Abstract
It is anticipated that effective vaccines will enable the resumption of social and economic normalcy. Current calls for masking, social distancing and other restrictive measures for the public-good are difficult to enforce and are unstainable. As ~2–4% of the 50 million SARS-CoV2-infected have succumbed to Covid-19, the US department of Health and Human Services has organized a public-private partnership called Operation Warp Speed (OWS) to develop, produce and deliver 300 million doses of safe and effective vaccines with a January 2021 target. While a majority of the 300+ Covid-19 vaccine candidates are in various stages of preclinical and early-stage clinical testing, 6 clinical candidates are supported with over 10 billion USD plus integrated resources under the OWS agenda. This unprecedented approach is investing in the manufacture of product candidates ahead of product approval. It is enabled by new gene and recombinant pharmaceutical platform technologies that are accelerating the clinical study timeline from ~10 to less than 1 year. It is anticipated that one or more of the 6 candidates under the OWS initiative will be safe, effective and provide a sustained immune response to prevent infection and disease progression. This way, social and economic activities could return to normalcy.
Keywords: Covid-19, SARS-CoV2, Vaccines, Gene therapy, Formulation, Biopharmaceutics, Biotechology
Until SARS-CoV2 is completely wiped-out, the current worldwide call for social distancing is unlikely to end the Covid-19 pandemic or allow a return to social-economic normalcy. A recent flare-up, even in the most restricted countries, clearly supports this notion. Many in the community are calling for fast-track vaccine development to prevent infection and progression to Covid-19 disease. According to a Johns Hopkins University database, a small fraction, ~2–4% of over 50 million infected people, will manifest severe acute respiratory syndrome (SARS), followed by multiple organ failure and death. There is currently no predictable disease course while a profile of people who will succumb to this disease is unavailable. This unpredictability, along with the deadly consequence of disease progression, has prompted drastic public health measures by implementing a world-wide call for a generalized shut-down of social activities along with masking and social distancing as a means to curb the speed of virus spread in communities. At the same time, it is hoped that heavy investment in vaccine discovery and development may accelerate the process of finding an effective vaccine and leading the world out of a pandemic.
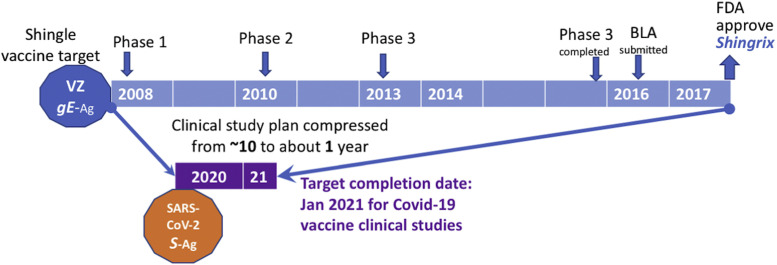
Other than the product development path for the seasonal influenza flu vaccine, typical vaccine development for a new or novel pathogen (i.e., novel coronavirus) is expected to take many years of research to fully understand the viral structure-function relationship as well as the key protective host immunities that can define vaccine antigen targets. There are examples where certain host immune responses may be harmful as some of the antibodies induced in early vaccine development appear to produce antibody dependent enhancement effects including impacts on blood vessels like those for the mosquito-borne Dengue virus.1 Typically, viral antigen delivery platforms and adjuvants are developed to formulate vaccines to increase potency while balancing safety. For example, as shown in Fig. 1 , Herpes Zoster (which causes reactivation later in life presented as shingles in elderly subjects) is a much better understood chicken pox virus. Even knowing that the immune response to Zoster glycoprotein-E (gE) antigen is the vaccine antigen target, it has taken a GSK pharmaceutical company sponsored clinical study program about 10 years (begun in 2008) to complete a study in 2017 and gain FDA approval of a Shingrix vaccine product under a biologic licensing application (or BLA).2
Fig. 1.
Accelerated vaccine development timeline for current Covid-19 vaccine candidates compared to a typical vaccine clinical study to product approval exemplified by a shingles vaccine called Singrix, which gained FDA licensing approval in 2017. Shingles, a painful outbreak on skin is caused by the reactivation of a previous exposure to a Herpes virus called Varicella Zoster or VZ. An immune response against viral glycoprotein antigen E or gE has been shown to provide protection. Current general consensus is that a SARS-CoV2 virus antigen called Spike protein or S Antigen, referred to as S–Ag, is a candidate antigen for most vaccine candidates under development. The timeline and 3 phases of shingles vaccine clinical development is presented along with a submission of the data assembled in the Biologic Licensing Application or BLA leading to the final approval of a product license by the Food and Drug Administration (FDA) presented for Shingrix. Based on the Operation Warp Speed (OWS) schedule, which targets a January 2021 completion of clinical studies for the funded Covid-19 vaccine candidates, the clinical development timeline is compressed and anticipated to be less than 1 year or 10-times the speed for average new vaccine development, particularly for candidates using a novel vaccine delivery platform.
However, due to the worldwide impact of the Covid-19 pandemic—with over 50 million infected and 1 million deaths—there is an urgent call to fast-track vaccine development under warp speed. Currently, with public investment and collaboration with small and large pharmaceutical companies, it is anticipated that the clinical development (including safety and efficacy evaluations) of vaccines will be completed with a January 2021 target timeline2 , 3; about 10 times the speed of a normal clinical vaccine development program (Fig. 1).
Accumulated data indicate that the time from viral infection to detectable virus and symptomatic Covid-19 SARS disease progression is somewhat unpredictable. While some risk factors such as obesity, diabetes and age are reported, the divergence of who progresses to SARS is less than certain. Also, infected individuals who are apparently healthy can progress to SARS with little or no warning within 24–48hr. The unpredictability of disease progression, even with only a small but significant fraction of people succumbing to Covid-19, has prompted a world-wide call for investing significant effort and resources to develop a safe and effective vaccine. In response, the government has assembled health experts, scientists and regulators as well as private companies to develop vaccines for Covid-19 while the understanding that knowledge of protective immunity is still evolving. Since the first case confirmed in the US in January 2020 (along with the revelation of the genetic sequence), the US department of Health and Human Services (HHS) has organized a public-private partnership called Operation Warp Speed (OWS) to develop, produce and deliver 300 million doses of safe and effective vaccines with a January 2021 target. This is a highly ambitious goal and the approach of the public-private partnership strategy is truly unprecedented with billions of dollars in upfront investment by the government while the vaccine is still in development and clinical testing (Table 1 ). OWS is charged to invest funds and resources for vaccine research and development while stockpiling before any vaccine product is demonstrated to work. The participation of regulatory agencies like the FDA along with the logistical planning of OWS for rapid vaccine deployment exhibit the urgency and priority of finding an effective vaccine to combat SARS-CoV2 and returning to normalcy.
Table 1.
A Select List of Covid-19 Vaccine Candidates in Clinical Development Under Public-Private-Partnership and Significant Public Investmenta.
| Candidate | Innovator | Large Pharma Partner | Public Investment (Millions in USD) | Clinical Status |
|---|---|---|---|---|
| mRNA-1273 | Moderna/NIAID | Catalent | $ 2455 | Phase 3 |
| AD1222 (ChAdOx1) | Oxford University | AstraZeneca | $ 1200 | Phase 3 |
| Ad26.CoV2.S | Harvard University | J & J | $ 1000 | Phase 1/2 |
| BNT162b1(2) (RNA-lipid vehicle) | BioNTech | Pfizer | $ 1950b | Phase 2/3 |
| CoVLP-Adjuvanted | Medicago | Sanofi/GSK | $ 2000 | Phase 1/2 |
| NVX-Co2373 Adjuvanted | Novavax | Not Disclosed | $2000 | Phase 1/2 |
The data in this table is constructed based on the published information under OWS (US Health and Human Services-HHS, 2020) and the ClinicalTrials.gov database as of Nov 2020.
The investment is in the form of 100 + 1300 million doses of vaccine purchase commitment while BNT162b1 is still under clinical development.
OWS's public-private partnership structure is notable. According to the published information, not only are most major pharmaceutical companies actively participating, so are key government organizations: NIH is leading the science, CDC is leading on infection and public health, FDA is providing regulatory pathways for product licensing, BARDA (a research and development organization of the department of defense or DoD) is providing additional resources. This support is being integrated into OWS for collectively planning and implementing cohesive strategies to allocate resources and find a Covid-19 vaccine by January 2021. To date, through OWS effort, over $10 billion has been invested in 6 distinct candidates from 12 companies in research, clinical development and/or stockpiling candidate vaccines ahead of FDA product approval.3 As summarized in Table 1, at least 3 are in phase 3 clinical testing while the FDA and its advisory panel is yet to finalize criteria for Covid-19 vaccine approval. Given the national and worldwide urgency, it is clear that the vaccine for Covid-19 will be reviewed under an Emergency Use Authorization (EUA). However, it is likely that vaccine efficacy will be about 50%, which is similar to the target for the seasonal flu vaccine that will be the basis of EUA approval. Regardless of the exact vaccine registration and approval pathway, all parties in OWS are committed to ensuring the safety and efficacy of vaccines before product licensing and the launch of a public vaccination campaign. In addition to the plan to stockpile candidate vaccines and support the cost of manufacturing, while still under clinical testing, the US HHS (department of health and human services) has also invested significant funding and support to ensure rapid deployment of vaccination under OWS.3
One may ask how the 6 Covid-19 vaccines in late clinical stage trials presented in Table 1 came about. They may also ask what distinctions in technological and pharmaceutical characteristics as well as scientific consideration prompted investment decisions even as these 6 candidates are still undergoing clinical testing for effectiveness and efficacy. There are at least 300 candidates in exploratory stages, 100 or so in preclinical testing and about 33 in various stages of clinical testing being reported worldwide.4 There are a few inactivated and live-attenuated SARS-CoV2 being considered as vaccine formulation platforms. However, public concern about the potential escape of pathogenic effects may have been the key to their exclusion in OWS's initial vaccine investment list. While there are multiple viral proteins expressed by SARS-CoV2, it is generally accepted that viral surface spike or S protein is the vaccine target. The viral S protein has been demonstrated to facilitate viral entry into host cells, initiating infection, making progeny and spreading. Thus, the scientific rationale is that eliciting antibodies and immune rsponses that can neutralize or prevent the viral entry and infection process is the key to the Covid-19 vaccine strategy. Thus, regardless of variation in the platform and formulation as well as molecular characteristics of vaccine candidates, lead candidate vaccine formulations are intended to elicit neutralizing antibodies. Making sufficient neutralizing viral antibody concentrations (or high titers) is believed to be the key to vaccine success and efficacy. Based on this product concept, vaccines using genetic, viral vectors or protein carriers are constructed with enabling technologies intended to elicit immune responses against Covid-19 by a number of mechanisms as listed in Table 2 .
Table 2.
The Characteristics, Enabling Technology and Proposed Mechanisms of Action for the Lead Covid-19 Vaccine Candidates Intended to Elicit Neutralizing Antibodies Against SARS-CoV2 S Protein.
| Candidate [Sponsors] | Enabling Technology Platform | Dosing Regimen | Mechanism of Action |
|---|---|---|---|
| mRNA-1273 [Moderna/NIAID Catalent] | SARS-CoV2 RNA + cell penetration excipient | Intramuscular 100 μg dose; 2 doses at d 1 & 28 | Viral mRNA seqeunce delivered to host cells to express viral spike protein antigen |
| AD1222-ChAdOx1 [Oxford U/AstraZeneca] | Recombinant Adeno virus with SARS-CoV2 sequence | Intramuscular 5 × 1010 virus; 2 doses at d 1 & 28 | Adeno virus infected host cell is direct to express viral spike protein antigen |
| Ad26.CoV2.S [Harvard Johnson & Johnson] | Recombinant Adeno virus with SARS-CoV2 sequence | Intramuscular; 2 to 3 doses, every 2 or 6 month | Recombinant Adeno virus infected host cell is directed to express viral spike protein antigen |
| BNT162b1(2) [BioNTech Pfizer] | SARS-CoV2 mRNA + cell penetration lipid excipient complex | Intramuscular 10,20,30 or 100μg; 2 doses d 1 & 21. | mRNA fragment of virus that directs host cell to express viral spike protein antigen |
| CoVLP-adjuvanted [Medicago Sanofi/GSK] | Virus like particles coated with SARSCoV2 protein | Intramuscular; 1 or 2 doses with 2 adjuvant variables; d 1 & 22 | Virus spike protein antigen is presented as protein particles without genetic materials; adjuvanted to enhance immune response |
| NVX-Co2373 Adjuvanted [Novavax] | Recombinant SARSCoV2 protein in nanoparticles | Intramuscular; 5 or 25 μg 2 doses; d 1 & 21 | Recombinant virus spike protein antigen is assembled in small nanoparticle adjuvant to boost immune response |
Given that the overall vaccination strategy is to induce SARS-CoV2 viral neutralization antibodies, mainly based on blocking the S protein to prevent initiation of host cell infection, there is great interest in learning whether a virus neutralization antibody would be helpful or harmful. Based on the results of convalescent antibody transfer to naïve subjects (using serum from individuals with strong antibody signals after recovering from Covid-19 infection), neutralization antibodies are likely to be safe and could be helpful (FDA basis for convalescent antibody product EUA approval). However, the correlate of higher neutralization antibody titer and time to recovery is not strong.
Building on this knowledge, recent phase 2 vaccines studies that evaluate vaccine antibody characteristics are being evaluated in those receiving 2 doses of AD1222-ChAdOx1 recombinant adenovirus vaccine. Vaccine recipients exhibit neutralization antibody titer at nearly the 218 range.5 Another recombinant adenoviral vector (Ad5-vectored Covid-19 vaccine) produced neutralization titer in 18–19.6 The two vaccine candidates appeared to produce about a 10-fold difference in neutralization antibody response in the study participants. As higher neutralization antibody titer suggests a higher degree of protection against viral infection, vaccines that provide higher neutralization titer can serve as outcome measures and surrogate indicators. However, neutralization antibody titer can vary depending on the assay configuration in which the dose of virus deployed evaluates the ability of a serum to neutralize viral infection. Thus, a standardized viral neuralization titer assay method for use across different vaccine candidate outcomes may be necessary to evaluate the comparative potency of neutralization antibodies induced by each vaccine candidate. In addition, given current regulatory approval calling for 50% efficacy for preventing other seasonal viruses such as flu vaccines, it is likely that a Covid-19 vaccine will be considered for approval under Emergency Use Authorization (EUA) with a similar expectation. Whether and what levels of neutralization antibody titer would indicate 50% protection in the population or prevent disease progression outcomes remains to be experimentally determined.
In order for any Covid-19 vaccine candidate to be a viable product that can be rapidly and consistently scaled up for manufacturing and regulatory approval, there are several key metrices of scientific know-how: maturation in technological progress, reproducible and robust pharmaceutical processes, as well as advances in regulatory science for judging the quality and performance of the product for safety as well as efficacy as an injectable vaccine dosage form. Clearly, advances in DNA/RNA sequencing and predictions about the protein products of SARS-CoV2 have allowed the creation of RNA sequences that code for S protein to produce recombinant protein as an immunogen or vaccine antigen. Recombinant protein can also be expressed by host-cells directed to produce virus-like protein particles (NVX-Co2373) and VLP as immunogens (CoVLP). Viral RNA sequences could also be stabilized with excipients to form vaccine formulations (mRNA-1273 and BNT162b1), which are intended to direct host cells to express S protein as immunogens.
Compared to protein or RNA/DNA formulated in carriers, genetic codes programed in virus is more efficient in directing host cells to express SARS-CoV2 S protein, virologists and scientists working on gene therapy have been evaluating adeno viruses and defining how to insert foreign genes (in this case SARS-CoV2 S protein sequences) to direct host cells to express the protein. Unfortunately, most serotypes of adenovirus are highly immunogenic and thus quickly absorb and clear the virus due to pre-existing neutralization antibodies in most people. With many years of research by scientists to prevent the early abortion of adenovirus, two (of more than 50 distinct serotypes) unique adenoviruses—human recombinant AD26 (Ad26.CoVS1) and Chimp OX1 (AD1222-ChAdOx1) are able to induce neutralization antibodies against inserted exogenous protein sequences. Thus, two of the lead Covid-19 candidates listed in Table 2 are the beneficiaries of these accumulated gene delivery carriers and viral constructs as well as manufacturing control and knowledge. These innovations, discoveries and technical readiness levels have paved the way for an accelerated path to design and evaluate various molecular platforms as well as adjuvants to enhance cell penetration and immune induction with adjuvanted particles or accessory molecules that combine with respective immunogens (Table 2).
In addition to the above mentioned promising immunogens that are vaccine product candidates, vaccine candidates must be scalable and able to be manufactured with quality and stability attributes for injectable fit-for-purpose uses. In this case, the vaccine product must be sterile, free of known or unknown contaminants (such as other viruses or cell proteins typically could be co-purified in the process in laboratory scale) and stable in storage for at least 1 year. For example, adenovirus particles and RNA vaccines may not be stable in room temperature and could lose potency to infect/transfect cells, thus requiring storage in −20 to 80 °C. While accommodating the storage conditions appropriate for each vaccine platform, it is highly desirable to develop a vaccine that will be heat-stable if possible as the logistics of cold-chain transport and storage in remote areas may be neither practical nor feasible.
With a number of gene therapeutics tested in pharmaceutical scale up and human studies, the FDA has historical data on how to address these issues. The FDA also has preparations of sterile recombinant adenovirus products such as those built on AD26 and ChOX1 intended to induce cells to express Covid-19 S protein antigen. Thus, the maturation of the pharmaceutical, manufacturing and regulatory sciences enables the anticipation of these issues to incorporate vaccine attributes that include scalability, stability and manufacturing ability as well as considerations of regulatory requirements in both preclinical and clinical development. This has translated into an unprecedented speed from the discovery of Covid-19 causative agents to 6 novel vaccine candidates in clinical testing, with half (3) of them in the late stage studies in preparation for product scaling and distribution.
Whether any of the heavily invested Covid-19 vaccine candidates will provide sufficient protective immune responses against SARS-CoV2 infection or disease progression, current data indicates that all of the candidates in late stage clinical studies appeared to be safe. Thousands of people enrolled worldwide and study data indicates that most of the vaccines produce significant neutralization antibody levels based on viral nucleic acid sequence assembly or engineered virus as well as recombinant protein platforms. Whether vaccine-elicited neutralization antibody levels are sufficient for preventing or ameliorating Covid-19 disease progression remains to be seen. If higher neutralization levels and extended duration of both antibody and cell mediated responses are needed, one could consider primary immunization with a gene-mediated RNA or recombinant viral vaccine such as mRNA-1273, AD1222-ChAdOx1 and Ad26.CoVS1, followed by boosting a protein-based vaccine such as CoVLP CoVL and NVX-Co2373. Genetic based vaccine priming, followed by a protein based vaccine boosting strategy has been shown to provide a significantly higher and more durable HIV vaccine response. This strategy is being implemented in the development of an effective HIV vaccine.7 Already in a recent interim news report using a SARS-CoV2 mRNA vaccine tested in 44,000 people, studies appeared to provide 90% protection according to the sponsor BioNTech-Pfizer (which is a part of OWS initiative for the advance purchase of 100 million initially, and at a later point, 1300 million doses of Covid-19 vaccine product although Pfizer has communicated that no OWS funds were used in the R&D of this vaccine candidate).1 2 8 Hopefully, by January 2021, the safety information for these vaccine candidates will be in place. If a higher, more durable immune response is needed to find an effective Covid-19 vaccine, a prime-boost vaccine strategy could be considered using products developed by two different teams/sponsors to respond to this urgent global health issue. Perhaps such strategies could be facilitated under the OWS.
Acknowledgements
Rodney JY Ho and His research programs are supported in part by NIH, United States grants U01AI48055; AI149665; UM1AI120176 and UNITAID grant 2020-39-GLAD. The editorial assistance of Matthew Hartman is greatly appreciated.
References
- 1.Halstead S., O'Rourke E. Antibody-enhanced dengue virus infection in primate leukocytes. Nature. 1977:739–741. doi: 10.1038/265739a0. [DOI] [PubMed] [Google Scholar]
- 2.GSK Shingrix: zoster vaccine recombinant, adjuvanted. 2018. https://www.fda.govhttps://www.fda.gov/media/112428/download [DOI] [PMC free article] [PubMed]
- 3.US Health and Human Services-HHS Department of health and human services. 2020. https://www.hhs.gov/sites/default/files/fact-sheet-operation-warp-speed.pdf HHS.gov.
- 4.Le T.T., Cramer J.P., Chen R., Mayhew S. Evolution of the COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(10):667–668. doi: 10.1038/d41573-020-00151-8. [DOI] [PubMed] [Google Scholar]
- 5.Folegatti P.M., Ewer K.J., Aley P.K. Safety and immunogenicity of the Chad0x1 nCoV-19 vaccine against SARS-CoV2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020:1–13. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu F.C., Guan X.H., Li Y.H. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020:1–10. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michael N.L. Simplified steps to heterologous prime-boost HIV vaccine development? J Clin Invest. 2019;129(11):4572–4573. doi: 10.1172/JCI132440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson L.A., Neergaard L. Associated Press; 2020. Pfizer Says COVID-19 Vaccine Is Looking 90% Effective.https://apnews.com/article/pfizer-vaccine-effective-early-data-4f4ae2e3bad122d17742be22a2240ae8 [Google Scholar]