Natural killer (NK) cells play a key role in both antibacterial and antitumor immunity. Pseudomonas aeruginosa infection has already been reported to alter NK cell functions. We studied in vitro the effect of P. aeruginosa on NK cell cytotoxic response (CD107a membrane expression) to a lymphoma cell line. Through positive and negative cell sorting and adoptive transfer, we determined the influence of monocytes, lymphocytes, and regulatory T cells (Treg) on NK cell function during P. aeruginosa infection.
KEYWORDS: immunity, infection, cancer, Pseudomonas aeruginosa, regulatory T cells, NK cells
ABSTRACT
Natural killer (NK) cells play a key role in both antibacterial and antitumor immunity. Pseudomonas aeruginosa infection has already been reported to alter NK cell functions. We studied in vitro the effect of P. aeruginosa on NK cell cytotoxic response (CD107a membrane expression) to a lymphoma cell line. Through positive and negative cell sorting and adoptive transfer, we determined the influence of monocytes, lymphocytes, and regulatory T cells (Treg) on NK cell function during P. aeruginosa infection. We also studied the role of the activating receptor natural killer group 2D (NKG2D) in NK cell response to B221. We determined that P. aeruginosa significantly altered both cytotoxic response to B221 and NKG2D expression on NK cells in a Treg-dependent manner and that the NKG2D receptor was involved in NK cell cytotoxic response to B221. Our results also suggested that during P. aeruginosa infection, monocytes participated in Treg-mediated NK cell alteration. In conclusion, P. aeruginosa infection impairs NK cell cytotoxicity and alters antitumor immunity. These results highlight the strong interaction between bacterial infection and immunity against cancer.
INTRODUCTION
The physiologic immune process to fight severe infections consists of first an inflammatory response in order to clear the pathogen and heal the affected tissue and second an anti-inflammatory response that aims to restore immune homeostasis. The second phase leaves an immunological scar that can impair antitumor immunity (1, 2). There is increasing evidence that antitumor and anti-infectious immunity involve shared pathways—notably those involving immune evasion (3). In this setting, Pseudomonas aeruginosa infection has already been reported to alter antitumor immunity through natural killer (NK) cell function impairment (4).
NK cells are innate lymphoid cells that exhibit two main functions as follows: cytokine production and cytotoxic response via perforin/granzyme release through granule exocytosis in the extracellular milieu. NK cells patrol in the circulation and in tissues to sense and differentiate normal or abnormal cells (infected or tumor cells) through activating and inhibitory receptors. NK cell response usually depends on the balance between activating and inhibitory signals. The latter are mainly represented by major histocompatibility complex (MHC) class I molecules detected by killer cell immunoglobulin-like receptor (KIR) and allow self-tolerance. NK cells can also recognize activating ligands expressed on both tumor cells and pathogens (5–7).
P. aeruginosa is a Gram-negative opportunistic bacterium that is a major cause of severe pneumonia in immunocompromised patients. P. aeruginosa infection is an interesting model to study antitumor immunity alteration because it can interfere with signalization pathways in order to evade immunity (8). Moreover, its hypermutable genome explains its ability to persist in the host.
The mechanisms that explain how P. aeruginosa can alter antitumor immunity have to be addressed in order to orient future research and development to reduce the impact of infection on the occurrence of cancer. The aim of our study was to evaluate the effect of P. aeruginosa infection on NK cell cytotoxicity in response to a tumor cell line.
RESULTS
P. aeruginosa infection altered NK cell cytotoxic response to B221 cell line.
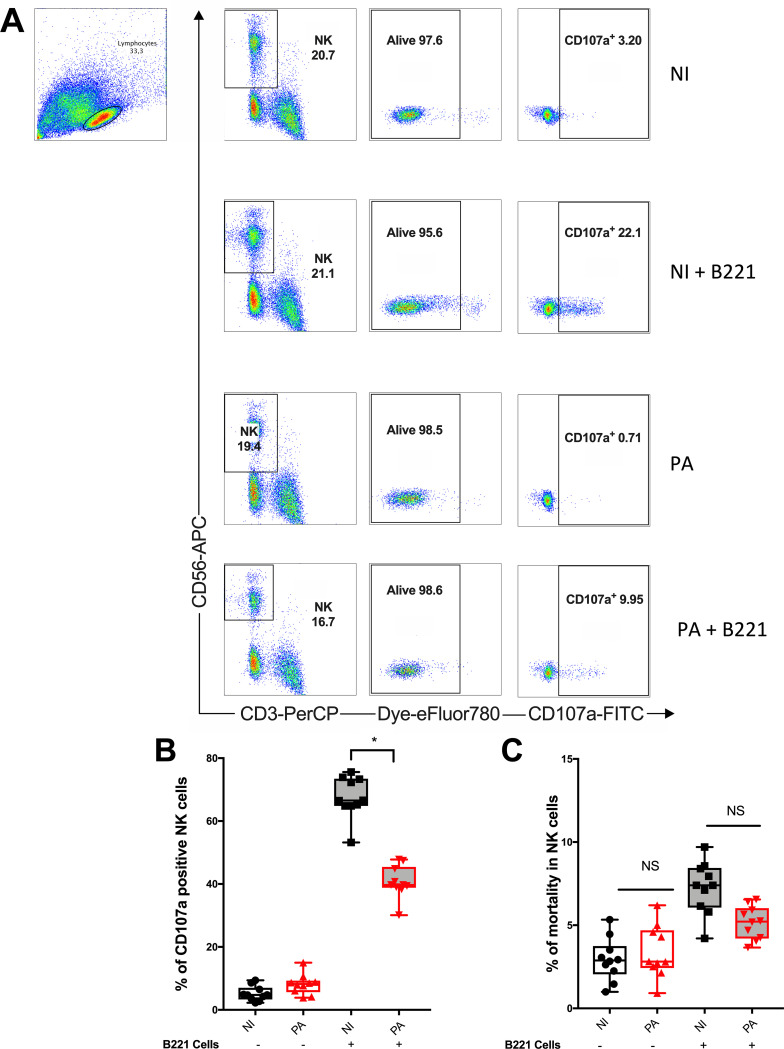
NK cells play a key role in immunity against bacteria (9) and tumors (4) through two main functions as follows: cytokine activity and the release of cytotoxic granules. We previously studied NK cell cytokine response after P. aeruginosa infection and found a direct modulation of the gamma interferon (IFN-γ) response induced by the bacteria (8). Focusing on cytotoxic response during antitumor immunity, we then evaluated the consequences of peripheral blood mononuclear cell (PBMC) infection with P. aeruginosa on NK cell cytotoxic activity toward an MHC class I-negative Epstein-Barr virus (EBV)-transformed human B lymphoma cell line (referred to as B221 cells). As a surrogate marker of cytotoxicity, we analyzed CD107a membrane expression, which corresponded to perforin and granzyme B granule release (10). In the absence of B221 cells, the cytotoxic activity of NK cells was low with or without P. aeruginosa infection (Fig. 1A and B; see also Fig. SA1 in the supplemental material). When exposed to B221 cells, CD107a activity significantly increased in noninfected NK cells. Interestingly, P. aeruginosa infection before B221 cell exposition significantly decreased CD107a+ NK cells (Fig. 1A and B) compared with the noninfected cells. NK cell viability between infected and noninfected conditions was similar, ruling out a toxic effect of P. aeruginosa infection to explain these results (Fig. 1C). Overall, these results show that P. aeruginosa infection significantly impaired NK cell cytotoxic response to a tumor cell line, which encouraged us to further investigate the underlying mechanism. Unless otherwise stated, mobilization assays (i.e., CD107a staining) in the following experiments were all performed with B221 cells as target cells.
FIG 1.
Pseudomonas aeruginosa infection-impaired NK cell cytotoxicity in response to B221 cell line. NK cell cytotoxic activity (CD107a) among PBMCs was assessed with or without infection and with (+) or without (−) B221 cell exposition (see Fig. SA1 in the supplemental material). (A) Representative density plots illustrating CD107a expression in NK cells (PerCp-CD3− APC-CD56+ eFluor780− in lymphocyte gate) by flow cytometry. Histograms illustrating CD107a+ (B) and the mortality rate of NK cells (C) in noninfected (NI) or 24-h Pseudomonas aeruginosa-infected (PA) conditions. Data are shown as the median and interquartile range of 10 distinct healthy donors. *, P < 0.05; NS, nonsignificant difference; B221, 5-h exposition of PBMC to B221 cells with a B221/PBMC ratio of 1:1; APC-H7 eFluor 780, viability assessment.
NKG2D is involved in NK cell cytotoxic response to B221 cell line.
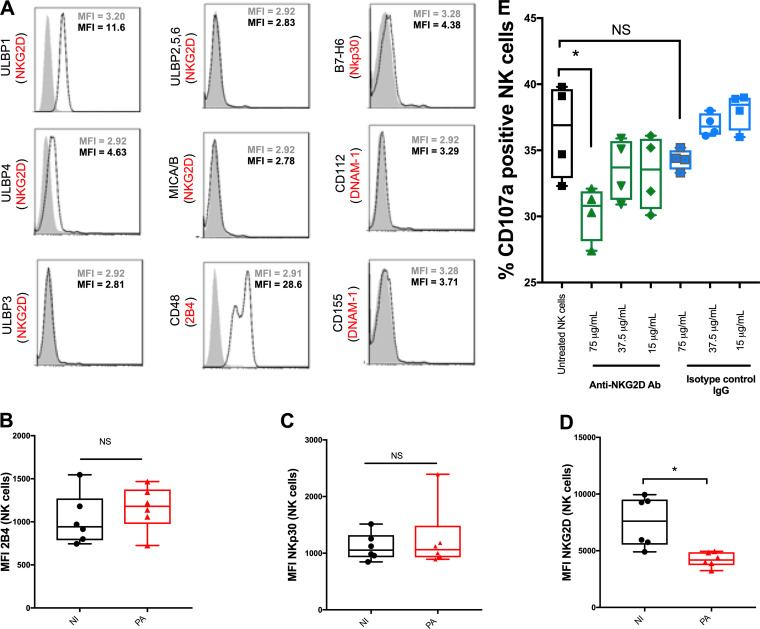
NK cells sense their environment through inhibitory or activating receptors. The cytotoxic response of NK cells is usually driven by an imbalance between inhibitory and activating ligands. In our model, since B221 cells do not express MHC class I molecule (inhibitory ligands), the cytotoxic response mainly depends on activating receptors. To explain the reduction of NK cell cytotoxicity, we hypothesized that P. aeruginosa infection altered the membrane expression of activating receptors. We first assessed whether B221 cells expressed specific ligands for NK cell activating receptor. The B221 cell line expressed one ligand for the 2B4 receptor (CD244, a transmembrane receptor belonging to the CD2 family) and one ligand for the NKp30 receptor (Fig. 2A). These 2 receptors have been reported to play a key role in cytotoxic response to antitumor immunity (11, 12). Interestingly, B221 cells also express 2 specific ligands (ULBP1 and ULBP4) of the natural killer group 2D (NKG2D) receptor, which has been reported to be involved in immune response against both tumors and P. aeruginosa infection (13, 14). Contrary to 2B4 and NKp30, the expression of NKG2D significantly decreased after P. aeruginosa infection (Fig. 2B to D), which could explain the reduction of cytotoxicity after P. aeruginosa infection. Moreover, blocking NKG2D in sorted NK cells before exposition to B221 cells significantly decreased cytotoxic response to B221 cells (Fig. 2E) without altering the viability of NK cells (see Fig. SA3A for viability). As a result, the reduction of NKG2D expression could account for the altered cytotoxicity of NK cells toward B221 cells after P. aeruginosa infection.
FIG 2.
NKG2D-activating receptor is involved in NK cell cytotoxic response against B221 cells. (A) Histogram profiles for different ligands of activating NK cell receptors expressed on B221 cells as follows: ULPB1 to ULPB6 and MICA/B (ligands of NKG2D), B7-H6 (ligand of NKp30), CD48 (ligand of 2B4), and CD112 and CD155 (ligands of DNAM-1). Isotype-matched negative controls are shown as gray-filled curves and ligands as white-filled curves. (B, C, and D) 2B4, NKp30, and NKG2D mean fluorescence intensity (MFI) on NK cells in noninfected or 24-h Pseudomonas aeruginosa-infected (PA) PBMCs (without B221 cell exposition). (E) Representative histograms of CD107a activity in sorted NK cells in response to B221 cells with or without anti-NKG2D blocking antibody or its isotype. Data are shown as the median and interquartile range of 6 (B, C, and D) and 4 (E) distinct healthy donors. NK cells were analyzed in lymphocyte gate by flow cytometry after PerCp-CD3− APC-CD56+ eFluor780− staining. *, P < 0.05; NS, nonsignificant difference; B221, 5-h exposition to B221 cells with a B221/NK ratio of 10:1 in order to keep the same ratio as in PBMCs (assuming 10% of NK cells in PBMC).
Alteration of NK cell cytotoxicity after P. aeruginosa infection is mediated by CD3+ cells.
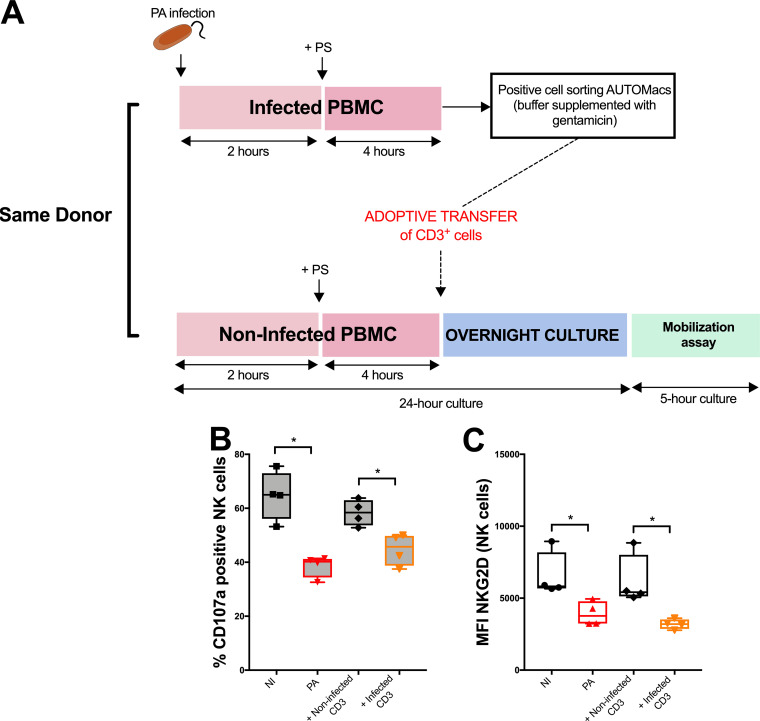
Accessory cells have been reported to participate in NK cell functions (15). In order to determine their role in the impairment of NK cell cytotoxicity toward B221 cells after P. aeruginosa infection, we developed an adoptive transfer strategy (Fig. 3A). Transfer of sorted CD3+ cells from infected PBMCs onto noninfected PBMCs altered NK cell cytotoxicity and NKG2D expression compared with that of their noninfected counterparts (Fig. 3B and C). These data suggested that NK cell cytotoxicity impairment after P. aeruginosa infection is a T cell-dependent mechanism.
FIG 3.
Involvement of T cells in NK cell cytotoxic impairment after Pseudomonas aeruginosa infection. (A) Comprehensive diagram explaining adoptive transfer strategy of infected (or noninfected) CD3+ cells. (B) CD107a activity of NK cells after CD3+ adoptive transfer when exposed to B221 cell line. (C) NKG2D mean fluorescence intensity (MFI) of NK cells after CD3+ adoptive transfer (no exposition to B221 cell line for this analysis). Data are shown as the median and interquartile range of 4 distinct healthy donors. NK cells were analyzed in lymphocyte gate by flow cytometry after PerCp-CD3− APC-CD56+ eFluor780− staining. *, P < 0.05; NI, noninfected PBMC; PA, PBMC after 24-h infection with Pseudomonas aeruginosa; B221, 5-h exposition to B221 cells with a B221/PBMC ratio of 1:1.
Treg participate in NK cell cytotoxicity impairment after P. aeruginosa infection.
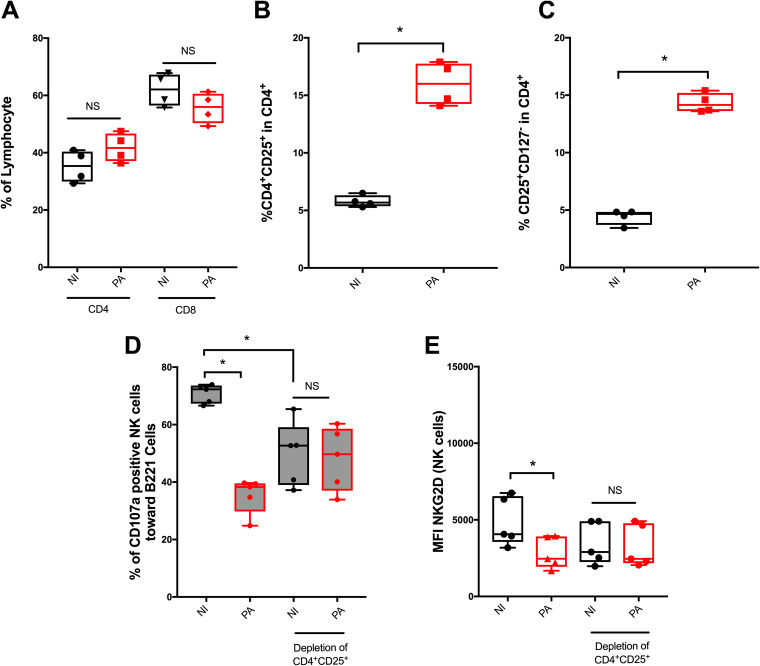
Among T lymphocytes, regulatory T cells (Treg) have been reported to regulate the cytotoxic activity of NK cells toward abnormal cells (16, 17). Treg were initially described as CD4+ CD25+ T cells (18) and more recently as CD4+ CD25+ CD127− cells (19). P. aeruginosa infection did not significantly modify the percentage of CD4+ and CD8+ T cells among lymphocytes (Fig. 4A) but increased the percentages of both CD25+ and CD25+ CD127− cells among CD4+ T cells (Fig. 4B and C; see also Fig. SA3B to D for numbers). Thus, we hypothesized that CD4+ CD25+ depletion (see Fig. SA2 in the supplemental material) before infection would prevent the induction of CD4+ CD25+ CD127− cells (Treg) and, therefore, the alteration of NK cell cytotoxicity. After CD4+ CD25+ depletion, P. aeruginosa infection did not alter NK cell cytotoxicity (Fig. 4D) or NKG2D expression (Fig. 4E) compared with that of the nondepleted condition. These results suggested that CD4+ CD25+ cells are key players in P. aeruginosa-mediated NK cell cytotoxicity alteration.
FIG 4.
Regulatory T cell involvement in the reduction of NK cell cytotoxicity after Pseudomonas aeruginosa infection. Histograms illustrating the percentage of CD4+ and CD8+ cells among lymphocytes (A), CD4+ CD25+ cells (B), and CD4+ CD25+ CD127− cells among CD4+ cells (C) in noninfected (NI) or 24-h Pseudomonas aeruginosa-infected (PA) conditions. Histograms illustrating CD107a activity (D) and NKG2D mean fluorescence intensity (MFI) (E) in noninfected (NI) or 24-h Pseudomonas aeruginosa-infected (PA) PBMCs depleted in CD4+ CD25+ cells before infection (see Fig. SA1 and SA2 in the supplemental material). Data is shown as the median and interquartile range of 4 (A, B, and C) and 5 (D and E) distinct healthy donors. NK cells were analyzed in lymphocyte gate by flow cytometry after PerCp-CD3− APC-CD56+ eFluor780− staining. *, P < 0.05; NS, nonsignificant difference; B221, 5-h exposition to B221 cells with a B221/PBMC ratio of 1:1.
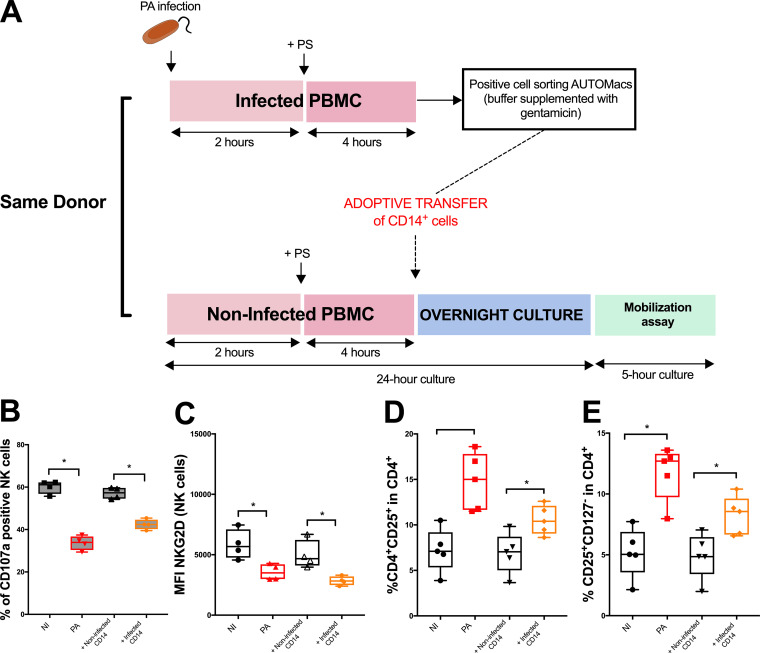
Infected CD14+ cells are involved in regulatory T cell-mediated NK cell cytotoxicity impairment.
In PBMCs, CD14+ cells are mainly monocytes (with <2% circulating dendritic cells) (20). Thus, in the following experiments, the sorted CD14+ cells are referred to as monocytes. The expression of costimulation molecules (CD80/86) on antigen presenting cells (APC), such as monocytes, is essential for the induction and the survival of Treg in the periphery (21). Transfer of CD14+ cells (Fig. 5A) from infected PBMCs onto noninfected PBMCs altered NK cell cytotoxicity toward B221 cells and NKG2D expression (Fig. 5B and C) compared with that of their noninfected counterparts. This alteration was concomitant of an increase in the percentage of CD4+ CD25+ and CD25+ CD127− cells among CD4+ cells after CD14+ infected cell transfer (Fig. 5D and E; see also Fig. SA3E, F, and G for numbers). These results support the involvement of monocytes in Treg-induced NK cell cytotoxicity impairment after P. aeruginosa infection.
FIG 5.
Effect of CD14+ cell transfer on NK cell cytotoxicity and Treg frequency after Pseudomonas aeruginosa infection. (A) Comprehensive diagram explaining adoptive transfer strategy of infected (or noninfected) CD14+ cells. (B) CD107a activity of NK cells after CD14+ adoptive transfer when exposed to B221 cell line. (C) NKG2D mean fluorescence intensity (MFI) of NK cells after CD14+ adoptive transfer (no exposition to B221 cell line for this analysis). Histograms illustrating the effect of CD14+ adoptive transfer on the percentage of CD4+ CD25+ (D) and CD4+ CD25+ CD127− (E) cells among CD4+ cells. *, P < 0.05; NS, nonsignificant difference; NI, noninfected PBMC; PA, PBMC after 24-h infection with Pseudomonas aeruginosa; B221, 5-h exposition of PBMC to B221 cells with a B221/PBMC ratio of 1:1. Data is shown as the median and interquartile range of 4 (B and C) and 5 (D and E) distinct healthy donors. NK cells were analyzed in lymphocyte gate by flow cytometry after PerCp-CD3− APC-CD56+ eFluor780− staining.
Overall, the present results suggest that NK cell cytotoxicity in response to the B221 cell line is altered after P. aeruginosa infection by complex interactions involving CD4+ CD25+ and CD14+ cells. The reduction of cytotoxic response was at least in part related to the alteration of NKG2D expression on NK cells.
DISCUSSION
Our study provides novel insight into our understanding of how bacterial infection could impair tumor immunity. Notably, we highlighted that P. aeruginosa infection led to Treg expansion involving monocytes, with subsequent reduction of NKG2D expression on NK cells, which altered NK cell cytotoxicity against a human B lymphoma cell line.
NK cells play a key role in both antibacterial and antitumor immunity. Our team has already reported the key role of NK cells to defend against P. aeruginosa in vitro (8) or in vivo (9). There is increasing evidence of complex interaction between antitumor and antibacterial immunity (22). For example, in a murine model of melanoma, P. aeruginosa infection has been reported to depress tumor control and enhance its metastatic diffusion through NK cell function impairment (4). The authors suggested that P. aeruginosa can provoke a direct phagocytosis-induced apoptosis of NK cells via caspase 9 activation, whereas in the present study, P. aeruginosa infection altered NK cell cytotoxicity without markedly impairing NK cell viability (Fig. 1C). These differences could be explained by 8-h infections in antibiotic-free medium of the NK92 cell line with high multiplicity of infection (MOI) (500 bacteria for 1 NK cell) and with a different P. aeruginosa strain in the Chung et al. model (4). However, taken together, these results strongly suggest the need to conduct further research on the interaction between bacteria and tumor immunity.
NK cell antitumor function is correlated with the level of expression of activating receptors and the amount of circulating tumor-infiltrating NK cells (23). NK cell response usually depends on the balance between inhibitory and activating receptors. Nevertheless, natural killer group 2D (NKG2D), a C-type lectin-like molecule, is considered to be a “dominant” activating receptor since it can trigger cytotoxic activity despite simultaneous inhibitory signals (13). NKG2D is involved in antitumor as well as antibacterial immunity. Regarding antibacterial defenses, NKG2D has been reported to participate in the bacterial clearance in a murine model of P. aeruginosa pneumonia (24). Regarding antitumor immunity, NKG2D recognizes various activating ligands (NKG2D-L) derived from nonclassical MHC class I molecules expressed on several histologic types of tumors including melanoma (25) and lacking on normal tissues. Interestingly, a tumor can release transforming growth factor β (TGF-β) (26) or soluble NKG2D-L (27) leading to NKG2D internalization to escape NK cell immunity. Thus, NKG2D is a candidate receptor to study the interaction between bacterial infection and tumor immunity.
Accessory cells are key regulators of NK cell function (17). Consistent with our results, the increased number and frequency of Treg was correlated to cancer progression and inversely correlated to the function of NK cells and NKG2D expression (28, 29). The underlying mechanisms remain uncertain. Interleukin-2 (IL-2) neutralization via CD25 receptor or membrane-bound TGF-β on Treg were reported to reduce NKG2D expression (30, 31) and alter NK cell immunity against cancer expressing NKG2D ligands (32). IL-2 supplementation at increasing doses did not prevent the drop of cytotoxicity after infection and did not support CD25 involvement in our model (data not shown). The role of TGF-β has not yet been addressed. In the same way, monocytes increase regulatory T cell activation through cytokine or reactive oxygen species (ROS) release during inflammatory response (33, 34). We confirmed the role of infected monocytes in regulatory T cell expansion.
Our in vitro study has limitations. The CD107a mobilization assay is a surrogate marker of degranulation, but its detection is correlated with target lysis assessed by 51Cr release (35). Considering coculture as a tumor model may be questionable. However, the study of circulating NK cell function from PBMCs is an accurate approach given their critical roles in preventing metastasis diffusion (36). In our model, the involvement of NKG2D in the cytotoxic response must be tempered considering that blocking antibodies did not suppress but only partly decreased CD107a expression. Our results pled for a predominant accessory cell-dependent alteration of NK cell functions, but specific pathways were not addressed and will require further studies. In the noninfected conditions, the proportion of CD107a-positive cells was significantly lower after CD4+ CD25+ depletion. The sorting process could explain this difference, but a specific effect of this subset on CD107a activity cannot be excluded. However, after CD4+ CD25+ depletion, the similar proportion of CD107a-positive cells in infected or noninfected conditions suggests that the reduction of CD107a activity after P. aeruginosa infection involves CD4+ CD25+ cells.
Regarding Treg characterization, we did not study FOXP3 intracellular staining. However, the CD4+ CD25+ CD127− T cells are known to express the highest level of FOXP3 (37). Finally, despite systematic control of the supernatant on agar plates, we cannot exclude undetectable residual bacterial pathogen-associated molecular patterns (PAMPs) that could have participated in the results during adoptive transfer experiments.
In conclusion, we found that P. aeruginosa infection can alter the cytotoxic function of NK cells against tumor cells by a CD4+ CD25+-dependent activation loop, which is initiated by monocytes. Hence, manipulating CD4+ CD25+ cells or monocytes stands as an interesting therapeutic target to enhance antitumor immunity and to correct postinfectious immune defects (38).
MATERIALS AND METHODS
PBMCs from healthy donors, 721.221 cell line, and cell sorting.
All volunteer blood donors were recruited at the blood transfusion center (Nantes, France). Human peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood by gradient centrifugation on Ficoll-Hypaque and frozen (Lymphoprep, Norway). Before sorting or infection, PBMCs were cultured at 37°C in 5% CO2 overnight in RPMI 1640 medium (Gibco) containing glutamine (Gibco) with 10% fetal bovine serum (Gibco; <10 endotoxin units [EU]/ml endotoxin contamination), penicillin-streptomycin (PS) free, and supplemented with 100 U/ml IL-2 (Proleukin [aldesleukin]; Chiron). This medium is referred to as “IL-2 medium” throughout.
Human PBMCs were sorted with an autoMACS pro separator (Miltenyi Biotec, Germany) and the corresponding isolation kits as follows: untouched NK cells, CD14+, CD3+, and CD4+ CD25+ cells. Cell sorting assessment yielded to a mean (±standard deviation [SD]) purity of 86% (±6%) for NK cells and 90% (±4%) for CD14+ or CD3+ cell isolation. After CD4+ CD25+ cell selection, the negative cell fraction contained <0.5% of residual CD4+ CD25+ cells. The cells were kept in IL-2 medium during cell sorting.
The 721.221 cell line (referred to as B221 cells; from EFS-PL laboratory, Nantes, France) is an MHC class I-negative EBV-transformed human B lymphoma cell line. This cell line was cultured at 37°C in 5% CO2 in the same medium as PBMCs without IL-2 supplementation and was used as a positive control to assess NK cell cytotoxic granule release (39).
Infection.
Before infection, PBMCs or sorted cells were seeded in 24-well plates (1 × 106 per well in 1 ml) in IL-2 medium.
Infections were performed with the PAO1 strain, which is a clinical strain of Pseudomonas aeruginosa (number 15692) whose genome has been fully sequenced (40). The PAO1 strain was grown overnight in brain heart infusion medium at 37°C. Bacterial inoculum was calibrated by nephelometry. After 2 h of coculture in IL-2 medium with a multiplicity of infection (MOI) for bacteria/PBMC of 25:1, the wells were supplemented with 10 mM PS to prevent bacterial overgrowth until the 24th hour (8). After 24 h of coculture, the NK cell mobilization assay was performed. Noninfected conditions followed the same protocol (see Figure SA1 in the supplemental material). Bacterial loads were checked by inoculation of serial dilution on agar plates.
NK cell mobilization assay.
CD107a mobilization assay was detected by flow cytometry. This assay reflects NK cell cytotoxicity given that CD107 is localized in a lipid layer surrounding cytotoxic granules and remains attached to the surface of cells after granule release (41). The cells from 24-h P. aeruginosa-infected and noninfected wells were numbered, resuspended in fresh IL-2 medium with PS, seeded in 96-well plates (0.5 × 106 per well in 100 μl), and exposed to B221 (IL-2 medium with PS, 0.5 × 106 per well in 100 μl) for 5 h at 37°C in 5% CO2. The B221/PBMC ratio was 1:1 (indicating a B221/NK ratio of 10:1 assuming 10% NK cells among PBMC). Similarly, the control wells (without B221 cells) were supplemented with 100 μl of IL-2 medium with PS (see Figure SA1 in the supplemental material).
NKG2D blocking.
The anti-hNKG2D antibody (IgG1, clone 149810; R&D) or its isotype (IgG1, clone NCG01; Invitrogen) were incubated with sorted NK cells (untouched NK cell isolation kit) for 1 h (4°C) in 100 μl of IL-2 medium with PS for final concentrations of 75, 37.5, and 15 μg/ml. The mobilization assay was subsequently performed as described above.
CD3+ or CD14+ adoptive transfer.
After 6 h of infection with P. aeruginosa (including PS supplementation after 2 h) in IL-2 medium, PBMCs were treated with gentamicin for 1 h (final concentration of 2 mg/ml), which was adjusted to 1,000-fold the MIC of the antibiotic for the PAO1 strain (MIC = 2 mg/liter). The cells were then sorted (CD3+ or CD14+) using a positive selection kit, resuspended in fresh IL-2 medium with PS, and adoptively transferred onto noninfected PBMCs. The lack of residual viable bacteria was controlled by pure inoculation of the supernatant on agar plates, and cultures were followed for 48 h. Adoptive transfer of noninfected CD3+ or CD14+ cells followed the same protocol.
Regulatory T cell depletion.
PBMCs were depleted from CD4+ CD25+ cells using a positive selection kit. Then the negative fraction was infected for 24 h (see Fig. SA2 in the supplemental material).
Cell labeling.
Antibodies were purchased from BD Biosciences unless otherwise stated. Data were collected with a 4-color FACSCalibur (BD Biosciences) and LSR II cytometer (Beckton Dickinson, Le Pont de Claix, France) and analyzed using FlowJo 6.2 software (FlowJo, LLC, Ashland, OR, USA). For PBMCs, NK cell gating was performed with anti-CD56-APC (NCAM16.2), anti-CD3-PerCP (SK7), and the corresponding isotype-matched control monoclonal antibody (MAb). NK cell-activating receptor phenotyping was performed with anti-NKp30 (Z21), anti-NKG2D (1D11), and anti-2B4 (2-69) MAbs. The expression of the ligands of NK cells activating receptors on B221 cells was determined using anti-ULBP1 (170818), anti-ULBP2,5,6 (165903), anti-ULBP3 (166510), anti-ULBP4 (709116), anti-B7-H6 MAbs (8750001; R&D Systems, Minneapolis, MN, USA), anti-MICA/B (6D4), anti-CD112 (R2.525, BD Biosciences, Le Pont de Claix, France), anti-CD48 (BJ40), and anti-CD155 (SKII.4; BioLegend, San Diego, CA, USA).
For mobilization assay, cells were stained during the 5-h exposition to B221 cells with an anti-CD107 MAb (clone H4A3). Viability was assessed by APC-fixable viability dye kit eFluor 780 staining (eBioscience).
Statistical analysis.
Statistical analyses were performed with GraphPad prism software (La Jolla, CA, USA). Continuous nonparametric variables were expressed as the median (25th to 75th percentile). The Kruskal-Wallis test was used to compare multiple groups. Dunn’s post hoc test was used to perform comparisons between the 2 groups. A P value of <0.05 was considered to be statistically significant.
Ethics statement.
Written informed consent was obtained from all individuals (Biocollection Authorization Number DC-2012-1555). All experiments were approved by the Ethics Committee of Ouest IV, France (11/13) and performed in accordance with relevant guidelines and regulations.
Data availability.
The data sets generated and/or analyzed during the current study are available at https://data.mendeley.com/datasets/2jrwyy4c28/draft?a=5b1c6245-9aea-4d91-b4eb-05b3e4a31d8e.
Supplementary Material
ACKNOWLEDGMENTS
We thank all of the members of the EA 3826 laboratory and the center de ressources biologiques for banking all of the blood samples of the healthy volunteers. We also thank the EFS PL laboratory members for phenotyping 721.221 cells and allowing us to use this lymphoma cell line to perform mobilization assays. We thank the scientific members of la ligue contre le cancer for their advice on writing the manuscript.
M.V. and K.A. designed all of the experiments. M.V., A.B., G.D., A.R., C.J., T.C., B.G., J.C., C.R., and K.A. wrote the main manuscript text. All authors reviewed the manuscript. M.V., A.B., C.J., T.C., J.C., and G.D. performed the experiments.
We have no conflicts of interest to declare.
M.V. was supported by an award from the Ligue Nationale Contre le Cancer.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Hotchkiss RS, Moldawer LL. 2014. Parallels between cancer and infectious disease. N Engl J Med 371:380–383. doi: 10.1056/NEJMcibr1404664. [DOI] [PubMed] [Google Scholar]
- 2.Smith HA, Kang Y. 2013. Acute infection induces a metastatic niche: a double menace for cancer patients. Clin Cancer Res 19:4547–4549. doi: 10.1158/1078-0432.CCR-13-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacqueline C, Tasiemski A, Sorci G, Ujvari B, Maachi F, Missé D, Renaud F, Ewald P, Thomas F, Roche B. 2017. Infections and cancer: the “fifty shades of immunity” hypothesis. BMC Cancer 17:257. doi: 10.1186/s12885-017-3234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung JW, Piao Z-H, Yoon SR, Kim MS, Jeong M, Lee SH, Min JK, Kim JW, Cho Y-H, Kim JC, Ahn JK, Kim KE, Choi I. 2009. Pseudomonas aeruginosa eliminates natural killer cells via phagocytosis-induced apoptosis. PLoS Pathog 5:e1000561. doi: 10.1371/journal.ppat.1000561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sivori S, Carlomagno S, Pesce S, Moretta A, Vitale M, Marcenaro E. 2014. TLR/NCR/KIR: which one to use and when? Front Immunol 5:105. doi: 10.3389/fimmu.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.López-Soto A, Gonzalez S, Smyth MJ, Galluzzi L. 2017. Control of metastasis by NK cells. Cancer Cell 32:135–154. doi: 10.1016/j.ccell.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Esin S, Batoni G, Counoupas C, Stringaro A, Brancatisano FL, Colone M, Maisetta G, Florio W, Arancia G, Campa M. 2008. Direct binding of human NK cell natural cytotoxicity receptor NKp44 to the surfaces of mycobacteria and other bacteria. Infect Immun 76:1719–1727. doi: 10.1128/IAI.00870-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vourc'h M, Roquilly A, Broquet A, David G, Hulin P, Jacqueline C, Caillon J, Retière C, Asehnoune K. 2017. Exoenzyme T plays a pivotal role in the IFN-γ production after pseudomonas challenge in IL-12 primed natural killer cells. Front Immunol 8:1283. doi: 10.3389/fimmu.2017.01283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broquet A, Roquilly A, Jacqueline C, Potel G, Caillon J, Asehnoune K. 2014. Depletion of natural killer cells increases mice susceptibility in a Pseudomonas aeruginosa pneumonia model. Crit Care Med 42:e441–e450. doi: 10.1097/CCM.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 10.Bryceson YT, March ME, Barber DF, Ljunggren H-G, Long EO. 2005. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med 202:1001–1012. doi: 10.1084/jem.20051143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altvater B, Landmeier S, Pscherer S, Temme J, Juergens H, Pule M, Rossig C. 2009. 2B4 (CD244) signaling via chimeric receptors costimulates tumor-antigen specific proliferation and in vitro expansion of human T cells. Cancer Immunol Immunother 58:1991–2001. doi: 10.1007/s00262-009-0704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiegler N, Textor S, Arnold A, Rölle A, Oehme I, Breuhahn K, Moldenhauer G, Witzens-Harig M, Cerwenka A. 2013. Downregulation of the activating NKp30 ligand B7-H6 by HDAC inhibitors impairs tumor cell recognition by NK cells. Blood 122:684–693. doi: 10.1182/blood-2013-02-482513. [DOI] [PubMed] [Google Scholar]
- 13.Wesselkamper SC, Eppert BL, Motz GT, Lau GW, Hassett DJ, Borchers MT. 2008. NKG2D is critical for NK cell activation in host defense against Pseudomonas aeruginosa respiratory infection. J Immunol 181:5481–5489. doi: 10.4049/jimmunol.181.8.5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nausch N, Cerwenka A. 2008. NKG2D ligands in tumor immunity. Oncogene 27:5944–5958. doi: 10.1038/onc.2008.272. [DOI] [PubMed] [Google Scholar]
- 15.Kerdiles Y, Ugolini S, Vivier E. 2013. T cell regulation of natural killer cells. J Exp Med 210:1065–1068. doi: 10.1084/jem.20130960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmer J, Andrès E, Hentges F. 2008. NK cells and Treg cells: a fascinating dance cheek to cheek. Eur J Immunol 38:2942–2945. doi: 10.1002/eji.200838813. [DOI] [PubMed] [Google Scholar]
- 17.Smyth MJ, Teng MWL, Swann J, Kyparissoudis K, Godfrey DI, Hayakawa Y. 2006. CD4+CD25+ T regulatory cells suppress NK cell-mediated immunotherapy of cancer. J Immunol 176:1582–1587. doi: 10.4049/jimmunol.176.3.1582. [DOI] [PubMed] [Google Scholar]
- 18.Jonuleit H, Schmitt E, Stassen M, Tuettenberg A, Knop J, Enk AH. 2001. Identification and functional characterization of human CD4+CD25+ T cells with regulatory properties isolated from peripheral blood. J Exp Med 193:1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simonetta F, Chiali A, Cordier C, Urrutia A, Girault I, Bloquet S, Tanchot C, Bourgeois C. 2010. Increased CD127 expression on activated FOXP3+CD4+ regulatory T cells. Eur J Immunol 40:2528–2538. doi: 10.1002/eji.201040531. [DOI] [PubMed] [Google Scholar]
- 20.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJM, Liu Y-J, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. 2010. Nomenclature of monocytes and dendritic cells in blood. Blood 116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 21.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 22.Kerin Povšič M, Ihan A, Beovič B. 2016. Post-operative infection is an independent risk factor for worse long-term survival after colorectal cancer surgery. Surg Infect (Larchmt) 17:700–712. doi: 10.1089/sur.2015.187. [DOI] [PubMed] [Google Scholar]
- 23.Delahaye NF, Rusakiewicz S, Martins I, Ménard C, Roux S, Lyonnet L, Paul P, Sarabi M, Chaput N, Semeraro M, Minard-Colin V, Poirier-Colame V, Chaba K, Flament C, Baud V, Authier H, Kerdine-Römer S, Pallardy M, Cremer I, Peaudecerf L, Rocha B, Valteau-Couanet D, Gutierrez JC, Nunès JA, Commo F, Bonvalot S, Ibrahim N, Terrier P, Opolon P, Bottino C, Moretta A, Tavernier J, Rihet P, Coindre J-M, Blay J-Y, Isambert N, Emile J-F, Vivier E, Lecesne A, Kroemer G, Zitvogel L. 2011. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med 17:700–707. doi: 10.1038/nm.2366. [DOI] [PubMed] [Google Scholar]
- 24.Borchers MT, Harris NL, Wesselkamper SC, Zhang S, Chen Y, Young L, Lau GW. 2006. The NKG2D-activating receptor mediates pulmonary clearance of Pseudomonas aeruginosa. Infect Immun 74:2578–2586. doi: 10.1128/IAI.74.5.2578-2586.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vetter CS, Groh V, Thor Straten P, Spies T, Bröcker E-B, Becker JC. 2002. Expression of stress-induced MHC class I related chain molecules on human melanoma. J Invest Dermatol 118:600–605. doi: 10.1046/j.1523-1747.2002.01700.x. [DOI] [PubMed] [Google Scholar]
- 26.Coudert JD, Held W. 2006. The role of the NKG2D receptor for tumor immunity. Semin Cancer Biol 16:333–343. doi: 10.1016/j.semcancer.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Groh V, Wu J, Yee C, Spies T. 2002. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 28.Orentas RJ, Kohler ME, Johnson BD. 2006. Suppression of anti-cancer immunity by regulatory T cells: back to the future. Semin Cancer Biol 16:137–149. doi: 10.1016/j.semcancer.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Doubrovina ES, Doubrovin MM, Vider E, Sisson RB, O'Reilly RJ, Dupont B, Vyas YM. 2003. Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J Immunol 171:6891–6899. doi: 10.4049/jimmunol.171.12.6891. [DOI] [PubMed] [Google Scholar]
- 30.Pedroza-Pacheco I, Madrigal A, Saudemont A. 2013. Interaction between natural killer cells and regulatory T cells: perspectives for immunotherapy. Cell Mol Immunol 10:222–229. doi: 10.1038/cmi.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghiringhelli F, Ménard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, Lecesne A, Robert C, Blay J-Y, Bernard J, Caillat-Zucman S, Freitas A, Tursz T, Wagner-Ballon O, Capron C, Vainchencker W, Martin F, Zitvogel L. 2005. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med 202:1075–1085. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghiringhelli F, Ménard C, Martin F, Zitvogel L. 2006. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunol Rev 214:229–238. doi: 10.1111/j.1600-065X.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 33.Walter GJ, Evans HG, Menon B, Gullick NJ, Kirkham BW, Cope AP, Geissmann F, Taams LS. 2013. Interaction with activated monocytes enhances cytokine expression and suppressive activity of human CD4+CD45ro+CD25+CD127low regulatory T cells. Arthritis Rheum 65:627–638. doi: 10.1002/art.37832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang D, Shui T, Miranda JW, Gilson DJ, Song Z, Chen J, Shi C, Zhu J, Yang J, Jing Z. 2016. Mycobacterium leprae-infected macrophages preferentially primed regulatory T cell responses and was associated with lepromatous leprosy. PLoS Negl Trop Dis 10:e0004335. doi: 10.1371/journal.pntd.0004335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alter G, Malenfant JM, Altfeld M. 2004. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, Shi M, Zhang H, Yang Y, Wu H, Tien P, Wang F-S. 2008. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol 129:428–437. doi: 10.1016/j.clim.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Yu N, Li X, Song W, Li D, Yu D, Zeng X, Li M, Leng X, Li X. 2012. CD4+CD25+CD127low/− T cells: a more specific Treg population in human peripheral blood. Inflammation 35:1773–1780. doi: 10.1007/s10753-012-9496-8. [DOI] [PubMed] [Google Scholar]
- 38.Hotchkiss RS, Monneret G, Payen D. 2013. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.David G, Djaoud Z, Willem C, Legrand N, Rettman P, Gagne K, Cesbron A, Retière C. 2013. Large spectrum of HLA-C recognition by killer Ig-like receptor (KIR)2DL2 and KIR2DL3 and restricted C1 specificity of KIR2DS2: dominant impact of KIR2DL2/KIR2DS2 on KIR2D NK cell repertoire formation. J Immunol 191:4778–4788. doi: 10.4049/jimmunol.1301580. [DOI] [PubMed] [Google Scholar]
- 40.Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, Garber RL, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody LL, Coulter SN, Folger KR, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong GK, Wu Z, Paulsen IT, Reizer J, Saier MH, Hancock RE, Lory S, Olson MV. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 41.Zaritskaya L, Shurin MR, Sayers TJ, Malyguine AM. 2010. New flow cytometric assays for monitoring cell-mediated cytotoxicity. Expert Rev Vaccines 9:601–616. doi: 10.1586/erv.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated and/or analyzed during the current study are available at https://data.mendeley.com/datasets/2jrwyy4c28/draft?a=5b1c6245-9aea-4d91-b4eb-05b3e4a31d8e.