The animals were acclimatized to laboratory conditions (23°C, 12h/12h light/dark, 50% humidity, ad libitum access to food and water) for two weeks prior to experimentation. All experiments and subsequent tissue collection were approved by and performed according to The University of California, San Diego Institutional Animal Care and Use Committee (protocol number S07088).
3. Results
3.1. FZHY attenuates development of CCl4-induced liver fibrosis
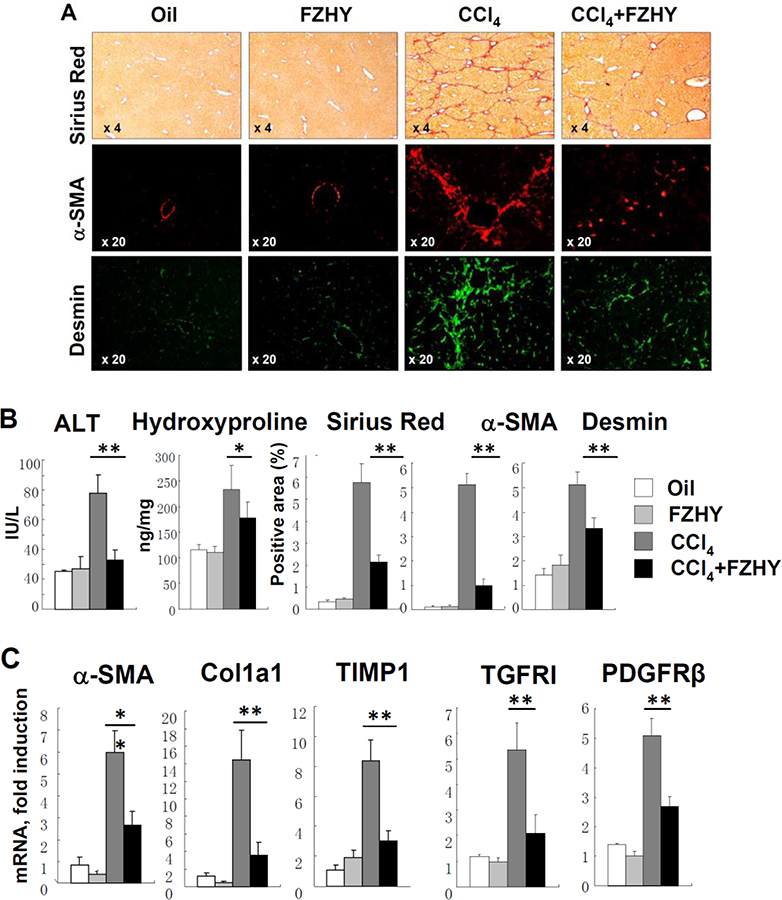
To study the effect of FZHY on liver fibrosis induced by toxic liver injury, wild type mice (males, 10 weeks old) were subjected to CCl4-liver injury (or received corn oil), and were treated with FZHY (or vehicle, see study design). As expected, CCl4-injured mice developed severe liver injury and liver fibrosis as demonstrated by increased levels of serum ALT (4 fold increase), elevated content of hepatic hydroxyproline (2.5 fold increase), increased Sirius Red staining (12 fold increase), and staining for myofibroblast markers, α-SMA (14 fold) and Desmin (3.2 fold, Figure 1A–B). Development of CCl4-induced liver fibrosis was associated with increased expression of fibrogenic genes α-SMA (6 fold), Col1a1 (15 fold), TIMP1 (9 fold), TGF-β1 (4.5 fold), TGFRI (5.3 fold), and PDGFRβ (3.1 fold) mRNA (compared with control mice). Treatment with FZHY (4.0 g/kg body weight, twice a week, by oral gavage) greatly decreased liver injury in CCl4-treated mice. Thus, the serum ALT level was decreased in FZHY treated CCl4-mice (33.1 ± 6 U/L compared to CCl4-mice, 78 ± 12 U/L, p<0.05). Similarly, administration of FZHY dramatically reduced development of liver fibrosis in CCl4-treated mice, as shown by reduced hydroxyproline (177 ± 30 ng/mg vs 234 ± 46 ng/mg in CCl4-mice, p <0.05), decreased area of Sirius Red staining (2.1 ± 0.3 % vs 5.7 ± 0.8 % in CCl4-mice p<0.01), and α-SMA (1.0 ± 0.2 % vs 5.1 ± 0.4 %, p<0.01), and Desmin (3.3 ± 0.4 % vs 5.0 ± 0.5 %, p<0.01) staining (Figure 1A–B). In concordance, expression of fibrogenic genes α-SMA, Col1a1, TIMP1, TGF-β1, TGFRI, and PDGFRβ was downregulated on average by more than 50% by FZHY compared to vehicle in CCl4-treated mice. In addition, when administered to oil-treated control mice, FZHY did not affect liver function or liver histology (Figures 1B–C), and did not cause an immune response (such as spleenomegaly, enlargement of lymph node, or abnormal lymphoid infiltration in parenchymal organs). Overall, our data suggest that FZHY is a non-toxic compound with anti-fibrotic properties which may prevent development of toxic liver injury in mice.
Figure 1:
FZHY compound attenuates development of CCl4-induced liver fibrosis.
Development of liver fibrosis was evaluated in CCl4-injured mice ± FZHY (4.0 g/kg body weight, n=10 per group). The potential side effect of FZHY was evaluated in uninjured mice ± FZHY. (A) Sirius Red staining (×4) and immunofluorescence staining for α-SMA +and Desmin+ myofibroblasts, representative images are taken using objectives x4 and x20, respectively. (B) Serum ALT levels (IU/L) and Hepatic hydroxyproline content (ng/mg liver) were measured. The positive area for Hepatic Sirius Red staining, immunostaining for α-SMA (red) and Desmin (green) calculated as percent, *p<0.05, **p<0.01. (C) Hepatic levels of hepatic mRNA expression of pro-fibrogenic genes were measured using quantitative RT-PCR *p<0.05, **p<0.01.
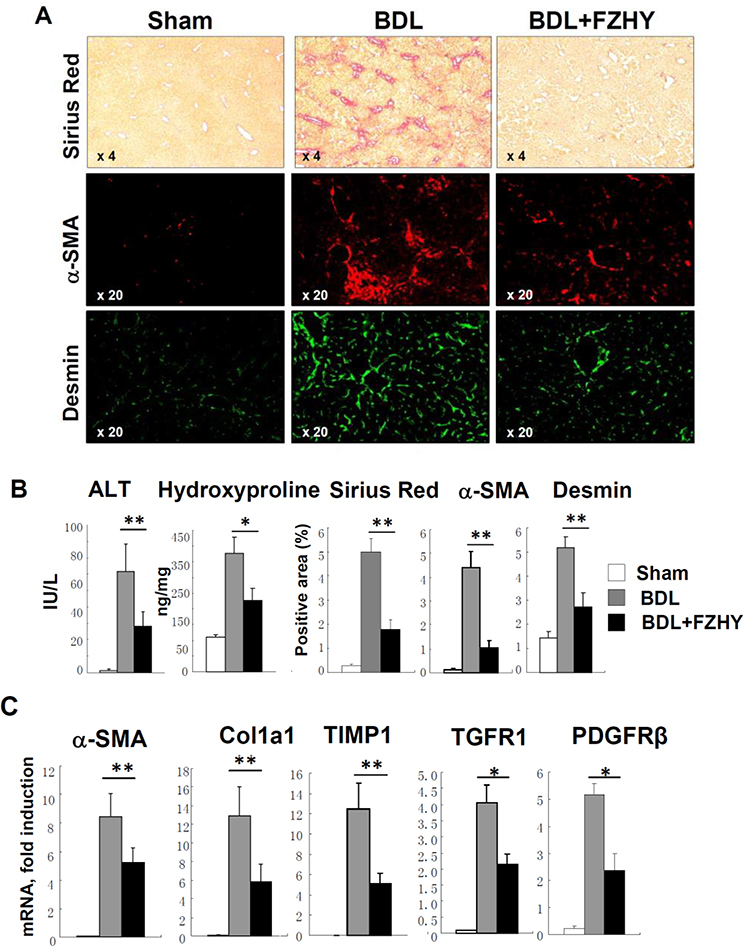
3.2. FZHY inhibits development of BDL-induced liver fibrosis
For this purpose, wild type mice (males, 10 weeks old) were subjected to cholestatic liver injury induced by ligation of common bile duct (BDL, 3 weeks) or sham operated (control), and were treated with FZHY (or vehicle, see study design). Similar to CCl4, FZHY attenuated development of liver fibrosis in BDL-operated mice (compared with control mice), as indicated by improved ALT level in FZHY treated BDL-mice (54 ± 5 U/L vs 73 ± 6 U/L in BDL-mice, p<0.01, Figure 2A–B), reduced hydroxyproline content (226 ± 38 ng/mg vs 378 ± 50 ng/mg in BDL-mice, p < 0.01), reduced area of Sirius Red staining (1.7 ± 0.4 % vs 5.0 ± 0.56 % in BDL-mice, p < 0.01) and staining for hepatic α-SMA (1.0 ± 0.3 % vs 4.4 ± 0.6 %) and Desmin (2.7 ±0.5 % vs 5.1 ± 0.4 %, p<0.01, Figure 2A–B). Our data suggest that FZHY is effective in prevention of liver fibrosis of different etiologies.
Figure 2:
Administration of FZHY compound attenuates BDL-induced liver fibrosis.
Development of liver fibrosis was evaluated in sham- or BDL-operated mice ± FZHY (4.0 g/kg body weight, n=10 per group). (A) Sirius Red staining (×4) and immunofluorescence staining for α-SMA +and Desmin+ myofibroblasts, representative images are taken using objectives x4 and x20, respectively. (B) Serum ALT levels (IU/L) and Hepatic hydroxyproline content (ng/mg liver) were measured. The positive area for Hepatic Sirius Red staining, immunostaining for α-SMA (red) and Desmin (green) calculated as percent, *p<0.05, **p<0.01. (C) Hepatic levels of hepatic mRNA expression of pro-fibrogenic genes were measured using quantitative RT-PCR *p<0.05, **p<0.01.
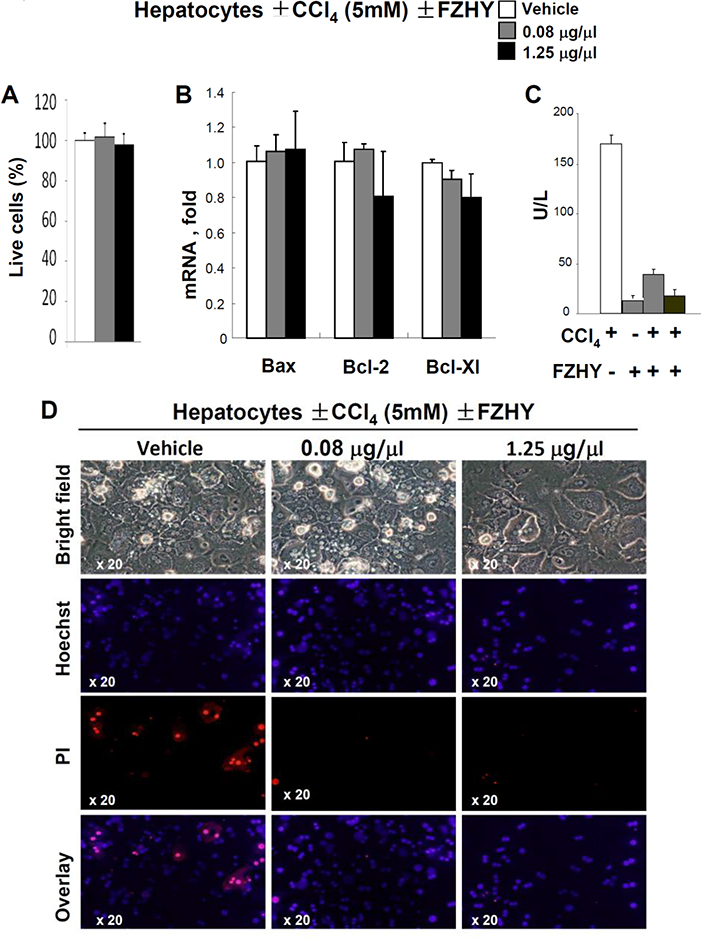
3.3. FZHY protects hepatocytes from toxic injury
First, we tested a range of concentrations of FZHY for toxicity and stimulation of cultured primary murine hepatocytes. Based on assessment of cell viability and expression of survival genes Bax, Bcl-2, Bcl-XL (Figure 3A–B), these functions of primary hepatocytes (1 × 105 cells, 6h) were unaltered by FZHY, and, therefore, FZHY concentrations of 0.08 μg/ml and 1.25 μg/ml of FZHY were used to assess the biological activity of FZHY on cultured hepatocytes. For this purpose, primary murine hepatocytes (1 × 105 cells, 6h) were pretreated with 0.08 μg/ml and 1.25 μg/ml of FZHY (for 15 min) or w/vol of PBS (control) and cultured in the presence of CCl4 (5mmol/L). The hepatotoxic effect of CCl4 on hepatocytes was measured by release of ALT into culture supernatant. As expected, CCl4 injured cultured hepatocytes, as indicated by elevated ALT (170 ± 11 U/L) and rapid death of hepatocytes (detected by a combination of nuclei fragmentation (Hoehst) and intake of PI in 21 ± 5 % of hepatocytes, p<0.02, Figure 3C–D). The hepatotoxic effect of CCl4 was inhibited by addition of 0.08 μg/ml of FZHY, and even further reduced (to the level of ALT in uninjured hepatocytes, 23 ± 2 U/L) when cultured in the presence of 1.25 μg/ml of FZHY. In support of this notion, FZHY-treated hepatocyte were completely protected from CCl4-induced cell death (Figure 3D).
Figure 3:
FZHY compound possesses hepatoprotective effect.
Murine hepatocytes were isolated from uninjured C57BL6 mice, plated and cultured ±CCl4 (5mM) ± FZHY (0.08 or 1.25 μg/μl). (A) The effect of FZHY on viability of cultured primary hepatocytes was evaluated by the number of live cells, shown as percent, compared with hepatocytes cultured in the presence of vehicle (PBS), *p<0.05, **p<0.01. (B) mRNA expression levels of Bax, Bcl-2, or Bcl-XI mRNA in hepatocytes ± FZHY, *p<0.05, **p<0.01. (C) The levels of ALT were measured in the supernatants of CCl4-treated hepatocytes ± FZHY (0.08 or 1.25 μg/μl), *p<0.05, **p<0.01. The data is average of at least three independent experiments (C). FZHY recipe suppresses CCl4-induced apoptosis of hepatocytes. The effect of FZHY on CCl4-induced apoptosis of hepatocytes was evaluated by co-staining of cultured hepatocytes with Propidium Iodide (PI, to detect nuclei of apoptotic cells) and Hoechst (to visualize the total cell nuclei). The representative images of hepatocytes ± FZHY (0.08 or 1.25 μg/μl) are shown using objective x20.
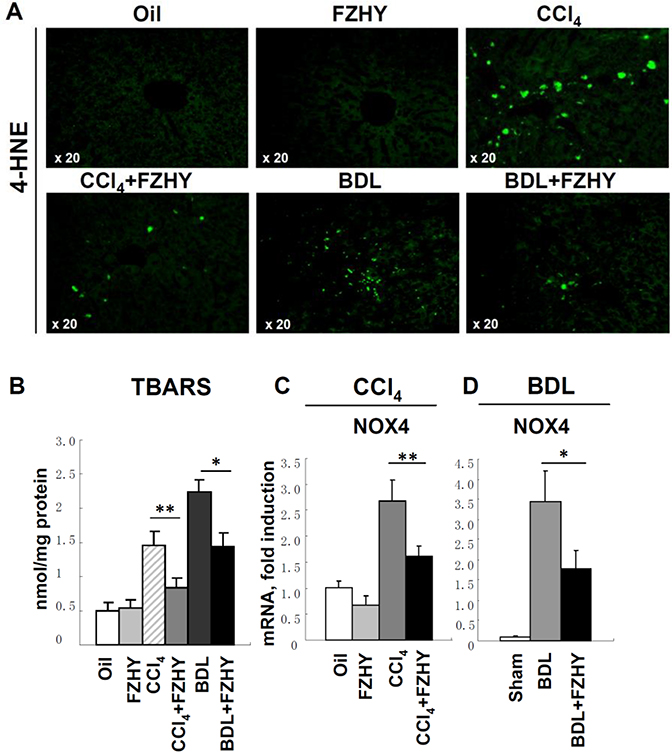
3.4. FZHY compound decreases ROS production and hepatic lipid peroxidation
Next, the mechanism by which FZHY protects CCl4- and BDL-treated mice was investigated in further details. Hepatocyte damage is usually caused by the generation of Reactive Oxygen Species (ROS) and oxidative stress. ROS reacts with membrane lipids causing accumulation of 4-hydroxynonenal (4-HNE), the end product of lipid peroxidation, which is commonly used as ROS marker [57] [1]. The expression of 4-HNE using immunohistochemistry (Figure 3A) revealed that 4-HNE expression was strongly induced in CCl4- and BDL-mice (that received vehicle) but not in CCl4- and BDL-mice treated with FZHY, suggesting that FZHY protected mice from fibrogenic injury via inhibition of ROS production (Figure 4A). In agreement, the assay for thiobarbituric acid-reactive substances (TBARS) demonstrated that hepatic levels of malondialdehyde (MDA) equivalents of lipid peroxidation were also reduced by FZHY treatment in CCl4 mice (0.8 ± 0.1 vs 1.4 ± 0.2 nmol/mg in PBS-treated) and in BDL-mice (1.4 ± 0.2 vs 2.2 ± 0.1 nmol/mg in PBS-treated) (Figure 3B), indicating that FZHY possesses strong hepatoprotective properties. To test this hypothesis, we evaluated hepatic levels of NADPH oxidases (NOXs) which serve as a major source of ROS in the fibrotic liver [2]. Thus, both NOX1 (expressed in aHSCs and hepatocytes) and NOX2 (expressed in Kupffer cells) mediate pro-fibrogenic effects in endogenous liver cells [3], while NOX4 (highly expressed in hepatocytes and HSCs [8]) is additionally implicated in mediation of TGF-β-inducible hepatocyte apoptosis [4, 5]. Quantitative RT-PCR analysis have shown that administration of FZHY inhibited induction of NOX4 in livers of CCl4-mice (1.6 ± 0.2 vs 2.6 ± 0.4 PBS-treated CCl4-mice, Figure 3C) and in BDL-treated mice (17.8 ± 4.6 vs 34.4 ± 7.5 PBS-treated BDL-mice, Figure 3D), but not other NOXs (NOX1 or NOX2, not shown). Therefore, we concluded that FZHY mediates its hepatoprotective functions in part by inhibiting NOX4-dependent ROS production in hepatocytes. Since NOX4 has been suggested to facilitate activation of HSCs [3], we next examined if FZHY may directly inhibit activation of HSCs by studying primary cultures of isolated mouse HSCs.
Figure 4:
FZHY compound suppresses hepatic lipid peroxidation and ROS production via inhibition of NOX expression in hepatocytes.
Liver tissues from Oil- or CCl4-injured mice ± FZHY (4.0g/kg body weight, n=10 per group); and from sham- or BDL-operated mice ± FZHY (4.0g/kg body weight, n=10 per group) were analyzed for the presence of lipid peroxidation and NOX expression. (A) Immunofluorescence staining of 4-HNE (green, ×20), representative images are shown. (B) MDA levels were measured in liver tissues using TBARS assay, the results are average of three independent experiments, and presented as nmol/mg protein, p<0.05, **p<0.01. (C) Expression of NOX4 was measured by RT-PCR in livers of Oil- or CCl4-injured mice ± FZHY, (D) and sham- or BDL-operated mice ± FZHY, *p<0.05, **p<0.01. Expression of NOX1 and NOX2 in these mice, but was not changed upon FZHY administration (data not shown).
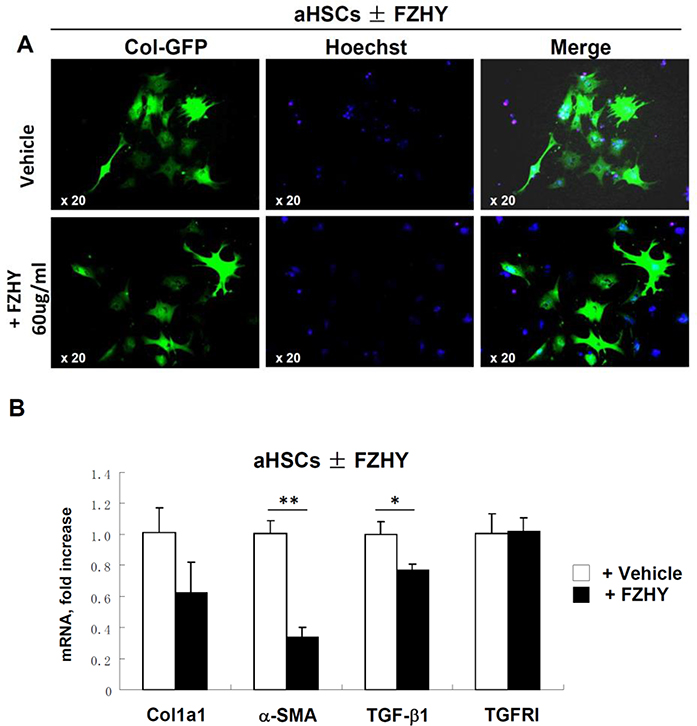
3.5. FZHY does not have significant effect on inhibition of HSC activation in vitro
Primary mouse qHSCs were isolated from untreated Col-GFP reporter mice (that express GFP under control of collagen-α1(I) promoter/enhancer [6]), plated and activated on plastic (18 h) in the presence of different concentration of FZHY (range from 0.08 μg/μl to 60 μg/μl) or vehicle (PBS). Expression of collagen-α1(I) was assessed in this experiment by upregulation of GFP in HSCs upon activation. Surprisingly, administration of high doses of FZHY (60 μg/μl) only slightly reduced GFP expression (10%) in aHSCs (vs PBS-treated aHSCs, Figure 4A). Similarly, RT-PCR analysis revealed that FZHY-treated aHSCs minimally downregulated Col1a1, α-SMA and TGF-β1 mRNA (compared to PBS-treated aHSCs), while expression of TGFRI remained unchanged (Figure 4B). Overall our results suggest that FZHY does not prevent HSC activation in vitro, indicating that HSCs might not be a direct FZHY target. Therefore, we next examined if FZHY can indirectly affect HSC activation via regulation of cytokine secretion by inflammatory cells recruited to injured liver.
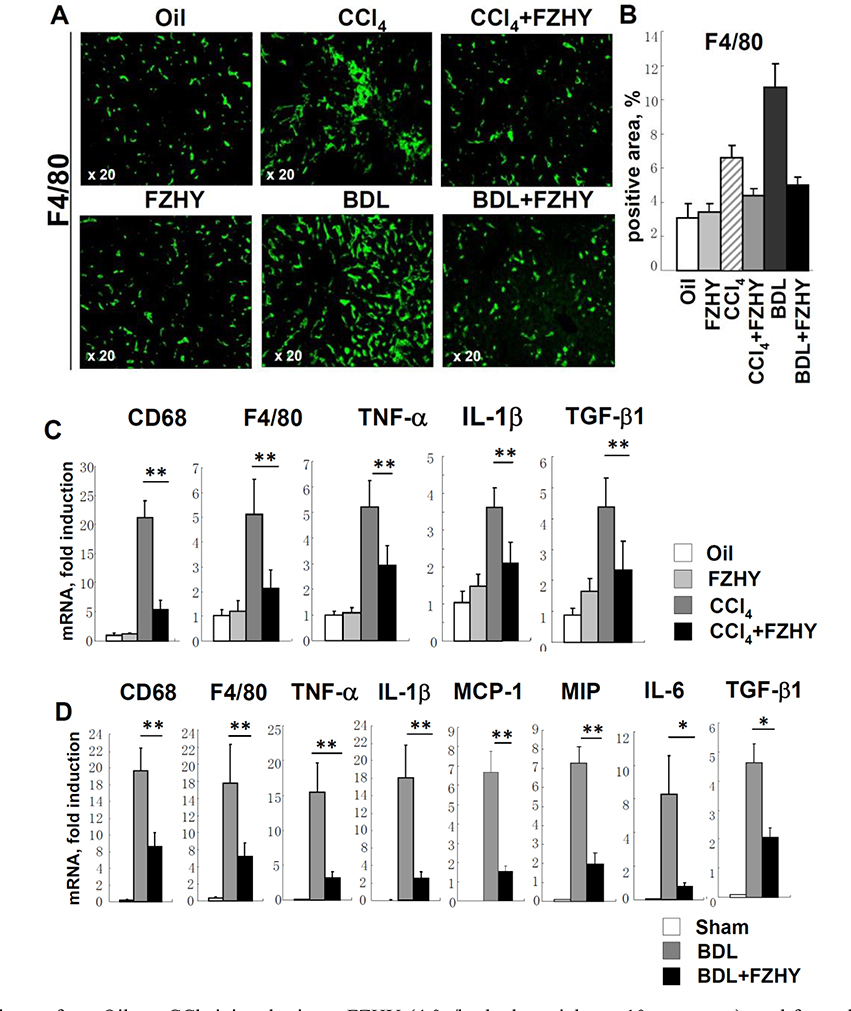
3.6. FZHY compound inhibits recruitment and activation of inflammatory cells
Development of CCl4- and BDL- liver injury caused recruitment and activation of hepatic macrophages (Kupffer cells), as demonstrated by immunostaining for macrophage marker F4/80+ (Figure 6A–B), and was associated with the increased expression of CD68+ and F4/80+ mRNA in fibrotic liver (Figure 6C–D). Remarkably, administration of FZHY strongly decreased the numbers of F4/80+ Kupffer cells, and CD68+ and F4/80+ mRNA expression in livers of CCl4 and BDL-mice (but not in non-injured mice), suggesting that FZHY inhibits the flux of macrophages to fibrotic liver. In support of this notion, expression of MIP-1 and MCP1, the factors critical for macrophage recruitment, were strongly suppressed (by >3 fold) in BDL-operated mice upon FZHY administration (Figure 6D). We next tested if reduced numbers of Kupffer cells in FZHY-treated CCl4 and BDL-mice were also associated with a change in hepatic cytokine expression profile. Indeed, expression level of TGF-β1, the major profibrogenic cytokine in fibrotic livers, was reduced (by ≈2 fold) in FZHY-treated CCl4 and BDL-mice. Furthermore, expression level of pro-inflammatory cytokines, such as TNF-α, IL-1β and IL-6, were downregulated in CCl4 and BDL-mice (by ≈1.5 fold and >7 fold, respectively). Overall, our data suggest that, in addition to hepatocprotective functions, FZHY compound possesses strong anti-fibrotic and anti-inflammatory properties, it inhibits macrophage recruitment to fibrotic livers and their activation, and this way attenuates activation of hepatic HSCs/myofibroblasts.
Figure 6:
FZHY inhibits recruitment and activation of inflammatory macrophages in fibrotic liver.
Liver tissues from Oil- or CCl4-injured mice ± FZHY (4.0g/kg body weight, n=10 per group); and from sham- or BDL-operated mice ± FZHY (4.0g/kg body weight, n=10 per group) were analyzed for the expression of myeloid cell markers and inflammatory and fibrogenic cytokines. (A) Immunofluorescent staining for F4/80 (green, ×20), representative images are shown. (B) Positive area for F4/80 staining is calculated as percent, *p<0.05, **p<0.01. (C) Expression of myeloid genes and pro-inflammatory mediators was measured by RT-PCR in liver tissues from Oil- CCl4-injured mice ± FZHY and (D) sham- or BDL-operated mice ± FZHY, *p<0.05.
4. Discussion
Liver fibrosis is the common outcome of many chronic liver injuries. Despite increasing understanding of the molecular pathways involved in pathogenesis of liver fibrosis, therapeutic options for patients with liver fibrosis are often limited. Traditional Chinese medicine has been used in China since 2800 BC. Clinical practices and experimental results have suggested that some herbal medicines had anti-fibrotic effects, including Salvia miltiorrhiza and salvianolic acid B [22, 23]. FZHY compound is composed of several herbs including Salvia miltiorrhiza and salvianolic acid B, which historically were suggested to be the most effective when taken in combination (see Table 1). The present study was designed to test if oral administration of FZHY compound can attenuate development of experimental liver fibrosis of different etiologies. In addition, we explored the mechanism by which FZHY compound may mediate its anti-fibrotic properties. The complimentary use of herbal supplements may improve the treatment in patients with liver fibrosis by increasing the immune response or the anti-oxidant level [24].
Here we demonstrated that FZHY is a non-toxic compound that has therapeutic potential to improve liver function and reduce inflammation. Administration of FZHY compound produced an anti-fibrotic effect in mice subjected to two models of liver fibrosis of different etiologies, CCl4 and BDL, as confirmed by decreased hepatic collagen Type I deposition, reduction of activated α-SMA +and Desmin+ hepatic myofibroblasts, and suppression of pro-fibrogenic gene expression, including α-SMA, collagen α1(I), TGF-β1, TGFRI, TIMP-1 and PDGF-β. These results are in concordance with previous reports provided by Chinese investigators [16, 25]. To further understand the mechanism of FZHY action, we examined the effect of FZHY on hepatic cells that play a critical role in pathogenesis of liver fibrosis.
Following hepatic injury, several events have been identified to be critical in the pathogenesis of liver fibrosis [11]. They include: damage to hepatocytes, recruitment of inflammatory cells, release of TGF-β1, the major fibrogenic cytokine, induction of ROS, and activation of collagen producing myofibroblasts. Many cell types contribute to the pathogenesis of liver fibrosis, including hepatocytes, inflammatory cells, Kupffer cells, endothelial cells, and myofibroblasts [26, 27]. Hence, sustained hepatic inflammation is generally accepted to represent the key prerequisite of fibrogenesis [27]. Kupffer cells are liver-resident macrophages. They express myeloid markers such as F4/80, CD68, CD11b, CCR2, and CX3CR1 [28, 29]. Kupffer cells are believed to participate actively in the development of liver fibrosis by secretion of TGF-β1, IL-6 and other profibrogenic cytokines. HSCs are the major contributors to myofibroblasts in the fibrotic liver and are considered the most important cell type for the production of collagens [4]. Taken together, liver injury induces cross-talk between these cell types to induce fibrogenic signals such as TGF-β1, TNF-α, and ROS.
To investigate the mechanism of FZHY anti-fibrotic action, we demonstrated that FZHY possesses a strong hepatoprotective effect. The present study showed in vivo and in vitro that FZHY exhibit no cytotoxicity to hepatocytes, or immune cells. Moreover, FZHY significantly decrease apoptosis of hepatocytes in the course of hepatic injury. Injury of hepatocytes, which is often associated with production of ROS, plays a critical role in the development of liver fibrosis. Oxidative stress results from an inappropriate balance between production and clearance of ROS, leads to aberrant tissue repair in the liver, and contributes to the development of liver fibrosis. To investigate the mechanism by which FZHY prevents hepatocyte apoptosis, we measured ROS mediated hepatic lipid peroxidation products 4-HNE and MDA, as indicators of oxidative stress in the liver. Our study showed that FZHY inhibits lipid peroxidation, and it mediates its anti-oxidant effects in part via blocking NOX4 expression in damaged hepatocytes. Thus, hepatoprotective effect of FZHY is an important mechanism for its anti-fibrotic action.
In addition to its hepatoprotective and anti-oxidant properties, FZHY inhibits recruitment and activation of myeloid cells, as demonstrated by suppression of hepatic inflammatory mediators such as TNF-α, IL-6 and IL-1β, and reduction of F4/80, CD68, MCP-1, and MIP-1 expression levels. Liver inflammation is characterized by activation of distinct chemokine pathways in the liver and the circulation allowing distinct immune cell populations to enter the liver via sinusoids and postsinusoidal venules. Recent investigations have shed light on the intimate interactions between myofibroblasts and infiltrating immune cells, which drive liver scarring. Experimental models of liver fibrosis have demonstrated that disruption of signaling pathways regulated by such chemokines as MCP-1 and its receptor, or CCL5 (RANTES) or CCR1 / CCR5 and others, may effectively prevent collagen deposition, by targeting migration of monocytes and macrophages [30]. Our results indicate that FZHY inhibits macrophage recruitment to fibrotic liver by suppressing the intrahepatic expression of chemoattractant chemokines (MCP-1 and MIP) in fibrotic liver.
The activation of HSCs plays a critical role in liver fibrogenesis [31]. In the present study, we investigated the effect of FZHY on HSC activation in vivo and in vitro. We demonstrated that treatment with FZHY decreased the number of hepatic α-SMA+ and Desmin+ myofibroblasts in vivo, but did not significantly inhibit HSC activation in vitro. One explanation of this result would suggest that specific microenvironment (which is not reproduced using the in vitro culture conditions) is required for successful FZHY-aHSC interaction. Thus, the presence of surrounding cells which may increase solubility of FZHY, activation of biologically active ingredients of FZHY by proteolytic cleavage, availability of active chelators, and etc. may be required for FZHY-aHSC interaction. In support of this concept, high concentrations of FZHY weakly attenuate in vitro HSC activation by ≈20%. Alternatively, and more likely, FZHY may not directly influence HSC activation, but function via inhibition of fibrogenic and inflammatory cytokines. Consistently with this notion, expression of TGF-β1, the major fibrogenic cytokine required for HSC/myofibroblast activation, was reduced in the injured livers of mice that were treated with FZHY.
These results indicate that FZHY compound inhibits the development of liver fibrosis of different etiologies in mice. In addition to its hepatoprotective and anti-oxidant properties, FZHY recipe can effectively inhibit recruitment and activation of inflammatory cells, but it does not directly inhibit HSCs activation in vitro. Our data provides a scientific validation of the therapeutic potential of the FZHY compound using preclinical models of liver fibrosis, Our study for the first time provides a mechanism of FZHY action, and supports the clinical trials (phase II trial) of supplementary administration of Fuzheng Huayu capsules to patients with HCV liver fibrosis.
Figure 5:
FZHY recipe does not inhibit HSC activation in vitro.
qHSCs were isolated from Collagen-α1(I)-GFP mice and plastic activated. (A) GFP fluorescence (green, ×20) of HSCs incubated ± FZHY (60ug/ml). The same number of GFP+ cells were detected in HSCs cultured HSCs incubated ± FZHY, indicating that FZHY has minimal effect on HSC activation. Representative images are shown using objective x20. (B) Expression of fibrogenic cytokines including collagen-α1(I), α-SMA, TNF-β1and TGFRI in HSCs incubated ± FZHY (60ug/ml), *p<0.05, **p<0.01
Acknowledgments
Grant Support: Supported by the National Institutes of Health R01DK101737, U01AA022614, and R01DK099205, R01DK111866 (T.K.), R01DK101737, U01AA022614, R01DK09920, P50AA011999, AI043477 (D.A.B.)
Abbreviations:
- NADPH
Nicotinamide adenine dinucleotide phosphate
- TGF-β1
Transforming growth factor-β1
- ROS
Reactive oxygen species
- α-SMA
α-smooth muscle actin
- RAS
Rennin angiotensin system
- FZHY
Fuzheng Huayu
- CCl4
Carbon tetrachloride
- BDL
Bile duct ligation
- PBS
Phosphate-buffered saline
- TIMP-1
Tissue inhibitor of matrix metalloproteinase-1
- PDGF-β
Plateletderived growth factor-β
- MCP-1
Monocyte chemoattractant protein-1
- MIP-1
Macrophage inflammatory protein-1
- ALT
Alanine aminotransferase
- MDA
Malondialdehyde
- 4-HNE
4-hydroxynonenal
- NASH
Non-alcoholic steatohepatitis
- AIH
Autoimmune hepatitis
- HCV
Hepatitis C virus
- HBV
Hepatitis B virus
- ECM
Extracellular matrix
- HSC
Hepatic stellate cell
- TNF-α
Tumor necrosis factor-α
References
- 1.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 115 (2005): 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun Beicheng, Karin Michael. Obesity, inflammation, and liver cancer. J Hepatol 56 (2012): 704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Exp Biol Med 233 (2008): 109–122. [DOI] [PubMed] [Google Scholar]
- 4.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology 134 (2008): 1655–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kisseleva T, Brenner DA. Hepatic stellate cells and the reversal of fibrosis. J Gastroenterol Hepatol 21 (2006): 84–87. [DOI] [PubMed] [Google Scholar]
- 6.Cheung KF, Ye DW, Yang ZF, et al. Therapeutic efficacy of Traditional Chinese Medicine 319 recipe on hepatic fibrosis induced by carbon tetrachloride in rats. J Ethnopharmacol 124 (2009): 142–150. [DOI] [PubMed] [Google Scholar]
- 7.Friedman SL, Bansal MB. Reversal of hepatic fibrosis: fact or fantasy? Hepatology 43 (2006): 82–88. [DOI] [PubMed] [Google Scholar]
- 8.Subeq YM, Ke CY, Lin NT, et al. Valsartan decreases TGF-beta1 production and protects against chlorhexidine digluconate-induced liver peritoneal fibrosis in rats. Cytokine 53 (2011): 223–230. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Wang Z, Kwong SQ, et al. Inhibition of PDGF, TGF-beta and Abl signaling and reduction of liver fibrosis by the small molecule Bcr-Abl tyrosine kinase antagonist Nilotinib. J Hepatol 55 (2011): 612–625. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Bataller R, Dulyx J, et al. Attenuated hepatic inflammation and fibrosis in angiotensin type 1a receptor deficient mice. J Hepatol 43 (2005): 317–323. [DOI] [PubMed] [Google Scholar]
- 11.Yokohama S, Yoneda M, Haneda M, et al. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology 40 (2004): 1222–1225. [DOI] [PubMed] [Google Scholar]
- 12.Kisseleva T, Brenner DA. Anti-fibrogenic strategies and the regression of fibrosis. Best Pract Res Clin Gastroenterol 25 (2011): 305–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chor SY, Hui AY, To KF, et al. Anti-proliferative and pro-apoptotic effects of herbal medicine on hepatic stellate cell. Journal of Ethnopharmacology 100 (2005): 180–186. [DOI] [PubMed] [Google Scholar]
- 14.Luk JM,Wang XL, Liu P, et al. Traditional Chinese herbal medicines for treatment of liver fibrosis and cancer: from laboratory discovery to clinical evaluation. Liver International 27 (2007): 879–890. [DOI] [PubMed] [Google Scholar]
- 15.Liu P, Liu C, Xu LM, et al. Effects of Fuzheng Huayu 319 recipe on liver fibrosis in chronic hepatitis B. World J Gastroenterol 4 (1998): 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia YH, Wang RQ, Mi HM, et al. Fuzheng Huayu recipe prevents nutritional fibrosing steatohepatitis in mice. Lipids Health Dis 11 (2012): 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paik YH, Kim JK, Lee JI, et al. Celecoxib induces hepatic stellate cell apoptosis through inhibition of Akt activation and suppresses hepatic fibrosis in rats. Gut 58 (2009): 1517–1527. [DOI] [PubMed] [Google Scholar]
- 18.Seki E, de Minicis S, Inokuchi S, et al. CCR2 promotes hepatic fibrosis in mice. HEPATOLOGY 50 (2009): 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiskirchen R, Gressner A. Isolation and culture of hepatic stellate cells. Methods Mol Med 117 (2005): 99–113. [DOI] [PubMed] [Google Scholar]
- 20.Magness ST, Bataller R, Yang L, et al. A dual reporter gene transgenic mouse demonstrates heterogeneity in hepatic fibrogenic cell populations. Hepatology 40 (2004): 1151–1159. [DOI] [PubMed] [Google Scholar]
- 21.Lu Y, Wang X, Cederbaum AI. Lipopolysaccharide-induced liver injury in rats treated with the CYP2E1 inducer pyrazole. Am J Physiol Gastrointest Liver Physiol 289(2005): 308–319. [DOI] [PubMed] [Google Scholar]
- 22.Lee TY, Wang GJ, Chiu JH, et al. Long-term administration of Salvia miltiorrhiza ameliorates carbon tetrachloride-induced hepatic fibrosis in rats. Journal of Pharmacy and Pharmacology 55 (2003): 1561–1568. [DOI] [PubMed] [Google Scholar]
- 23.Lin YL,Wu CH, Luo MH, et al. In vitro protective effects of salvianolic acid B on primary hepatocytes and hepatic stellate cells. Journal of Ethnopharmacology 105 (2006): 215–222. [DOI] [PubMed] [Google Scholar]
- 24.Liu C, Hu Y, Xu L, et al. Effect of Fuzheng Huayu formula and its actions against liver fibrosis. Chin Med 4 (2009): 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang QL, Tao YY, Shen L, et al. Chinese herbal medicine Fuzheng Huayu recipe inhibits liver fibrosis by mediating the transforming growthfactor-β1/Smads signaling pathway. Zhong Xi Yi Jie He Xue Bao 10 (2012): 561–568. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Lafdil F, Kong X, et al. Signal transducer and activator of transcription 3 in liver diseases: a novel therapeutic target. International Journal of Biological Sciences 7 (2011): 536–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zimmermann HW, Tacke F. Modification of chemokine pathways and immune cell infiltration as a novel therapeutic approach in liver inflammation and fibrosis. Inflamm Allergy Drug Targets 10 (2011): 509–536. [DOI] [PubMed] [Google Scholar]
- 28.Aoyama T, Inokuchi S, Brenner DA, et al. CX3CL1-CX3CR1 interaction prevents carbon tetrachloride-induced liver inflammation and fibrosis in mice. Hepatology 52 (2010): 1390–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seki E, De Minicis S, Inokuchi Seki ES, et al. CCR2 promotes hepatic fibrosis in mice. Hepatology 50 (2009): 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baeck C, Wehr A, Karlmark KR, et al. Pharmacological inhibition of the chemokine CCL2 (MCP-1) diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. Gut 61 (2012): 416–426. [DOI] [PubMed] [Google Scholar]
- 31.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology 134 (2008): 1655–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]