Abstract
Context
The methanol extracts from Hippeastrum reticulatum (L'Hér.) Herb. (Amaryllidaceae) (HR) display acetylcholinesterase inhibitory (AChEI) activity.
Objective
AChEI of alkaloids isolated from HR bulbs and the ameliorating effects of the alkaloid fraction (AHR) on memory and cognitive dysfunction in scopolamine-treated mice were investigated.
Materials and methods
Alkaloids were isolated by column chromatography and identified by spectroscopy. AChEI was evaluated using the modified Ellman’s method. Sixty Swiss male mice were randomly divided into six groups, received samples for 15 days. Normal group received saline, scopolamine-treated group scopolamine (1.5 mg/kg, intraperitoneal injection). Test groups received AHR (5, 10 and 15 mg/kg, per os) and positive control group donepezil (5 mg/kg, per os), administered 1 h before the test, scopolamine was injected 30 min prior to testing. The cognitive-enhancing activity of AHR against scopolamine-induced memory impairments was investigated using Y-maze, the novel object recognition test (NORT) and the Morris water maze (MWM) test.
Results
Seven alkaloids were isolated for the first time from the genus Hippeastrum: trans-dihydronarciclasine (1), N-chloromethylnarcissidinium (2), narciprimin (3), narciclasine-4-O-β-d-xylopyranoside (4), N-methyltyramine (5), 3β,11α-dihydroxy-1,2-dehydrocrinane (6) and brunsvigine (7); three are new compounds (2, 5, 6). Among them, 2–3 and 5–6 showed AChEI in vitro with IC50 values of 29.1, 46.4, 70.1 and 104.5 µg/mL, respectively. The anti-AChEI of 2, 5 and 6 are reported for the first time. In in vivo test, AHR (15 mg/kg) significantly increased in spontaneous alternation performance in the Y-maze test (p < 0.01), it significantly increased the time spent exploring the novel object (p < 0.05) comparison with scopolamine-treated group. The administration of AHR at doses 10 and 15 mg/kg significantly decreased escapes latency and swimming distance to the platform on day 6 compared to these in day 1 (p < 0.01 and p < 0.05, respectively).
Conclusions
AHR could be a potential candidate of future trials for treatment of memory and cognitive dysfunction in Alzheimer’s disease.
Keywords: Anti-AChE activity, Y-maze test, novel object recognition test, Morris water maze test
Introduction
Alzheimer’s disease (AD) is a disease of dementia that usually starts slowly and gradually worsens over time and usually occurs in older people (Burns and Iliffe 2009). In recent years, the prevalence of patients with dementia is increasing, which might be related to the increase in life expectancy of people in the world. According to the World Health Organization (WHO 2019), there were around 50 million people with dementia, and there are nearly 10 million new cases every year. It is estimated that the number of people with dementia may increase to 82 million in 2030 and 152 million in 2050, and the prevalence of people with AD could account for 60–70% among all people (Duthey 2013). Due to the acetylcholinesterase (AChE) inhibitory (AChEI) activity, drugs with such activity have been accepted to treat memory deficits in patients with AD (Yiannopoulou and Papageorgiou 2013). Thus, studies are concentrating on finding AChE inhibitors as new substances to treat AD.
Hippeastrum (Amaryllidaceae) is a large genus with over 90 species recorded (The Plant List 2020). The species belonging to this genus possess several important biological properties, such as antibacterial, antioxidant, antiviral and AChE inhibitors (Andradea et al. 2012). In Vietnam, this genus has two species: Hippeastrum equestre Herb. and Hippeastrum reticulatum (L.Hér.) Herb. (HR). Screening studies indicated that both species have the potent inhibitory effect of AChE, particularly HR (Andradea et al. 2012). Galanthamine and other alkaloids from galanthamine group (montanine, hippeastrine, pretazettine) have activity against AD (Pagliosa et al. 2010; Ding et al. 2017). However, there are still very few studies on the HR species.
This study contributes knowledge on the chemical composition and AChEI activity of isolated compounds from HR. We also investigated the effect of the alkaloid fraction of HR on cognitive and memory deficits in a scopolamine-induced rodent model of AD.
Materials and methods
General experimental procedures
NMR spectra were recorded using a Bruker Avance 500 spectrometer (500 MHz for 1H NMR, 125 MHz for 13C NMR) (Bruker, Billerica, MA) with TMS as an internal reference. Column chromatography was performed with silica gel (60 N, spherical, neutral, 40–50 μm, Kanto Chemical Co., Inc., Tokyo, Japan), YMC RP-18 (Fuji Silysia Chemical Ltd., Kasugai, Japan) and Sephadex LH-20 (Dowex® 50WX2-100, Sigma-Aldrich, St. Louis, MO). Analytical TLC separations were performed on pre-coated silica gel 60F254 and RP-18 F254 plates (0.25 or 0.50 mm thickness, Merck KGaA, Darmstadt, Germany). Acetylcholinesterase, acetylthiocholine (ACTI), 5,5-dithio-bis(2-nitrobenzoic acid) (DTNB) and galanthamine hydrobromide were purchased from Sigma-Aldrich (St. Louis, MO). Dimethyl sulphoxide (DMSO) was purchased from Merck (Darmstadt, Germany). All chemicals used were of the highest grade available.
Plant material
The bulbs of HR were collected in May 2018 from Thua Thien Hue province (16°44′30″N 107°23′48″E), Vietnam. Its identification was confirmed by Dr. Vu Tien Chinh, Vietnam National Museum of Nature, Vietnam Academy of Science and Technology. A voucher specimen (TTH-01) was deposited at the Faculty of Pharmacy, Hue University of Medicine and Pharmacy, Vietnam.
Extraction and isolation of constituents
The bulbs of HR were washed, dried at 50 °C (12.5 kg) then pulverized into powder, extracted with methanol (20 L × three times) by immersion at room temperature to yield an extract. Due to its large volume, the extract was divided into six parts. Each part was subjected to Diaion HP-20 column chromatography (15 cm × 60 cm, diameter × length). Water was passed through the column to remove the water-soluble components, then the compounds were eluted with methanol. After that, the solution was collected and the solvent evaporated to obtain 150 g of extract.
The methanol extract was acidified with 2% HCl to pH 2 and then extracted with ethyl acetate (EtOAc) (1 L × three times) to obtain an EtOAc fraction (60 g). The remaining acid solution was alkalinized with ammonium hydroxide to pH 10 and then extracted with dichloromethane (CH2Cl2) (1 L × 3 times) to obtain alkaloid fraction (AHR, 30 g).
The AHR (30 g) was chromatographed on a normal phase silica gel column (8 cm × 60 cm, diameter × length) and eluted with CH2Cl2:methanol:H2O (5:1:0.1, v/v/v) to obtain five fractions, frAHR1–fr.AHR5. Fraction AHR2 (8 g) was subjected to reverse-phase RP-18 silica gel column chromatography and eluted with methanol:H2O (3:1, v/v) to obtain seven fractions, fr.AHR2.1–fr.AHR2.7. Fraction AHR2.2 (600 mg) was further purified by Sephadex LH-20 and eluted with methanol to yield five fractions, fr.AHR2.2.1–fr.AHR2.2.5. The purification of fr.AHR2.2.3 (30 mg) yielded compound 1 (5.2 mg). Fr.C2.5 (150 mg) was applied to reverse-phase RP-18 silica gel column and eluted with methanol:H2O (1:1, v/v) to obtain compound 2 (8 mg).
Fraction AHR3 (10 g) was chromatographed on a reverse-phase RP-18 silica gel column and eluted with methanol:H2O (1:3, v/v) to give six fractions, fr.AHR3.1–fr.AHR3.6. Fraction AHR3.3 (450 mg) was further applied to silica gel chromatography and eluted with CH2Cl2:EtOAc:methanol (5:3:1, v/v/v) to obtain four fractions, fr.AHR3.3.1–fr.C3.3.4. Fraction AHR3.3.2 (150 mg) was purified by Sephadex LH-20 column to obtain compound 3 (8 mg).
Fraction AHR4 (8 g) was applied to reverse-phase RP-18 silica gel column chromatography and eluted with methanol:H2O (3:1, v/v) to yield five fractions, fr.AHR4.1–fr.AHR4.5. Fraction AHR4.3 (1 g) was then subjected to silica gel column chromatography and eluted with CH2Cl2:methanol (10:1, v/v) to obtain four fractions, fr.AHR4.3.1–fr.AHR4.3.4. Fraction AHR4.3.3 (60 mg) was further purified by Sephadex LH-20 and eluted with methanol to obtain compound 4 (15 mg).
Fraction AHR1 (4 g) was chromatographed on reverse-phase RP-18 silica gel column and eluted with acetone:H2O (5:1, v/v) to give six fractions, fr.AHR1.1–fr.AHR1.6. Fraction AHR1.3 (600 mg) was applied to silica gel chromatography and eluted with CH2Cl2:methanol:NH3 (10:1:0.1, v/v/v) to obtain six fractions, fr.AHR1.3.1–fr.C1.3.6. Fraction AHR1.3.2 (120 mg) was further separated on a reverse-phase RP-18 silica gel column chromatography and eluted with methanol:H2O (3:1, v/v) to yield compound 5 (8 mg). Fraction AHR1.6 (1.5 g) was chromatographed on reverse-phase RP-18 silica gel column and eluted with methanol:H2O (1:1, v/v) to obtain six fractions, fr.AHR1.6.1–fr.C1.6.6. Fraction AHR1.6.2 (450 mg) was purified by Sephadex LH-20 column and eluted with methanol to yield four fractions, fr.AHR1.6.2.1–fr.AHR1.6.2.4. Fraction C1.6.2.1 (270 mg) was applied to normal phase silica gel column and eluted with CH2Cl2:methanol:NH3 (5:1:0.1, v/v/v) to obtain compound 6 (7 mg). The purification fr.AHR1.6.2.3 (100 mg) yielded compound 7 (10 mg).
Acetylcholinesterase inhibition assay
The AChE inhibiting activity of the extracts and isolated compounds was measured using the modified Ellman’s method (Ellman et al. 1961). Acetylthiocholine was used as a substrate to examine the inhibitory effect of the sample. The test method procedure: pH 8 buffered buffer, samples (extracts and isolated compound of Hippeastrum reticulatum) and 0.25 IU/mL AChE enzyme solution was added, successively, to each well of the 96-well plate. The mixture was well mixed and incubated for 15 min at room temperature. After that, DTNB test solution (5,5′-dithiobis (2-nitrobenzoic acid) 2.4 mM and ATCI substrate solution (ACTI iodide) 2.4 mM) were added to the mixture and mixed well. The mixture was incubated for a further 15 min at room temperature, then the solution was measured at 405 nm for 10 s. The absorption was measured with the ELISA Microplate Reader EMR 500 (San Jose, CA). We used galantamine as a positive control. The inhibition percentage was calculated using the following equation: (1 – S/E)×100, where E and S are the enzymatic activities with and without the sample tested, respectively. The AChEI activity of each sample was expressed as the concentration (in µg/mL) required to inhibit AChE hydrolysis by 50% (the IC50 value), which was calculated from the logarithmic dose–inhibition curve.
Behavioural experiments
Animal groups and drug treatments
Sixty Swiss male mice were randomized into six groups (n = 10 per group): (1) control (saline), (2) scopolamine 1.5 mg/kg/day, (3) AHR 5 mg/kg/day + scopolamine 1.5 mg/kg/day, (4) AHR 10 mg/kg/day + scopolamine 1.5 mg/kg/day, (5) AHR 200 mg/kg/day + scopolamine 1.5 mg/kg/day and (6) positive control group (donepezil 5 mg/kg/day + scopolamine 1.5 mg/kg/day). These groups were used for experiments below (Y-maze test, novel object recognition test (NORT) and Morris water maze (MWM) test). The same set of animals was used for all three behavioural tests since the intervals between two behavioural tests were three days. AHR is the alkaloid fraction of HR. The method of extracting the AHR fraction is described above. The AHR was resuspended in saline and administered orally (per os, p.o.). Scopolamine was also dissolved in saline and administered by intraperitoneal (i.p.) injection. AHR was administered 1 h before the behaviour test, whereas scopolamine was injected 30 min prior to testing. The mice were treated in strict compliance with The Animal Center Guidelines for the Care and Use of Laboratory Animals at the Vietnam Military Medical University, consistent with EU Directive 2010/63/EU. This study was approved by the Committee for Animal Experiments and Ethics (Institutional Animal Care and Use Committee, IACUC) at the Vietnam Military Medical University (approval no. IACUC-063/19).
Y-maze test
The Y-maze test was performed according to the procedures described by Lam et al. (2016). Drugs and scopolamine have been administered for 14 days before the Y maze trial. The Y-maze, made of dark grey polyvinyl plastic, consists of three arms with equal angles between the arms. Each arm is 35 cm long, 5 cm wide and 10 cm high. Mice were placed in one arm and allowed to freely move in and explore the Y-maze for around 10 min. The behaviour of each animal was recorded individually and analysed by AnyMaze software (Stoelting, Kiel, WI). The arms of the maze were cleaned with 10% ethanol between each exercise to eliminate the odour and waste of mice. One hour before each trial, mice were treated with saline (p.o., in control group and scopolamine-treated group) or AHR (5, 10 or 15 mg/kg/day, p.o., in AHR-treated group) or donepezil (5 mg/kg/day, p.o., in positive control group). Thirty minutes before the trial, animals also administered saline (i.p., control group) or scopolamine (1.5 mg/kg, i.p., in scopolamine-treated group, AHR-treated group and positive control group). Alternative behaviour is determined based on the number of successes going into three different arms in succession. Access to the arms is determined by mice placing all four legs within range of the arms. The alternation score (%) for each mouse was defined as the ratio of the actual number to the possible number of alternations multiplied by 100, as shown by the following equation: spontaneous alternation (%)= [(number of alternations)/(total arm entries – 2)] × 100. The number of arm entries was used as an indicator of locomotor activity.
Novel object recognition test
The NORT was performed according to the procedures described by Lam et al. (2016). The exploration time and frequencies were recorded (n = 10 per group). This test is performed in an open square box with dimensions of 45 × 45 × 50 cm3. First, the mice were placed in an open square box with objects and were free to explore within 5 min. Twenty-four hours later, the mouse was given an exercise in the sample phase. In this phase, the mice were placed in an open box with two identical objects, A1 and A2, placed symmetrically and approximately 10 cm away from the wall for 5 min. The mice were free to explore within 5 min. Exploration is defined as the moment the mice touch the object with their nose and/or front legs. After 24 h, mice received a test phase in which one of the two objects was replaced by a new one. Animals were free to explore in an open box with one familiar (object A1 or A2) and one novel (B) object within 5 min. All objects have the same texture and size but differ in shape. After each exercise, all test subjects were wiped with 10% alcohol to remove odours and waste. One hour before a trial, mice were treated with saline (p.o., in control group and scopolamine-treated group) or AHR (5, 10 or 15 mg/kg/day, p.o., in AHR-treated group) or donepezil (5 mg/kg/day, p.o., in positive control group). Thirty minutes before the trial, animals also administered saline (i.p., control group) or scopolamine (1.5 mg/kg, i.p., in scopolamine-treated group, AHR-treated group and positive control group).
Morris water-maze test
The Morris water-maze test (Morris 1981) was performed according to the procedures described by Lam et al. (2016). The Morris water-maze is a black barrel (80 cm in diameter and 35 cm high) with a black painted inner surface. The container is filled with water and fresh milk to make the water turbid. The water temperature varies between 20 °C ± 1 °C. The water tank is divided into four quadrants of equal area. A small transparent platform (4 cm in diameter and 18 cm high) is placed at centre of one of four quadrants and submerged 1 cm below the surface of the water to be invisible. The water tank is placed in a quiet room with landmarks placed around the water basin so that the animal can be oriented. The position of the platform and the landmarks do not change throughout the experiment. Before the experiment, mice were allowed to swim for around 60 s without the platform in the water tank. After six consecutive days, each mouse was tested four times daily for practice, the interval between each trial was approximately 2 min. During each exercise, the mice were placed in the water opposite the wall of the tank in one of the four parts of the tank. The escape latencies and swimming distances of each mouse from the starting position to the target quadrant is recorded by camera and analysed by AnyMaze software (Stoelting, Kiel, WI). The average of these parameters was calculated for each session per mouse. The mice were allowed to swim for a maximum of 1 min at a time. If the mice were not on the platform after 1 min, the mice were caught and placed on the platform for 10 s to remember the safe place. On the seventh day, the mice performed the exercise without the platform in the tank. The swimming time in the quadrant of platform location (time spent in target quadrant) was determined. One hour before a trial, mice were treated with saline (p.o., in control group and scopolamine-treated group) or AHR (5, 10 or 15 mg/kg/day, p.o., in AHR-treated group) or donepezil (5 mg/kg/day, p.o., in positive control group). Thirty minutes before the trial, animals also administered saline (i.p., control group) or scopolamine (1.5 mg/kg, i.p., in scopolamine-treated group, AHR-treated group and positive control group).
Statistical analysis
The results of the behavioural studies are expressed as mean ± SEM, Y-maze test for spontaneous alternation (%), object recognition test for distance travelled and MWM test for probe-trial swimming times were analysed by one-way analysis of variance (ANOVA) followed by Tukey’s post hoc for multiple comparisons. Time spent exploring a familiar and novel object in the sample and test phases in the object recognition test was normalized by dividing the values by the total time spent exploring. Normalized values of the time spent exploring familiar and/or novel objects were analysed by repeated-measures two-way ANOVA with object as a within-subject variable and treatment (experimental group) as a between-subject variable, followed by Tukey's post hoc analysis. Escape latencies and swimming distance in the training trials in the MWM test were analysed by repeated measures two-way ANOVA with day as a within-subject variable and treatment as a between-subject variable, followed by Tukey's post hoc analysis. The differences were considered as statistically significant at p < 0.05.
Results
Identification of isolated constituents
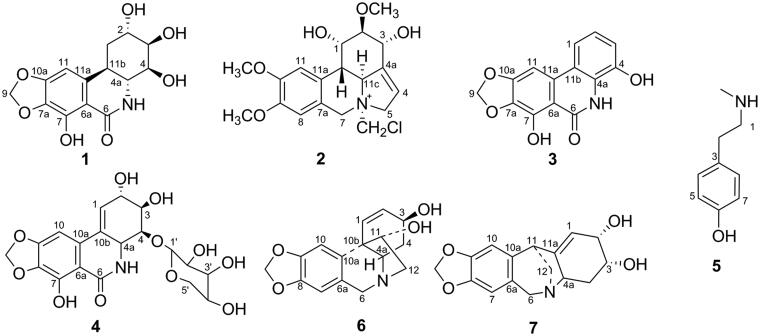
Further separation and purification of the C fraction led to the isolation of seven alkaloids isolated for the first time from the genus Hippeastrum, including trans-dihydronarciclasine (1) (Varró et al. 2017), N-chloromethylnarcissidinium (2) (Hoshino 1998), narciprimin (3) (Nair et al. 2011), narciclasine-4-O-β-d-xylopyranoside (4) (Katoch et al. 2020), N-methyltyramine (5) (Kikuchi et al. 2010), 3β,11α-dihydroxy-1,2-dehydrocrinane (6) (Sener et al. 1993) and brunsvigine (7) (Hong et al. 2008) (Figure 1). Detailed NMR spectroscopic data of all previously known isolates are in publications cited above.
Figure 1.
Structure of compounds isolated from Hippeastrum reticulatum.
Acetylcholinesterase inhibition
The Hippeastrum reticulatum extracts have been tested for AChEI. The results showed that the AHR had the best activity with an IC50 value of 39.85 µg/mL (Table 1).
Table 1.
Acetylcholinesterase inhibitory activity in vitro of extracts of Hippeastrum reticulatum.
| Fractions | IC50±SD (µg/mL) |
|---|---|
| Methanol | 98.71 ± 1.75 |
| EtOAc | − |
| AHR | 39.85 ± 0.94 |
| Galanthamine | 0.36 ± 0.01 |
(–) no inhibition.
IC50 values were reported as means ± SD of triplicates.
The isolated compounds have been tested for AChEI in vitro. Among them, compound 3 showed the most potent inhibitory activity against AChE with an IC50 value of 29.1 µg/mL (Table 2).
Table 2.
Acetylcholinesterase inhibitory activity in vitro of isolated compounds.
| Compounds | IC50±SD (µM) |
|---|---|
| trans-Dihydronarciclasine (1) | 1593.73 ± 39.09 |
| N-chloromethylnarcissidinium (2) | 264.86 ± 0.23 |
| Narciprimin (3) | 107.31 ± 2.45 |
| Narciclasine-4-O-β-d-xylopyranoside (4) | − |
| N-methyltyramine (5) | 463.97 ± 9.65 |
| 3β,11α-Dihydroxy-1,2-dehydrocrinane (6) | 161.68 ± 7.16 |
| Brunvigine (7) | 801.17 ± 17.88 |
| Galanthamine | 1.15 ± 0.03 |
(–) no inhibition.
IC50 values were reported as means ± SD of triplicates.
Effect of AHR on scopolamine-induced memory impairment in mice
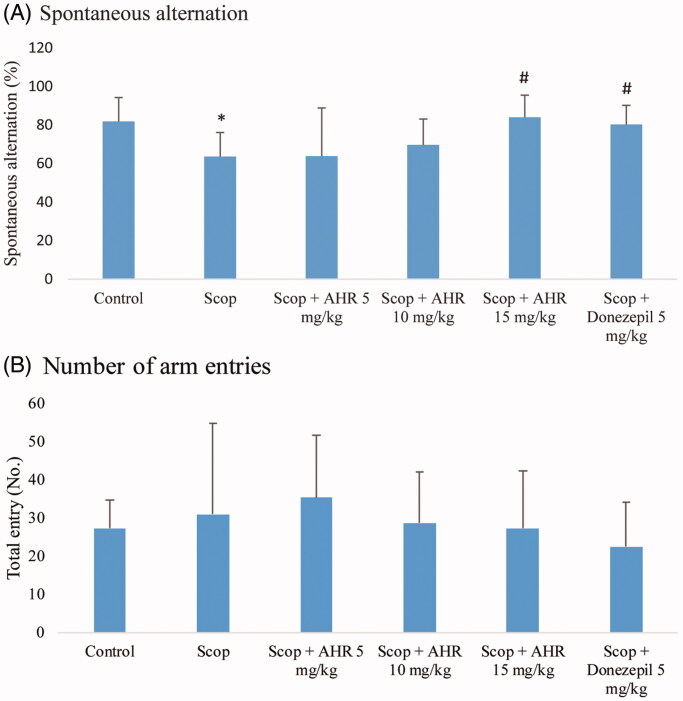
The results from the Y-maze test are illustrated in Figure 2. The percentage of spontaneous alternation in the scopolamine-treated group was significantly lower than the control group (p < 0.01). Treatment of mice given scopolamine with AHR at 15 mg/kg and donepezil at 5 mg/kg, the percentage of spontaneous alternation significantly increased compared with the scopolamine-treated group (p < 0.01 and p < 0.05, respectively). In addition, there were no significant differences in the number of arm entries among the six experimental groups (p > 0.05). These results suggested that administration of AHR prevents memory deficits caused by scopolamine without affecting locomotor activity.
Figure 2.
The effect of AHR on memory and cognitive impairments induced by scopolamine in mice as measured by the Y-maze test. Spontaneous alternation behaviour (%) (A) and numbers of arm entries (B) during a 10 min test session were recorded. Data are presented as mean ± SEM (n = 10 per group).
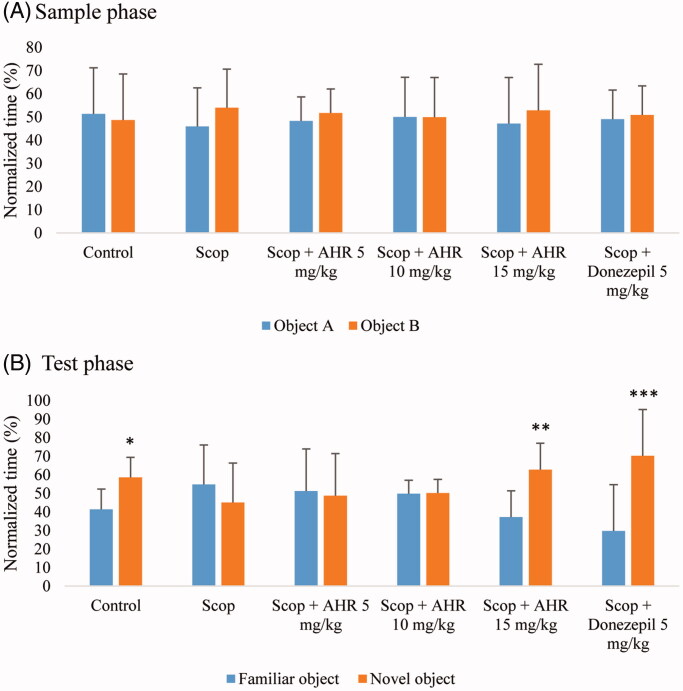
The results from NORT are illustrated in Figure 3. Figure 3(A) shows the mean normalized time spent exploring two identical objects (objects A and B) during the sample phase by the six experimental groups. Repeated-measures two-way ANOVA indicated no significant main effects of object (p > 0.05) or treatment (experimental groups) (p > 0.05). Furthermore, there was no significant interaction between object and treatment (p > 0.05). These findings indicate that neither scopolamine nor HR affect the behaviour of mice during this phase.
Figure 3.
The effect of AHR on the time spent exploring objects in the sample phase (A) and the test phase (B) during novel object recognition test. The time spent exploring objects was normalized to the total duration of exploration. Data are presented as mean ± SEM (n = 10). Significant difference compared to a familiar object at *p < 0.05, **p < 0.01 and ***p < 0.001, respectively.
Figure 3(B) shows the mean normalized time spent exploring two different objects (familiar versus novel) during the test phase by six experimental groups. In the control group, the normalized time to discover a novel object was significantly longer than that of the familiar object (p < 0.05). In the scopolamine-treated group, there was no statistically significant difference in the time discoveries between novel and familiar objects (p > 0.05). Treatment with AHR at 15 mg/kg and donepezil at 5 mg/kg in mice given scopolamine, the time to discover novel objects was significantly longer than that of familiar objects (p < 0.01 and p < 0.001, respectively).
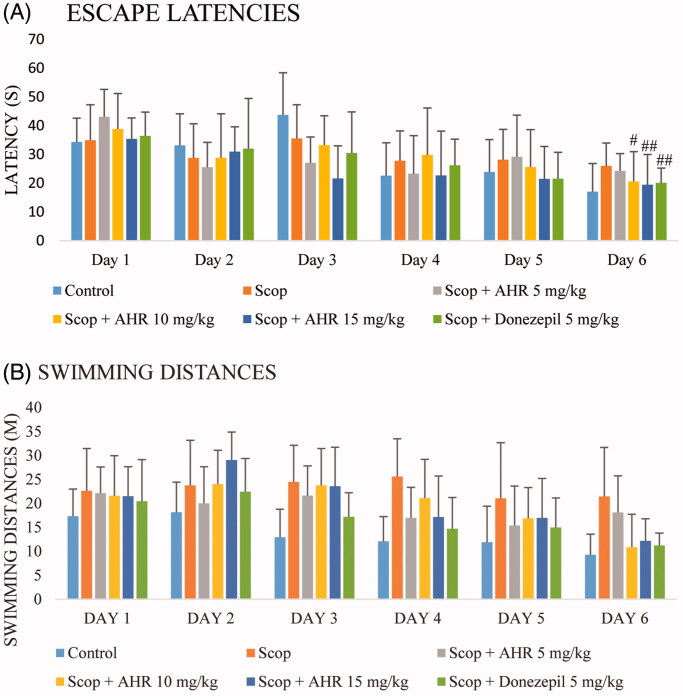
The results on the escape latency and swimming distances during spatial learning in the MWM test are illustrated in Figure 4. The test showed no statistically significant difference in escape latency between the experimental groups both before and after treatment (p > 0.05). Upon comparison between days in the experimental groups, the results showed that in the control group, the mean escape latency and the swimming distances to the platform on day 6 were statistically shorter than that on the day 1 (p < 0.05). A comparison between the group of treatment AHR at 5 mg/kg in mice given scopolamine and the scopolamine-treated group, there was no statistically significant difference in the mean escape latency time between experimental days (p > 0.05). Treatment of mice receiving scopolamine with AHR at 10 and 15 mg/kg and donepezil at 5 mg/kg, the mean escape latency and swimming distances to the platform on day 6 was significantly shorter than on day 1 (p < 0.01, p < 0.05 and p < 0.05, respectively).
Figure 4.
The effect of AHR on escape latencies (A) and swimming distance (B) during the training-trial sessions of the Morris water maze task in mice with scopolamine-induced memory dysfunction. Data are presented as mean ± SEM (n = 10 per group). Significant difference from day 1 (#p < 0.01, ##p < 0.05, respectively).
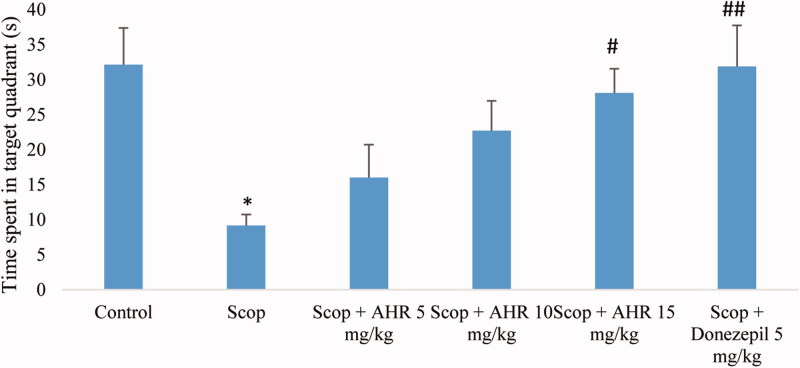
Figure 5 shows the mean time spent swimming in the target quadrant during the probe trial. The test showed that the time spent in the target quadrant by the scopolamine-treated group was significantly lower than the control group (p < 0.01). According to the results, the treatment of mice given scopolamine with AHR at 15 mg/kg and donepezil at 5 mg/kg, the time in the target quadrant significantly increased compared with the scopolamine group (p < 0.05 and p < 0.01, respectively).
Figure 5.
The effect of AHR on the time spent swimming in the target quadrant during the probe-trial session of the Morris water maze test. Data are presented as mean ± SEM (n = 10 per group). *Significant difference from the control group (p < 0.05); significant difference from the scopolamine-treated group (#p < 0.05, ##p < 0.01, respectively).
Discussion
To the best of our knowledge, compounds 1–7 were isolated for the first time from the genus Hippeastrum. From our understanding, the anti-AChEI of compounds 2, 5 and 6 was reported for the first time in this study.
Recognition and memory in rodents such as mice are investigated using many behavioural tests. The Y-maze test, Morris water-maze test and recognition tests are usually applied in previous studies (Wolf et al. 2016; Lueptow 2017; Kraeuter et al. 2019). Thus, in the present study, we also employed these tests to investigate the effects of HR extracts in ameliorating memory and recognition deficits in experimental animals.
The main outputs from Y-maze analysis are the percentage of spontaneous alternation and the number of arm entries. The percentage of spontaneous alternation can be used to measure short-term spatial memory in mice. The number of entries per arm is a measurement of activity and locomotion during the testing session and is also be used to calculate the percent alternations. There was no correlation between spontaneous alternation and the number of arm entries made. Our results showed that AHR affected short-term spatial memory, but did not affect locomotor activity in mice. It does not increase or decrease the mice's locomotor activity. Therefore, the total number of arm entries of mice did not change significantly among the study groups. In the Y-maze test, alternation behaviour was determined from successive entries into three different arms. Rodents tend to explore new places that mice have not visited (Morellini 2013). Thus, alternation behaviour might be used to investigate spatial memory in rodents (Sarter et al. 1988). In the present study, after scopolamine injection, spontaneous alternation (%) significantly decreased compared with that in vehicle-treated mice. In contrast, after AHR treatments, the mean spontaneous alternation (%) of mice was significantly increased at a dose of 15 mg/kg compared with that in scopolamine-treated mice. These results indicated that AHR at a dose of 15 mg/kg ameliorated deficits in memory and recognition.
In the Morris water test, mean latencies and swimming distances in the control decreased gradually from day 1 to day 6. These indicated learning and spatial memory abilities in mice. In the scopolamine-treated group, there were no significant differences in latencies and travel distances from day 1 to day 6. These suggested that there is a deficit in learning and spatial memory in scopolamine-treated mice. After treatments of AHR, we found that there was a tendency for latencies and travel distances in mice to decrease, especially at doses of AHR 10 and 15 mg/kg. This indicated the effect of AHR to ameliorate memory deficits of AHR-treated mice. To confirm this suggestion, we continued to more thoroughly analyse mice’s behaviour in the MWM test. On day 7, the platform was removed from the water maze. The memory ability of mice was evaluated by time spent in the target quadrant. A longer time spent in the target quadrant indicated better memory (Wolf et al. 2016). Our results indicated that time spent in the target quadrant decreased in scopolamine-treated mice. This also indicated deficits in spatial memory in scopolamine-treated mice. After treatments of AHR extract, time spent in the target quadrant increased significantly in mice treated with 15 mg/kg. This suggested that AHR extract strongly ameliorated memory deficits in the mouse model of AD.
Recognition deficits in mice were investigated by the object recognition test. Rodents prefer to explore novel objects longer than familiar objects (Leger et al. 2013). The object recognition test contains two continuative phases: a sample phase and a test phase. In the sample phase, mice explored two similar objects. As both objects were novel for mice, exploring time was equal for the two objects in all experimental groups. In the test phase, one of the two objects in the sample phase was displaced by the novel object. Thus, there were differences in the exploring time of the objects in experimental groups. These were as follows: in the control group, the exploring time of the novel object was significantly longer than that of the familiar object. This is normal behaviour in mice. In the scopolamine group, there was no significant difference in the exploring time of the novel object compared with that of the familiar object. This indicated that scopolamine induced a recognition deficit in mice. After treatments, the time spent exploring the novel object was significantly longer than that of the familiar object in AHR-treated mice at a dose of 15 mg/kg. This suggested that HR at a dose of 15 mg/kg ameliorated deficits in object recognition.
In summary, our results showed that AHR extract and donepezil exhibited similar trend in behavioural analysis. However, AHR or its constituents may have better bioavailability and fewer side effects than donepezil. Therefore, future studies are needed to develop this potential species into a drug.
Conclusions
From AHR, seven alkaloidal compounds were isolated. Among them, narciprimin, 3β,11α-dihydroxy-1,2-dehydrocrinane, N-methyltyramine and N-chloromethylnarcissidinium showed anti-AChE activity with range of IC50 values from 29.1 to 104.49 µg/mL. In the in vivo experiment, the administration of AHR at 15 mg/kg in mice given scopolamine effectively prevented memory and cognitive deficits.
Funding Statement
This work was supported by the Ministry of Education and Training, Vietnam, under Grant [number B2018-DHH-63].
Disclosure statement
The authors report no conflicts of interest.
References
- Andradea JP, de Pignia NB, Torras-Claveriaa L, Guoa Y, Berkovb S, Reyes-Chilpac R, Amranid AE, Zuanazzie JAS, Codinaa C, Viladomata F, et al. 2012. Alkaloids from the Hippeastrum genus: chemistry and biological activity. Rev Lat Quim. 40:83–98. [Google Scholar]
- Burns A, Iliffe S.. 2009. Alzheimer's disease. BMJ. 338:b158. [DOI] [PubMed] [Google Scholar]
- Ding Y, Qu D, Zhang K-M, Cang X-X, Kou Z-N, Xiao W, Zhu J-B.. 2017. Phytochemical and biological investigations of Amaryllidaceae alkaloids: a review. J Asian Nat Prod Res. 19(1):53–100. [DOI] [PubMed] [Google Scholar]
- Duthey B. 2013. Alzheimer's disease. World Health Organization; [accessed 2020 Feb 28]. https://www.who.int/medicines/areas/priority_medicines/BP6_11Alzheimer.pdf.
- Ellman GL, Courtney KD, Andres V, Feather-Stone RM.. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 7:88–95. [DOI] [PubMed] [Google Scholar]
- Hong AW, Cheng TH, Raghukumar V, Sha CK.. 2008. An expedient route to montanine-type Amaryllidaceae alkaloids: total syntheses of (–)-brunsvigine and (–)-manthine. J Org Chem. 73(19):7580–7585. [DOI] [PubMed] [Google Scholar]
- Hoshino O. 1998. The Amaryllidaceae alkaloids. In: Cordell GA, editor. The alkaloids: chemistry and biology. Vol. 51. San Diego: Academic Press; p. 323–424. [Google Scholar]
- Katoch D, Kumar D, Padwad YS, Singh B, Sharma U.. 2020. Narciclasine-4-O-β-d-xylopyranoside, a new narciclasine glycoside from Zephyranthes minuta. Nat Prod Res. 34(2):233–240. [DOI] [PubMed] [Google Scholar]
- Kikuchi H, Uchiyama N, Ogata J, Kikura-Hanajiri R, Goda Y.. 2010. Chemical constituents and DNA sequence analysis of a psychotropic herbal product. Forensic Toxicol. 28(2):77–83. [Google Scholar]
- Kraeuter AK, Guest PC, Sarnyai Z.. 2019. The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol Biol. 1916:105–111. [DOI] [PubMed] [Google Scholar]
- Lam LM, Nguyen MT, Nguyen HX, Dang PH, Nguyen NT, Tran HM, Nguyen HT, Nguyen NM, Min BS, Kim JA, et al. 2016. Anticholinesterases and memory improving effects of Vietnamese Xylia xylocarpa. Chem Cent J. 10(48):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, Freret T.. 2013. Object recognition test in mice. Nat Protoc. 8(12):2531–2537. [DOI] [PubMed] [Google Scholar]
- Lueptow LM. 2017. Novel object recognition test for the investigation of learning and memory in mice. J Vis Exp. 2017:55718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morellini F. 2013. Spatial memory tasks in rodents: what do they model? Cell Tissue Res. 354(1):273–286. [DOI] [PubMed] [Google Scholar]
- Morris RG. 1981. Spatial localization does not require the presence of local cues. Learn Motiv. 12(2):239–260. [Google Scholar]
- Nair J, Aremu AO, van Staden J.. 2011. Isolation of narciprimine from Cyrtanthus contractus (Amaryllidaceae) and evaluation of its acetylcholinesterase inhibitory activity. J Ethnopharmacol. 137(3):1102–1106. [DOI] [PubMed] [Google Scholar]
- Pagliosa LB, Monteiro SC, Silva KB, de Andrade JP, Dutilh J, Bastida J, Cammarota M, Zuanazzi JAS.. 2010. Effect of isoquinoline alkaloids from two Hippeastrum species on in vitro acetylcholinesterase activity. Phytomedicine. 17(8–9):698–701. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bodewitz G, Stephens DN.. 1988. Attenuation of scopolamine-induced impairment of spontaneous alteration behaviour by antagonist but not inverse agonist and agonist beta-carbolines. Psychopharmacology (Berl). 94(4):491–495. [DOI] [PubMed] [Google Scholar]
- Sener B, Könükol S, Kruk C, Pandit UK.. 1993. New crinine-type alkaloids from Pancratium maritimum L. growing in Turkey. Nat Prod Lett. 1(4):287–291. [Google Scholar]
- The Plant List . 2020. A working list of all plant species; [accessed 2020 Feb 28]. http://www.theplantlist.org/.
- Varró G, Hegedűs L, Simon A, Balogh A, Grün A, Leveles I, Vértessy BG, Kádas I.. 2017. The first enantioselective total synthesis of (–)-trans-dihydronarciclasine. J Nat Prod. 80(6):1909–1917. [DOI] [PubMed] [Google Scholar]
- WHO . 2019. Dementia; [accessed 2020 Sep 9]. https://www.who.int/news-room/fact-sheets/detail/dementia.
- Wolf A, Bauer B, Abner EL, Ashkenazy-Frolinger T, Hartz AM.. 2016. A comprehensive behavioral test battery to assess learning and memory in 129S6/Tg2576 mice. PLoS One. 11(1):e0147733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiannopoulou KG, Papageorgiou SG.. 2013. Current and future treatments for Alzheimer's disease. Ther Adv Neurol Disord. 6(1):19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]