Abstract
Using data from a large Australian twin sample we examined the extent to which genetic variation in the Big Three personality dimensions (positive emotionality, negative emotionality, and constraint) and their lower-order components explained genetic variation in the risk for disordered gambling (DG) among men and women. Genetic influences contributing to individual differences in normal-range personality traits explained over 40% of the genetic risk for DG, with a larger contribution among women than among men. The largest and most robust contributions came from the higher-order personality dimension of negative emotionality and its two lower-order dimensions of alienation and aggression. Surprisingly, low self-control was associated with the genetic risk for DG only among women, and risk-taking/sensationseeking did not explain genetic risk for DG in either sex. The results of this study have implications for the causes of comorbidity between DG and other psychiatric disorders, the search for genes associated with DG risk, and the possibility of sex differences in the etiology of DG. Using a broad-band inventory of personality supports the conclusion that there probably is a substantial proportion of genetic variation in DG that cannot be explained by individual differences in personality.
Keywords: disordered gambling, personality, genetic influences, twin study, sex differences
There is now replicated evidence from two large twin studies that genetic influences may account for a substantial proportion of the variation in risk for disordered gambling1 (DG) (Slutske et al., 2000; Slutske et al., 2010). A series of follow-up analyses from the all-male Vietnam Era Twin Registry study have suggested that DG may have common genetic underpinnings with both externalizing and internalizing psychopathology; for example, DG shared genetic risk factors with alcohol dependence (Slutske et al., 2000), antisocial personality disorder (Slutske et al., 2001), and major depression (Potenza et al., 2005), with genetic correlations of 0.45, 0.43, and 0.58, respectively.
Another approach to characterizing the genetic risk for DG is to decompose the variation based on more fundamental behavioral tendencies, that is, by focusing on the extent to which the genetic risk for DG is shared with genetic variation contributing to individual differences in normal-range personality characteristics. This could yield insights about whether personality might be an important intermediate phenotype through which the genetic risk for DG is expressed (Gottesman & Gould, 2003), and provide important clues to the shared genetic risk for DG and comorbid psychiatric disorders.
Two studies of nontreatment-seeking individuals from the community have used omnibus personality inventories to describe young (Slutske et al., 2005) and middle-aged adults (Bagby et al., 2007) with and without DG. Among 939 18-year-olds from a New Zealand birth cohort there were significant associations with two of the Big Three higher-order personality dimensions of negative emotionality and constraint (Slutske et al., 2005). Among 283 newspaper-recruited Canadian adults there were significant associations with two of the Big Five higher-order personality dimensions of conscientiousness and neuroticism (Bagby et al., 2007). The results of these two community-based studies are broadly consistent with the conclusion that low behavioral control and high negative emotionality are associated with DG in adulthood. A genetically informative study of the association between personality and DG would yield valuable information about the extent to which genetic variation in individual differences in personality can explain the genetic risk for DG. Taken together, the results of the previous multivariate twin studies demonstrating common genetic underpinnings of DG with externalizing and internalizing disorders, and the results of the personality studies demonstrating the associations of two of the major dimensions of personality with DG suggest that the personality dimensions of behavioral control and negative emotionality may explain a portion of the genetic risk for DG.
However, it is not clear the extent to which these conclusions apply to women. The previous multivariate twin studies of DG were all based on a single male twin sample (Potenza et al., 2005; Slutske et al., 2000; Slutske et al., 2001), and in neither of the community-based personality and DG studies were differences between men and women explored (Bagby et al., 2007; Slutske et al., 2005). When sex differences were examined in a previous paper based on a univariate twin study of DG, the proportion of genetic variation in DG risk was comparable and there was evidence that the DG susceptibility genes overlapped considerably in men and women (Slutske et al., 2010). It is still possible, however, that previously undiscovered sex differences in the etiologic pathways to DG may emerge within the context of a more powerful multivariate twin study that explores the genetic linkages between personality and DG in men vs. women.
In the present study, we examined in a large Australian sample of male, female, and unlike-sex twin pairs the extent to which genetic variation in the Big Three personality dimensions (positive emotionality, negative emotionality, and constraint) and their lower-order components explain genetic variation in the risk for DG. We also examined whether there were differences between men and women in the role of personality variation in the genetic risk for DG.
Method
Participants
The participants for this study were 4,764 members of the national community-based Australian Twin Registry (ATR) Cohort II (Slutske et al., 2009). In 2004–2007, a telephone interview containing a thorough assessment of gambling behaviors was conducted with the ATR Cohort II members (individual response rate of 80%). These 4,764 individual twins included 1,875 complete twin pairs (867 MZ [520 female, 347 male], 1,008 DZ [367 female–female, 227 male–male, and 414 female–male]), and 1,014 individual twins from incomplete pairs (304 MZ [151 female, 153 male], 710 DZ [181 female–female, 216 male–male, and 313 female–male]).
Those participants who completed the structured diagnostic telephone interview were mailed a paper-and-pencil personality questionnaire with a postage-paid return envelope. Of the 4,764 participants who completed the telephone interview, 4,355 (91%) returned the personality questionnaire. The mean age of the participants was 37.7 years (range = 32–43) and 57.2% of the sample was female (for more details, see Slutske et al., 2009).
Measures
Personality.
Participants completed a modified 177-item version of the Multidimensional Personality Questionnaire (MPQ; Tellegen & Waller, 2008). The personality scales comprising the MPQ can be viewed at the higher-order level as defining three distinct superfactors (positive emotionality, negative emotionality, and constraint) and at the lower-order level as defining 10 more basic aspects of personality variation. The positive emotionality scale is a combination of scores from the lower-order MPQ scales of well-being, social potency, achievement, and social closeness. The negative emotionality scale is a combination of scores from the lower-order MPQ scales of stress reaction, alienation, and aggression. The constraint scale is a combination of scores from the lower-order MPQ scales of self-control, harm avoidance, and traditionalism. (Complete descriptions of the MPQ scales can be found in the online supplemental materials.)
Two of the lower-order MPQ scales from the constraint dimension, self-control and harm avoidance, are of particular interest as correlates of DG because the personality traits of impulsivity or risk-taking/sensation-seeking have been featured in theories of the development of DG (Blaszczynski & Nower, 2002; Sharpe, 2002) and addictions in general (e.g., Kreek et al., 2005). MPQ self-control can be thought of as a reversed measure of impulsivity. Low scorers on this scale are impulsive, spontaneous, reckless, and careless. MPQ harm avoidance can be thought of as a reversed measure of a risk-taking or sensation-seeking disposition. Low scorers on this scale go for risky activities and adventures and enjoy the excitement of dangerous situations.
Disordered gambling.
DG was assessed using the National Opinion Research Center DSM–IV Screen for Gambling Problems (NODS; Gerstein et al., 1999). The test–retest reliability of the lifetime diagnosis of DG from the NODS was high (κ= 0.67; Yule’s Y = 0.79). The NODS Diagnostic and Statistical Manual for Mental Disorders-Fourth Edition (DSM–IV) diagnostic criteria were assessed for all participants who reported that they had ever gambled at least five times within a single 12 month period; the majority of participants, 77.5%, surpassed this gambling threshold. Among the 4,764 participants in the study, 595 (12.5%) had experienced at least one DG symptom in their lifetime; 322(6.8%), 90 (1.9%), 52 (1.1%), 27(0.6%), 38 (0.8%), 23 (0.5%), 18(0.4%), 11 (0.2%), 7 (0.1%), and 7 (0.1%) participants had experienced from 1 to 10 DG symptoms, respectively.
Data Analysis
Biometric models were fitted by the method of robust weighted least squares directly to the raw twin data using the Mplus program (version 5.2; Múthen & Múthen, 2007). Biserial correlations of the associations between the continuous personality measures and the underlying normally distributed latent DG liability were estimated. The DG symptom count was dichotomized at one or more symptoms to maximize the statistical power in multivariate biometric models (see Slutske et al., 2010). Although this threshold conforms most closely to the idea of problem gambling, the assumption of the liability-threshold model that we used means that the results will apply equally to all levels of disordered gambling behavior, including pathological gambling disorder. This definition of DG evidenced high reliability (test–retest r = .95) and validity, as indicated by significant associations with DG as measured by the South Oaks Gambling Screen (r = .72; Slutske et al., 2011). (For a more complete discussion of the rationale underlying the choice of the DG phenotype and comparisons with alternative approaches, see the online supplemental materials.)
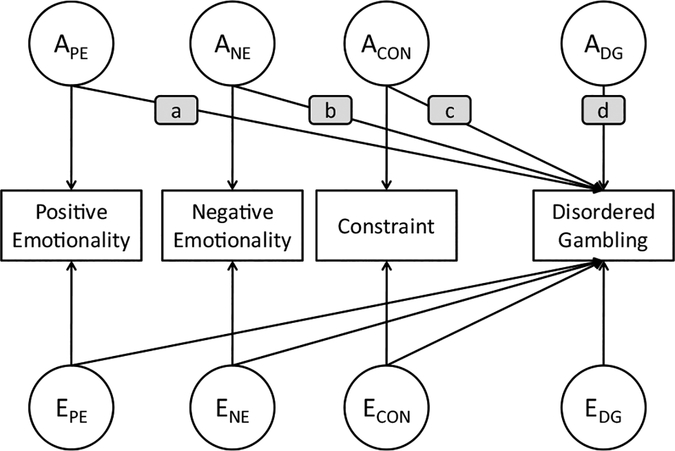
The multivariate biometric model is presented in Figure 1. In this model, there were separate sources of variation (additive genetic [APEM, ANEM, ACON] and nonshared environmental [EPEM, ENEM, ECON] influences) estimated for each of the three Big Three personality dimensions and for DG (ADG, EDG). In addition, there were paths from these two sources of variation for each of the Big Three personality dimensions to DG. These paths represent the contribution of genetic and environmental sources of individual differences in personality that also contribute to variation in DG risk. (Because the estimate of shared family environment was zero for the outcome of interest, DG risk, and nearly zero for all of the Big Three personality dimensions, their inclusion sometimes led to empirically underidentified models. Therefore, shared family environmental influences were not included in the bivariate or multivariate models.)
Figure 1.
Multivariate biometric model of Big Three personality dimensions and disordered gambling (shown for a single twin). Shaded boxes with a, b, and c indicate the paths leading from personality-related sources of genetic variation contributing to DG risk; the shaded box with the letter d represents the path leading from residual sources of genetic variation contributing to DG risk (PE = Positive Emotionality; NE = Negative Emotionality; CON = Constraint; DG = disordered gambling; A = additive genetic influences; E = unique (nonshared) environmental influences).
Bivariate models were fit to each of the Big Three personality dimensions (considered in isolation) and DG. The purpose of these models was to estimate: (a) the magnitude of the genetic correlation between the personality trait and DG, and (b) the proportion of genetic variation in DG risk that was explained by the personality trait. A multivariate model including all three of the Big Three personality traits and DG was fit to estimate the proportion of the genetic variation in DG risk that was explained by all three of the Big Three personality dimensions considered in aggregate. Using the path labels in Figure 1, this was estimated as (a2 + b2 + c2) ÷ (a2 + b2 + c2 + d2).
Results
All three Big Three personality dimensions were significantly associated with DG risk. The correlation of negative emotionality and DG risk was moderate, and the correlations of positive emotionality and constraint with DG risk were modest (see Table 1). Although the lower-order dimensions loading on a given Big Three higher-order dimension were all correlated with each other by design, they evidenced differential associations with DG risk. Of the four positive emotionality lower-order dimensions, two were negatively associated (well being and social closeness) and one was positively associated (social potency) with DG risk, (although all of the associations were modest). All three negative emotionality lower-order personality dimensions were positively associated with DG risk. Of the three constraint lower-order dimensions, only two were significantly (negatively) associated with DG risk (self-control and harm avoidance).
Table 1.
Within- and Cross-Twin Correlations Between Big Three Higher-Order and Lower-Order Personality Traits and Disordered Gambling Risk
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Full sample | Cross-twin | Cross-twin | Cross-twin | |||||
| Within-twin | Within-twin | MZ | DZ | Within-twin | MZ | DZ | OSDZ | |
| Big 3 higher-order personality dimensions | ||||||||
| Positive emotionality | −.06 | −.05 | .03 | .00 | −.07 | −.09 | .02 | −.02 |
| Negative emotionality | .32 | .31 | .18 | .14 | .32 | .28 | .16 | .12 |
| Constraint | −.11 | −.06 | .07 | .03 | −.15 | −.14 | −.03 | −.06 |
| Lower-order dimensions of positive emotionality | ||||||||
| Well being | −.08 | −.04 | −.03 | .05 | −.13 | −.11 | .06 | .03 |
| Social potency | .06 | .05 | .08 | .06 | .08 | .08 | .06 | .03 |
| Achievement | −.05 | −.09 | .06 | −.01 | .00 | .05 | .06 | −.04 |
| Social Closeness | −.10 | −.05 | .02 | −.10 | −.15 | −.26 | −.12 | −.05 |
| Lower-order dimensions of negative emotionality | ||||||||
| Stress reaction | .26 | .26 | .15 | .01 | .26 | .14 | .15 | .08 |
| Alienation | .24 | .24 | .17 | .19 | .24 | .29 | .13 | .10 |
| Aggression | .24 | .20 | .08 | .13 | .25 | .23 | .07 | .03 |
| Lower-order dimensions of constraint | ||||||||
| Self-control | −.17 | −.13 | −.02 | .01 | −.20 | −.26 | −.05 | −.09 |
| Harm avoidance | −.07 | −.01 | .04 | .08 | −.10 | −.13 | .08 | −.03 |
| Traditionalism | .00 | .03 | .17 | −.02 | −.03 | .07 | −.06 | .03 |
Note. Cell entries are biserial correlations, underlined correlations are significantly different from zero at p < .05. MZ = monozygotic; DZ = dizygotic; OSDZ = opposite-sex dizygotic.
The within-twin correlation provides a theoretical upper bound on the cross-trait cross-twin correlation between a given personality dimension and DG risk because the association across twins should not exceed the association within an individual. Thus, it was not surprising that the cross-twin correlations were generally modest (see Table 1). A comparison of the MZ cross-trait cross-twin correlation to the corresponding within-twin association provides an indication of the extent to which the phenotypic association between a given personality dimension and DG risk is because of familial factors. For example, the within-twin and MZ cross-twin correlations among women between negative emotionality and DG risk of 0.32 and 0.28, respectively, suggests that familial factors common to negative emotionality and DG risk account for a large portion of their association, and the DZ cross-twin correlation of 0.16 suggests that most of this association is explained by overlapping genetic influences.
Bivariate model-fitting has low power to estimate a genetic (or unique environmental) correlation when the within-twin correlations are small. Therefore, the focus of the remaining analyses were the Big Three higher-order personality dimensions and the four lower-order dimensions that evidenced meaningful within-twin associations with DG risk (r > |.15|), that is, stress reaction, alienation, aggression, and self-control. Bivariate models were fit to the seven MPQ scales (considered in isolation) and DG for the purpose of estimating the genetic and unique environmental correlations between the personality trait and DG risk (see Table 2). Genes contributing to variation in only one of the Big Three personality dimensions, negative emotionality, were significantly associated with genes contributing to variation in DG risk. There were also significant genetic correlations for all four of the lower-order personality dimensions and DG risk, with relatively large genetic correlations between the traits of alienation and aggression and DG risk. The genetic correlations were generally larger among women than among men; there were substantial differences between men and women for the Big Three higher-order factor of constraint and its lower-order component of self-control. There were unique environmental correlations for three personality dimensions (negative emotionality, stress reaction, and self-control) and DG risk that were significant only among men.
Table 2.
Genetic and Unique Environmental Correlations Between Big Three Higher-Order and Selected Lower-Order Personality Traits and Disordered Gambling Risk
| Mena | Womena | Combinedb | ||||
|---|---|---|---|---|---|---|
| rG | rE | rG | rE | rG | rE | |
| Big Three higher-order personality dimensions | ||||||
| Positive emotionality | .05 [−.16, .26] | .07 [−.27, .13] | .13 [−.35, .09] | −.06 [−.27, .15] | −.05 [−.20, .11] | −.07 [−.21, .07] |
| Negative emotionality | .44 [.22, .67] | .21 [.02, .39] | .57 [.37, .76] | .03 [−.16, .22] | .52 [.38, .66] | .15 [.02, .28] |
| Constraint | .15 [−.12, .42] | −.21 [−.42, .01] | −.25 [−.46, −.04] | −.10 [−.29, .10] | −.12 [−.28, .04] | −.10 [−.24, .04] |
| Selected lower-order personality dimensions | ||||||
| Stress reaction | .30 [.07, .53] | .25 [.07, .43] | .34 [.10, .57] | .18 [−.01, .37] | .34 [.18, .50] | .21 [.08, .33] |
| Alienation | .46 [.24, .68] | .08 [−.10, .26] | .58 [.38, .77] | −.13 [−.30, .05] | .53 [.39, .67] | .01 [−.12, .12] |
| Aggression | .26 [−.02, .54] | .12 [−.06, .31] | .54 [.32, .77] | .02 [−.15, .19] | .46 [.28, .65] | .11 [−.01, .24] |
| Self-control | −.03 [−.27, .22] | −.20 [−.39, −.04] | −.45 [−.68, −.21] | −.04 [−.22, .14] | −.29 [−.46, −.13] | −.09 [−.21, .04] |
Note. Underlined correlations are significantly greater than zero at p < .05; numbers in brackets are 95% confidence intervals.
Includes same-sex twin pairs.
includes same- and unlike-sex twin pairs.
The bivariate models also yielded information about the proportion of genetic variation in DG risk that was explained by genetic variation in each personality trait (considered in isolation and in aggregate). As expected from the magnitude of the genetic correlations, the largest contributor was negative emotionality. Two different multivariate models were examined to estimate the joint effect of personality-related genetic variation on genetic liability to DG. One model incorporated the Big Three personality dimensions of positive emotionality, negative emotionality, and constraint (as depicted in Figure 1). Because these three higherorder dimensions were designed to be orthogonal (Tellegen & Waller, 2008) there was no need to account for correlations between them. The other model incorporated the four lower-order personality dimensions of stress reaction, alienation, aggression, and self-control. Because the first three of these scales were lower-order components of negative emotionality they were correlated by design. Therefore, additional paths between the corresponding latent genetic and environmental factors and stress reaction, alienation, and aggression were added to account for this (e.g., from ASR and ESR to the manifest indicators for alienation and aggression). Genetic variation in the Big Three personality dimensions explained 29% of the genetic liability for DG (22% among men, 40% among women), and genetic variation in the four lower-order dimensions explained 41% of the genetic liability for DG (22% among men, 62% among women). The proportion of genetic liability for DG explained by personality-related genetic variation was substantially larger among women than among men. (More details of the results of fitting these models are included in the online supplemental materials.)
Discussion
In a large community-based twin study we examined the extent to which genetic variation in the Big Three personality dimensions and their lower-order components explained genetic variation in the risk for DG. Genetic influences contributing to individual differences in normal-range personality traits explained over 40% of the genetic risk for DG, with the largest and most robust contributions from the higher-order personality dimension of negative emotionality and its two lower-order dimensions of alienation and aggression. Surprisingly, the higher-order dimension of constraint was associated with the genetic risk for DG only among women. Of the lower-order components of constraint, the strongest genetic association was with low self-control. Low harm avoidance did not explain genetic risk for DG in either sex.
The genetic overlap between negative emotionality and DG risk is consistent with and brings together two lines of research demonstrating significant genetic associations between DG and major depression (Potenza et al., 2005) and significant phenotypic associations between DG and the Big Three personality dimension of negative emotionality (Slutske et al., 2005) and the Big Five personality dimension of neuroticism (Bagby et al., 2007). Given the prominence of the personality traits of impulsivity and risk-taking/sensation-seeking in theories of the development of DG (Blaszczynski & Nower, 2002; Sharpe, 2002) and addictions in general (e.g., Kreek et al., 2005), we expected to find even stronger evidence that genetic influences on the Big Three higher-order personality dimension of constraint and two of its lower-order dimensions of self-control and harm avoidance (reversed measures of impulsivity and risk-taking/sensation-seeking, respectively) would account for a substantial portion of the genetic variation in risk for DG. Although there was modest evidence for an important role of the Big Three higher-order dimension of constraint in explaining a substantial portion of the genetic variation in DG risk overall, this finding was qualified by a significant sex interaction in the strength of the associations. That is, the genetic overlap between constraint and its lower-order dimension of self-control was larger among women than among men. The most striking example was the genetic correlation between self-control and DG risk, which was −0.45 among women and essentially nil among men. Although the association between self-control and DG risk were similar between men and women at the phenotypic level, this association was largely attributable to common genes among women but entirely because of common unique environments among men. This surprising result requires replication.
Failure to find a substantial association between harm avoidance and DG in the present study differs from a previous study (based on the same personality assessment) that obtained significant prospective associations of DG at age 21 with both low self-control and low harm avoidance measured at age 18 (Slutske et al., 2005), but is consistent with a previous cross-sectional study of adults that found significant associations of DG with impulsiveness, but not with excitement-seeking (Bagby et al., 2007). It will be important to further explore the genetic associations between DG and self-control and between DG and risk-taking because this has major implications for theories of the etiology of DG. For example, some theorists posit that these two personality constructs have distinct neural underpinnings (e.g., Steinberg, 2010).
The interpretation of the cross-sectional genetic associations reported here is ambiguous because there are several plausible interpretations. A significant genetic correlation between a measure of personality, say negative emotionality, and DG risk might arise because (a) genetically influenced negative emotionality constitutes part of the vulnerability to develop DG, (b) genetically influenced DG may change one’s relative level on the trait of negative emotionality, or (c) negative emotionality and DG have common genetic underpinnings (pleiotropy); it is quite possible that all three interpretations are correct. The first interpretation would be consistent with individual differences in negative emotionality being an intermediate phenotype for DG, whereas the other two would not. A recent study showing that indicators of low self-control and high negative emotionality that are observable as early as 3 years of age are predictive of DG in adulthood (Slutske et al., 2012) suggests that a combination of low behavioral and emotional control may be a genetically influenced vulnerability factor to develop DG, or that there are genes that are associated with both individual differences in behavioral and emotional control in childhood and the later risk for DG in adulthood.
Most of the multivariate twin studies of DG and associated psychopathology have considered the relation between DG and each psychiatric disorder in isolation, without taking into account the possibility that each bivariate relation may be describing the same sources of genetic covariation. The only exception comes from an analysis of the all-male Vietnam Era Twin Registry study dataset demonstrating that the genetic variation in the risk for alcohol dependence did not explain any additional genetic variation in DG risk after genetic variation in child and adult antisocial behavior disorders were included in the model (Slutske et al., 2001). In other words, they were all accounting for the same genetic sources of overlap. Altogether, 28% of the variation in DG risk among men was explained by genetic variation in the risk for these other co-occurring behavioral disorders (Slutske et al., 2001). The use of a comprehensive personality assessment in the present study allowed for broader coverage in potentially explaining genetic variation in DG risk. Using two different strategies for selecting personality traits as predictors of DG risk (the three Big Three dimensions or the top four lower-order dimensions), we were able to account for 30–40% of the genetic variation in DG risk, with a substantially larger amount of genetic variation explained by genetic differences in personality among women (40–60%) than among men (~20% using both strategies).
Limitations
This study has at least four limitations. First, it is unclear how the results of this Australian twin study will generalize to other cultures. Second, the age range of the sample was relatively narrow (32–43 years). The extent to which these results can be generalized to other age groups such as adolescents and older individuals remains an unanswered question. Third, a broad DG phenotype was used to have adequate numbers of affected twins to fit multivariate and sex-limitation models. Previous analyses of these data (and new analyses reported in the online supplemental materials) supports the assumption of the liability threshold model (Slutske et al., 2010; Slutske et al., 2011) and suggests that the results apply to a narrower pathological gambling disorder phenotype as well as the broader DG phenotype that was used in this article. Fourth, we did not explore potential DG subtypes (e.g., Blaszczynski & Nower, 2002) that might have distinct personality correlates. This remains an important direction for future research.
Summary and Conclusions
Despite limitations, this study represents an important step forward. It is the largest community-based study of the personality correlates of DG among men and women, and the first genetically informed investigation of personality and DG. Overall, genetic influences contributing to individual differences in normal-range personality traits explained over 40% of the genetic risk for DG, with a larger contribution among women (62%) than among men (22%). The largest and most robust contributions of genetic risk for DG among both men and women came from the higher-order personality dimension of negative emotionality and its two lower-order dimensions of alienation and aggression. Surprisingly, low self-control was associated with the genetic risk for DG only among women, and risk-taking/sensation-seeking did not explain genetic risk for DG in either sex.
One implication of these results is that some of the genes associated with DG risk will also be genes associated with variation in normal-range personality characteristics, particularly the traits of alienation(i.e., feeling mistreated, victimized, and betrayed) and aggression (i.e., hurting others for one’s own advantage, and being willing to frighten and cause discomfort to others). Another implication is that the causes of the comorbidity between DG and the externalizing and internalizing psychiatric disorders may in part be explained by the effects of genes that also contribute to variation in personality. Because this study was based on a comprehensive inventory of personality, the results suggest that there is a substantial portion of the genetic variation in DG that cannot be explained by individual differences in normal-range personality. Finally, this study provides provocative new evidence consistent with sex differences in the etiology of DG. This novel and unanticipated finding awaits replication.
Supplementary Material
Acknowledgments
Supported by National Institutes of Health Grant MH66206.
Footnotes
The term “disordered gambling” was coined to describe the full spectrum of gambling-related problems, including pathological gambling as defined by the Diagnostic and Statistical Manual of Mental Disorders as well as subclinical problems (Shaffer, Hall, & Vander Bilt, 1999). This is the definition of disordered gambling that has been used in this report.
Supplemental materials: http://dx.doi.org/10.1037/a0029999.supp
Contributor Information
Wendy S. Slutske, Department of Psychology, University of Missouri;
Seung Bin Cho, Department of Psychology, University of Missouri;.
Thomas M. Piasecki, Department of Psychology, University of Missouri;
Nicholas G. Martin, Genetic Epidemiology Laboratory, Queensland Institute of Medical Research, Brisbane, Australia.
References
- Bagby RM, Vachon DD, Bulmash EL, Toneatto T, Quilty LC, & Costa PT (2007). Pathological gambling and the five-factor model of personality. Personality and Individual Differences, 43, 873–880. doi: 10.1016/j.paid.2007.02.011 [DOI] [Google Scholar]
- Blaszczynski A, & Nower L (2002). A pathways model of problem and pathological gambling. Addiction, 97, 487–499. doi: 10.1046/j.1360-0443.2002.00015.x [DOI] [PubMed] [Google Scholar]
- Gerstein D, Murphy S, Toce M, Hoffmann J, Palmer A, Johnson R, … Sinclair S (1999). Gambling Impact and Behavior Study: Report to the National Gambling Impact Study Commission. Chicago: National Opinion Research Center. [Google Scholar]
- Gottesman II, & Gould TD (2003). The endophenotype concept in psychiatry: Etymology and strategic intentions. The American Journal of Psychiatry, 160, 636–645. doi: 10.1176/appi.ajp.160.4.636 [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Nielsen DA, Butelman ER, & LaForge KS (2005). Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nature Neuroscience, 8, 1450–1457. doi: 10.1038/nn1583 [DOI] [PubMed] [Google Scholar]
- Muthén LK, & Muthén B (2004). Mplus User’s Guide. Los Angeles, CA: Author. [Google Scholar]
- Potenza MN, Xian H, Shah K, Scherrer JF, & Eisen SA (2005). Shared genetic contributions to pathological gambling and major depression in men. Archives of General Psychiatry, 62, 1015–1021. doi: 10.1001/archpsyc.62.9.1015 [DOI] [PubMed] [Google Scholar]
- Shaffer HJ, Hall MN, & Vander Bilt J (1999). Estimating the prevalence of disordered gambling behavior in the United States and Canada: A research synthesis. American Journal of Public Health, 89, 1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe L (2002). A reformulated cognitive-behavioral model of problem gambling: Biopsychosocial perspective. Clinical Psychology Review, 22, 1–25. doi: 10.1016/S0272-7358(00)00087-8 [DOI] [PubMed] [Google Scholar]
- Slutske WS, Caspi A, Moffitt TE, & Poulton R (2005). Personality and problem gambling: A prospective study of a birth cohort of young adults. Archives of General Psychiatry, 62, 769–775. doi: 10.1001/archpsyc.62.7.769 [DOI] [PubMed] [Google Scholar]
- Slutske WS, Eisen SA, True WR, Lyons MJ, Goldberg J, & Tsuang MT (2000). Common genetic vulnerability for pathological gambling and alcohol dependence in men. Archives of General Psychiatry, 57, 666–673. doi: 10.1001/archpsyc.57.7.666 [DOI] [PubMed] [Google Scholar]
- Slutske WS, Eisen SA, Xian H, True WR, Lyons MJ, Goldberg J, & Tsuang MT (2001). A twin study of the association between pathological gambling and antisocial personality disorder. Journal of Abnormal Psychology, 110, 297–308. doi: 10.1037/0021-843X.110.2.297 [DOI] [PubMed] [Google Scholar]
- Slutske WS, Meier MH, Zhu G, Statham DJ, Blaszczynski A, & Martin NG (2009). The Australian twin study of gambling (OZ-GAM): Rationale, sample description, predictors of participation, and a first look at sources of individual differences in gambling involvement. Twin Research and Human Genetics, 12, 63–78. doi: 10.1375/twin.12.1.63 [DOI] [PubMed] [Google Scholar]
- Slutske WS, Moffitt TE, Poulton R, & Caspi A (2012). Behavioral observations of under-control at age 3 predict disordered gambling at age 32: A longitudinal study of a complete birth cohort. Psychological Science, 23, 510–516. doi: 10.1177/0956797611429708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS, Zhu G, Meier MH, & Martin NG (2010). Genetic and environmental influences on disordered gambling in men and women. Archives of General Psychiatry, 67, 624–630. doi: 10.1001/archgenpsychiatry.2010.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutske WS, Zhu G, Meier MH, & Martin NG (2011). Disordered gambling as defined by the DSM-IV and the South Oaks Gambling Screen: Evidence for a common etiologic structure. Journal of Abnormal Psychology, 120, 743–751. doi: 10.1037/a0022879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L (2010). A dual systems model of adolescent risk-taking. Developmental Psychobiology, 52, 216–224. [DOI] [PubMed] [Google Scholar]
- Tellegen A, & Waller NG (2008). Exploring personality through test construction: Development of the Multidimensional Personality Questionnaire In Boyle GJ, Matthews G, & Saklofske DH (Eds.), The SAGE handbook of personality theory and assessment: Personality measurement and testing (Vol. 2, pp. 261–292). Thousand Oaks, CA: Sage Publications. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.