Recently, Chakhtoura et al. discussed the possible role of vitamin D supplements in Coronavirus disease 2019 (COVID-19) in this journal [1]. COVID-19 is characterized by a systemic inflammatory response [2]. Based on anti-inflammatory and anti-microbial properties of vitamin D metabolites, protective effects of vitamin D supplementation in COVID-19 are suggested. This hypothesis is supported by some [3], but not all [4] cohort and case-control studies describing low 25-hydroxyvitamin D (25(OH)D) levels (i.e. vitamin D deficiency) in patients with COVID-19. However, an important issue which has not been addressed by Chakhtoura et al. is reverse causality. Because upregulation of the enzyme 25(OH)D 1-alpha hydroxylase (CYP27B1) has been demonstrated in activated immune cells, circulating 25(OH)D levels could be lowered by the COVID-19-associated systemic inflammatory response. We investigated whether systemic inflammation lowers circulating 25(OH)D levels using the experimental human endotoxemia model, a standardized, controlled, and reproducible model of systemic inflammation in humans in vivo.
Nine healthy non-smoking male volunteers (median [interquartile range] age of 22 [21−23] years and BMI of 23.7 [22.7–24.9] kg/m2) received a bolus of 1 ng/kg E. Coli-derived lipopolysaccharide (LPS), followed by a continuous infusion of 1 ng/kg/h LPS for 3 h, in order to induce systemic inflammation. Study procedures are described in detail elsewhere [5]. The study was approved by the local ethics committee of the Radboud university medical center and complied with the Declaration of Helsinki including current revisions and the Good Clinical Practice guidelines. All subjects provided written informed consent. Circulating concentrations of 25(OH)D, were measured in lithium-heparin plasma using liquid chromatography-tandem mass spectrometry [6]. Blood cytokine levels were measured in ethylenediaminetetraacetic acid plasma using a Luminex assay (Milliplex, Millipore).
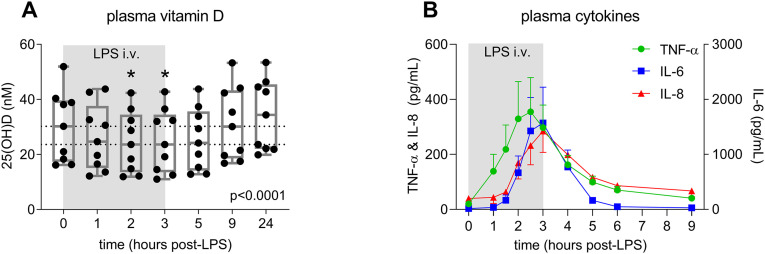
Baseline circulating 25(OH)D levels were generally low in participants of our study (30.2 [18.3–38.9] nM), and decreased significantly following the start of LPS administration (Fig. 1A). The lowest 25(OH)D concentrations were observed 2–3 h after initiation of LPS infusion (23.6 [13.5–34.5] nM), coinciding with peak levels of pro-inflammatory cytokines tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-8 (Fig. 1B). 25(OH)D levels recovered to baseline 6 h following cessation of LPS infusion.
Fig. 1.
Plasma 25(OH)D levels (panel A) and cytokine concentrations (panel B) in response to LPS infusion in healthy volunteers. In panel A, data are depicted as individual datapoints and corresponding box-plots (whiskers: min-max), and dashed lines indicate median values at baseline (T = 0) and nadir (T = 2 and T = 3). P-value was calculated using Friedman test. *p < 0.05 vs. baseline, calculated using Dunn's post-hoc test. In panel B, data are presented as mean and SEM. Data of 9 subjects are shown in both panels.
Our data reveal that systemic inflammation lowers circulating 25(OH)D levels in humans. This mechanism may contribute to the low circulating 25(OH)D concentrations observed in patients suffering from infectious diseases, including COVID-19. In virtually all of these patients, onset of disease precedes hospital admission by at least several days. Our results show that 25(OH)D levels already decrease within hours of initiation of a systemic inflammatory response. As such, the developing inflammatory response in COVID-19 patients may have decreased 25(OH)D levels before in-hospital measurements were performed. Although these findings do not exclude possible beneficial effects of vitamin D supplementation, they emphasize that reverse causality could explain the observed associations between low 25(OH)D concentrations and COVID-19. We support Chakhtoura et al. in their call for clinical trials [1]. Because a protective effect of vitamin D supplementation remains uncertain at present, taking vitamin D supplements should nevertheless not discourage the public from adhering to routine protective measures such as social distancing and wearing of face masks.
Author contributions
JS, PP, and MK designed the study. JS, JvdO, and CG were responsible for data collection. JS and MK performed the statistical analysis and drafted the manuscript. JvdO, CG, and PP critically revised the manuscript. All authors read and approved the final manuscript.
Access to data
JS, JvdO, CG, and MK performed and are responsible for data analysis. JS and MK had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
No funding was obtained for this work.
Declaration of competing interest
None.
References
- 1.Chakhtoura M., Napoli N., El Hajj Fuleihan G. Commentary: myths and facts on vitamin D amidst the COVID-19 pandemic. Metabolism. 2020;109:154276. doi: 10.1016/j.metabol.2020.154276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kox M., Waalders N.J.B., Kooistra E.J., Gerretsen J., Pickkers P. Cytokine levels in critically ill patients with COVID-19 and other conditions. JAMA - J Am Med Assoc. 2020;324(15):E1–E3. doi: 10.1001/jama.2020.17052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pizzini A., Aichner M., Sahanic S. Impact of vitamin d deficiency on covid-19—a prospective analysis from the covild registry. Nutrients. 2020;12(9):1–9. doi: 10.3390/nu12092775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hastie C.E., Mackay D.F., Ho F. Vitamin D concentrations and COVID-19 infection in UK biobank. Diabetes Metab Syndr Clin Res Rev. 2020;14(4):561–565. doi: 10.1016/j.dsx.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geven C., van Lier D., Blet A. Safety, tolerability and pharmacokinetics/pharmacodynamics of the adrenomedullin antibody adrecizumab in a first-in-human study and during experimental human endotoxaemia in healthy subjects. Br J Clin Pharmacol. 2018;84(9):2129–2141. doi: 10.1111/bcp.13655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Ouweland J.M.W., Beijers A.M., van Daal H. Overestimation of 25-hydroxyvitamin D3 by increased ionisation efficiency of 3-epi-25-hydroxyvitamin D3 in LC–MS/MS methods not separating both metabolites as determined by an LC–MS/MS method for separate quantification of 25-hydroxyvitamin D3, 3-epi-25-hydroxyvitamin D3 and 25-hydroxyvitamin D2 in human serum. J Chromatogr B. 2014;967:195–202. doi: 10.1016/j.jchromb.2014.07.021. [DOI] [PubMed] [Google Scholar]