Abstract
Aims
During the coronavirus disease 2019 (COVID-19) pandemic, organisations have produced management guidance for cancer patients and the delivery of cytotoxic chemotherapy, but none has offered estimates of risk or the potential impact across populations.
Materials and methods
We combined data from four countries to produce pooled age-banded case fatality rates, calculated the sex difference in survival and used data from four recent studies to convert case fatality rates into age/sex-stratified infection fatality rates (IFRs). We estimated the additional risk of death in cancer patients and in those receiving chemotherapy. We illustrate the impact of these by considering the impact on a national incident cancer cohort and analyse the risk–benefit in some clinical scenarios.
Results
We obtained data based on 412 985 cases and 41 854 deaths. The pooled estimate for IFR was 0.92%. IFRs for patients with cancer ranged from 0 to 29% and were higher in patients receiving chemotherapy (0.01–46%). The risk was significantly higher with age and in men compared with women. 37.5% of patients with a new diagnosis of cancer in 2018 had an IFR ≥5%. Survival benefits from adjuvant chemotherapy ranged from 5 to 10% in some common cancers, compared with the increased risk of death from COVID-19 of 0–3%.
Conclusions
Older male patients are at a higher risk of death with COVID-19. Patients with cancer are also at a higher risk, as are those who have recently received chemotherapy. We provide well-founded estimates to allow patients and clinicians to better balance these risks and illustrate the wider impact in a national incident cohort.
Key words: Cancer, chemotherapy, COVID-19, risk
Introduction
The world is experiencing a pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Although the overall case fatality rate (CFR) is lower than some other recent respiratory infections, the widespread pattern of infection puts many more people at risk [1]. Patients with a range of comorbidities are at increased risk of harm [2], including those with cancer who are more susceptible to infection because of their systemic immunosuppressive state, owing to the malignancy and anticancer treatments [3]. Although there are now a range of guidelines and prioritisation approaches [4,5], none of these has addressed the issue of estimating risks in cancer patients.
The available data indicate a clear age effect in the risk of death from coronavirus disease 19 (COVID-19) [6,7] and a higher risk of death in men compared with women. Data from 72 314 cases from the Chinese Center for Disease Control and Prevention report a CFR of 5.6% in patients with cancer [1]. There is one small series of 18 patients with cancer that suggests higher risks of intubation or death, but it is too small to draw robust conclusions. One very recent case–control study also suggested higher risks of death or intensive therapy unit (ITU) admission in patients with COVID-19 who have a history of cancer compared with non-cancer controls with COVID-19 [8]. There are several studies indicating an increased risk of death in patients with cancer or those who are immunosuppressed who are infected during influenza pandemics [9]. The risk estimates vary considerably, and probably represent both biological and methodological variation.
Most recent work has presented outcomes as CFRs (defined as number of deaths/number of cases). These are easy to calculate, but are subject to ascertainment bias (when only more ill patients are tested for the disease) and the effect of the delay between infection and death, which makes early estimates of CFR prone to underestimating true death rates [10]. We would ideally like to know the infection fatality rate (IFR; i.e. the proportion of those infected who die) but a robust estimate requires near-universal testing of ‘cases’ (i.e. those who are obviously unwell), those with mild symptoms and those who are asymptomatic. This typically requires a ‘closed’ population where we can assess both cases and infection rates. Such situations occur when patients are screened after travel home from an infected area or when an outbreak occurs in an isolated population. Where possible, IFRs should be preferred to CFRs because they provide a better estimate of the true population risk, accepting that most of the population are probably infected, but not all become ‘cases’ (i.e. not become unwell enough to come to medical attention).
Here we estimate age/sex-based IFRs for COVID-19 and the additional risk for cancer patients. We use that to develop a model to calculate age/sex-specific IFRs in cancer patients and compare those to benefits from chemotherapy in some common clinical scenarios. We assess the impact of COVID-19-associated mortality in a national incident cancer population across different tumour types.
Materials and Methods
Estimating Infection Fatality Rate
We identified all national data that provided CFRs in 10-year (or more specific) age bands, where there were national reports of ≥100 deaths and a reference national age distribution. For each nation, we identified the age group with the highest case ascertainment rate (i.e. highest proportion of that age band diagnosed as COVID-19 cases). In line with previous work [6] we assumed a uniform infection rate across age groups and adjusted CFRs in other age groups assuming that lower proportions of testing represented under-ascertainment of cases, but not deaths, to generate adjusted CFRs, and then combined these, weighted by number of deaths, to produce pooled adjusted CFRs (see Appendix A). We calculated the sex-associated difference in risk of death due to COVID-19, calculating an odds ratio for risk of death due to COVID-19 for males (relative to females) based on pooled international data [11], which had a 1:1 male:female ratio of COVID-19 cases. There was also a 1:1 male:female ratio in the combined general population of the countries included so the proportion of case ascertainment was about equal in males and females. Assuming a 1:1 male:female ratio among COVID-19 cases, which is supported by worldwide data, and that differential risk between sexes was independent of age, which we confirmed by calculating age-stratified odds ratios for sex where age/sex-stratified data on cases and deaths were available, we calculated age/sex-stratified estimates of CFR. We converted CFRs into IFRs by combining CFR:IFR ratios from sources that either used closed populations with near-universal ascertainment rates, or attempted to close the population through statistical methods, and which provided rates of both cases and infections ascertained using swab-based polymerase chain reaction (rather than serological) methods to obtain final age/sex-stratified estimates of IFR for death due to COVID-19 (see Appendix B).
Estimating Risks in Patients with Cancer
We conducted a systematic review to estimate the risk of harm (including death) in patients with cancer during previous viral outbreaks. We searched three bibliographic databases on 13 March 2020 (see Appendix C). We conducted a second systematic review on 1 May 2020 to identify the risk of severe harm and/or death in patients with cancer during the COVID-19 pandemic (see Appendix D) ensuring we covered the same terms as a recent review [12]. For both reviews, we supplemented with additional resources found through grey literature and web searches, including preprints, removed duplicates and screened titles and abstracts for relevance; those thought to be relevant had full-text extracted and reviewed by two reviewers (LPS and JC). Studies were included in the final analysis if they reported some measure of risk (normally as an odds ratio) from cancer or chemotherapy in the context of a viral pandemic. We extracted data on study population, methodology and measures, and conducted a random-effects meta-analysis of the odds ratio for death. We used age/sex-stratified IFR and the estimate of additional risk of death with cancer alone and cancer with chemotherapy to generate expected age/sex-stratified IFRs for COVID-19 patients who also had cancer and chemotherapy. We plotted estimated IFRs based on age/sex alone, age/sex with cancer and age/sex with cancer and chemotherapy.
Population-level Impact
We obtained data on all new diagnoses of invasive cancer in the UK between 2016 and 2018 inclusive, and calculated average annual figures. We calculated age/sex IFRs for every tumour site and examined the numbers and proportions of patients with each cancer where the IFR exceeded 2.5%, 5%, 10% and 20% (see Appendix E).
Clinical Scenarios and Risk–Benefit Analysis
We constructed clinical scenarios by identifying clinical decision tools or key clinical trials in the curative setting in three tumour sites. We extracted data on overall survival and absolute difference in overall survival at timepoints specified. We analysed the risk–benefit of giving chemotherapy in these clinical scenarios, comparing the survival benefit from chemotherapy with the increased risk of death from COVID-19 due to chemotherapy if the patients in these scenarios did acquire COVID-19 (i.e. risk of infection of 1). The increased risk of death from COVID-19 was calculated from the excess risk of death from COVID-19 due to chemotherapy adjusted for total risk of death from COVID-19 with cancer and chemotherapy and overall survival from cancer with chemotherapy. We extended the risk–benefit analysis by applying it to a putative population of cancer patients of defined age, sex, cancer site and treatment intent, informed by one of these clinical scenarios. Details of the derivation of the equation used and the worked population example can be found in Appendix F.
Sensitivity Analysis
Where we estimated proportions, rates or risks, we calculated 95% confidence intervals using standard statistical approaches.
Results
We included data on 412 985 COVID-19 cases and 41 854 COVID-19 deaths from four different countries (China, Italy, the Netherlands and Spain) to generate our estimates of age-related CFR for COVID-19. The age group with the highest ascertainment ratio was the >90-year-old group. Adjustment of age-stratified CFRs for under-ascertainment reduced the CFRs considerably, especially in the 50–70-year-old age group (Table 1 ). The odds ratio for death from COVID-19 for males (versus females) was 1.77 (95% confidence interval 1.73–1.80), based on data on 819 145 COVID-19 cases and 57 814 COVID-19 deaths from 30 countries [11]. This odds ratio was used to convert adjusted age-stratified CFRs to age/sex-stratified CFRs (Table 2 ). We identified four studies that reported both CFRs and IFRs in populations with near-complete case ascertainment based on modelling infection rates in travellers, travellers returning from Wuhan to Japan, infections on the Princess Diamond cruise ship and a COVID-19 outbreak in a Seattle care home [6,[13], [14], [15]]. Based on these, we calculated an IFR:CFR ratio of 0.48 (95% confidence interval 0.47–0.48). This led to the calculation of age- and age/sex-stratified IFRs in Table 1, Table 2. IFRs ranged from 0% to >15% depending on the age and sex of the patient.
Table 1.
Deaths, crude case fatality rate (CFR) and adjusted CFR by age band for four countries individually and pooled
| Age (years) | Italian deaths | Crude Italian CFR | Adjusted Italian CFR | Dutch deaths | Crude Dutch CFR | Adjusted Dutch CFR | Spanish deaths | Crude Spanish CFR | Adjusted Spanish CFR | Chinese deaths | Adjusted Chinese CFR | Combined CFR | Combined IFR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–19 | 0 | 0.0% | 0.00% | 1 | 0.21% | 0.00% | 5 | 0.36% | 0.00% | 1 | 0.02% | 0.00% | 0.00% |
| 20–29 | 0 | 0.0% | 0.00% | 3 | 0.09% | 0.01% | 25 | 0.31% | 0.03% | 7 | 0.06% | 0.03% | 0.02% |
| 30–39 | 44 | 0.3% | 0.04% | 8 | 0.24% | 0.02% | 50 | 0.35% | 0.05% | 18 | 0.15% | 0.06% | 0.03% |
| 40–49 | 209 | 0.9% | 0.13% | 19 | 0.44% | 0.04% | 138 | 0.61% | 0.10% | 38 | 0.30% | 0.13% | 0.06% |
| 50–59 | 834 | 2.6% | 0.54% | 112 | 1.57% | 0.22% | 401 | 1.44% | 0.32% | 130 | 1.25% | 0.52% | 0.25% |
| 60–69 | 2529 | 9.8% | 2.07% | 388 | 7.54% | 0.92% | 1152 | 4.87% | 1.22% | 309 | 3.99% | 1.88% | 0.90% |
| 70–79 | 6723 | 24.3% | 6.79% | 1308 | 23.40% | 4.24% | 3375 | 15.01% | 4.85% | 312 | 8.61% | 5.99% | 2.85% |
| 80–89 | 9413 | 30.8% | 16.27% | 1966 | 30.19% | 14.42% | 5502 | 24.05% | 13.34% | 208 | 13.40% | 15.08% | 7.18% |
| 90+ | 3409 | 27.7% | 27.65% | 760 | 29.60% | 29.60% | 2457 | 24.95% | 24.95% | NA | NA | 26.87% | 12.80% |
| Total | 23 161 | 13.1% | 2.28% | 4565 | 11.89% | 1.30% | 13 105 | 8.57% | 1.56% | 1023 | 1.38% | 1.93% | 0.92% |
Table 2.
Age/sex case fatality rates (CFRs) and infection fatality rates (IFRs) in baseline populations, in patients with cancer and in patients with cancer receiving chemotherapy
| Age/sex group | CFR by age and sex | IFR by age and sex | IFR with cancer | IFR with cancer and chemotherapy |
|---|---|---|---|---|
| 0–19 Male | 0.01% | 0.00% | 0.01% | 0.01% |
| 0–19 Female | 0.00% | 0.00% | 0.00% | 0.01% |
| 20–29 Male | 0.04% | 0.02% | 0.05% | 0.09% |
| 20–29 Female | 0.02% | 0.01% | 0.03% | 0.05% |
| 30–39 Male | 0.07% | 0.03% | 0.07% | 0.15% |
| 30–39 Female | 0.04% | 0.02% | 0.04% | 0.09% |
| 40–49 Male | 0.17% | 0.08% | 0.18% | 0.36% |
| 40–49 Female | 0.09% | 0.05% | 0.10% | 0.20% |
| 50–59 Male | 0.66% | 0.32% | 0.70% | 1.41% |
| 50–59 Female | 0.37% | 0.18% | 0.40% | 0.81% |
| 60–69 Male | 2.39% | 1.14% | 2.51% | 4.97% |
| 60–69 Female | 1.37% | 0.65% | 1.44% | 2.89% |
| 70–79 Male | 7.57% | 3.60% | 7.69% | 14.51% |
| 70–79 Female | 4.42% | 2.10% | 4.57% | 8.89% |
| 80–89 Male | 18.67% | 8.89% | 17.87% | 30.70% |
| 80–89 Female | 11.48% | 5.47% | 11.43% | 20.80% |
| 90+ Male | 32.42% | 15.44% | 28.93% | 45.32% |
| 90+ Female | 21.32% | 10.15% | 20.13% | 33.91% |
| Total Male | 2.45% | 1.17% | 2.57% | 5.09% |
| Total Female | 1.40% | 0.67% | 1.47% | 2.96% |
The first systematic review on risk of death from previous viral pandemics in cancer patients identified 850 distinct articles. We used data from the four included studies comprising 1318 patients to calculate a pooled odds ratio for death of 3.7 (95% confidence interval 1.5–9.1) [[16], [17], [18], [19]] (see Appendix C). The second, COVID-19-specific systematic review identified 418 distinct articles from three search engines and an additional one through a web search; we identified one case–control study that reported the odds ratios for the risk of death in cancer patients infected with COVID-19 (see Appendix D). This study reported an elevated risk of death and ITU admission in 105 cancer patients compared with 536 matched controls, with an odds ratio for death in cancer patients of 2.34 (95% confidence interval 1.15–4.77), an odds ratio of death in cancer patients without active treatment of 2.23 (0.98–5.11) and an odds ratio of death in cancer patients with chemotherapy of 4.54 (1.21–16.99) [8]. We used these latter two odds ratios to convert baseline age/sex-stratified IFRs to age/sex-stratified IFRs in cancer patients ± chemotherapy in Table 2. IFRs ranged from 0 to 29% when age/sex were combined with cancer and 0.01 to >45% when the effect of chemotherapy was added.
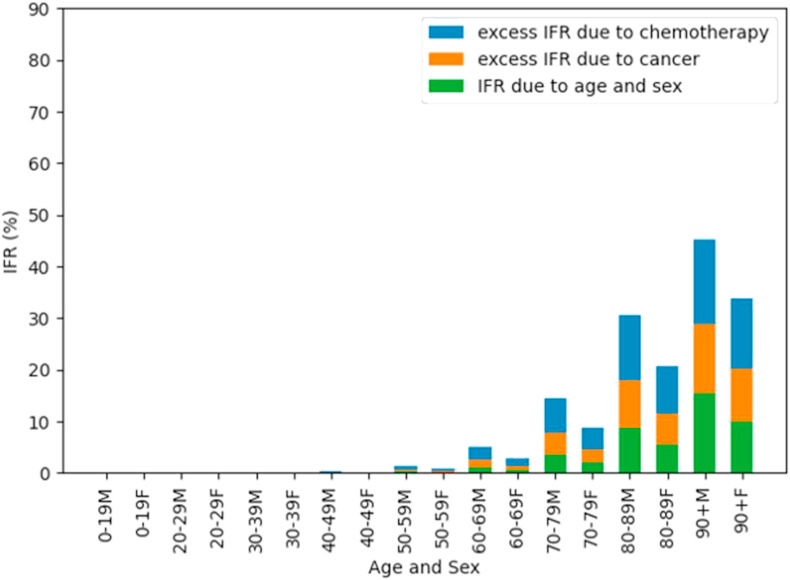
The risk of death with COVID-19 increases substantially with age, and is higher in men. The risks are higher in patients with cancer and chemotherapy, and this risk is multiplicative, and so the absolute increase in risk is larger in older and male patients. Figure 1 shows the estimated IFRs for death due to COVID-19 by age/sex band, divided by contribution from age/sex, cancer and chemotherapy.
Fig 1.
Age/sex infection fatality rates (IFRs) and contribution by age/sex, cancer and chemotherapy.
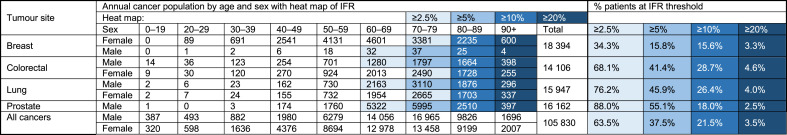
Table 3 shows age/sex distributions of the absolute number of patients per year for the four most common cancer types in the UK, and for all cancers combined (details for other cancers can be found in Appendix E). Additional shading shows where risk exceeds prespecified IFR thresholds (2.5–20%). Proportions of all patients within each tumour group that exceed each risk threshold are summarised towards the right-hand side of the table. Given the age of incidence, 16% of all breast cancer patients have an IFR of 5% or greater, whereas 41% of those with colorectal, 46% of those with lung and 55% of those with prostate cancer have an IFR ≥5%. Across tumour sites, 37.5% of all patients who will be diagnosed with cancer this year have an IFR ≥5%.
Table 3.
Age/sex-stratified incident cancer population for four common cancers, with percentage of patients who exceeded different infection fatality rate (IFR) thresholds
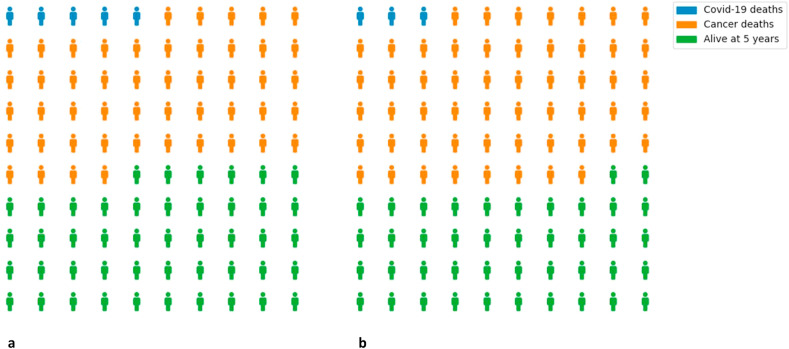
Table 4 shows three clinical scenarios in which chemotherapy is given in the curative setting to patients (of specified age and sex) with breast, lung and brain tumours. In general, survival benefits for cancer due to chemotherapy were 5–10% at 5–10 years, compared with an increased risk of death from COVID-19 due to chemotherapy ranging from 0 to 3% if the patients acquired COVID-19. Extending this to a population, Figure 2 presents expected death and survival among 100 men aged 60–69 years with lung cancer treated with adjuvant chemotherapy to further illustrate the trade-off between survival benefit from cancer and increased COVID-19 mortality conferred by chemotherapy. Figure 2 shows that although expected deaths from COVID-19 are greater (five versus three) when patients are given chemotherapy, the overall number of patients surviving at 5 years is also greater (46 versus 42), owing to the reduced number of deaths from cancer.
Table 4.
Clinical scenarios to illustrate balance of survival benefit from cancer and increased risk of death from COVID-19 with chemotherapy
| Tumour Site | Clinical decision tool/evidence | Case history | Treatments and benefit | Risk–benefit analysis |
|---|---|---|---|---|
| Breast | NHS PREDICT (breast cancer) | 71-year-old post-menopausal woman with a symptomatic pT3 (60 mm) N0 M0 ER+ HER2- G3 IDC who has a WLE + SLNB. | Survival at 10 years: Surgery only = 45% + hormone therapy = 54% + chemotherapy = 54% + bisphosphonates = 63% |
There is a 4.8% benefit at 10 years with adjuvant FEC (second generation) chemotherapy. This compares with the increased risk of death if she acquires COVID-19 of 2.4% |
| Lung | LACE Meta-analysis | 68-year-old male with resected stage IIIA NSCLC, EGFR wild-type; PDL1 <1% | Survival at 5 years: Surgery only = 43.4% + chemotherapy = 48.8% |
There is a 5.4% survival benefit at 5 years with cisplatin-based chemotherapy. This compares with the increased risk of death if he acquires COVID-19 of 1.2% |
| Brain | Stupp 2009 | 63-year-old male with WHO grade IV resected, MGMT methylated glioblastoma multiforme | Survival at 5 years: Surgery and radiotherapy = 0% + chemotherapy = 11% |
There is an 11% survival benefit at 5 years with concurrent and adjuvant temozolamide This compares with the increased risk of death if he acquires COVID-19 of 0.3% |
IDC: Invasive Ductal Carcinoma; WLE: Wide local excision; SLNB: Sentinel Lymph Node Biopsy; NSCLC: Non-small cell lung cancer; EGFR: Epidermal Growth Factor Receptor; PDL-1: Programmed Death Ligand-1; MGMT: Methyl-guanine-methly-transferase.
Fig 2.
Projected deaths and survivals in 100 males aged 60–69 years with lung cancer who are (a) given and (b) not given adjuvant chemotherapy, who all contract COVID-19.
Discussion
There is now clear evidence that age and sex are significant risk factors for death in patients infected with COVID-19. However, most of the published data are based on CFRs. Our work presents the first estimates of IFR based on pooled international data and we used those to estimate the IFR in patients with cancer and the additional risk when receiving chemotherapy. We used that to estimate the IFR in a national incident cancer cohort and showed that a significant minority of newly diagnosed cancer patients had a risk of COVID-19-related death ≥5%. Although there are now many COVID-19-related cancer guidelines, this is the first to provide estimates of risk based on simple patient characteristics.
Previous work has largely presented age-based CFRs; one work calculated age-based IFRs, based on just over 1000 deaths in patients from China. In contrast, we drew on data from >40 000 deaths in >400 000 cases from four countries, addressed the sex difference in risk and used a pooled estimate of the additional risk of death in patients with cancer. Our approach of adjusting CFRs to account for under-ascertainment of mild cases is similar to that used in previous work, and the conversion from CFR to IFR is simple but supported by estimates from multiple sources. Accurately estimating IFR is challenging, and is biased by under-testing of mild cases, time delay between infection, clinical symptoms and death, and under-ascertainment of deaths in the community. Our current model attempts to adjust for this, but the eventual solution relies on widespread testing of both symptomatic and asymptomatic individuals. Nonetheless, the estimates of age/sex IFR are very robust, with very small confidence intervals. Risk of death with cancer is unlikely to be already accounted for by age/sex-stratified IFRs as the prevalence of cancer among all COVID-19 cases and deaths is negligible (1–2%).
Although we used odds ratios for increased risk of death in cancer patients from the only COVID-19-specific study, the direction and magnitude of effect is consistent with other work. Previous studies during viral pandemics report an increased risk of death for cancer patients [[16], [17], [18], [19]] and a very recent large UK COVID-19-specific study also showed an increased risk of death in cancer patients [20]. The estimate used in this work lies within the range of other estimates, and therefore there are now multiple sources of evidence that support the fact that patients with cancer are at a higher risk of death from COVID-19. Although the exact figures depend on accurate parameter estimation, the clinical implications are less sensitive. The IFR due to age/sex alone in patients aged ≥70 years is such that any of the estimates of elevated risk in cancer patients, whether COVID-19-specific or not, suggest that the IFR in cancer patients aged over 70 years are likely to be ≥2% for women and 3% for men. For many patients, this is a significant increase in accepted risk levels; as a comparison, in a large national adjuvant chemotherapy cohort the 30-day mortality was 1% [21].
Other COVID-19-specific data also support our conclusions. In one study of 138 patients, 40% of those with a history of cancer required ITU admission, compared with a baseline risk of 26% [22], but this included only 10 patients with cancer. A review of 18 cancer patients who developed COVID-19 showed a higher risk of harm (defined as requiring ventilation in ITU or death) (7/18; 39%) than the general population (124/1572; 8%). Twelve of these 18 were being followed up after surgery, whereas the four who had undergone chemotherapy or surgery within the last month had a higher risk still [23]. However, these studies do not report how many patients in each age group had each comorbidity and do not allow us to generate measures of risk (such as odds ratios) [24]. The exact reasons for this higher risk are unclear. Patients with cancer might be at a higher risk of infection [25], and there are well-described immunosuppressive effects of cancer, and chemotherapy; the relative impact of each as yet is difficult to distinguish.
Although more COVID-19-specific data will emerge, this will take time: of data on 8250 patients who have been admitted to intensive care in the UK, only 1.9% had either metastatic cancer or haematological malignancies [26]. The systematic reviews we have carried out highlight the lack of information. In particular, there is a need to distinguish between the risk due to cancer, the risk in different groups of cancer patients and the risks associated with treatment. This will require detailed, large-scale prospective data collection, and there are a variety of projects aiming to deliver this.
Our model provides age/sex-stratified IFRs, based on large pooled multinational data from a variety of sources. We only require users to be able to specify overall survival with treatment, absolute improvement in overall survival with treatment and the patient's age and sex in order to balance risk against benefit. For most younger patients, especially women, the benefits continue to outweigh the risks. However, considering the entire incident population, there is a significant risk of COVID-19-related death, especially in older patients. This risk depends on infection and emphasises the importance of reducing the risk of infection in newly referred patients. Given that newly diagnosed cancer patients often require significant investigations to establish a diagnosis, it is important to rationalise these and weigh them against patient-specific IFRs.
Informed consent relies on understanding the balance between risk and benefit; given that the risk associated with treatment has changed, we would suggest reconfirming informed consent in patients who started chemotherapy before the COVID-19 pandemic and are continuing. Palliative chemotherapy may be given primarily for symptom relief, rather than to improve survival, and there are some situations where palliative radiotherapy might be substituted for chemotherapy. Radiotherapy and non-cytotoxic agents (e.g. immunotherapy and hormones) almost certainly have different risk profiles, but there are common concerns given the nosocomial pattern of disease transmission for COVID-19 [27].
There may be additional factors to consider, such as additional comorbidities, ethnicity or differences in risk between different primary tumour groups. There are data to believe these may be important, but at present, there are not enough data to model these. However, our approach is flexible enough to incorporate these as they become available. In addition, although we have focused on death as an outcome, there are increasing reports of morbidity in survivors.
Although most of Western Europe is now in a second wave of infection, total numbers infected still represent a minority of the population [28]. There is, therefore, a considerable ongoing risk from a second wave, which will probably persist for many months. In contrast to outbreaks of seasonal infections, there is no pre-existing immunity or vaccine, and the CFR is about five-fold higher. For those reasons, decision-making in the investigation and treatment of cancer patients in the context of a COVID-19 pandemic needs to be done carefully, and in light of the available data.
Conclusions
In summary, IFR for COVID-19 is about 1% in the general population but is probably significantly higher among cancer patients (up to 30%) and even greater in those who are undergoing active treatment with cytotoxic chemotherapy (with estimates up to 45%). The magnitude of increased risk conferred by cancer during the current pandemic is comparable with that in past influenza pandemics. Up to 40% of patients diagnosed with cancer this year may have a IFR from COVID-19 of more than 5%. However, the survival benefit conferred by adjuvant chemotherapy is expected, in most cases, to exceed the increased risk of death from COVID-19.
Data Availability
All code and data are available online at our GitLab repository: https://gitlab.com/computational.oncology/covidcancerrisk.
Conflicts of interest
M. Williams receives funding from the Imperial/NIHR Biomedical Research Centre; S. Dadhania receives funding from the Imperial College/Institute of Cancer Research CRUK Major Centre; L. Pakzad-Shahabi receives funding from Brain Tumour Research and the Brain Tumour Research Campaign. J. Chen is supported by the Guangdong International Young Research Talents Training Programme for Postdoctoral Researchers.
Role of funding source
The authors of the study receive funding from Imperial/NIHR Biomedical Research Centre; Imperial College/Institute of Cancer Research CRUK Major Centre; Brain Tumour Research and the Brain Tumour Research Campaign and Guangdong International Young Research Talents Training Programme for Postdoctoral Researchers. This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors. No funding source had a role in study design, collection, analysis or interpretation of data, writing of the report or decision to submit for publication.
Acknowledgements
The authors thank Katie Spencer and Alice Dewdney for highlighting errors in calculations in an earlier version; to Alison Falconer for discussions about palliative chemotherapy; to those who commented on an earlier draft, and to all of those who have provided data on which this work is based.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clon.2020.10.021.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. J Am Med Assoc. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Jain V., Yuan J.-M. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: a systematic review and meta-analysis. Int J Publ Health. 2020;65:533–546. doi: 10.1007/s00038-020-01390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamboj M., Sepkowitz K.A. Nosocomial infections in patients with cancer. Lancet Oncol. 2009;10:589–597. doi: 10.1016/S1470-2045(09)70069-5. [DOI] [PubMed] [Google Scholar]
- 4.European Society for Medical Oncology Cancer patient management during the COVID-19 pandemic. 2020. https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic Available at:
- 5.Marron J., Joffe S., Jagsi R., Spence R., Hlubocky F.J. Ethics and resource scarcity: ASCO recommendations for the oncology community during the COVID19 pandemic. J Clin Oncol. 2020;38:2201–2205. doi: 10.1200/JCO.20.00960. [DOI] [PubMed] [Google Scholar]
- 6.Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai M.Y., Liu D., Liu M., Zhou F.X., Li G.L., Chen Z. Patients with cancer appear more vulnerable to SARS-CoV-2: a multi-center study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribeiro A.F., Pellini A.C.G., Kitagawa B.Y., Marques D., Madalosso G., Nogueira Figueira G.D.C. Risk factors for death from influenza a (H1N1)pdm09, State of São Paulo, Brazil, 2009. PLoS One. 2015;10 doi: 10.1371/journal.pone.0118772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghani A.C., Donnelly C.A., Cox D.R., Griffin J.T., Fraser C., Lam T.H. Methods for estimating the case fatality ratio for a novel, emerging infectious disease. Am J Epidemiol. 2005;162:479–486. doi: 10.1093/aje/kwi230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Global Health 50/50 Sex, gender and COVID-19: overview and resources. 2020. https://globalhealth5050.org/covid19/ Available at:
- 12.Desai A., Sachdeva S., Parekh T., Desai R. COVID-19 and cancer: lessons from a pooled meta-analysis. JCO Glob Oncol. 2020;6:557–559. doi: 10.1200/GO.20.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimball A., Hatfield K.M., Arons M., James A., Taylor J., Spicer K. Asymptomatic and presymptomatic SARS-COV-2 infections in residents of a long-term care skilled nursing facility - King County, Washington, March 2020. Morbid Mortal Weekly Rep. 2020;69:377–381. doi: 10.15585/MMWR.MM6913E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance. 2020;25:2000180. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishiura H., Kobayashi T., Yang Y., Hayashi K., Miyama T., Kinoshita R. The rate of underascertainment of novel coronavirus (2019-nCoV) infection: estimation using Japanese passengers’ data on evacuation flights. J Clin Med. 2020;9:419. doi: 10.3390/jcm9020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chao C.M., Lai C.C., Chan K.S., Cheng K.C., Chou W., Yuan K.S. Outcomes of patients with severe influenza infection admitted to intensive care units: a retrospective study in a medical centre. J Med Microbiol. 2017;66:1421–1428. doi: 10.1099/jmm.0.000593. [DOI] [PubMed] [Google Scholar]
- 17.Ji H., Gu Q., Chen L.L., Xu K., Ling X., Bao C.J. Epidemiological and clinical characteristics and risk factors for death of patients with avian influenza a H7N9 virus infection from Jiangsu province, Eastern China. PLoS One. 2014;9 doi: 10.1371/journal.pone.0089581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kusznierz G., Uboldi A., Sosa G., Torales S., Colombo J., Moyano C. Clinical features of the hospitalized patients with 2009 pandemic influenza A (H1N1) in Santa Fe, Argentina. Influenza Other Resp Virus. 2013;7:410–417. doi: 10.1111/j.1750-2659.2012.00405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson D.L., Jungk J., Hancock E., Smelser C., Landen M., Nichols M. Risk factors for 2009 pandemic influenza A (H1N1)-related hospitalization and death among racial/ethnic groups in New Mexico. Am J Public Health. 2011;101:1776–1784. doi: 10.2105/AJPH.2011.300223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallington M., Saxon E.B., Bomb M., Smittenaar R., Wickenden M., McPhail S. 30-day mortality after systemic anticancer treatment for breast and lung cancer in England: a population-based, observational study. Lancet Oncol. 2016;17:1203–1216. doi: 10.1016/S1470-2045(16)30383-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang W., Guan W., Chen R., Wang W., Li J., Xu K. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CDC China Coronovirus statistics. 2020. http://rs.yiigle.com/yufabiao/1181998.htm Available at:
- 25.Yu J., Ouyang W., Chua M.L., Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6:1108–1110. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ICNARC. Reports. 2020. https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports Available at: [Google Scholar]
- 27.Wang Y., Wang Y., Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol. 2020;92:568–576. doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallapaty S. Antibody tests suggest that coronavirus infections vastly exceed official counts. Nature. 2020 doi: 10.1038/d41586-020-01095-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All code and data are available online at our GitLab repository: https://gitlab.com/computational.oncology/covidcancerrisk.