Supplemental Digital Content is available in the text.
Keywords: coronavirus disease 2019, immunothrombosis, intravenous immunoglobulin, pneumonia
Objectives:
Dysregulated neutrophil and platelet interactions mediate immunothrombosis and cause lung injury in coronavirus disease 2019. IV immunoglobulin modulates neutrophil activation through FcγRIII binding. We hypothesized that early therapy with IV immunoglobulin would abrogate immunothrombosis and improve oxygenation and reduce progression to mechanical ventilation in coronavirus disease 2019 pneumonia.
Design:
Prospective randomized open label.
Setting:
Inpatient hospital.
Patients and Intervention:
Hypoxic subjects with coronavirus disease 2019 pneumonia were randomized 1:1 to receive standard of care plus IV immunoglobulin 0.5 g/kg/d with methylprednisolone 40 mg 30 minutes before infusion for 3 days versus standard of care alone.
Main Results:
Sixteen subjects received IV immunoglobulin and 17 standard of care. Median ages were 51 and 58 years for standard of care and IV immunoglobulin, respectively. Acute Physiology and Chronic Health Evaluation II and Charlson comorbidity scores were similar for IV immunoglobulin and standard of care. Seven standard of care versus two IV immunoglobulin subjects required mechanical ventilation (p = 0.12, Fisher exact test). Among subjects with A-a gradient of greater than 200 mm Hg at enrollment, the IV immunoglobulin group showed: 1) a lower rate of progression to requiring mechanical ventilation (2/14 vs 7/12, p = 0.038 Fisher exact test), 2) shorter median hospital length of stay (11 vs 19 d, p = 0.01 Mann Whitney U test), 3) shorter median ICU stay (2.5 vs 12.5 d, p = 0.006 Mann Whitey U test), and 4) greater improvement in Pao2/Fio2 at 7 days (median [range] change from time of enrollment +131 [+35 to +330] vs +44·5 [–115 to +157], p = 0.01, Mann Whitney U test) than standard of care. Pao2/Fio2 improvement at day 7 was significantly less for the standard of care patients who received glucocorticoid therapy than those in the IV immunoglobulin arm (p = 0.0057, Mann Whiney U test).
Conclusions:
This pilot study showed that IV immunoglobulin significantly improved hypoxia and reduced hospital length of stay and progression to mechanical ventilation in coronavirus disease 2019 patients with A-a gradient greater than 200 mm Hg. A phase 3 multicenter randomized double-blinded clinical trial is under way to validate these findings.
Coronavirus disease 2019 (COVID-19) infection, as is common with many types of viral and atypical infections, is characterized by a biphasic illness of a relatively mild protean phase driven by viral replication, and second phase, driven by the immune response. The second phase may lead to potentially catastrophic disease manifestations requiring hospitalization and high-level medical care characterized by acute respiratory distress syndrome (ARDS), vasculitis with thrombotic complications, and multiorgan involvement (1–4). Therefore, from a pathophysiological standpoint, clinically meaningful therapies for COVID-19 will likely emerge from immunomodulation.
IV immunoglobulin (IVIG) has been found to have broad therapeutic applications for the treatment of a variety of inflammatory, infectious, autoimmune, and viral diseases including Kawasaki disease and may modulate the immune response via multiple mechanisms including blocking a wide array of proinflammatory cytokines that potentially lead to severe inflammatory responses as well as Fc-gamma receptor binding of activated macrophages (5). There are published reports retrospectively showing potential benefit of IVIG treatment for COVID-19 ARDS in adults and the associated Kawasaki-like illness in children (6–11).
It has been recently demonstrated that the end-organ damage seen in severe cases of COVID-19 infection, including severe lung injury, may be mediated by microvascular “immmunothrombosis” driven by activated neutrophil and platelet interactions, including neutrophil extracellular trap (NET) formation (12). It has been previously demonstrated that IVIG may attenuate neutrophil activation and NET formation, thereby mitigating vascular injury by these mechanisms (13–15). This is the first study to evaluate prospectively the addition of IVIG to otherwise standard treatment for adults with moderate-to-severe hypoxemia secondary to COVID-19.
MATERIALS AND METHODS
Study Design
This was an open-label randomized controlled trial performed at two hospital centers: Sharp Memorial Hospital (San Diego, CA) and Sharp Grossmont Hospital (La Mesa, CA). The research protocol was approved by the Internal Review Board of Sharp Healthcare (protocol number 2004192, April 23, 2020) prior to patient enrollment and was registered on clinicaltrials.gov (April 28, 2020; NCT04411667).
Study Population
Adult patients greater than 18 years of age hospitalized with COVID-19 infection confirmed by positive polymerase chain reaction testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) genome in nasopharyngeal or oropharyngeal swab sample were considered for inclusion if they demonstrated moderate-to-severe hypoxia (sPo2 ≤ 96% on ≥ 4 L O2 by nasal cannula) but not on mechanical ventilation. Patients were considered for enrollment when treating hospitalists and/or intensivists notified the study team for consideration.
Randomization and Treatments
After informed consent was obtained electronically by DocuSign (San Francisco, CA), subjects were randomized 1:1 into treatment arm or standard of care (SOC) control arm. Author M.G., the randomizing investigator, used a web-based computer-generated randomization (A study [IVIG] group, B control [SOC] group) in blocks of 10 (https://www.sealedenvelope.com/simple-randomiser/v1/lists). When the randomization list was generated, M.G. placed the codes into individual sealed and sequentially numbered envelopes. The batch of sealed envelopes was stacked in sequential order and retained in a locked drawer in the Investigational Research Pharmacy. After informed consent was obtained from a potential study subject, M.G. obtained the next numbered envelope (i.e., #001, #002, #003, etc.) to obtain the A, B randomization code for treatment arm allocation. SOC consisted of the subject remaining on or being eligible for any treatment (e.g., glucocorticoids, convalescent plasma, and remdesivir) not part of a randomized clinical trial. The IVIG treatment arm consisted of the subject receiving IVIG (Octagam 10% provided by Octapharma USA) 0.5-g/kg IV daily for 3 days beginning on the day of enrollment, in addition to SOC. For subjects not already receiving glucocorticoid therapy, enrolled treatment arm subjects received methylprednisolone 40-mg IV once 30 minutes before IVIG to mitigate headache commonly experienced after IVIG therapy. Enrollment in other clinical trials and the use of off-label agents (e.g., tocilizumab) was not allowed, whereas the subject was enrolled and monitored for progression to the endpoint of: 1) respiratory failure requiring receipt of mechanical ventilation (a composite of either receiving ventilation or the subject status changed to a do not resuscitate/do not intubate resulting in progressive respiratory failure and death) or 2) death from nonrespiratory causes prior to receipt of mechanical ventilation. If the subject progressed to mechanical ventilation, receipt of off-label agents and/or enrollment in other clinical trials was allowed. Subject hospital course was followed until hospital discharge for the purpose of total and ICU days of hospital days.
Clinical Data Extraction and Analysis
Relevant clinical and laboratory information was captured to allow for group comparisons, including the calculation of Charlson comorbidity index and Acute Physiology and Chronic Health Evaluation (APACHE) II acute illness severity score. The alveolar-arterial (A-a) gradient was calculated for each subject at the time of enrollment based on arterial blood gases when available or based on Pao2 extrapolated from Spo2 and fraction of inspired oxygen (Fio2). Conversion of Sao2 to Pao2 as well as Fio2 from oxygen therapy was estimated from accepted conversion table available through web-based resources (https://www.intensive.org/epic2/Documents/Estimation%20of%20PO2%20and%20FiO2.pdf). Interleukin (IL)-6 was measured from blood 24–48 hours after enrollment (https://ltd.aruplab.com/Tests/Pub/0051537).
Statistical Analysis
The initial goal of the pilot study was to enroll 20 patients (10 per arm). Based on the initial open-label results of this study in May 2020, Octapharma USA, the supplier of Octagam 10% decided to seek guidance from the U.S. FDA for a Phase 3 examining Octagam 10% for use on COVID-19 respiratory disease. After 20 patients had reached the composite ventilation endpoint, death, or discharged from the hospital, the data were reviewed by a data safety monitoring board (DSMB) consisting of two hospitalist physicians, one critical care physician, and one pharmacist/statistician. The DSMB voted to continue the study until phase 3 randomized placebo-controlled multicenter study of IVIG in COVID-19 (Octagam 10% therapy in COVID-19 patients with severe disease; clinicaltrials.gov, NCT04400058, May 22, 2020) became available. Statistical differences in rates of receipt of mechanical ventilation and other categorical or ordinal variables were calculated using Fisher exact test, and differences in continuous variables were calculated using Mann Whitney U test.
RESULTS
Baseline Patient Demographics and Clinical Characteristics
Thirty-four patients were randomized into the study, as shown in Supplemental Figure 1 (http://links.lww.com/CCX/A422) (17 SOC, 17 IVIG). Immediately after randomization and notification of the principal investigator, one subject was immediately deemed unevaluable by the principal investigator and excluded due to a high risk of bacterial superinfection (elevated absolute neutrophil count of 9,900/mm3 and procalcitonin of 1.45 ng/mL) (1). The subjects were well matched with respect to age, underlying comorbidities, concomitant therapies (Table 1), and laboratory characteristics (Table 2). One IVIG and four SOC subjects had normal d-dimer concentrations at enrollment. Median (range) d-dimer concentrations at the time of enrollment were 827 (386–7028) and 691 (283–1657) for IVIG and SOC, respectively (normal < 500 ng/mL).
TABLE 1.
Enrolled Patient Demographics, Characteristics, and Coronavirus Disease 2019 Therapies
| Characteristic | IV Immunoglobulin (n = 16) | Standard of Care (n = 17) |
|---|---|---|
| Mean age (yr) | 54 | 54 |
| Median age (yr) | 58 | 51 |
| Male, n (%) | 10 (63) | 10 (59) |
| Ethnicity, n (%) | ||
| Hispanic/Latino | 13 (81) | 15 (88) |
| White | 3 (19) | 2 (12) |
| Mean body mass index | 32.8 | 34.8 |
| Comorbidities | ||
| Diabetes mellitus, n (%) | 6 (38) | 6 (35) |
| Mean HgbA1c, % | 10.1 | 6.4 |
| Hypertension, n (%) | 4 (25) | 7 (41) |
| Chronic kidney disease, n (%) | 0 (0) | 1 (6) |
| Coronary artery disease, n (%) | 1 (6) | 1 (6) |
| Congestive heart failure, n (%) | 1 (6) | 1 (6) |
| Asthma/chronic obstructive pulmonary disease, n (%) | 2 (12) | 2 (12) |
| Current smoker, n (%) | 1 (6) | 1 (6) |
| Former smoker, n (%) | 2 (12) | 1 (6) |
| Immunocompromised, n (%) | 1 (6) | 0 (0) |
| Other coronavirus disease 2019 therapies, n (%) | ||
| Remdesivir | 8 (50) | 9 (53) |
| Convalescent plasma | 2 (12) | 3 (18) |
| Glucocorticoids | 16 (100) | 10 (59) |
aAll IV Immunoglobulin patients received at least methylprednisolone 40 mg IV 30–60 min before each dose (3 d) per protocol.
TABLE 2.
Mean Values of Relevant Laboratory Data for Study Groups
| Laboratory Value | IV Immunoglobulin (n = 16) | Standard of Care (n = 17) |
|---|---|---|
| WBC (× 1,000/mm3) | 7.9 | 8.3 |
| Absolute lymphocyte count | 0.8 | 1.0 |
| Platelet (× 1,000/mm3) | 256 | 247 |
| Hemoglobin (g/dL) | 13.0 | 13.4 |
| Creatinine (mg/dL) | 0.78 | 0.85 |
| C-reactive protein (mg/L) | 142 | 140 |
| Procalcitonin(ng/mL) | 0.25 | 0.25 |
| Ferritin (ng/mL) | 990 | 1,014 |
| d-dimer (ng/mL) | 1,456 | 758 |
Clinical Outcomes
Median Charlson index was 2 for both groups. Median APACHE II score was 7 for SOC and 7.5 the IVIG study group. Figure 1 demonstrates the subjects in the control SOC and treatment IVIG group that developed a need for mechanical ventilation after study enrollment, denoted as the red data points.
Figure 1.
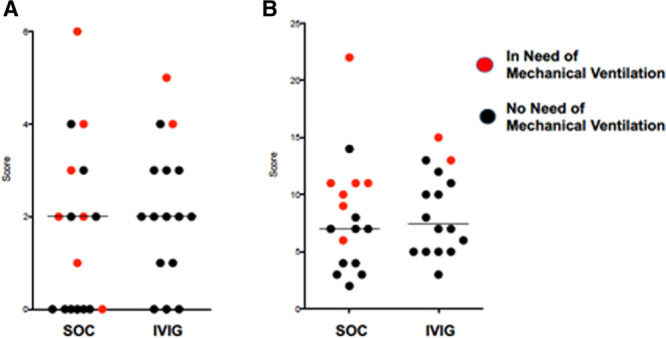
Distribution of (A) Charlson comorbidity index and (B) Acute Physiology and Chronic Health Evaluation II scores of enrolled study subjects in both treatment arms, showing even distribution of chronic illness and acute severity of illness. Horizontal bars denote median values. Red points indicate patients who ultimately required a need for mechanical ventilation. IVIG = IV immunoglobulin, SOC = standard of care.
Among the entire enrolled and evaluated subjects in each arm, two patients in the IVIG arm and seven patients in the control SOC arm developed a need for mechanical ventilation. Of these SOC subjects, six received ventilation and one was made a “do not intubate” and the patient expired within 24 hours. The difference in the receipt of mechanical ventilation was not statistically significant between the two groups (p = 0.12, Fisher exact test). Among subjects whose respiratory failure progressed to the need for mechanical ventilation, one of two IVIG subjects and two of seven control subjects also received concomitant convalescent plasma. Concomitant glucocorticoid therapy was given to five of the seven control subjects who progressed to mechanical ventilation. Remdesivir was given to one of two IVIG patients and three of seven SOC subjects that required mechanical ventilation.
ICU and total length of stay were numerically shorter in the IVIG group than the SOC group but did not achieve statistical significance. Median (range) of ICU stays were 1 day (range, 0–16 d) for IVIG and 9 days (range, 0–45 d) for SOC (p = 0.08, Mann Whitney U test). Median (range) of total hospital stay was 12 days (range, 6–29 d) for IVIG and 17 days (range, 4–60 d) for SOC (p = 0.48, Mann Whitney U test).
Post Hoc Analysis of Subjects Stratified by Hypoxia
After the phase 3 study (Octagam 10% therapy in COVID-19 patients with severe disease; clinicaltrials.gov, NCT04400058) was open for enrollment and this study was closed, a post hoc analysis was performed examining the respiratory morbidity (receipt of mechanical ventilation and oxygenation at 7 d postenrollment) based on alveolar-arterial (A-a) gradients. A-a gradients were calculated directly from arterial blood gas (9/17 SOC subjects, 6/16 IVIG subjects) or estimated based on Sao2 and Fio2 measurement for the rest. Based on an increase in APACHE II severity of illness scoring associated with achieving and A-a gradient of greater than 200 mm Hg (+2 points), subjects were stratified into those with A-a gradient less than or equal to 200 mm Hg or greater than 200 mm Hg at enrollment, corresponding to a Pao2/Fio2 of greater than 140, or the approximate requirement of 6 L O2 by nasal cannula for a Sao2 of less than or equal to 92%. As shown in Figure 2A, none of the seven subjects (five SOC, two IVIG) with A-a gradient less than 200 mm Hg progressed to mechanical ventilation. For subjects with A-a gradient of greater than 200 mm Hg, the progression to mechanical ventilation was 7/12 (58%) in the SOC control arm versus 2/14 (14%) in the IVIG, a difference that was statistically significant (p = 0.038, Fisher Exact test).
Figure 2.
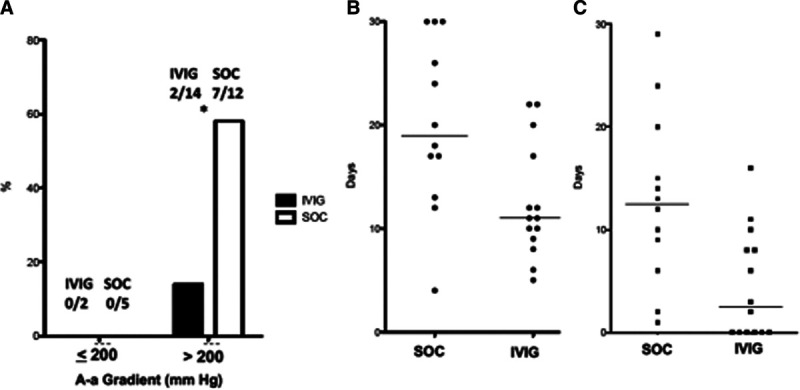
A, Rates of mechanical ventilation in study subjects stratified by alveolar-arterial (A-a) gradient. Among patients with A-a gradient > 200 mm Hg, receipt of IV immunoglobulin (IVIG) reduced rates of mechanical ventilation (*p = 0.038, Fisher exact test). B, Total length of hospital stay (d) among patients in standard of care (SOC) vs IVIG with A-a gradient > 200 mm Hg. Median stay (horizontal bar) SOC 19 d vs IVIG 11 d, p = 0.01 Mann Whitney U test. C, Length of ICU stay (d) among patients in SOC vs IVIG with A-a gradient > 200 mm Hg. Median (horizontal bar) stay SOC 12.5 d vs IVIG 2.5 d, p = 0.006 Mann Whitney U test.
Among the seven patients with A-a gradient less than or equal to 200 mm Hg, no patient required ICU stay during their illness and length of hospital stay ranged for 3–8 days. For the subjects with A-a gradient greater than 200, median length of hospital stay was 19 days (range, 4–30 d) and 11 days (range, 5–22 d) for the SOC and IVIG groups, respectively (p = 0.013, Mann Whitney U test) (Fig. 2B). Median ICU stay was 12.5 days (range, 1–29 d) and 2.5 days (range, 0–16 d) for SOC and IVIG, respectively (p = 0.006, Mann Whitney U test) (Fig. 2C). Total ventilator patient-days were 98 days for SOC (5.8 d/patient enrolled) and 23 days for the IVIG group (1.4 d/patient enrolled).
Improvement in oxygenation was evaluated by examining the Pao2/Fio2 ratio at the day of enrollment and 7 days after enrollment in individual subjects for SOC (Fig. 3A) and IVIG (Fig. 3B). Differences in day 7 Pao2/Fio2 minus enrollment Pao2/Fio2 are shown in Figure 3C for both groups, with negative numbers representing worsening in oxygenation. Among the entire subject population, median improvement Pao2/Fio2 was greater for IVIG [+153 (range, +35 to +330)] than SOC [+90 (range, –115 to +280)] but did not reach statistical significance (p = 0·057, Mann Whitney U test). However, when refocusing on the patients with A-a gradient greater than 200 at enrollment, median improvement of Pao2/Fio2 for IVIG was significantly greater than SOC, with median (range) of +131 (+35 to +330) versus +44·5 (–115 to +157) (p = 0·01, Mann Whitney U test).
Figure 3.
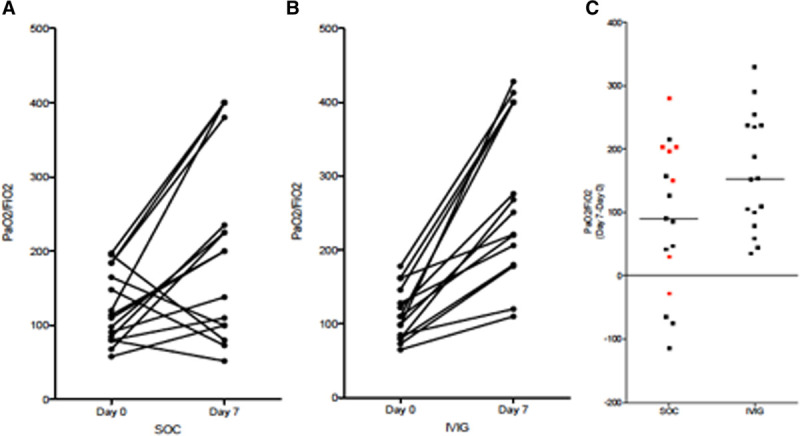
Individual patient progress of Pao2/Fio2 at day of enrollment (day 0) and 7 d later in control standard of care (SOC) group (A) and IV immunoglobulin (IVIG) group (B). Patients who were discharged and the one patient that died before day 7 had values placed from the last available day. C, The absolute differences for each group. Red data points denote patients who did not receive any glucocorticoid therapy. Differences in 7-d Pao2/Fio2 showed greater improvement oxygenation in IVIG-treated patients when compared with the entire SOC cohort (p = 0·057, Mann Whitney U test) but became significant after considering only those patients with alveolar-arterial gradient at enrollment of > 200 (p = 0.01, Mann Whitney U test) and when comparing IVIG vs only SOC patients who received glucocorticoid therapy (p = 0.025, Mann Whitney U test).
Effect of Glucocorticoids
After this study was designed and completed, data emerged demonstrating survival benefits of dexamethasone in hypoxic COVID-19 patients (16). Figure 3C denotes subjects in the SOC group who did not receive any glucocorticoid therapy as red data points (n = 7). The 10 SOC subjects receiving glucocorticoid therapy received them for a median of 11 days (range, 4–20 d), with daily dose ranging from 20 to 125 mg of methylprednisolone or equivalent. Of the remaining seven SOC subjects that did not receive glucocorticoids, four were in the low-risk group with A-a gradient less than or equal to 200. Of the remaining three subjects with A-a gradient greater than 200, two progressed to requiring mechanical ventilation, one of which was made do not intubate and died. In the IVIG group, seven of 16 subjects did not receive methylprednisolone premedication before IVIG, because they received glucocorticoid therapy as part of their standard treatment. The median duration as 9 days (range, 5–14 d) with daily dose ranges 20–125 mg methylprednisolone or equivalent.
Pao2/Fio2 changes between enrollment and day 7 of the 10 SOC patients who received glucocorticoid therapy were median +53 (range, –115 to +216), a difference that remained significantly lower than the IVIG group (p = 0.0057, Mann Whiney U test).
Interleukin-6
A subset of enrolled subjects had serum IL-6 concentrations sent out to a commercial laboratory (ARUP Labs, Salt Lake City, UT) on blood drawn 24–48 hour postenrollment. These would be reflective of having received no IVIG in the SOC control arm and one or two doses of IVIG in the IVIG treatment arm. Supplemental Figure 2 (http://links.lww.com/CCX/A423) shows significantly reduced IL-6 serum concentrations in the IVIG group (median, 5; range, 2–9 pg/mL) compared with the SOC group (median, 18; range, 3.6–141 pg/mL).
Adverse Events, Safety, and Tolerability
Three subjects (18%) in the SOC group and one subject (6%) in the IVIG group died. The death in the IVG group occurred after the patient developed a Staphylococcus aureus pneumonia and then Escherichia coli bacteremia 4 and 6 days after mechanical ventilation, respectively. The SOC deaths included one subject who developed progressive respiratory failure in the need of mechanical ventilation who was then made “do not intubate” and expired, one subject had care withdrawn after failing to make progress 20 days on mechanical ventilation, and one subject developed Pseudomonas aeruginosa and Enterobacter cloacae ventilator-associated pneumonia and died after being on mechanical ventilation for 17 days. All subjects in the IVIG study arm tolerated IVIG 3 doses without any adverse events being reported to the clinical study team.
DISCUSSION
Prior retrospective studies have shown that the use of IVIG in COVID-19 infection may improve clinical outcomes, including reduced length of stay and mortality (6–11). The benefit of IVIG appears to be more based on earlier administration than on dose (7). In this first study to evaluate IVIG in a randomized prospective format, 3 days of IVIG with methylprednisolone 40 mg reduced the rate of progression of respiratory failure requiring mechanical ventilation in COVID-19 patients (13% with IVIG vs 41% without IVIG, p = 0.12). Although this difference did not achieve statistical significance among the collective subject cohorts, a post hoc analysis of patients with A-a gradient greater than 200 mm Hg showed: 1) reduction in progression to mechanical ventilation with IVIG to be statistically significant (14% with IVIG vs 58% without IVIG, p = 0.038), 2) shorter of duration of length hospital stay, 3) ICU length of stay, and 4) improvement in oxygenation (Pao2/Fio2) at day 7.
Recent findings have shown that dysregulated neutrophils and platelets become activated and cooperate in driving “immunothrombosis” in tissue microvasculature, driving end-organ damage in severe COVID-19 infection (12). This includes formation of NETs, an immune defense that has been proposed to assist in entrapment of pathogens for immune-mediated clearance (12). However, endovascular NET formation induces vascular inflammation and may drive morbidity and mortality in infections like malaria, dengue fever, and now it appears in COVID-19 as well (12, 17, 18). Therefore, therapies that target immunothrombosis may be extremely useful in treating respiratory failure and vascular complications in COVID-19. Indeed, IVIG has been shown to modulate neutrophil activation and NET formation through IVIG activity on FcγRIII receptors on neutrophils (13, 19, 20). This effect was found to be mediated by Src homology 2-containing tyrosine phosphatase-1 (13). Therefore, a solid mechanism exists to explain, at least in part, the therapeutic effects of IVIG in COVID-19 observed in this study.
IVIG was well tolerated and the 3-day course was completed by all subjects. The hypercoaguability conferred by IVIG raised theoretical concerns when superimposed on the recently observed COVID-19 induced thrombotic events, but no cases of arterial or venous thrombosis associated with IVIG were identified in this study. This is particularly notable given that 15 of the 16 subjects in the IVIG treatment arm had elevated d-dimer concentrations at the time of enrollment, suggestive of some form of intravascular thrombosis and fibrinolysis consistent with the pathophysiology of more severe COVID-19 cases. Indeed, the mechanism of thrombotic events in COVID-19 and the effects of IVIG suggests that IVIG would mitigate rather than aggravate COVID-19 coagulopathy.
The importance of IL-6 in the cytokine release syndrome in COVID-19 has been suggested by the Th17 lymphocyte populations found in patients with alveolar injury (21). In this study, the subset of patients who had IL-6 concentrations measured within 24–48 hours of study enrollment showed significantly lower IL-6 levels among those who received IVIG compared with the SOC group. Although preenrollment IL-6 levels were not measured, reduction of IL-6 production by IVIG has been previously shown and further validates the potential role of IVIG in severe COVID-19 infection (22).
As a blood product derived from healthy donors, efficacy of IVIG may in fact improve over time as a higher percentage of the population of donors develops neutralizing antibodies to the SARS-CoV-2 virus. Convalescent plasma may offer some benefit in a subset of COVID-19 patients per a recent randomized study, so these benefits could theoretically be added to the immunomodulatory effects of IVIG as the donor pool of IVIG develops increased immunity to SARS-CoV-2 (23).
This study has some important limitations. First, this study was performed in two hospitals in one U.S. city, resulting in a fairly homogenous population of younger Hispanic/Latino patients where results may not automatically translate to other patient settings. Second, the study was not blinded and therefore subject to bias. This was reduced by the fact that the clinical investigators had minimal clinical decision-making on these patients, particularly regarding the endpoints of mechanical ventilation and discharge from the hospital. Third, the concomitant use of methylprednisolone therapy may have confounded the results. At the time of study initiation, the use of glucocorticoid therapy was controversial, believed to possibly causing harm in COVID-19. We decided to give methylprednisolone 40 mg (equivalent to approximately 7·5-mg dexamethasone) to mitigate any potential adverse effects of IVIG such as headache, theorizing that benefit would outweigh risk by increasing tolerability of IVIG. However, the benefit of glucocorticoid therapy was subsequently shown with 6 mg of dexamethasone for 10 days (16). However, our comparison of IVIG patients with the SOC patients who received glucocorticoids gives some evidence that benefits of IVIG treatment group were not due to premedication with methylprednisolone. Fourth, the sample size was small, which markedly reduced the power and strength of the positive findings, particularly given that COVID-19 treatment standards have been a moving target. The 2-month study enrollment period saw a shifting attitude toward favoring glucocorticoids (16), the incorporation of remdesivir into treatment standards (24–26), and the movement away from early intubation/mechanical ventilation in favor of more aggressive self-proning protocols (27, 28).
CONCLUSIONS
In summary, this pilot study showed that IVIG 0.5 g/kg daily for 3 days with concomitant methylprednisolone 40 mg reduced progression of respiratory failure requiring mechanical ventilation, total length of hospital stay, and ICU length of stay, and improved oxygenation at 7 days in COVID-19 patients with a calculated or estimated A-a gradient of greater than 200 mm Hg (Pao2/Fio2 < 140 and Sao2 ≤ 92% on ≥ 6 L nasal cannula). This study served as the foundation of a larger phase 3, multicenter, double-blind placebo-controlled trial evaluating IVIG in COVID-19, which is currently enrolling patients and which will hopefully validate these findings.
ACKNOWLEDGMENTS
We thank Octapharma USA, who did not influence the design or execution of the study and did not have a role in data analysis or article preparation.
Supplementary Material
Footnotes
Supported, in part, by IV Immunoglobulin (Octagam 10%) provided by Octapharma USA, Hoboken, NJ.
NCT04411667, Clinicaltrials.gov, April 28, 2020
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020; 395:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siddiqu HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical- therapeutic staging proposal. J Heart Lung Transplant. 2020; 39:405–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020; 71:762–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Lau YF, Lamirande EW, et al. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J Virol. 2010; 84:1289–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galeotti C, Kaveri SV, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017; 29:491–498 [DOI] [PubMed] [Google Scholar]
- 6.Shao Z, Feng Y, Zhong L, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical patients with COVID-19: A multicenter retrospective cohort study. Clin Transl Immunol. 2020; 9:e1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie Y, Cao S, Li Q, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020; 81:318–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou AG, Xie SM, Zhang J, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. Preprint posted online March 4, 2020. doi: 10.20944/preprints202003.0065.v1 [Google Scholar]
- 9.Cao W, Liu X, Bai T, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect Dis. 2020; 7:ofaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: An observational cohort study. Lancet. 2020; 395:1771–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020 May 17. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Nicolai L, Leunig A, Brambs S, et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020; 142:1176–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang JE, Hidalgo A, Frenette PS. Intravenous immunoglobulins modulate neutrophil activation and vascular injury through FcγRIII and SHP-1. Circ Res. 2012; 110:1057–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cicco S, Cicco G, Racanelli V, et al. Neutrophil extracellular traps (NETs) and damage-associated molecular patterns (DAMPs): Two potential targets for COVID-19 treatment. Mediators Inflamm. 2020; 2020:7527953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uozumi R, Iguchi R, Masuda S, et al. Pharmaceutical immunoglobulins reduce neutrophil extracellular trap formation and ameliorate the development of MPO-ANCA-associated vasculitis. Mod Rheumatol. 2020; 30:544–550 [DOI] [PubMed] [Google Scholar]
- 16.Horby P, Lim WS, Emberson JR; Recovery Collaborative Group. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020 Jul 17. [online ahead of print] [Google Scholar]
- 17.Knackstedt SL, Georgiadou A, Apel F, et al. Neutrophil extracellular traps drive inflammatory pathogenesis in malaria. Sci Immunol. 2019; 4:eaaw0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opasawatchai AO, Amornsupawat P, Jiravejchakul N, et al. Neutrophil activation and early features of NET formation are associated with dengue virus infection in humans. Front Immuno. 2019; 9:3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aloulou M, Ben Mkaddem S, Biarnes-Pelicot M, et al. IgG1 and IVIg induce inhibitory ITAM signaling through FcγRIII controlling inflammatory responses. Blood. 2012; 119:3084–3096 [DOI] [PubMed] [Google Scholar]
- 20.Ben Mkaddem S, Aloulou M, Benhamou M, et al. Role of FcγRIIIA (CD16) in IVIg-mediated anti-inflammatory function. J Clin Immunol. 2014; 34Suppl 1S46–S50 [DOI] [PubMed] [Google Scholar]
- 21.Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020; 127:104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Purswani M, Johann-Liang R, Neeley M, et al. Effect of intravenous immune globulin (IVIG) on interleukin-6 (IL-6) production by whole blood ♦ 894. Pediatr Res. 1998; 43:154 [Google Scholar]
- 23.Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: A randomized clinical trial. JAMA. 2020; 324:460–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19 - preliminary report. Reply. N Engl J Med. 2020; 383:992–994 [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020; 395:1569–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 days in patients with severe covid-19. N Engl J Med. 2020 May 27. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elharrar X, Trigui Y, Dols AM, et al. Use of prone positioning in nonintubated patients with covid-19 and hypoxemic acute respiratory failure. JAMA. 2020; 323:2336–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, non-intubated patients in the emergency department: A single ED’s experience during the COVID-19 pandemic. Acad Emerg Med. 2020; 27:375–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


