Abstract
The disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Coronavirus Disease-2019 (COVID-19), is a global emergency. The first case of COVID-19 was confirmed in Japan in January 2020, a second outbreak of infection occurred in mid-March and a third peak at the beginning of August. The COVID-19 phenotype was milder in Japan than in other countries, although the restrictive measures applied in the country have not been as strict as in other places. Factors related to a possible reduced susceptibility to the pulmonary manifestations of SARS-CoV-2 may have contributed to better outcomes and lower mortality in Japan.
Keywords: COVID-19, Pandemic, Japan, SARS-CoV-2
Introduction
The disease caused by a new strain of beta coronavirus, known as the severe acute respiratory syndrome coronavirus 2, or SARS-CoV-2, Coronavirus Disease-2019, (COVID-19), is a global emergency more likely to cause a severe infection in older individuals and patients with underlying medical conditions [1].
The first case of SARS-CoV-2 infection was confirmed in Japan on January 16, 2020, in a resident of Kanagawa Prefecture, located on the central coast of Japan's largest island, Honshu, who had returned from Wuhan, China. In addition, early February passengers aboard the Diamond Princess cruise ship were quarantined after its return to Yokohama, the capital of Kanagawa Prefecture located half an hour south of Tokyo, because a passenger who disembarked in Hong Kong was confirmed to have COVID-19. By the end of February, multiple cases of COVID-19 were identified nationwide. To prevent and mitigate the COVID-19, the Japanese government decided the temporary closure of all Japanese elementary, junior high, and high schools at the end of February.
The first wave of SARS-CoV-2 infection from China was detected in Japan at a very early state leading to a gradual transmission curve and apparently controlled through implementation of active surveillance. There were no strict quarantine measures. COVID-19 spreads by forming clusters, and Japan developed a cluster-based response consisted in prospective and retrospective tracing to identify common sources of infection. A three-pronged basic strategy was implemented consisting in 1) early detection and early response to clusters; 2) early patient diagnosis ensuring intensive care and a medical service system for very ill patients; and 3) behaviour modification of citizens. The latter focused on avoiding 1) closed spaces with poor ventilation; 2) crowders places with many people nearby, and 3) close-contact setting such as short distance conversations, defining the concept called the “Three Cs”. Later, the concept has been expanded to “Three Cs Plus” which include behaviours such as loud talking and singing. The strategy of avoiding the “Three Cs” helped to control the spread of the disease.
In early March the number of cases of COVID-19 increased across Europe and the United States (USA). A second outbreak of infection occurred in Japan around mid-March and the SARS-CoV-2 haplotype network analysis suggested that these new cases could have been imported via travelers and returnees from Europe, North America, or other countries [2]. On April 7, it was proclaimed a 1-month state of emergency in Japan for several prefectures and later extended to the rest of the country for an indefinite period.
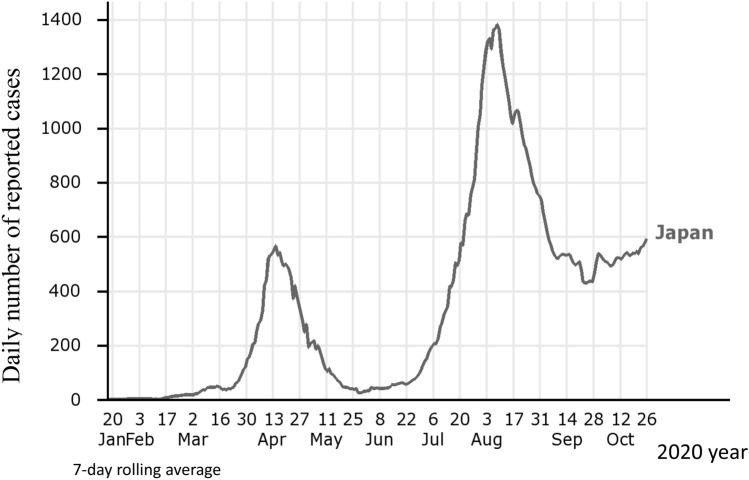
The cumulative number of testing for SARS-CoV-2 conducted in Japan during the two initial outbreaks of COVID-19 was fewer than in other countries, and centered on high-risks groups and people related to the clusters. Later, the number of diagnostic tests was extended to larger population, and a third peak was reached at the beginning of August (Fig. 1).
Fig. 1.
Trends in daily number of COVID-19 cases reported in Japan. Chart elaborated at https://www.iancampbell.co.uk/covid-19.php with data from Johns Hopkins University. Last accessed 27 October 2020
The total number of cases of COVID-19 reported in Japan by the Ministry of Health, Labor, and Welfare (MHLW) are 97.074 [3] with one of the lowest death rate per capita from SARS-CoV-2 (13.56 deaths/million habitants) [4, 5]. There is no enough information yet to interpret the low mortality rate in Japan during COVID-19 pandemic, but several possible explanations have been suggested. The Japanese population may have relative immunity conferred by the mandatory Bacille Calmette–Guerin (BCG) tuberculosis vaccine or may have been exposed to a milder strain of the virus prior to the spread of a more virulent strain. The possibility of reduced expression of angiotensin-converting enzyme 2 (ACE2) receptors, the SARS-CoV-2 cell entry receptor, in the respiratory tract was also noted. Other possible explanations include Japanese cultural traits characterized for social distance, use of face mask or bowing [6].
SARS-Co-V-2 and rheumatic diseases
Cases reported of COVID-19 in Japan by the MHLW are cumulative cases confirmed by a positive SARS-CoV-2 polymerase chain reaction (PCR) test [3]. During the two initial outbreaks of SARS-CoV-2 infection in Japan, extensive PCR testing was not performed and the low rate of confirmed SARS-CoV-2 cases makes it impossible to know the extent of the infection in the country.
There are no reports on the incidence of COVID-19 among patients with rheumatic diseases in Japan and whether patients using immunosuppressive therapies are at increased risk of contracting SARS-CoV-2. Data from transplant patients treated with immunosuppressants in Italy suggest that these patients do not have a higher frequency or more severe COVID-19 [7]. Similar to other countries, age and comorbidities such as hypertension, diabetes mellitus, cardiovascular disease and lung involvement would be risks for poor outcomes of COVID-19.
Autoimmune disease flare triggered by COVID-19 was reported in a 58-year-old Japanese woman with a 20-year history of systemic lupus erythematosus. The patient had a severe relapse of immune thrombocytopenia during the course of COVID-19, successfully treated with intravenous immunoglobulins. She did not develop thrombosis, but a new positive test for the lupus anticoagulant was detected. COVID-19 remained mild throughout the clinical course on treatment with ciclesonide inhalation [8]. Tocilizumab discontinuation during the course of COVID-19 was described in a 51-year-old Japanese woman with rheumatoid arthritis (RA) without exacerbation of respiratory symptoms. She was confirmed positive for SARS-CoV-2 by PCR 2 weeks after her last tocilizumab injection. She developed nasal discharge, mild cough and respiratory distress and a chest computer tomography (CT) showed a ground glass opacity mainly in the peripheral region in both lungs. The patient did not receive specific treatment for COVID-19. Arthralgia recurred during tocilizumab withdrawal, and after confirmation of negative results for SARS-CoV-2 and disappearance of ground glass opacity on the chest CT, tocilizumab was resumed [9]. Moreover, Mokuda S et al. reported upregulation of ACE2 expression in the active synovium of patients with RA, suggesting that RA activity may alter the efficiency of SARS-CoV-2 entry into synovial cells and that non-planned preventive withdrawal of disease-modifying anti-rheumatic drugs (DMARDs) could lead to increase the risk of COVID-19 [10].
Rheumatologists around the world are looking for strategies to optimize the care of patients with rheumatic diseases during the pandemic. A position statement for the care for patients with rheumatic diseases during COVID-19 pandemic was developed by the Asia–Pacific League Against Rheumatism (APLAR) COVID-19 task force comprising rheumatologists from several Asia–Pacific countries including Japan [11]. The statement is based on best available evidence up to 26 April 2020 and expert opinion and provides guidance regarding clinical-decision making in patients with rheumatic diseases.
Preventing patients with rheumatic disease from contracting COVID-19 is essential and the “mitigation approach”, which includes including social distancing, frequent hand washing and quarantining strategies are the primary interventions to hamper the spread of infection. The task force stated that non-steroidal anti-inflammatory drugs (NSAIDs) should not be recommended as the first line of option for the treatment of symptoms of COVID-19, but patients with arthritis taking NSAIDs for symptomatic relief should continue their treatment as needed. Patients with stable rheumatic diseases should continue their treatment. In case of COVID-19 infection, treatment of infection takes priority and immunosuppressive treatment may be de-escalated or temporarily withheld. In patients with rheumatic diseases on long-term steroids, glucocorticoid therapy must not be stopped even if they developed symptoms suggestive of COVID-19. Use of hydroxychloroquine and sulfasalazine should be continued. The APLAR could not recommend any specific treatment for patients with COVID-19 but indicate that worsening of the rheumatic diseases may induce a systemic inflammatory state, which may represent an additional risk factor for a major susceptibility to viral infection.
The Japanese rheumatology community [12], which is facing new challenges in these exceptional pandemic times, has reduced the number of routine clinical visits to rheumatology departments, and increased telephone consultations to minimize exposure to SARS-CoV-2. Consideration has been given in patients with rheumatic diseases with biological or immunosuppressive therapies to raising the dosing interval between intravenous drugs and, if necessary, switching to different biologic agents. Rheumatology services are providing a safe continuous medical care, tailoring the treatment to the individual, protecting the patient and staff and adopting measures to reduce the risk of viral transmission.
Patients with rheumatic diseases are at increased risk of developing comorbid conditions. An international study (COMORA) that included seventeen countries from five different continents evaluated the prevalence and comorbidities in patients with RA and showed wide variability, not only in prevalence but also in the recommendations for preventing and managing these comorbidities between countries [13]. Common pulmonary diseases, especially chronic obstructive pulmonary disease, were observed less frequently in Asian countries (Japan 1.4%, Korea 1.3%, Taiwan 0.3%) than in European countries or the USA (Hungary 8%, USA 7.5%). Rheumatologists should be mindful of the assessment and treatment of comorbidities to improve morbidity and mortality, but also to reduce the risk of COVID-19 in patients with rheumatic diseases.
Interestingly, the Japanese population had a high prevalence of interstitial lung disease (ILD) after treatment with leflunomide (LEF), a drug licensed for the treatment of RA in the USA in 1998 and launch in Japan in 2003. Since its launch in Japan, adverse pulmonary events were reported with accelerated ILD leading to several deaths [14, 15]. The annual reported incidence of LEF-induced pneumonitis in Asia is 0.5–1.2% [16, 17], while the incidence in the West is under 0.1% [18]. In Japan, the licensed doses of DMARDs are lower than those in Western practice; however, LEF was introduced in Japan at a relatively high dose, equivalent to that in Western countries, and this higher dose may have partially contributed to the high rates of lung disease. Also, genetic differences might explain the apparent increased susceptibility of Japanese and Korean patients to pneumonitis from LEF.
The major phenotype of COVID-19 is the acute respiratory distress syndrome (ARDS), a lethal syndrome due to ‘‘cytokine storms,’’ in which immune cells and non-immune cells release large amounts of proinflammatory cytokines that cause damage to the host. To enter the cell, it is necessary the binding of the S1 region of the virus spike protein to the cell surface receptor followed by the fusion of the viral and cellular membranes mediated by the S2 subunit of spike protein. SARS-CoV-2 cell entry depends on surface molecules such as ACE2 and transmembrane serine protease TMPRSS2 [19]. Alveola type 2 cells highly express both ACE2 and TMPRSS2 in the steady state, and these cells might be the primary entry cells for SARS-CoV-2 in the lung. In Japan, the low mortality rate indicates that the number of cases with lung involvement has been relatively low compared to other populations. Differential characteristics in the frequency of pulmonary manifestations across populations following exposure to LEF or SARS-CoV-2 cannot be yet explained but might be related to environmental risks factors which could contribute to the regulation of expression of as yet unknown molecules or receptors in the lung in susceptible individuals.
COVID-19 treatment
There is no specific and effective antiviral treatment for COVID-19. Based on available evidence, the Japanese Association for Infectious Disease has made an approach to drug treatment for COVID-19 [20]. Remdesivir a nucleotide analogue prodrug that inhibits viral RNA polymerases, with in vitro activity against SARS-CoV-2, is the only drug approved in Japan for COVID-19, but several drugs can be used off-label according to physician’s judgement. Results from international trials on remdesivir, including patients from Japan, indicated that an antiviral drug alone is not likely to be sufficient for the treatment of COVID-19 [21, 22]. Faviparivir is an antiviral agent approved in Japan for the treatment of new or re-emerging influenza virus infections, but not yet approved for the treatment of COVID-19. A multicenter, randomized, open-label study has been conducted at 25 hospitals across Japan. Eighty-nine asymptomatic and mildly ill patients with COVID-19 were randomly assigned to early (from day 0) or late (from day 6) faviparivir therapy. The study did not yield statistically significant differences between the two treatment arms in viral clearance by day 6 and time to fever disappearance [23]. Faviparivir in combination with nafamostat mesylate, a TMPRSS2 inhibitor, was effective in reducing mortality in a series of 11 critically ill patients with COVID-19 [24]. Finally, treatment with ciclesonide inhalation led to favorable outcomes in three cases of COVID-19 pneumonia [25].
In conclusion, Japan has a milder COVID-19 phenotype than other countries, even though the Japanese population is older, has comorbidities and the restrictive measures applied in the country have not been as strict as elsewhere. The number of diagnostic tests performed in Japan is lower compared to other populations and focused on clusters, so the total number of infections in Japan cannot be known. Factors possibly related to a lower susceptibility to SARS-CoV-2 pulmonary manifestations could have favoured better outcomes and lower mortality in Japan.
Acknowledgements
Authors thanks Dr Sharon JB Hanley, Department of Obstetrics and Gynaecology, Hokkaido University, Sapporo, Japan, for her support in the preparation of the figure and helpful discussion.
Funding
The authors declare that no founding was involved in supporting this work.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sawalha AH, Manzi S. Coronavirus Disease-2019: Implication for the care and management of patients with systemic lupus erythematosus. Eur J Rheumatol. 2020;7:S117–S120. doi: 10.5152/eurjrheum.2020.20055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuroda M (2020) An epidemiological study of the SARS-CoV-2 genome in Japan. https://www.niid.gojp/niid/en/basic-science/467-genome/9598-genome-2020-1ehtml Accessed 27 October 2020
- 3.Ministry of Health, Labor, and Welfare About Coronavirus Disease 2019 (COVID-19). https://www.mhlw.go.jp/stf/covid-19/kokunainohasseijoukyou_00006.html#1-1 Accessed 27 October 2020
- 4.Coronavirus resource center. COVID-19 Dashboard by the center for Systems Science and Engineering (CSSE) at Johns Hopkins University of Medicine. https://coronavirus.jhu.edu/map.html Accessed 27 October 2020
- 5.Coronavirus (COVID-19) deaths worldwide per one million population as of October 27, 2020, by country. https://www.statista.com/statistics/1104709/coronavirus-deaths-worldwide-per-million-inhabitants/ Accessed 27 October 2020
- 6.Iwasaki A, Grubaugh ND. Why does Japan have so few cases of COVID-19? EMBO Mol Med. 2020;12:e12481. doi: 10.15252/emmm.202012481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl. 2020;26:832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 8.Kondo Y, Kaneko Y, Oshige T, Fukui H, Saito S, Okayama M, et al. Exacerbation of immune thrombocytopaenia triggered by COVID-19 in patients with systemic lupus erythematosus. Ann Rheum Dis. 2020 doi: 10.1136/annrheumdis-2020-218157. [DOI] [PubMed] [Google Scholar]
- 9.Yamada Z, Nanki T. COVID-19 in a patient with rheumatoid arthritis during tocilizumab treatment. J Clin Rheumatol. 2020;26:240–241. doi: 10.1097/RHU.0000000000001576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mokuda S, Tokunaga T, Masumoto J, Sugiyama E. Angiotensin-converting enzyme 2, a SARS-CoV-2 receptor, is upregulated by interleukin 6 through STAT3 signaling in synovial tissues. J Rheumatol. 2020;47:1593–1595. doi: 10.3899/jrheum.200547. [DOI] [PubMed] [Google Scholar]
- 11.Tam LS, Tanaka Y, Handa R, Chang CC, Cheng YK, Isalm N, et al. Care for patients with rheumatic diseases during COVID-19 pandemic: a position statement from APLAR. Int J Rheum Dis. 2020;23:717–722. doi: 10.1111/1756-185X.13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oku K, Atsumi T. Rheumatology practice in Japan: challenges and opportunities. Rheumatol Int. 2019;39:1499–1505. doi: 10.1007/s00296-019-04281-0. [DOI] [PubMed] [Google Scholar]
- 13.Dougados M, Soubrier M, Antunez A, Balint P, Balsa A, Buch MH, et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA) Ann Rheum Dis. 2014;73:62–68. doi: 10.1136/annrheumdis-2013-204223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito S, Sumida T. Interstitial lung disease associated with leflunomide. Intern Med. 2004;43:1103–1104. doi: 10.2169/internalmedicine.43.1103. [DOI] [PubMed] [Google Scholar]
- 15.Kamata Y, Nara H, Kamimura T, Haneda K, Iwamoto M, Masuyama J, et al. Rheumatoid arthritis complicated with acute interstitial pneumonia induced by leflunomide as an adverse reaction. Intern Med. 2004;43:1201–1204. doi: 10.2169/internalmedicine.43.1201. [DOI] [PubMed] [Google Scholar]
- 16.Sawada T, Inokuma S, Sato T, Otsuka T, Saeki Y, Takeuchi T, et al. Leflunomide-induced interstitial lung disease: prevalence and risk factors in Japanese patients with rheumatoid arthritis. Rheumatology (Oxford) 2009;48:1069–1072. doi: 10.1093/rheumatology/kep052. [DOI] [PubMed] [Google Scholar]
- 17.Ju JH, Kim SI, Lee JH, Lee SI, Yoo WH, Choe JY, et al. Risk of interstitial lung disease associated with leflunomide treatment in Korean patients with rheumatoid arthritis. Arthritis Rheum. 2007;56:2094–2096. doi: 10.1002/art.22666. [DOI] [PubMed] [Google Scholar]
- 18.Suissa S, Hudson M, Ernst P. Leflunomide use and the risk of interstitial lung disease in rheumatoid arthritis. Arthritis Rheum. 2006;54:1435–1439. doi: 10.1002/art.21806. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280 e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Japanese Association for Infectious Diseases. Approach to drug treatment for COVID-19. https://www.kansensho.or.jp/uploads/files/topics/2019ncov/covid19_drug_200817.pdf Accessed 27 October 2020
- 21.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19—Preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 22.Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med. 2020;382:2327–2336. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doi Y, Hibino M, Hase R, Yamamoto M, Kasamatsu Y, Hirose M, et al. A prospective, randomized, open-label trial of early versus late favipiravir in hospitalized patients with COVID-19. Antimicrob Agents Chemother. 2020 doi: 10.1128/AAC.01897-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doi K, Ikeda M, Hayase N, Moriya K, Morimura N, the COVID-UTH study group Nafamostat mesylate treatment in combination with favipiravir for patients critically ill with Covid-19 a case series. Crit Care. 2020;24:392. doi: 10.1186/s13054-020-03078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwabuchi K, Yoshie K, Kurakami Y, Takahashi K, Kato Y, Morishima T. Therapeutic potential of ciclesonide inahalation for COVID-19 pneumonia: report of three cases. J Infect Chemother. 2020;26:625–632. doi: 10.1016/j.jiac.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]