ABSTRACT
Biofilm is an important virulent marker attributed to the development of urinary tract infections (UTIs) by uropathogenic E. coli (UPEC). Drug-resistant and biofilm-producing UPEC are highly problematic causing catheter-associated or recurrent UTIs with significant morbidity and mortality. The aim of the current study was to investigate the prevalence of biofilm formation and phylogenetic groups in drug-resistant UPEC to predict their ability to cause disease. This prospective study was conducted at the Department of Microbiology, University of Karachi from January to June 2019. A total of 50 highly drug-resistant UPEC were selected for this study. UPEC isolates were screened to form biofilm by Congo-red agar (CRA) and microtiter plate (MTP) technique. The representative biofilm-producing isolates were analysed by scanning electron microscopy (SEM) monitoring. Phylogenetic analysis was done by PCR method based on two preserved genes; chuA, yjaA and TspE4-C2 DNA fragment. On CRA 34 (68%) UPEC were slime producers, while on MTP 20 (40%) were strong biofilm producers, 19 (38%) moderate and 11 (22%) were low to negligible biofilm producers. Molecular typing confirmed that phylogenetic group B2 was prevalent in drug resistant UPEC strains. Pathogenic strains belonged to phylogenetic group B2 and D were found to have greater biofilm forming ability as compare to non-pathogenic commensal strains that belonged to phylogenetic group A. Our results indicate that biofilm formation vary in drug resistant UPEC belonged to different phylogenetic groups. This study indicates possible link between in vitro biofilm formation and phylogenetic groups of UPEC, therefore this knowledge might be helpful to predict the pathogenic potential of UPEC and help design strategies for controlling UTIs.
KEYWORDS: Urinary tract infections (UTIs), biofilm formation, uropathogenic E. coli, phylogenetic groups
1. Introduction
Urinary tract infections (UTIs) affect approximately 150 million individuals annually worldwide with significant health care expenditures [1]. It is the third most detected bacterial infection and a crucial health problem following respiratory and gastrointestinal tract infections [1–3]. Being a prevalent etiologic agent E. coli is responsible for 90% community acquired and 50% nosocomial UTIs affecting all age groups [4,5]. E. coli has the ability to form microcolonies in lining of mucosa of urinary bladder and surface of urinary catheters [6]. Biofilm like intracellular bacterial communities (IBCs) permits UPEC to colonize in bladder and resist expulsion hence play important role in pathogenicity [7].
Biofilms are structurally and dynamically complex biological systems comprising sessile community of microorganisms that are usually concentrated at solid–liquid interface. These are typically surrounded by an extracellular polymeric substance referred as slime consists of exopolysaccharide, protiens and DNA that facilitate adherence to the abiotic or biotic surfaces and microbial aggregation [8,9]. Biofilm also makes organisms more virulent, resistant to antimicrobial drugs and host immune responses [10–12].
It is estimated that biofilm is accountable for 80% of all microbial infections and over 65% of nosocomial infections [7,13,14]. Biofilm also plays an important role in horizontal gene transfer (HGT) facilitated by highly dense cells in close proximity [15] which facilitate movement of resistance genes and virulence factors, especially under selective pressure of antibiotic (s) [10,16]. Bacteria in biofilm are metabolically less active, resistant to exogenous environmental stress, like antibacterial agents, due to mass transfer limitations in the biofilm matrix [17].
Clermont’s original typing scheme generally divided E. coli isolates into four categories; group A, group B1, group B2, and group D [18]. These groups can be determined with the help of genetic markers (chuA, yjaA and TspE4.C2 DNA fragment). chuA encodes outer membrane hemin receptor gene that involves in heme transport. yjaA encodes for gene responsible for cellular response to hydrogen peroxide and acid stress and TspE4.C2 DNA encodes for putative lipase esterase gene [19,20].
It is presumed that microbial biofilm is associated with bacterial pathogenicity and imparts a great impact in the progression of urological infections and treatment outcome [21]. Moreover, phylogenetic background also plays a significant role in virulence of E. coli strains [22]. To the best of our knowledge, the biofilm-forming ability of UPEC in association with phylogenetic background has not been reported from Pakistan. Therefore in this study we aimed to find out association between phylogenetic groups and biofilm-forming ability of UPEC from Pakistan. Understanding the relationship between biofilm formation and phylogenetic groups might prove critical to predict the pathogenic potential and to establish novel strategies for controlling UTIs.
2. Materials and methods
2.1. Bacterial strains
The study included 50 drug-resistant UPEC strains obtained from urine specimens (taken from patients with recurrent infections and suffering from urosepsis) with bacterial count ≥105 CFU/mL submitted to clinical laboratory of tertiary care hospital in Karachi, Pakistan for routine culture and antimicrobial susceptibility testing. All UPEC were isolated on MacConkey’s agar (Oxoid) and subjected to further confirmation by conventional biochemical tests [23].
2.2. Qualitative screening of biofilm formation on CRA
UPEC strains were grown on Congo-red agar (CRA) for slime production by method modified by Freeman et al. [24]. CRA was prepared by Brain Heart infusion agar with addition of sucrose (5 g/100 ml) and Congo-red (0.8 g/l). Aqueous solution of Congo-red was prepared separately, autoclaved at 121°C for 15 min, and added to agar when cooled down at 55°C. After inoculation of bacterial strains, plates were incubated for 24 to 48 hours at 37°C. CRA permits the detection of slime-producing bacteria by distinction of colour change of colonies. The colour of colonies was evaluated by six-colour reference scale for fine classification given by Arciola et al. [25]. Like; very black, black, and almost black colonies indicative of slime production considered as positive results, and Bordeaux, red, and very red classified as negative results for strains unable to produce slime.
2.3. Biofilm formation by micro titer plate (MTP) technique
Biofilm assay was performed by the method described by George A. O’Toole et al. [26]. UPEC isolates were inoculated in Luria Bertani (LB) broth overnight at 37°C. Aliquots of 20 µL of culture were inoculated in 180 µL LB broth in flat bottomed 96 well MTP in triplicate at 37°C, in static condition. After 48 h incubation, wells were gently washed, three times with water and dried at 65°C. Subsequently 200 µL of Crystal violet (0.1%) was added in each well of MTP and left for 30 min, at room temperature. MTP wells were washed three times and dried at room temperature overnight. Crystal violet was solubilized in 200 µL of 30% glacial acetic acid and absorbance was measured at 590 nm using microplate reader (Synergy, HTX Multimode reader). The optical density (OD) of the negative control wells was subtracted from the OD of each tested well by using formula BF = AB-CW, where BF = Biofilm formation, AB is the OD at 590 nm of stained bacteria, and CW is the OD 590 nm of control well having medium without bacteria.
2.4. Biofilm formation on glass slide
Biofilm forming potential of all UPEC isolates was evaluated on glass slides, as described earlier by Mirani & Jamil [27]. Cultures were refreshed and inoculated in 3 mL LB broth. Three glass slides were submerged in 50 mL LB broth and were autoclaved. Overnight broth cultures (1:100 dilution) were inoculated in 50 mL LB broth containing 3 glass slides and incubated at 37°C. 1st, 2nd, and 3rd slides were taken out after 24 h, 48 h and 72 h, respectively. Slides were washed and stained with Crystal Violet (1%), eluted in 5 ml of 95% ethanol and absorbance was taken at 590 nm by using Spectrophotometer (UV visible Spectrophotometer, UV Pharmaspec 1700, Shimadzu).
2.5. Scanning electron microscopy (SEM) analysis
Selected strains of UPEC (biofilm positive and negative isolates) on glass slides were observed at 24, 48 and 72 h by SEM monitoring as described by [28]. Biofilms were negatively stained on glass slides with 0.2% uranyl acetate, washed with 70% ethanol solution, air-dried and the sample was gold-coated, upto 300°A. Biofilm formation was observed under an SEM (Jeol JSM-6380A, Japan).
2.6. Statistical analysis
Mean OD of biofilm formation among UPEC at 24 h, 48 h and 72 h (on glass slides) was determined by a repeated measure ANOVA with a Greenhouse-Geisser correction using SPSS version 16. P-value <0.005 was considered as statistically significant.
2.7. Determination of phylogenetic groups in UPEC
DNA from purified UPEC cultures was isolated by colony boiling method [29]. Triplex PCR was performed for molecular typing of UPEC isolates, targeting three genetic markers: chuA, yjaA and TspE4.C2, as described by Clermont et al. [18], Primer sequences and product sizes are listed in Table 1. PCR program was run as follows: initial denaturation at 95°C for 10 min, 30 cycles of 5 s at 95°C, 10 s at 59°C, 30 s at 72°C and a final extension step of 7 min at 72°C. E. coli (ATCC 25,922) was used as positive control. PCR products were observed on 2% agarose gel and saved using digital camera in Gel-Doc EZ Imager system (Bio-Rad).
Table 1.
Primers for the amplification of phylogenetic grouping of UPEC
| PCR reaction | Primer ID | Target Primer Sequence | PCR Product (bp) |
|---|---|---|---|
| Triplex |
ChuA.1 ChuA.2 |
GACGAACCAACGGTCAGGAT TGCCGCCAGTACCAAAGACA |
279 |
|
YjaA.1 YjaA.2 |
TGAAGTGTCAGGAGACGCTG ATGGAGAATGCGTTCCTCAAC |
211 | |
| TspE4C2.1 TspE4C2.2 |
GAGTAATGTCGGGGCATTCA CGCGCCAACAAAGTATTACG |
152 |
3. Results
3.1. Congo-red agar method
A total of 50, drug-resistant UPEC strains were assessed for their biofilm-forming ability in-vitro by slime production on CRA. Out of 50 strains 34 (68%) were found to be biofilm producers. Among these, 19 strains showed very black colonies, six isolates showed black while nine isolates showed almost black colonies. UPEC isolates unable to form black colonies are classified as no slime producers within 24–48 hours according to colour classification scheme described elsewhere [25].
3.2. Biofilm formation by micro titer plate (MTP) technique
Quantification of biofilm by standard MTP method has divided UPEC into three categories, strong, moderate and weak/negligible adherent based on crystal violet staining (OD at 590). The results indicated 20 (40%) as strong, 19 (38%) as moderate and 11 (22%) as weak or negligible biofilm producers (Table 3).
Table 3.
Prevalence of biofilm formation (virulence) associated with UPEC phylogenetic groups
| Virulence mechanism | UPEC strains | |||
|---|---|---|---|---|
|
Prevalence of biofilm formation by MTP |
Phylogenetic groups | |||
| A% (n = 15) | B1% (n = 1) |
B2% (n = 24) |
D% (n = 10) |
|
| Strong | 13.33%(2) | 100%(1) | 62.5%(15) | 20%(2) |
| Moderate | 26.66%(4) | 0%(0) | 33.33%(8) | 70%(7) |
| Low | 60%(9) | 0%(0) | 4.16%(1) | 10%(1) |
Strong (OD ≥0.240), Moderate (OD 0.120–0.240) and Weak (OD≤0.120) based on crystal violet staining (OD 590). OD = optical density
3.3. Biofilm formation on glass slides and scanning electron microscopy (SEM) analysis
Biofilm formation on glass slides gives more clear information about biofilm-forming ability of UPEC strains (Figure 1). All UPEC strains were evaluated by this method. Majority were found to be biofilm producers, only few isolates showed negligible biofilm formation at 24 h that were also found to be non-slime producers by CRA as well as by MTP method. OD at different time intervals (24, 48 and 72 h) showed considerable variability most of the UPEC strains achieved high level of biofilm formation at 48 h with the exception of some strains that achieved peak at 72 h (Figure 2).
Figure 1.
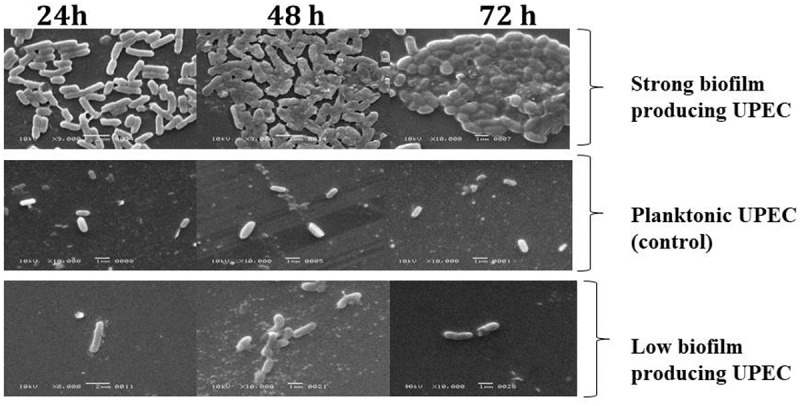
Scanning electron microscopy (SEM) analysis
Biofilm producing UPEC can be differentiated from planktonic control E. coli by production of extracellular polymeric substance. Planktonic cells characterized by their intact structure throughout 24, 48 and 72 h while biofilm production characterized by cell to surface attachment, cell to cell interaction, cells aggregation and microcolonies formation leading to biofilm formation.
Figure 2.

A repeated measures ANOVA with a Greenhouse-Geisser correction determined that mean OD of biofilm formation (on glass slides) differed statistically significantly between time points 24, 48 and 72 h (p < 0.0001) among E. coli (n = 50) isolated from urine specimen
Biofilm formations on glass slides were selected for SEM analysis at time intervals of 24, 48 and 72 h to observe biofilm pattern while E. coli grown in broth was used as control for planktonic natural pattern. SEM is valuable tool for analysis of biofilm. Biofilm producing UPEC showed higher surface adherence in comparison to biofilm negative strains at 24 h. At 48 h SEM showed change in bacterial arrangements making community, while at 72 h this microcolony is marked by the presence of extracellular matrix while E. coli in planktonic form maintained its cell surface integrity at different time intervals (Figure 1) (Figure 3).
Figure 3.

PCR amplification of chuA, yjaA and TSP.C2 genetic marker for UPEC strains
Distribution of phylogroups in 50 UPEC isolates. L = molecular weight marker of 100 bp, Lane 1, 2, 3, 4 and 10 group A, Lane 5 group B1, Lane 6, 7, 8 and 9 group D and Lane 11 and 12 group B2 and Lane 13 E. coli ATCC 25,922 (positive control)
3.4. Phylogenetic groups
In order to classify 50 drug-resistant UPEC strains Clemont’s original phylogenetic scheme was used which is based on 3 genetic determinants (chuA, yjaA and TspE4.C2 DNA fragment) (Figure 3). Among all the analysed UPEC strains the most pathogenic strains belonged to group B2 and to some extent group D (Table 2). Non-pathogenic commensal UPEC were classified as group A and B1.
Table 2.
UPEC phylogenetic grouping based on presence of DNA markers
| E. coli phylogenetic groups | No. of UPEC isolate (%) | Distribution on DNA markers (n) | chuA gene | yjaA gene | TSPE4.C2 |
|---|---|---|---|---|---|
| Group A | 15 (30%) | 15 | - | + | - |
| Group B1 | 1 (2%) | 01 | - | - | + |
| Group B2 | 24 (48%) | 24 | + | + | + |
| Group D | 10 (20%) | 10 | + | - | + |
3.5. Association between biofilm formation and Clemont’s phylogenetic scheme
Significant difference was examined in mean ODs of biofilm formation calculated at 48 h by MTP method in UPEC classified as per Clemont’s original phylogenetic scheme. Majority of UPEC strains found in phylogenetic groups B2 and D were strong and moderate biofilm formers. Only one isolate belonging to phylogenetic group B1 showed strong biofilm-forming ability. Over all phylogenetic group A carried the highest number of low biofilm producers (Table 3).
4. Discussion
The bacterial adherence, aggregation and the growth on solid surfaces to form biofilm is an ancient survival strategy found in nature [30]. According to an estimation, less than 0.1% of the total microbial biomass present on earth is in the plankton form of growth while majority is in the aggregate condition, surrounded by an extracellular matrix as biofilm [31]. According to a recent study, biofilm has now been considered as the default bacterial lifestyle, and it is thought that planktonic single cells are transitional lifestyle of bacteria [32]. Microbial biofilm is of great concern due to antibiotic treatments failure and host immunological defences [33].
As there is no standardized method for biofilm detection and because of multifactorial nature of biofilm we could not depend on single method. Therefore in the current study the ability of UPEC to produce biofilm was assessed by Congo-red agar, microtiter biofilm assay, glass slides in static un-induced condition and SEM analysis. Moreover, strain property, culture media and methodology have great impact on outcome of biofilm formation, in vitro conditions. CRA is a rapid method for screening of slime production in bacteria that gives clue about biofilm-forming ability of the strain. Congo red binds directly with polysaccharides and form colour complex [25]. Biofilm on glass slide has given the best results, especially in case of strains that are weak/non biofilm producers on CRA and MTP. This might be due to greater surface area available for biofilm growth on glass slides. SEM provides the facility to observe biofilm pattern closely in natural forms.
In successful biofilm development, the key event is the attachment to the surface leading to subsequent aggregation and mature biofilm formation. This enhances the stability to cause diseases and enhance its drug resistance capacity [34,35]. On glass slides, higher ODs of biofilm were obtained at 48 h while there is decrease in ODs at 72 h might be due to the process of biofilm shearing or erosion except for a few strains that showed higher ODs at 72 h [36]. At 72 h, biofilm was characterized by extracellular polymeric substance that is comparable with other findings [37]. Overall, all UPEC were able to form microcolonies on glass surface that showed different adhesion patterns clumps and chains were also visible. Frömmel et al. [38] had reported various adhesion patterns such as diffusely distributed bacteria, chain, clumps and micro-colonies while Gomes et al. [39], have reported that biofilm formed on glass showed a 37% higher elongation than those formed on silicon.
Phylogenetic background gives knowledge about ecological distribution and evolutionary history. Moreover phylogenetic analysis has also been reported to have important contribution in virulence of pathogens [40,41]. Generally variations in phylogenetic groups are associated with geographical region, site of infection and antibiotic resistance [42]. Moreover other factors include are health status of host, environmental and social conditions, dietary and host genetic factors and differences in sampling regions [43].
In this research study phylogenetic typing by Clermont’s original phylogenetic typing scheme was evaluated. This method is highly congruent (2429 citations in September 2019 at www.ncbi.nlm.nih.gov) and still method of choice because of its simplicity and rapidity as described by recent studies [44–46]. In the present study UPEC strains were mainly associated with phylogenetic groups, B2 and D; however, they have also been associated with phylogenetic group B1 [42]. These UPEC isolates might be considered community isolates with predisposing factors which assist UTIs particularly in immune compromised individuals [47]. While, commensal UPEC strain with ability to cause community-acquired UTIs have been associated with phylogenetic group B1, we identified only one UPEC isolate out of fifty that belonged to phylogenetic group B1. Phylogenetic group B2 was the most prevalent in UPEC strains as observed in other findings reported from Asia [40,41] and globally [48,49]. However, phylogenetic group A was second most prevalent group among drug-resistant UPEC strains that exhibit slight phylogenetic shift towards group A, such shift may possibly occur if resistance is more readily acquired or contained by certain phylogenetic group which may increase fitness for pathogen [50].
On combining all data together, majority of strong biofilm producers belonged to B2 phylogenetic group, while in group D isolates were found to be moderate biofilm producers. Overall, 68% of UPEC isolates belonged to B2 and D groups. It might be due to the presence of pathogenicity islands and expression of more virulence determinants like adhesion factors, cell surface hydrophobicity, siderophore and toxins production, etc. Moreover, E. coli phylogroup B2 is the most commonly associated with persistent infections. Majority of isolates from group A were low/negligible biofilm producers. Similar observation was seen in a previous study by Nielson et al. [7]. There seems to be a possible correlation between phylogenetic groups and biofilm phenotype. Chakraborty et al. also observed similar findings [51]. However in group A some strains also showed high to moderate biofilm capability.
Additionally data regarding antimicrobial resistance (accepted to be published in Pakistan journal of pharmaceutical sciences) indicated more than 90% to be multidrug resistant to three or more classes of antibiotics. However according to the susceptibility pattern, all strains were found sensitive to fosfomycin, imipenem and colistin and would be helpful to tackle these drug resistant biofilm-forming strains.
Drug-resistant UPEC strains are more likely to form biofilms that effect likelihood of risk biofilm-associated infections, dissemination of virulence factors and resistant determinants since the biofilm has potential role in recurrent and persistent infections.
5. Conclusion
The research work presented here indicated possible link between in vitro biofilm formation and phylogenetic typing in UPEC from Pakistan. Majority of drug-resistant UPEC strains belonging to phylogenetic group B2 and D (pathogenic strains) showed strong and moderate biofilm formation in vitro. Only one strain of UPEC belonging to group B1 and few strains in group A also showed strong biofilm-forming ability. Biofilm has the potential role in pathogenesis of recurrent UTIs, antibiotic resistance and also facilitates transfer of genetic material. In conclusion our findings contribute better understanding of biofilm-forming capabilities which is critical for designing novel strategies for controlling infectious diseases.
Acknowledgments
The results described in this paper are part of the PhD thesis of Ms Saima Javed.
Disclosure statement
All authors declare that there is no conflict of interest.
Ethical consideration
This study does not belong directly to any human or animal subject so the ethical approval is not required.
References
- [1].Qiao L-D, Chen S, Yang Y, et al. Characteristics of urinary tract infection pathogens and their in vitro susceptibility to antimicrobial agents in China: data from a multicenter study. BMJ Open. 2013;3(12):e004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shah DA, Wasim S.. Abdullah FE: antibiotic resistance pattern of Pseudomonas aeruginosa isolated from urine samples of Urinary Tract Infections patients in Karachi, Pakistan. Pak J Med Sci. 2015;31(2):341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nandihal NW. Profile of urinary tract infection and quinolone resistance among Escherichia coli and Klebsiella species isolates. Int J Curr Microbiol App Sci. 2015;4(7):749–7. [Google Scholar]
- [4].Tabasi M, Karam MRA, Habibi M, et al. Bouzari S: genotypic characterization of virulence factors in Escherichia coli isolated from patients with acute cystitis, pyelonephritis and asymptomatic bacteriuria. JCDR. 2016;10(12):DC01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wireko S, Frimpong E. Feglo P: molecular characterization of extended-spectrum beta-lactamase producing urinary Escherichia coli isolated in Brong-Ahafo regional hospital, Ghana. Eur Sci J. 2017;13:9. [Google Scholar]
- [6].Mittal S, Sharma M. Chaudhary U: biofilm and multidrug resistance in uropathogenic Escherichia coli. Pathog Glob Health. 2015;109(1):26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nielsen DW, Klimavicz JS, Cavender T, et al. Logue CM: the impact of media, phylogenetic classification, and E. coli pathotypes on biofilm formation in extraintestinal and commensal E. coli from humans and animals. Front Microbiol. 2018;9.:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Toval F, Köhler C-D, Vogel U, et al. Dobrindt U: characterization of Escherichia coli isolates from hospital inpatients or outpatients with urinary tract infection. JCM. 2014;52(2):407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Miquel S, Lagrafeuille R, Souweine B. Forestier C: anti-biofilm activity as a health issue. Front Microbiol. 2016;7:592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tajbakhsh E, Ahmadi P, Abedpour-Dehkordi E, et al. Khamesipour F: biofilm formation, antimicrobial susceptibility, serogroups and virulence genes of uropathogenic E. coli isolated from clinical samples in Iran. Antimicrob Resist Infect Control. 2016;5(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Vu B, Chen M, Crawford RJ. Ivanova EP: bacterial extracellular polysaccharides involved in biofilm formation. Molecules. 2009;14(7):2535–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mandakhalikar KD, Rahmat JN, Chiong E, et al. Tambyah PA: extraction and quantification of biofilm bacteria: method optimized for urinary catheters. Sci Rep. 2018;8(1):8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Soto SM. Importance of biofilms in urinary tract infections: new therapeutic approaches. Adv bio. 2014;2014 [Google Scholar]
- [14].Health NIo: NIH guide: research on microbial biofilms . https://grantsnihgov/grants/guide/pa-files/PA-03-047html2002
- [15].Bajpai T, Varma M, Bhatambare G. Pandey M: Escherichia coli biofilms: accepting the therapeutic challenges. Inter J Health Allied Sci. 2016;5(4):204. [Google Scholar]
- [16].Gruber CJ, Lang S, Rajendra VK, et al. Zechner EL: conjugative DNA transfer is enhanced by plasmid R1 partitioning proteins. Front Mol Biosci. 2016;3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Anderson G. O’toole G: innate and induced resistance mechanisms of bacterial biofilms. Bacteria Biofilms Springer. 2008:85–105. [DOI] [PubMed] [Google Scholar]
- [18].Clermont O, Bonacorsi S. Bingen E: rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol. 2000;66(10):4555–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Barzan M, Rad M, Tabar GH. Azizzadeh M: phylogenetic analysis of Escherichia coli isolates from healthy and diarrhoeic calves in Mashhad, Iran. Bulg J Vet Med. 2017;20:1. [Google Scholar]
- [20].Lee CCY. Genotyping Escherichia coli isolates from duck, goose, and gull fecal samples with phylogenetic markers using multiplex polymerase chain reaction for application in microbial source tracking. J Experiment Microbio Immuno. 2011;15:130–135. [Google Scholar]
- [21].Tenke P, Kovacs B, Jäckel M. Nagy E: the role of biofilm infection in urology. World J Urol. 2006;24(1):13. [DOI] [PubMed] [Google Scholar]
- [22].Gordon DM, Clermont O, Tolley H. Denamur E: assigning Escherichia coli strains to phylogenetic groups: multi‐locus sequence typing versus the PCR triplex method. Environ Microbiol. 2008;10(10):2484–2496. [DOI] [PubMed] [Google Scholar]
- [23].Holt JG, Krieg NR, Sneath PH, et al. Williams ST: bergey’s manual of determinative bacteriology 9th edition. A Waverly Company Williams and Wilkins Baltimore; 1994;786-788. [Google Scholar]
- [24].Freeman D, Falkiner F. Keane C: new method for detecting slime production by coagulase negative staphylococci. J Clin Pathol. 1989;42(8):872–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Arciola CR, Campoccia D. Montanaro L: detection of biofilm-forming strains of Staphylococcus epidermidis and S. aureus. Expert Rev Mol Diagn. 2002;2(5):478–484. [DOI] [PubMed] [Google Scholar]
- [26].O’Toole GA. Microtiter dish biofilm formation assay. J Vis Exp. 2011;47:e2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mirani ZA. Jamil N: effect of sub‐lethal doses of vancomycin and oxacillin on biofilm formation by vancomycin intermediate resistant Staphylococcus aureus. J Basic Microbiol. 2011;51(2):191–195. [DOI] [PubMed] [Google Scholar]
- [28].Ali Mirani Z, Fatima A, Urooj S, et al. Abbas T: relationship of cell surface hydrophobicity with biofilm formation and growth rate: A study on Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli. Iran J Basic Med Sci. 2018;21(7):760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Dallenne C, Da Costa A, Decré D, et al. Arlet G: development of a set of multiplex PCR assays for the detection of genes encoding important β-lactamases in Enterobacteriaceae. J Antimicrob Chemother. 2010;65(3):490–495. [DOI] [PubMed] [Google Scholar]
- [30].Choong S. Whitfield H: biofilms and their role in infections in urology. BJU Int. 2000;86(8):935–941. [DOI] [PubMed] [Google Scholar]
- [31].Bjarnsholt T, Ciofu O, Molin S, et al. Høiby N: applying insights from biofilm biology to drug development—can a new approach be developed? Nat Rev Drug Discov. 2013;12(10):791. [DOI] [PubMed] [Google Scholar]
- [32].Boudarel H, Mathias J-D, Blaysat B. Grédiac M: towards standardized mechanical characterization of microbial biofilms: analysis and critical review. NPJ Biofilms Microbiomes. 2018;4(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Boudeau J, Barnich N, Darfeuille-Michaud A. Type 1 pili-mediated adherence of Escherichia coli strain LF82 isolated from Crohn’s disease is involved in bacterial invasion of intestinal epithelial cells. Mol Microbiol. 2004;39(5):1272–1284. [DOI] [PubMed] [Google Scholar]
- [34].Vysakh A, Midhun SJ, Jayesh K, et al. Latha M: studies on biofilm formation and virulence factors associated with uropathogenic Escherichia coli isolated from patient with acute pyelonephritis. Pathophysiology. 2018. DOI: 10.1016/j.pathophys.2018.07.004 [DOI] [PubMed] [Google Scholar]
- [35].González MJ, Robino L, Iribarnegaray V, et al. Scavone P: effect of different antibiotics on biofilm produced by uropathogenic Escherichia coli isolated from children with urinary tract infection. Pathog Dis. 2017;75:4. [DOI] [PubMed] [Google Scholar]
- [36].Donlan RM. Biofilms: microbial life on surfaces. Emerg Infect Dis. 2002;8(9):881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Azeredo J, Azevedo NF, Briandet R, et al. Jaglic Z: critical review on biofilm methods. Crit Rev Microbiol. 2017;43(3):313–351. [DOI] [PubMed] [Google Scholar]
- [38].Frömmel U, Böhm A, Nitschke J, et al. Schröder C: adhesion patterns of commensal and pathogenic Escherichia coli from humans and wild animals on human and porcine epithelial cell lines. Gut Pathog. 2013;5(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gomes LC. Mergulhão FJ: SEM Analysis of Surface Impact on Biofilm Antibiotic Treatment. Scanning. 2017;2017:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kumar N, Nahid F. Zahra R: association of virulence factors, phylogenetic groups and antimicrobial resistance markers in Escherichia coli from Badin city, Pakistan. J Chemother. 2017;29(1):8–13. [DOI] [PubMed] [Google Scholar]
- [41].Bashir S, Haque A, Sarwar Y, et al. Anwar MI: virulence profile of different phylogenetic groups of locally isolated community acquired uropathogenic E. coli from Faisalabad region of Pakistan. Ann Clin Microbiol Antimicrob. 2012;11(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ochoa SA, Cruz-Córdova A, Luna-Pineda VM, et al. López-Martínez B: multidrug-and extensively drug-resistant uropathogenic Escherichia coli clinical strains: phylogenetic groups widely associated with integrons maintain high genetic diversity. Front Microbiol. 2016;7:2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Iranpour D, Hassanpour M, Ansari H, et al. Najafi A: phylogenetic groups of Escherichia coli strains from patients with urinary tract infection in Iran based on the new Clermont phylotyping method. Biomed Res Int. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Khairy RM, Mohamed ES, Abdel Ghany HM. Abdelrahim SS: phylogenic classification and virulence genes profiles of uropathogenic E. coli and diarrhegenic E. coli strains isolated from community acquired infections. PloS One. 2019;14(9):e0222441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Sheikh AF, Goodarzi H, Yadyad MJ, et al. Akhond MR: virulence-associated genes and drug susceptibility patterns of uropathogenic Escherichia coli isolated from patients with urinary tract infection. Infect Drug Resist. 2019;12:2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Stoppe N, Silva JS, Carlos C, et al. Torres TT: worldwide phylogenetic group patterns of Escherichia coli from commensal human and wastewater treatment plant isolates. Front Microbiol. 2017;8:2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Derakhshandeh A, Firouzi R, Motamedifar M, et al. Heidari S: distribution of virulence genes and multiple drug‐resistant patterns amongst different phylogenetic groups of uropathogenic Escherichia coli isolated from patients with urinary tract infection. Lett Appl Microbiol. 2015;60(2):148–154. [DOI] [PubMed] [Google Scholar]
- [48].Kõljalg S, Truusalu K, Stsepetova J, et al. Mikelsaar M: the Escherichia coli phylogenetic group B2 with integrons prevails in childhood recurrent urinary tract infections. Apmis. 2014;122(5):452–458. [DOI] [PubMed] [Google Scholar]
- [49].Luo Y, Ma Y, Zhao Q, et al. Yang J: similarity and divergence of phylogenies, antimicrobial susceptibilities and virulence factor profiles of Escherichia coli isolates causing persistence and reinfection of recurrent urinary tract infections. JCM. 2012;50(12):4002-4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Johnson JR, Kuskowski MA, Owens K, et al. Winokur PL: phylogenetic origin and virulence genotype in relation to resistance to fluoroquinolones and/or extended-spectrum cephalosporins and cephamycins among Escherichia coli isolates from animals and humans. J Infect Dis. 2003;188(5):759–768. [DOI] [PubMed] [Google Scholar]
- [51].Chakraborty A, Saralaya V, Adhikari P, et al. Hegde A: characterization of Escherichia coli phylogenetic groups associated with extraintestinal infections in South Indian population. Ann Med Health Sci Res. 2015;5(4):241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]


