Abstract
Trachypithecus, which currently contains 20 species divided into four groups, is the most speciose and geographically dispersed genus among Asian colobines. Despite several morphological and molecular studies, however, its evolutionary history and phylogeography remain poorly understood. Phayre’s langur (Trachypithecus phayrei) is one of the most widespread members of the genus, but details on its actual distribution and intraspecific taxonomy are limited and controversial. Thus, to elucidate the evolutionary history of Trachypithecus and to clarify the intraspecific taxonomy and distribution of T. phayrei, we sequenced 41 mitochondrial genomes from georeferenced fecal samples and museum specimens, including two holotypes. Phylogenetic analyses revealed a robustly supported phylogeny of Trachypithecus, suggesting that the T. pileatus group branched first, followed by the T. francoisi group, and the T. cristatus and T. obscurus groups most recently. The four species groups diverged from each other 4.5–3.1 million years ago (Ma), while speciation events within these groups occurred much more recently (1.6–0.3 Ma). Within T. phayrei, we found three clades that diverged 1.0–0.9 Ma, indicating the existence of three rather than two taxa. Following the phylogenetic species concept and based on genetic, morphological, and ecological differences, we elevate the T. phayrei subspecies to species level, describe a new species from central Myanmar, and refine the distribution of the three taxa. Overall, our study highlights the importance of museum specimens and provides new insights not only into the evolutionary history of T. phayrei but the entire Trachypithecus genus as well.
Keywords: Colobinae, Integrative zoology, Mitochondrial genome, Museum specimens, New species
INTRODUCTION
Trachypithecus is the most speciose and geographically widespread genus among Asian colobines (Anandam et al., 2013; Groves, 2001; Roos, 2021; Roos et al., 2014; Rowe & Myers, 2016; Zinner et al., 2013). Species of the genus are mainly found in Southeast Asia, from Bhutan, Assam (India), and Bangladesh in the west, through Myanmar, Thailand, Cambodia, and Laos to Vietnam and Southern China in the east, but also occur in large parts of the Sundaland region (Malay Peninsula, Sumatra, Borneo, Java, and some smaller islands). At present, 20 species of Trachypithecus are recognized (Anandam et al., 2013; Roos, 2021; Roos et al., 2014, 2019a; Rowe & Myers, 2016, Zinner et al., 2013), but until recently, different classifications with generally lower species numbers and varying species assemblies have been proposed (Brandon-Jones, 1984, 1995, 1996; Brandon-Jones et al., 2004; Groves, 2001; Napier, 1985; Napier & Napier, 1967, 1994; Oates et al., 1994; Roos et al., 2007; Weitzel & Groves, 1985). With increasing knowledge, particularly from genetic studies, a clearer picture of the evolutionary history of these primates has been obtained, which has also informed taxonomic revisions of the genus (Geissmann et al., 2004; He et al., 2012; Karanth, 2008, 2010; Karanth et al., 2008; Liedigk et al., 2009; Liu et al., 2013, 2020; Nadler et al., 2005; Osterholz et al., 2008; Perelman et al., 2011; Roos & Zinner, 2021; Roos et al., 2007, 2008, 2019a; Thant et al., 2013; Wang et al., 2012, 2015; Wangchuk et al., 2008; Zhang & Ryder, 1998).
Based on differences and similarities in genetics, phenotype, ecology, and behavior, members of the genus are classified into four species groups (Anandam et al., 2013; Osterholz et al., 2008; Roos, 2021; Roos et al., 2014; Rowe & Myers, 2016, Zinner et al., 2013). The T. pileatus group contains three species (T. pileatus, T. geei, and T. shortridgei), the T. francoisi group contains seven species (T. francoisi, T. delacouri, T. ebenus, T. hatinhensis, T. laotum, T. leucocephalus, and T. poliocephalus), the T. cristatus group contains six species (T. cristatus, T. auratus, T. germaini, T. margarita, T. mauritius, and T. selangorensis), and the T. obscurus group contains four species (T. obscurus, T. barbei, T. crepusculus, and T. phayrei) (Anandam et al., 2013; Roos, 2021; Roos et al., 2014; Rowe & Myers, 2016; Zinner et al., 2013). According to genetic data, the T. pileatus group diverged first, followed by the T. francoisi group, with the T. cristatus and T. obscurus groups most recently (Roos & Zinner, 2021; Roos et al., 2019a). Generally, mitochondrial and nuclear sequence data have provided consistent gene trees (Roos et al., 2019a), indicating limited gene flow, at least among the few species investigated so far. For the Indochinese gray langur (T. crepusculus), however, studies indicate that it is likely of hybrid origin (Liedigk et al., 2009; Roos et al., 2019a). Although various phylogenetic studies on Trachypithecus are available, they are generally limited to only a few species or individual species groups, or are based on short sequences of mitochondrial or nuclear DNA (Geissmann et al., 2004; He et al., 2012; Karanth, 2008, 2010; Karanth et al., 2008; Liedigk et al., 2009; Liu et al., 2013, 2020; Nadler et al., 2005; Osterholz et al., 2008; Perelman et al., 2011; Roos et al., 2007, 2008, 2019a; Thant et al., 2013; Wang et al., 2012, 2015; Wangchuk et al., 2008; Zhang & Ryder, 1998). Thus, a well-supported and complete species-level phylogeny for the genus is still missing.
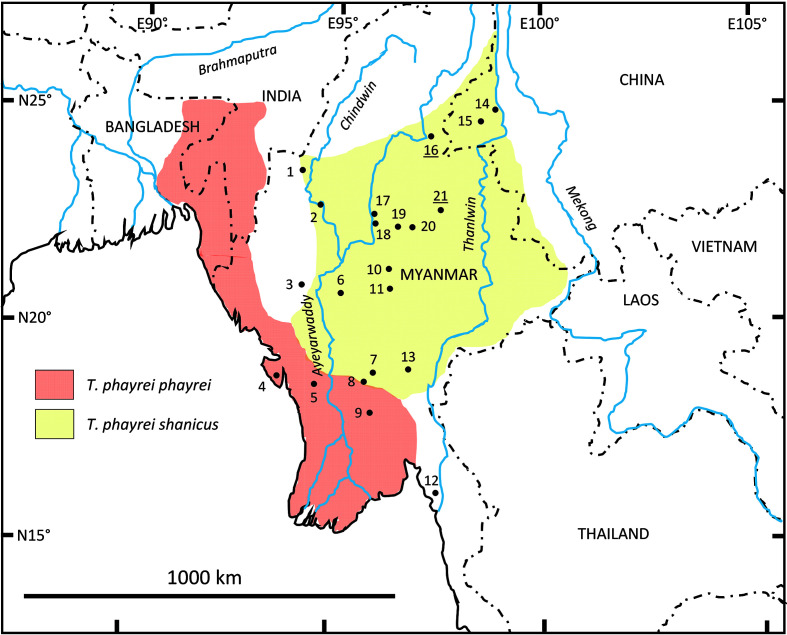
Phayre’s langur (T. phayrei) is a member of the T. obscurus group (Anandam et al., 2013; Roos, 2021; Roos et al., 2014; Rowe & Myers, 2016; Zinner et al., 2013). The species is one of the most widely distributed of the genus, but also one of the least studied in terms of ecology, behavior, genetics, and systematics. The species contains two subspecies, T. phayrei phayrei (Blyth, 1847) and T. p. shanicus (Wroughton, 1917) (Anandam et al., 2013; Roos, 2021; Roos et al., 2014; Rowe & Myers, 2016). Until recently (e.g., Groves, 2001), T. phayrei included a third subspecies, T. p. crepusculus (Elliot, 1909), but based on its putative hybrid status (Liedigk et al., 2009; Roos et al., 2019a), it has since been elevated to species level (Anandam et al., 2013; Roos, 2021; Roos et al., 2014; Rowe & Myers, 2016; Zinner et al., 2013). Nuclear sequence data suggest a closer relationship between T. crepusculus and T. barbei than T. phayrei (Roos et al., 2019a), hence supporting the separation of T. crepusculus from T. phayrei. The geographical distribution of the remaining subspecies of T. phayrei is poorly defined and based on only a few georeferenced museum specimens. Interestingly, according to the currently proposed distribution of T. phayrei (Bleisch et al., 2020; Figure 1), both subspecies seem to have crossed several large rivers (T. p. phayrei: west and east of the Ayeyarwaddy (=Irrawaddy) River; T. p. shanicus: west and east of the Chindwin, Ayeyarwaddy, and Thanlwin (=Salween) rivers). However, distribution across such large rivers is questionable as the ranges of other arboreal primates in the region are restricted by such barriers (e.g., T. leucocephalus and T. francoisi: Burton et al., 1995; Jiang et al., 1991; T. germaini and T. margarita: Nadler et al., 2005; Roos et al., 2008; T. geei and T. pileatus: Chetry et al., 2010a; Ram et al., 2016; Wangchuck et al., 2008; Pygathrix spp.: Nadler et al., 2003; Hylobatidae: Chetry et al., 2010b; Fan et al., 2017; Thinh et al., 2010a, 2010b). While DNA sequence data could potentially clarify whether these distribution ranges are real, few molecular genetic studies on the intraspecific relationships of T. phayrei have been reported. Based on mitochondrial DNA, He et al. (2012) showed a clear distinction between both subspecies, while Thant et al. (2013) revealed that a population from central Myanmar (location 6 in Figure 1) could neither be assigned to T. p. phayrei nor to T. p. shanicus, suggesting a potential third lineage of T. phayrei.
Figure 1.
Distribution of Trachypithecus phayrei according to IUCN Red List (Bleisch et al., 2020)
Numbers indicate sample locations for genetic analysis: 1: Letsegan, 2: Kin, 3: Dudaw-Taung, 4: Ramree Island, 5: near Mount Arakan, 6: Mount Popa, 7: 30 miles northwest of Toungoo, 8: Bago Yoma, 9: South Zamayi Reserve, 10: Myogyi Monastery, 11: Panlaung-Pyadalin Cave Wildlife Sanctuary, 12: Mount Yathae Pyan, 13: Yado, 14: Ho Mu Shu Pass, 15: Gaoligong Mountains National Park, 16: Cadu Ciaung, 17: Ngapyinin, 18: Lamaing, 19: Nattaung, 20: Gokteik, and 21: Se’en (for additional information see Supplementary Table S1). Underlined sites refer to type localities of examined holotypes (16: Presbytis melamera, 21: Pithecus shanicus).
In the current study, we aimed to establish a complete species-level phylogeny and time-calibrated tree for the genus Trachypithecus. We further investigated the taxonomic diversity and geographical distribution of the species T. phayrei. We generated 41 mitochondrial genomes (mitogenomes) via polymerase chain reaction (PCR) followed by Sanger or high-throughput shotgun sequencing using fecal samples from captive and wild animals and tissue samples from historical museum specimens.
MATERIALS AND METHODS
Ethics statement
We obtained tissue samples from museum specimens collected between 1886 and 1955 (Supplementary Table S1). Fecal material from captive and wild animals was collected during routine cage cleaning and field surveys, respectively, without disturbing, threatening, or harming the animals. Field surveys in Myanmar were permitted by the Forest Department, Myanmar. Body and craniodental measurements were taken solely from museum specimens. All research complied with protocols approved by the Animal Welfare Body of the German Primate Center (Germany) and adhered to the legal requirements of the habitat countries in which research was conducted. We conducted the study in compliance with the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) and the principles of the American Society of Primatologists for the ethical treatment of nonhuman primates.
Sample collection
Fecal samples from wild animals (n=6) were collected during fieldwork in Myanmar and fecal samples from captive, but wild-born animals were provided by the Endangered Primate Rescue Center, Vietnam (n=4), Singapore Zoo, Singapore (n=2), Dhaka Zoo, Dhaka, Bangladesh (n=1), and Mandalay Zoo, Mandalay, Myanmar (n=1). Fresh fecal samples were stored in 80% ethanol until further processing. Dried tissue samples (ca. 5×5 mm) from museum specimens were obtained from the Natural History Museum (NHMUK), London, UK (n=18), American Museum of Natural History (AMNH), New York, USA (n=5), Naturalis Biodiversity Center (RMNH), Leiden, The Netherlands (n=1), and the Zoological Reference Collection (ZRC) of the Lee Kong Chian Natural History Museum, Singapore (n=1). Specimens from the NHMUK included holotypes of Pithecus shanicus Elliot, 1909 (NHMUK.ZD.1914.7.8.5; T. p. shanicus) and Presbytis melamera Wroughton, 1917 (NHMUK.ZD.1888.12.1.64; synonym of T. p. phayrei), and a paratype of Presbytis geei Khajuria, 1956 (NHMUK.ZD.1956.379; T. geei). Furthermore, from the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA), we downloaded Illumina raw sequencing reads of two langur specimens, for which nuclear genomic data (Liu et al., 2020), but no published mitogenomes, were available. Details on specimens examined, including their origin, geographic coordinates, sample type, and sequencing data, are provided in Supplementary Table S1.
Mitogenome sequencing and assembly
DNA from fecal samples was extracted in a laboratory dedicated to handle fecal material with various precautions to avoid cross-sample contamination (e.g., separate and UV light decontaminated working areas, protective clothing, negative controls during DNA extraction and PCR amplifications). DNA extraction was performed with a First-DNA All Tissue kit (Gen-Ial, Germany) following Liedigk et al. (2015). DNA concentration was determined with a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, USA). Complete mitogenomes were amplified via 20 overlapping PCR products, each 1.0–1.2 kb in length. Details on PCR set-up and cycling conditions are outlined in Roos et al. (2011) and Liedigk et al. (2012, 2015). The PCR products were visualized on 1% agarose gels stained with ethidium bromide, then purified with a Qiagen PCR Purification kit (Qiagen, Germany), followed by Sanger sequencing on an ABI 3130xl sequencer (Applied Biosystems, USA) using a BigDye Terminator Cycle Sequencing kit (Applied Biosystems, USA) and both amplification primers. Sequence electropherograms were checked by eye with 4Peaks 1.8 (www.nucleobytes.com) and mitogenomes were manually assembled in SeaView 4.5.4 (Gouy et al., 2010). Annotation was conducted with Geneious 11.1.3 (https://www.geneious.com/).
DNA from museum samples was extracted using a column-based method that specifically recovers small DNA fragments (Dabney et al., 2013; Rohland et al., 2004). To reduce the risk of environmental (including human) and cross-sample contamination, DNA extraction and library preparation were performed in an ancient DNA laboratory, in which all standards for such laboratories were implemented (e.g., UV light decontamination before and after use, positive air pressure, separate sterile working areas, protective clothing, and negative controls during DNA extraction and sequencing library preparation). After extraction, the DNA concentration was measured with a Qubit 4.0 fluorometer (ThermoFisher Scientific, USA), and DNA quality and degradation status were checked on a Bioanalyzer 2100 (Agilent Technologies, USA). Genomic DNA (50 ng) was then subjected to shotgun library preparation with a NEBNext Ultra II DNA Library Prep kit (New England Biolabs, USA) following the standard protocols of the supplier. However, due to the degraded status of the DNA, DNA fragmentation prior to library preparation was omitted. After end repair, adapter ligation, and ligation cleanup (without size selection), libraries were indexed with multiplex oligos and then cleaned with the purification beads supplied in the kit. Libraries were also prepared from the pooled negative controls. Library concentration and size distribution were measured with a Qubit fluorometer and bioanalyzer, respectively, and molarity was quantified via quantitative PCR using the NEBNext Library Quant kit (New England Biolabs, USA). Sequencing was conducted on an Illumina HiSeq 4000 (50 bp or 100 bp single-end reads) at the NGS Integrative Genomics (NIG) unit of the University Medical Center Göttingen, Germany, or on an Illumina NextSeq (75 bp paired-end reads) at the University of Potsdam, Germany. Raw sequencing reads were demultiplexed with Illumina software. Subsequent bioinformatic analyses were performed with the Geneious package. First, we trimmed and quality-filtered the reads with BBDuk 37.64 in the BBTools package (https://jgi.doe.gov/data-and-tools/bbtools/) and removed duplicate reads with Dedupe 37.64 (BBTools package); both filtering steps were conducted with standard settings. For assembly, reads were mapped onto the mitogenome of a closely related Trachypithecus spp. (Supplementary Table S1) using the Geneious assembler with standard settings. All newly produced mitogenomes were manually checked and then annotated with Geneious.
To generate mitogenomes from published Illumina sequencing reads deposited in the NCBI SRA, we downloaded the data and randomly selected 20 million reads. Read processing, filtering, mitogenome assembly, and annotation were performed as described for museum samples.
Phylogenetic analyses
For phylogenetic reconstructions, our dataset was expanded with additional mitogenome sequences from GenBank (Supplementary Table S1). The final dataset was comprised of 72 sequences, including 53 Trachypithecus sequences and sequences from various other colobines (Semnopithecus, Presbytis, Rhinopithecus, Pygathrix, Nasalis, Simias, Colobus, Piliocolobus, and Procolobus) and non-colobines (Macaca, Papio, Theropithecus, Chlorocebus, Hylobates, Pongo, Gorilla, Pan, and Homo). Sequences were aligned with Muscle 3.8.31 (Edgar, 2010) in AliView 1.18 (Larsson, 2014) and manually checked. The generated alignment had a length of 16 969 bp, including 7 197 parsimony-informative and 1 499 parsimony-uninformative variable sites.
Phylogenetic trees were reconstructed with the maximum-likelihood (ML) algorithm in IQ-TREE 1.5.2 (Nguyen et al., 2015) and Bayesian inference (BI) in MrBayes 3.2.6 (Ronquist et al., 2012). For all reconstructions, the optimal substitution model (GTR+I+G), as determined with ModelFinder (Chernomor et al., 2016; Kalyaanamoorthy et al., 2017) in IQ-TREE under Bayesian Information Criterion (BIC), was applied. The BI tree was reconstructed via two independent Markov Chain Monte Carlo (MCMC) runs, each for one million generations with tree and parameter sampling every 100 generations and a burn-in of 25%. To check for convergence of all parameters and adequacy of burn-in, we investigated the uncorrected potential scale reduction factor (PSRF) (Gelman & Rubin, 1992), as calculated by MrBayes. The BI posterior probabilities (PP) and consensus phylogram with mean branch lengths from the posterior density of the trees were also calculated in MrBayes. Node support for the ML tree was obtained from 10 000 ultrafast bootstrap (BS) replications (Minh et al., 2013). All phylogenetic trees were visualized and edited in FigTree 1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/).
Divergence times were calculated with the BEAST 2.4.8 package (Bouckaert et al., 2014). We applied a relaxed log-normal clock model of lineage variation (Drummond et al., 2006) and used a Yule tree prior and the selected best-fit model of sequence evolution (GTR+I+G). To calibrate the molecular clock, we constrained 10 nodes with hard minimum and soft maximum bounds using gamma-distributed priors. These 10 nodes refer to the divergence of (1) Hominoidea vs. Cercopithecoidea, (2) Hominidae vs. Hylobatidae, (3) Pongo vs. Gorilla+Pan+Homo, (4) Gorilla vs. Pan+Homo, (5) Pan vs. Homo, (6) Cercopithecinae vs. Colobinae, (7) African vs. Asian Colobinae, (8) Chlorocebus vs. Papionini, (9) Macaca vs. African Papionini, and (10) Papio vs. Theropithecus. A detailed discussion on the selected node constraints is available in Roos et al. (2019b) and details on prior settings are listed in Supplementary Table S2. We ran BEAST analyses for 100 million generations with tree and parameter sampling every 5 000 generations. The adequacy of 10% burn-in and convergence of all parameters were assessed with Tracer 1.6 (http://tree.bio.ed.ac.uk/software/tracer/). We combined the sampling distributions of two independent runs with LogCombiner 2.4.8 and summarized trees with a burn-in of 10% in TreeAnnotator 2.4.8 (both programs are part of the BEAST package).
Morphometric analyses
External measurements (head-body length, tail length, hind foot length, and ear length) were taken from original museum specimen labels (15 adult males, 11 adult females, 14 young, subadults or adults of unknown sex), reflecting measurements taken on fresh specimens in the field (Supplementary Table S3). Eighteen cranial and dental measurements were taken on the skulls of 22 museum specimens (12 adult males, five adult females, five subadults) with hand-held calipers to the nearest 0.1 mm (Supplementary Table S3). A Kruskal-Wallis analysis of variance (ANOVA) by Ranks was conducted to determine significant differences between taxa (alpha=0.05), followed by post-hoc pair-wise population comparisons of traits (Mann-Whitney U test, with Bonferroni correction for multiple testing in StatisticaTM 13.5.0.17). Principal component analyses (PCAs) were computed using a combination of dental (molar lengths and widths), and cranial (skull length, condylobasal length, zygomatic width, orbit width, C-M3 length, upper canine width, upper palate breadth, anterior palatal foramina length and width, palatilar length, and braincase breadth and height) measurements in RStudio 1.2.5033 (RStudio Team, 2020). All measurement values were standardized by subtracting the mean and dividing by the standard deviation before multivariate analysis. Principal components were extracted from a covariance matrix.
RESULTS
Mitogenomic data
Of the 41 newly sequenced Trachypithecus mitogenomes, 14 were produced from fecal material via conventional PCR followed by Sanger sequencing, 25 were generated from museum samples via high-throughput shotgun sequencing, and two were assembled from published high-throughput shotgun sequencing reads (Liu et al., 2020). For museum samples, we obtained 7.0–53.9 million raw sequence reads per sample; after quality filtering and duplicate removal, we retained 2 299–30 433 reads mapped to the Trachypithecus spp. reference mitogenomes, resulting in 100% coverage and an average sequencing depth of 7–96 (for detailed information see Supplementary Table S1). For the two mitogenomes generated from published sequences, we obtained 100% coverage and an average sequencing depth of 97 and 342, respectively. All 41 mitogenomes contained 22 tRNA genes, 2 rRNA genes, 13 protein-coding genes, and a control region in the order typically found in mammals. All protein-coding genes were correctly transcribed without any premature stop codons and tRNAs exhibited typical secondary structure, indicating that our mitogenomes are likely free from nuclear mitochondrial DNA sequences (numts).
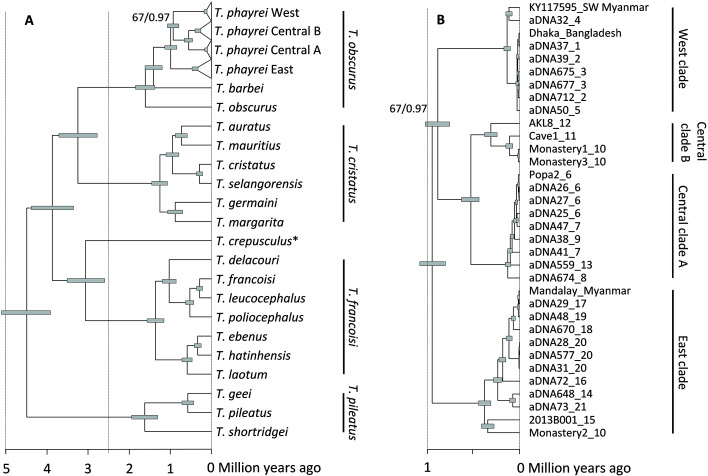
The ML and BI phylogenetic trees revealed identical branching patterns with strong node support (BS 100%, PP 1.0; Figure 2, Supplementary Figure S1). Only the relationships among the three clades found in T. phayrei were not well resolved (BS 67%, PP 0.97). Likewise, the phylogenetic position of Semnopithecus among Asian colobines and basal position of Rhinopithecus among odd-nosed monkeys were supported by BS values of 88% and 98%, respectively, with PP for both nodes of 1.0 (Supplementary Figure S1).
Figure 2.
Mitogenomic tree showing phylogenetic relationships and divergence times among mitochondrial lineages of Trachypithecus (A) and detailed view on T. phayrei (B)
Node bars indicate 95% highest posterior densities (HPDs). Node supports of <100% ML BS and <1.0 BI PP are given at respective nodes. In A, species group assignment is given on the right; *: T. crepusculus, a member of the T. obscurus group according to phenotype and nuclear sequence data. In B, sample labels contain individual ID and sample location number (as in Figures 1, 5, Supplementary Table S1). Clade assignment is given on the right. Complete ultrametric tree including all non-Trachypithecus taxa and details on estimated divergence times are provided in Supplementary Figure S1 and Table S4, respectively.
In Trachypithecus, the T. pileatus group branched first, ca. 4.49 million years ago (Ma) (95% highest posterior densities (HPDs): 3.91–5.10) (Figure 2, Supplementary Figure S1 and Table S4). The remaining taxa diverged 3.87 (3.35–4.38) Ma into a clade containing the T. obscurus and T. cristatus groups, and a clade subsuming the T. francoisi group and T. crepusculus. The T. obscurus and T. cristatus groups split 3.24 (2.78–3.70) Ma, and the T. francoisi group separated from T. crepusculus 3.06 (2.60–3.51) Ma. Speciation events within the four species groups occurred over a prolonged period, from 1.62 (1.31–1.94) Ma to 0.29 (0.22–0.36) Ma. In the T. pileatus group, T. shortridgei diverged from T. pileatus and T. geei 1.62 (1.31–1.94) Ma, and the latter two separated 0.57 (0.43–0.71) Ma. In the T. francoisi group, the southern taxa (T. laotum, T. hatinhensis, and T. ebenus) split from the central (T. delacouri) and northern taxa (T. francoisi, T. leucocephalus, and T. poliocephalus) 1.36 (1.15–1.56) Ma, and the central and northern taxa diverged 1.03 (0.86–1.20) Ma. Among the southern taxa, T. laotum separated from T. hatinhensis and T. ebenus 0.59 (0.47–0.72) Ma, while the latter two diverged 0.33 (0.24–0.42) Ma. Among the northern taxa, T. poliocephalus split from T. francoisi and T. leucocephalus 0.52 (0.42–0.63) Ma, followed by separation of T. francoisi and T. leucocephalus 0.29 (0.22–0.36) Ma. In the T. cristatus group, the mainland taxa (T. germaini and T. margarita) diverged from the central Sundaland (T. cristatus and T. selangorensis) and Javan taxa (T. auratus and T. mauritius) 1.25 (1.07–1.45) Ma and the latter two clades split 0.95 (0.79–1.11) Ma. Speciation events in these three clades occurred 0.87 (0.70–1.06) Ma (mainland clade), 0.29 (0.22–0.36) Ma (central Sundaland clade), and 0.72 (0.59–0.87) Ma (Javan clade). In the T. obscurus group, T. obscurus diverged first 1.60 (1.37–1.84) Ma and T. barbei separated from T. phayrei 1.40 (1.19–1.61) Ma.
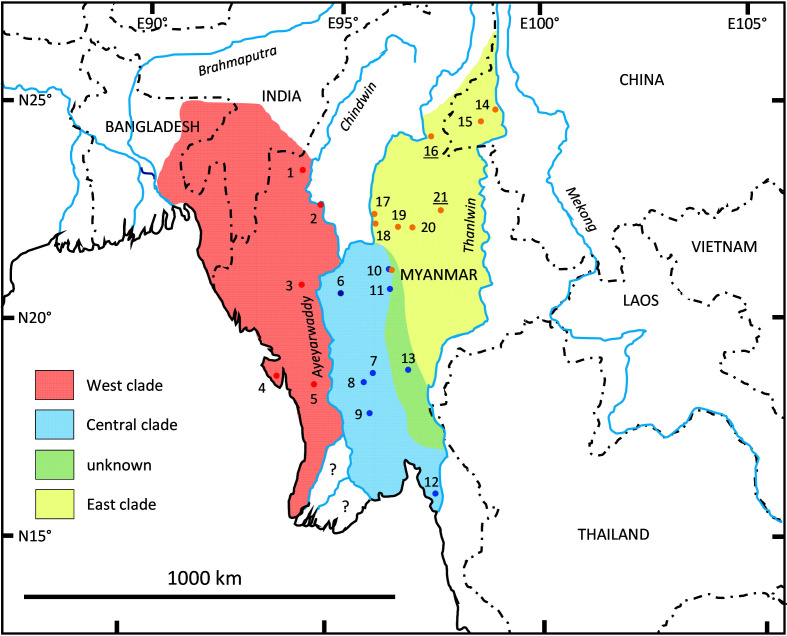
For T. phayrei, we obtained three major clades, which separated within a short period, 0.93–0.99 (0.79–1.13) Ma (Figure 2B). The samples from Bangladesh and Myanmar, west of the Ayeyarwaddy and Chindwin rivers (locations 1–5; Figures 1, 5), grouped in the West clade. Those from the central dry zone of Myanmar and neighboring Kayah-Karen Mountains, east of the Ayeyarwaddy River and west of the Thanlwin River (locations 6–13), formed the Central clade, and those from the Shan Plateau and neighboring China (locations 14–21) clustered in the East clade. In the Central clade, we found two subclades, with one containing samples from the central dry zone (locations 6–9; Central clade A) and the other containing samples from the western foothills of the Kayah-Karen Mountains (locations 10–12; Central clade B). One historical sample from Yado (location 13) clustered with Central clade A and not, as expected, with the geographically closer Central clade B. At location 10, the Myogyi Monastery, we found haplotypes of the Central B and East clades. The holotypes of Pithecus shanicus (location 21) and Presbytis melamera (location 16) both nested within the East clade.
Figure 5.
Geographical distribution of mitochondrial clades found in Trachypithecus phayrei
Sample locations are numbered as in Figures 1, 2 (see also Supplementary Table S1) and colored according to their mitochondrial clade assignment. Limits of the Central clade to the northeast and East clade to the southwest, depicted in light green, are not yet firmly resolved. Samples from locations 6–9 form Central clade A, while those from locations 10–12 cluster in Central clade B. Note, at location 10, haplotypes of the Central and East clades were found. Museum specimen from location 13 cluster unexpectedly with Central clade A (see Results).
Morphometric data
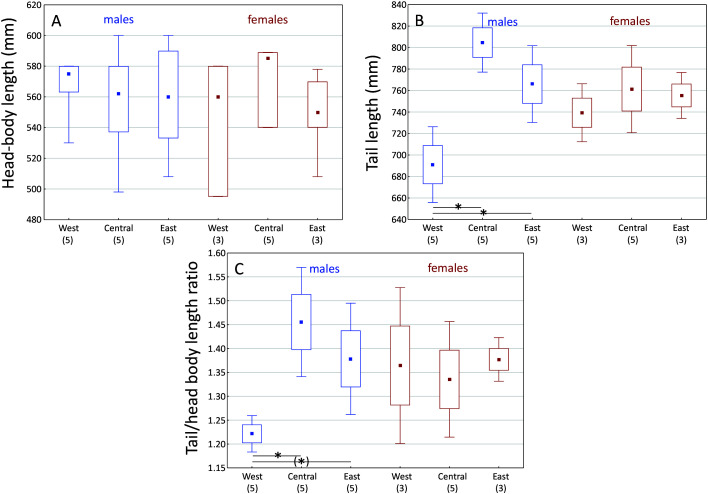
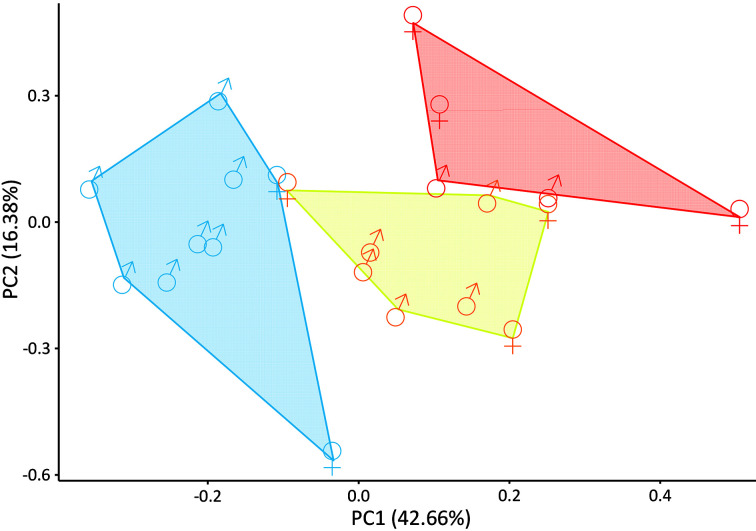
Based on the mitogenomic division of T. phayrei into three rather than two clades, we investigated whether morphological features supported such a division. We found that males, but not females, from the West clade had significantly shorter tails than those from the other two clades (post-hoc test: P<0.025;Figure 3, Supplementary Tables S5–S6). For molar measurements, ungrouped morphometric comparisons using PCA for all available specimens with full molar complements (both adults and subadults, comparable in this case because these teeth do not change in size as the cranium matures) demonstrated that all three clades occupied distinct molar-dimension morphospace (Figure 4, Supplementary Figure S2 and Table S7), even with sex and age variation within each group (Supplementary Figure S3). PCAs based on combined craniodental measurements also separated each clade (Supplementary Figure S3 and Table S8), with molar dimensions important in facilitating morphometric separation, even when both sexes and subadult skulls were included. Cranial measurements alone could not separate the three clades, thus demonstrating the importance of dental size and proportion in clade distinction, despite their overall cranial similarity. However, direct comparisons of skulls revealed useful, if subtle, skull characters in distinguishing the three groups (see Systematic biology, below).
Figure 3.
Head-body length (A), tail length (B), and tail/head-body length ratio (C) of adult male and female Trachypithecus phayrei representing West, Central, and East clades (median, quartiles, min-max)
Numbers in brackets: sample sizes; post-hoc pair-wise population comparisons of traits; Mann-Whitney U-test, with Bonferroni correction for multiple testing: *: P<0.025, (*): P<0.05; see Supplementary Table S6.
Figure 4.
Morphometric comparisons (principal component analysis performed on 12 molar measurements) among Trachypithecus phayrei individuals representing West (red), Central (blue), and East (yellow) clades
Shown is projection of specimen scores of first and second principal components, with variance explained by each component (graphical depictions of third principal component appear in Supplementary Figure S2 and underlying statistics are provided in Supplementary Table S7.)
DISCUSSION
In the current study, we inferred a robust mitochondrial species-level phylogeny of the genus Trachypithecus. In contrast to earlier studies, which examined only fragments of the mitogenome and/or a few species (Geissmann et al., 2004; He et al., 2012; Karanth, 2008, 2010; Karanth et al., 2008; Liedigk et al., 2009; Liu et al., 2013; Nadler et al., 2005; Osterholz et al., 2008; Roos et al., 2007, 2008; Thant et al., 2013; Wang et al., 2012, 2015; Wangchuk et al., 2008; Zhang & Ryder, 1998), we included full-length mitogenomes of all 20 currently recognized species (Anandam et al., 2013; Roos, 2021; Roos et al., 2014; Rowe & Myers, 2016; Zinner et al., 2013), including two name-bearing types. Based on this dataset, we resolved the branching patterns among and within species groups, except for the radiation within T. phayrei, with strong nodal support.
However, as mitochondrial DNA is only maternally inherited, the evolutionary history of the genus remains incomplete (Avise, 2000). Unfortunately, nuclear sequence data for species of Trachypithecus are still scarce, but the few available generally result in a tree topology identical to that obtained from mitogenomes (Liu et al., 2020; Perelman et al., 2011; Roos et al., 2019a). The only exception known so far is the phylogenetic position of T. crepusculus, which constitutes a distant relative of the T. francoisi group in mitogenome phylogenies (Figure 2), while nuclear sequence data support its membership in the T. obscurus group (Liedigk et al., 2009) and specifically as sister taxon to T. barbei (Roos et al., 2019a). This tree discordance is most likely the result of ancient hybridization (Liedigk et al., 2009; Roos et al., 2019a).
According to estimated divergence times, the four species groups (and the mitochondrial lineage of T. crepusculus) separated in the Pliocene, while speciation events within all species groups occurred on similar time scales in the Early Pleistocene, suggesting that Trachypithecus speciation in Southeast Asia has been largely influenced by extrinsic factors such as changes in forest cover and/or sea level (Hallet & Molnar, 2001; Heaney, 1986; Miller et al., 2005).
Distribution and taxonomy of “T. phayrei”
For T. phayrei, we obtained three geographically segregated mitochondrial clades, which does not reflect the current classification of T. phayrei into two subspecies across its distribution (Anandam et al., 2013; Bleisch et al., 2020; Roos, 2021; Roos et al., 2014; Rowe & Myers, 2016; Zinner et al., 2013) (compare Figures 1, 5). According to our data, the geographical distribution of the three clades appears to be delineated by large rivers and/or specific habitat types. The West clade is distributed in Bangladesh and Myanmar, west of the Chindwin and Ayeyarwaddy rivers (Figure 5). Although we had no genetic samples from India, specimens from this country would most likely fall into the West clade as well (on both geographical and morphological grounds). The region is dominated by tropical rainforests as well as tropical dry deciduous forests (Murray et al., 2020). The Central clade is restricted to the central dry zone of Myanmar and the western foothills of the Kayah-Karen Mountains between the Ayeyarwaddy and Thanlwin rivers. The region consists of open woodland in the northern part and tropical dry deciduous forest in the southern part (Murray et al., 2020). A specimen from Yado (location 13) in the Kayah-Karen Mountains clustered with the Central clade, but unexpectedly with Central clade A and not with the geographically closer Central clade B. We can exclude contamination in the laboratory as this specimen was not processed with any sample from the Central clade. There are also no indications of incorrect field records, so our findings remain obscure. Hence, the northeastern boundary of the Central clade remains poorly defined, though it might extend into the Kayah-Karen Mountains. The East clade is found on the Shan Plateau and in neighboring China, between the Ayeyarwaddy and Thanlwin rivers, with the southwestern limit probably extending into the Kayah-Karen Mountains. The region is dominated by typical Shan State tropical mixed forest (Murray et al., 2020). According to its currently assumed distribution (Bleisch et al., 2020; Figure 1), T. phayrei is also found east of the Thanlwin River, but we found no evidence for this, as specimens from east of the Thanlwin River from China and Thailand did not cluster with T. phayrei but instead fell into the T. crepusculus mitochondrial clade (C.R., unpublished data). Likewise, there is no known evidence for the presence of T. phayrei between the Chindwin and Ayeyarwaddy rivers, an area that may instead be occupied by T. shortridgei. At location 10, Myogyi Monastery, we found haplotypes of the Central clade B and East clade. The semi-habituated langurs at the monastery are fed by monks and visitors (Quyet et al., 2019), and exhibit phenotypical features of individuals of both the Central and East clades. We suspect that pet monkeys from further to the northeast were released at the site and interbred with the resident langurs, or less likely, that both populations overlap here naturally.
Taxonomically, the West clade corresponds to the nominate form T. p. phayrei (Presbytis phayrei Blyth, 1847) with the type locality “Arakan” (=Rakhine State, Myanmar), while the East clade corresponds to populations usually considered to represent subspecies T. p. shanicus (Pithecus shanicus Wroughton, 1917) with the type locality “Hsipaw, Northern Shan States”. For T. p. phayrei, Presbytis barbei Blyth, 1863 from the “interior of Tippera hills” (=Tripura State, India), Semnopithecus holotephreus Anderson, 1878 from an unknown locality, and Presbytis melamera Elliot, 1909 from “Cadu Ciaung, Bhamo, North Burma” are generally regarded or listed as synonyms (e.g., Groves, 2001; Napier, 1985; Pocock, 1939). However, the type locality of melamera (location 16) is east of the Ayeyarwaddy River and geographically close to that of shanicus (location 21). We sequenced the mitogenomes of these two holotypes and found that both clustered in the East clade, suggesting that melamera is not a synonym of T. p. phayrei, but instead is a senior synonym of T. p. shanicus. Wroughton (1918, 1921) also concluded that his shanicus (named in 1917) is morphologically identical with melamera. Pocock (1928) recognized similarities in the coloration of both holotypes and their close geographical distance but kept them separate because of the absence of a parting on the forehead in the melamera holotype, although this is probably because the individual was a subadult. The taxonomic name for the East clade is thus T. p. melamera (Elliot, 1909), with shanicus Wroughton, 1917 as a junior synonym. For the Central clade, however, no taxonomic name is yet available.
The three mitochondrial clades of T. phayrei diverged almost 1 Ma, a similar time scale as other speciation events within Trachypithecus, and are geographically confined by large rivers and/or different habitat types (ecological adaptation). Furthermore, the members of the three clades are diagnosably different in external morphology (see morphometric data). Following the phylogenetic species concept (Cracraft, 1983), we elevate the two recognized subspecies to species status, i.e., T. phayrei and T. melamera, and describe and name the taxon constituting our “Central clade” as a new species.
Systematic biology
Order Primates Linnaeus, 1758
Family Cercopithecidae Gray, 1821
Subfamily Colobinae Jerdon, 1867
Genus Trachypithecus Reichenbach, 1862
Trachypithecus phayrei (Blyth, 1847)
English name: Phayre’s langur.
Synonyms: Presbytis barbei Blyth, 1863; Semnopithecus holotephreus Anderson, 1878.
Distribution: East Bangladesh, Northeast India (Assam, Mizoram, and Tripura), and West Myanmar, west of the Chindwin and Ayeyarwaddy rivers (Figure 5).
Conservation status: Currently listed as Endangered (Bleisch et al., 2008a), but reassessment required.
Trachypithecus melamera (Elliot, 1909)
English name: Shan State langur.
Synonyms: Pithecus shanicus Wroughton, 1917.
Distribution: East Myanmar (Shan States) and Southwest China (West Yunnan), between the Ayeyarwaddy and Thanlwin rivers, with the southwestern limit probably extending into the Kayah-Karen Mountains (Figure 5).
Conservation status: Currently listed as Endangered (Bleisch et al., 2008b), but reassessment required.
Trachypithecus popa sp. nov.
Popa langur
Holotype: NHMUK ZD.1914.7.19.3 (adult male, stuffed skin and skull, left zygomatic arch slightly damaged; Figures S4–S6), collected by Guy C. Shortridge on 11 September 1913. Head-body length (HBL): 600 mm, tail length (TL): 800 mm, hindfoot length (HFL): 174 mm, ear length (EL): 33 mm, body mass (BM): 7.9 kg. Mitogenome GenBank accession No.: MT806047.
Type locality: Mount Popa, Myingyan District, Myanmar (N20°55’, E95°15’, 4 961 feet=1 512 m a.s.l.) (location 6 in Figures 1, 5).
Paratypes: NHMUK ZD.1914.7.19.4 (adult male, stuffed skin and skull) collected at the type locality by Guy C. Shortridge on 27 September 1913. HBL: 580 mm, TL: 795 mm, HFL: 161 mm, EL: 32 mm, BM: 8.2 kg. NHMUK ZD.1914.7.19.5 (adult female, stuffed skin) collected at the type locality by Guy C. Shortridge on 3 September 1913. HBL: 540 mm, TL: 780 mm, HFL: 152 mm, EL: 30 mm, BM: 7.0 kg. NHMUK ZD.1917.4.24.1 (adult male, stuffed skin and skull) collected at South Zamayi Reserve, 60 miles north of Pegu by J.M.D. Mackenzie on 10 March 1916. HBL: 498 mm, TL: 795 mm, HFL: 168 mm, EL: 33.5 mm, BM: 7.7 kg. NHMUK ZD.1937.9.10.4 (subadult male, stuffed skin and skull) collected 30 miles northwest of Toungoo by J.M.D. Mackenzie on 8 January 1928. HBL: 508 mm, TL: 785 mm, HFL: 165 mm, EL: 31 mm. NHMUK ZD.1937.9.10.5 (subadult male, stuffed skin and skull) collected 30 miles northwest of Toungoo by J.M.D. Mackenzie on 8 January 1928. HBL: 509 mm, TL: 795 mm, HFL: 165 mm, EL: 31 mm. AMNH M-54770 (juvenile male, skull) collected at Camp Pinmezali, Pegu Yoma by John C. Faunthorpe on 27 April 1924. RMNH MAM.59807 (adult male, stuffed skin with skull in situ) collected at Yado, Mount Cariani, Tounghoo (=Taungoo) District, Myanmar (800–1 000 m) by Leonardo Fea in December 1887 (field number: 40). HBL: 555 mm, TL: 750 mm.
Etymology: The English name for Trachypithecus popa is Popa langur. Mount Popa is a major landmark of the Myingyan District in Myanmar, and the place where the designated holotype was originally collected. The specific name “popa” is used as a noun in apposition.
Description: The species is dark brown or gray-brown on the dorsum, with a sharply contrasting gray or whitish venter. Hands and feet are black. From above the elbow, the arms on the dorsal side gradually darken to black hands. The pale underside extends onto the chin and down to the inner side of the arms and thighs. The tail is paler than the back, notably at the base and underside. The face is black with a wide fleshy-white muzzle and broad white rings fully encircling the eyes. The hairs on the head are raised to a crest or are at least long and irregularly structured, but with no parting or whorl behind the brows present. This crest of hair and the forward-facing whiskers give the head a rhomb-like shape (Figure 6, Supplementary Figures S4–S6). Body measurements (median and range) are: males (n=5) HBL: 562 (498–600) mm, TL: 795 (775–858) mm, HFL: 168 (144–178) mm, EL: 32 (30.0–33.5) mm, BM: 7.9 (7.7–8.2) kg; females (n=3) HBL: 585 (540–589) mm, TL: 780 (720–784) mm, HFL: 156 (152–160) mm, EL: 30 (20–32) mm, BM (n=1): 7.0 kg (Supplementary Table S3).
Figure 6.
Photos of Trachypithecus phayrei (A, B), Trachypithecus popa sp. nov. (C, D) and Trachypithecus melamera (formerly T. p. shanicus) (E, F)
A: Adult female T. phayrei at Yangon Zoo, Myanmar (photo by Tilo Nadler); B: Adult male T. phayrei from Lawachara National Park, Bangladesh (photo by Tanvir Ahmed); C, D: Subadult male T. popa from Mount Popa, Myanmar (photo by Lay Win); E: Adult female T. melamera at Mandalay Zoo, Myanmar (photo by Tilo Nadler), F: Adult female T. melamera with offspring from Gaoligong Mountains National Park, China (photo by Chi Ma).
Diagnosis: Overall, Trachypithecus popa sp. nov. is externally more similar to T. phayrei than to T. melamera. Body coloration in all three species is variable, but generally more fawn in T. melamera and more brownish to gray in Trachypithecus popa sp. nov. and T. phayrei. In Trachypithecus popa sp. nov. and T. phayrei, but not in T. melamera, the pale venter sharply contrasts with the back. The hands and feet are black in all three species. In Trachypithecus popa sp. nov., the arms (dorsal side) gradually darken to the hands from above the elbow, while in T. phayrei, they gradually darken from below the elbow. In T. melamera, the lower arms are not darker than the upper arms. In Trachypithecus popa sp. nov. and T. phayrei, the hairs on the head are raised to a crest or are at least long and irregularly structured, while T. melamera has a whorl or a parting behind the brows. Whiskers are laterally directed in T. phayrei, but forward directed in Trachypithecus popa sp. nov. and T. melamera. The direction of the whiskers in combination with the hairs on the head gives the head of T. phayrei a triangular shape, versus a rhomb-like shape for Trachypithecus popa sp. nov. and a round shape for T. melamera. All three species have a fleshy-white muzzle, which is wider in Trachypithecus popa sp. nov. and T. melamera. In T. melamera, the white around the eyes is restricted to the inner side, while in T. phayrei, the white normally encircles the eyes fully, although it is sometimes restricted to the inner side. In Trachypithecus popa sp. nov., the eyes are always fully encircled with broad white eye-rings. Males of T. phayrei have significantly shorter tails than males of the other two species (Figure 3, Supplementary Tables S5–S6).
Cranially, Trachypithecus popa sp. nov. has a slightly longer skull, especially relative to its width, than in T. phayrei and T. melamera; this is achieved by a slight anterior elongation of the facial region of the skull relative to these taxa, rendering Trachypithecus popa sp. nov. slightly more prognathic in lateral and dorsal views and creating a more rectangular shape of the bony palate in ventral view (vs. a more square palate in T. phayrei and T. melamera). The teeth are, on average, larger in Trachypithecus popa sp. nov. than in T. phayrei and T. melamera, and molar measurements are the clearest means for separating the skulls of this new taxon from its closest relatives (Supplementary Tables S3, S7; Figure 4, Supplementary Figures S2–S3); in particular, the third molar (M3/m3) appears larger overall in Trachypithecus popa sp. nov. when skulls are directly compared. PCAs using molar measurements and combined craniodental measurements separated T. phayrei, T. melamera, and Trachypithecus popa sp. nov., but cranial measurements alone did not separate them (Figure 4, Supplementary Figures S2, S3).
Distribution: Between the Ayeyarwaddy and Thanlwin rivers in the central dry zone of Myanmar and into the western foothills of the Kayah-Karen Mountains (Figure 5). The northeastern limit is undefined (see Discussion), but the species may occur throughout the Kayah-Karen Mountains. This species is endemic to Myanmar.
Conservation status: As evident from historical records (museum specimens and travel notes), the species was once widespread in the central dry zone of Myanmar. Only two of these populations are known to have survived (location 6: Mount Popa, location 8: Bago Yoma), while all others are considered possibly extirpated. However, during recent fieldwork, three new populations (locations 10–12) were discovered. At location 10, Myogyi Monastery, the langur population is estimated at 75–100 individuals (Quyet et al., 2019), but these langurs are probably hybrids between Trachypithecus popa sp. nov. and T. melamera. The populations at location 11, Panlaung-Pyadalin Cave Wildlife Sanctuary, and location 12, Mount Yathae Pyan, consist of 46–96 individuals (Quyet et al., 2019) and 20–30 individuals (A.K.L. and A.L. pers. observation), respectively. The population at Bago Yoma (location 8) contains about 22 individuals (A.K.L. pers. observation) and at Mount Popa (location 6), field surveys conducted in 2019 revealed a population size of 111 individuals (Thaung Win pers. communication). Mount Popa was declared a national park (Popa Mountain Park) in 1989 and has an area of 128.54 km2, including 26.97 km2 classified as suitable to highly suitable for langurs (Thant, 2013; Thant et al., 2013).
Throughout its range, Trachypithecus popa sp. nov. is threatened by hunting, habitat loss, degradation, and fragmentation caused by agricultural encroachment, illegal/unsustainable timber extraction, and disturbances caused by collection of non-timber products and free cattle grazing (Quyet et al., 2019; Thant et al., 2013). Considering a total population size of 199–259 individuals (excluding the possible hybrid population at Myogyi Monastery) in the four disjunct populations and the dramatic habitat loss over the last century, we propose to classify Trachypithecus popa sp. nov. as Critically Endangered (CR) as it meets the IUCN Red List criteria B1a and B1b (i-v) (IUCN, 2001). Furthermore, Trachypithecus popa sp. nov. needs to be added to the national and international lists of threatened species (IUCN, CITES). Improved protected area management, in particular improved law enforcement, in Popa Mountain Park and Panlaung-Pyadalin Cave Wildlife Sanctuary is essential to stabilize the two largest known populations. Mount Yathae Pyan is an isolated karst hill. This population could be protected through the designation of a community-protected area (CPA). The population status of the species in Bago Yoma is poorly understood and additional surveys are urgently required. The forests in Bago Yoma are severely degraded and fragmented, but could still provide the largest, contiguous habitat if deforestation and forest degradation are reversed through improved forest protection and restoration.
Comments: Except for species of the T. pileatus group, the natal coat of Trachypithecus spp. is generally yellowish, orange, or light brown (Anandam et al., 2013; Rowe & Myers, 2016). Trachypithecus popa sp. nov. may be an exception as photos show an infant with creamy white fur coloration (Supplementary Figure S7).
CONCLUSIONS
We present a robust mitogenomic species-level phylogeny of the genus Trachypithecus, thus providing new insights into the evolutionary history of the genus and forming a basis for future work. Based on our investigations of T. phayrei, we illuminated the intraspecific taxonomy of the species, resulting in the elevation of two known subspecies to species level, renaming of one subspecies, description of a new species, and largely refined distributional ranges for all three species. Including the proposed taxonomic changes, the genus Trachypithecus now contains 22 species, with Myanmar home to a total of 20 non-human primate species (Trachypithecus popa sp. nov., T. phayrei, T. melamera, T. barbei, T. obscurus, T. crepusculus, T. shortridgei, T. pileatus, Presbytis femoralis, Rhinopithecus strykeri, Macaca mulatta, M. fascicularis, M. arctoides, M. assamensis, M. leonina, Hoolock hoolock, H. leuconedys, H. tianxing, Hylobates lar, and Nycticebus bengalensis; Fan et al., 2017; Mittermeier et al., 2013; Roos et al., 2014; Rowe & Myers, 2016), of which Trachypithecus popa sp. nov. and probably H. leuconedys are endemic to the country. Trachypithecus germaini, commonly listed for Myanmar (e.g., Anandam et al., 2013; Groves, 2001; Roos et al., 2014; Rowe & Myers, 2016), is actually not present in the country. Its putative occurrence in Myanmar is based on the incorrect assignment of Pithecus pyrrhus atrior as a synonym of T. germaini instead of T. barbei (Geissmann et al., 2004; C.R., unpublished data).
Overall, our study reaffirms that museum collections are a valuable source for genetic and taxonomic investigations of primates, particularly as modern high-throughput sequencing technologies allow the analysis of highly damaged DNA, which is typically extracted from such material. Future studies on Trachypithecus should also include nuclear sequence data and multiple individuals per species and should focus on the three polytypic species of the genus, i.e., T. pileatus, T. cristatus, and T. obscurus.
DATA AVAILABILITY
Mitochondrial genome sequences were submitted to GenBank and are available under accession Nos. MT806030–MT806070.
SCIENTIFIC FIELD SURVEY PERMISSION INFORMATION
Permission for fieldwork in Myanmar was granted by the Forest Department, Myanmar.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
C.R. and F.M. conceived and designed the study. N.L., A.K.L., A.L., C.M., D.M., and L.K.Q. collected samples in the field. R.P.M., N.D., P.K., and M.A.H.C. provided valuable samples from their museum collections. N.M.L.T., N.L., A.K.L., A.L., K.M.Y., P.S., Z.M.H., M.N.N.M., T.A., D.C., L.K.Q., T.N., P.F., and F.M. provided field data. C.R., M.U., L.Y., M.L., Z.L., and M.H. generated the data. C.R., K.M.H., R.P.M., E.G.V., and D.Z. analyzed the data. C.R., K.M.H., and D.Z. wrote the paper. All authors discussed the data and read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We are grateful to the Myanmar Forest Department for permitting fieldwork. L.K.Q. and A.K.L. wish to thank the Management Board of the Panlaung-Pyadalin Cave Wildlife Sanctuary, and Kyaw Naing Oo and Win Hlaing for support during fieldwork. We thank the late Alan Mootnick and the staff of Singapore Zoo, Dhaka Zoo, and Mandalay Zoo for proving fecal samples as well as Christiane Schwarz and Michaela Preick for their excellent work in the laboratory. Many thanks also to Thaung Win and Chi Ma for langur photos, Alain Dubois for taxonomic advice, and two anonymous reviewers for their helpful comments on an earlier version of the manuscript.
Funding Statement
This study was supported by the Margot Marsh Biodiversity Foundation, Primate Action Fund, Helmsley Charitable Trust, and Critical Ecosystem Partnership Fund
References
- 1.Anandam MV, Bennett EL, Davenport TRB, Davies NJ, Detwiler KM, Engelhardt A, et al. 2013. Family Cercopithecidae (Old World monkeys) – Species accounts of Cercopithecidae. In: Mittermeier RA, Rylands AB, Wilson DE. Handbook of the Mammals of the World. Volume 3: Primates. Barcelona: Lynx Edicions, 628–753.
- 2.Anderson J. 1878. Anatomical and Zoological Researches: Comprising an Account of the Zoological Results of the Two Expeditions to Western Yunnan in 1868 and 1875, and a Monograph of the Two Cetacean Genera, Platanista and Orcella. Volume 1. London: B. Quaritch.
- 3.Avise JC. 2000. Phylogeography: The History and Formation of Species. Cambridge, MA: Harvard University Press.
- 4.Bleisch B, Brockelman W, Timmins RJ, Nadler T, Thun S, Das J, et al. 2008a [2020-11-03]. Trachypithecus phayrei ssp. phayrei. The IUCN red list of threatened species 2008: e.T136928A4350467. https://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T136928A4350467.en.
- 5.Bleisch B, Brockelman W, Timmins RJ, Nadler T, Thun S, Das J, et al. 2008b [2020-11-03]. Trachypithecus phayrei ssp. shanicus. The IUCN red list of threatened species 2008: e.T39863A10277688. https://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T39863A10277688.en.
- 6.Bleisch B, Brockelman W, Timmins RJ, Nadler T, Thun S, Das J, et al. 2020 [2020-07-22]. Trachypithecus phayrei. The IUCN red list of threatened species 2020: e.T22040A17960739. https://dx.doi.org/10.2305/IUCN.UK.2020-2.RLTS.T22040A17960739.en.
- 7.Blyth E Supplementary report of the curator of the Zoological Department. Journal of the Asiatic Society of Bengal. 1847;16(2):728–732. [Google Scholar]
- 8.Blyth E. 1863. Catalogue of the Mammalia in the Museum Asiatic Society. Calcutta: Savielle and Cranenburgh.
- 9.Bouckaert R, Heled J, Kühnert D, Vaughan T, Wu CH, Xie D, et al BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Computational Biology. 2014;10(4):e1003537. doi: 10.1371/journal.pcbi.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandon-Jones D. 1984. Colobus and leaf monkeys. In: MacDonald D. The Encyclopedia of Mammals. London: George Allen and Unwin, 398–410.
- 11.Brandon-Jones D A revision of the Asian pied leaf monkeys (Mammalia: Cercopithecidae: superspecies Semnopithecus auratus), with a description of a new subspecies . The Raffles Bulletin of Zoology. 1995;43(1):3–43. [Google Scholar]
- 12.Brandon-Jones D The Asian Colobinae (Mammalia: Cercopithecidae) as indicators of Quaternary climatic change. Biological Journal of the Linnean Society. 1996;59(3):327–350. doi: 10.1006/bijl.1996.0068. [DOI] [Google Scholar]
- 13.Brandon-Jones D, Eudey AA, Geissmann T, Groves CP, Melnick DJ, Morales JC, et al Asian primate classification. International Journal of Primatology. 2004;25(1):97–164. doi: 10.1023/B:IJOP.0000014647.18720.32. [DOI] [Google Scholar]
- 14.Burton FD, Snarr KA, Harrison SE Preliminary report on Presbytis francoisi leucocephalus . International Journal of Primatology. 1995;16(2):311–327. doi: 10.1007/BF02735484. [DOI] [Google Scholar]
- 15.Chernomor O, von Haeseler A, Minh BQ Terrace aware data structure for phylogenomic inference from supermatrices. Systematic Biology. 2016;65(6):997–1008. doi: 10.1093/sysbio/syw037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chetry D, Chetry R, Ghosh K, Bhattacharjee PC Status and conservation of Golden langur in Chakrashila Wildlife Sanctuary, Assam, India. Primate Conservation. 2010a;25:81–86. doi: 10.1896/052.025.0112. [DOI] [Google Scholar]
- 17.Chetry D, Chetry R, Ghosh K, Singh AK Status and distribution of the Eastern Hoolock Gibbon (Hoolock leuconedys) in Mehao Wildlife Sanctuary, Arunachal Pradesh, India . Primate Conservation. 2010b;25:87–94. doi: 10.1896/052.025.0113. [DOI] [Google Scholar]
- 18.Cracraft J. 1983. Species concepts and speciation analysis. In: Johnston RF. Current Ornithology. New York: Plenum Press, 159–187.
- 19.Dabney J, Knapp M, Glocke I, Gansauge MT, Weihmann A, Nickel B, et al Complete mitochondrial genome sequence of a Middle Pleistocene cave bear reconstructed from ultrashort DNA fragments. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(39):15758–15763. doi: 10.1073/pnas.1314445110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drummond AJ, Ho SYW, Phillips MJ, Rambaut A Relaxed phylogenetics and dating with confidence. PLoS Biology. 2006;4(5):e88. doi: 10.1371/journal.pbio.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edgar RC Quality measures for protein alignment benchmarks. Nucleic Acids Research. 2010;38(7):2145–2153. doi: 10.1093/nar/gkp1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elliot DG Descriptions of apparently new species and subspecies of monkeys of the genera Callicebus, Lagothrix, Papio, Pithecus, Cercopithecus, Erythrocebus, and Presbytis . Annals and Magazine of Natural History. 1909;4(21):244–274. doi: 10.1080/00222930908692668. [DOI] [Google Scholar]
- 23.Fan PF, He K, Chen X, Ortiz A, Zhang B, Zhao C, et al Description of a new species of Hoolock gibbon (Primates: Hylobatidae) based on integrative taxonomy. American Journal of Primatology. 2017;79(5):e22631. doi: 10.1002/ajp.22631. [DOI] [PubMed] [Google Scholar]
- 24.Geissmann T, Groves CP, Roos C The Tenasserim lutung, Trachypithecus barbei (Blyth, 1847) (Primates: Cercopithecidae): description of a live specimen, and a reassessment of phylogenetic affinities, taxonomic history, and distribution . Contributions to Zoology. 2004;73(4):271–282. doi: 10.1163/18759866-07304003. [DOI] [Google Scholar]
- 25.Gelman A, Rubin DB Inference from iterative simulation using multiple sequences. Statistical Science. 1992;7(4):457–472. doi: 10.1214/ss/1177011136. [DOI] [Google Scholar]
- 26.Gouy M, Guindon S, Gascuel O SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Molecular Biology and Evolution. 2010;27(2):221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- 27.Groves CP. 2001. Primate Taxonomy. Washington DC: Smithsonian Institution Press.
- 28.Hallet B, Molnar P Distorted drainage basins as markers of crustal strain east of the Himalaya. Journal of Geophysical Research: Solid Earth. 2001;106(B7):13697–13709. doi: 10.1029/2000JB900335. [DOI] [Google Scholar]
- 29.He K, Hu NQ, Orkin JD, Nyein DT, Ma C, Xiao W, et al Molecular phylogeny and divergence time of Trachypithecus: with implications for the taxonomy of T. phayrei . Zoological Research. 2012;33(E5–6):E104–E110. doi: 10.3724/SP.J.1141.2012.E05-06E104. [DOI] [PubMed] [Google Scholar]
- 30.Heaney LR Biogeography of mammals in SE Asia: estimates of rates of colonization, extinction and speciation. Biological Journal of the Linnean Society. 1986;28(1–2):127–165. [Google Scholar]
- 31.IUCN (International Union for Conservation of Nature). 2001. IUCN Red List Categories and Criteria: Version 3.1. 2nd ed. Gland and Cambridge: IUCN.
- 32.Jiang HS, Feng M, Wang J, Wu MC, Lai YM, Liu ZM The distribution and ecological habit of white-headed langur (Presbytis leucocephalus) . Acta Theriologica Sinica. 1991;11(3):236–237, 193. [Google Scholar]
- 33.Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods. 2017;14(6):587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karanth KP Primate numts and reticulate evolution of capped and golden leaf monkeys (Primates: Colobinae) Journal of Biosciences. 2008;33(5):761–770. doi: 10.1007/s12038-008-0096-6. [DOI] [PubMed] [Google Scholar]
- 35.Karanth KP Molecular systematics and conservation of the langurs and leaf monkeys of South Asia. Journal of Genetics. 2010;89(4):393–399. doi: 10.1007/s12041-010-0057-3. [DOI] [PubMed] [Google Scholar]
- 36.Karanth KP, Singh L, Collura RV, Stewart CB Molecular phylogeny and biogeography of langurs and leaf monkeys of South Asia (Primates: Colobinae) Molecular Phylogenetics and Evolution. 2008;46(2):683–694. doi: 10.1016/j.ympev.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 37.Khajuria H A new langur (Primates: Colobidae) from Goalpara District, Assam. Annals and Magazine of Natural History. 1956;9(98):86–88. doi: 10.1080/00222935608655728. [DOI] [Google Scholar]
- 38.Larsson A AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics. 2014;30(22):3276–3278. doi: 10.1093/bioinformatics/btu531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liedigk R, Thinh VN, Nadler T, Walter L, Roos C Evolutionary history and phylogenetic position of the Indochinese grey langur (Trachypithecus crepusculus) . Vietnamese Journal of Primatology. 2009;1(3):1–8. [Google Scholar]
- 40.Liedigk R, Kolleck J, Böker KO, Meijaard E, Md-Zain BM, Abdul-Latiff MAB, et al Mitogenomic phylogeny of the common long-tailed macaque (Macaca fascicularis fascicularis) . BMC Genomics. 2015;16(1):222. doi: 10.1186/s12864-015-1437-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liedigk R, Yang MY, Jablonski NG, Momberg F, Geissmann T, Lwin N, et al Evolutionary history of the odd-nosed monkeys and the phylogenetic position of the newly described Myanmar snub-nosed monkey Rhinopithecus strykeri . PLoS One. 2012;7(5):e37418. doi: 10.1371/journal.pone.0037418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu ZJ, Wang BS, Nadler T, Liu GJ, Sun T, Huang CM, et al Relatively recent evolution of pelage coloration in Colobinae: phylogeny and phylogeography of three closely related langur species. PLoS One. 2013;8(4):e61659. doi: 10.1371/journal.pone.0061659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu ZJ, Zhang LY, Yan ZZ, Ren ZJ, Han FM, Tan XX, et al Genomic mechanisms of physiological and morphological adaptations of limestone langurs to karst habitats. Molecular Biology and Evolution. 2020;37(4):952–968. doi: 10.1093/molbev/msz301. [DOI] [PubMed] [Google Scholar]
- 44.Miller KG, Kominz MA, Browning JV, Wright JD, Mountain GS, Katz ME, et al The Phanerozoic record of global sea-level change. Science. 2005;310(5752):1293–1298. doi: 10.1126/science.1116412. [DOI] [PubMed] [Google Scholar]
- 45.Minh BQ, Nguyen MAT, von Haeseler A Ultrafast approximation for phylogenetic bootstrap. Molecular Biology and Evolution. 2013;30(5):1188–1195. doi: 10.1093/molbev/mst024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mittermeier RA, Rylands AB, Wilson DE. 2013. Handbook of the Mammals of the World. Volume 3: Primates. Barcelona: Lynx Edicions.
- 47.Murray NJ, Keith DA, Tizard R, Duncan A, Htut WT, Hlaing N, et al. 2020. Threatened Ecosystems of Myanmar. An IUCN Red List of Ecosystems Assessment. Version 1.0. Yangon: Wildlife Conservation Society.
- 48.Nadler T, Momberg F, Dang NX, Lormee N. 2003. Vietnam Primate Conservation Status Review 2002. Part 2: Leaf Monkeys. Hanoi: Fauna & Flora International – Vietnam Program and Frankfurt Zoological Society.
- 49.Nadler T, Walter L, Roos C Molecular evolution, systematics and distribution of the taxa within the silvered langur species group (Trachypithecus [cristatus]) in Southeast Asia . Zoologischer Garten (NF) 2005;75(4):238–247. [Google Scholar]
- 50.Napier JR, Napier PH. 1967. A Handbook of Living Primates. London: Academic Press.
- 51.Napier JR, Napier PH. 1994. The Natural History of the Primates. London: MIT Press.
- 52.Napier PH. 1985. Catalogue of Primates in the British Museum (Natural History) and Elsewhere in the British Isles. Part III: Family Cercopithecidae, Subfamily Colobinae. London: British Museum (Natural History).
- 53.Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution. 2015;32(1):268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oates JF, Davies AG, Delson E. 1994. The diversity of living colobines. In: Davies AG, Oates JF. Colobine Monkeys: Their Ecology, Behaviour and Evolution. Cambridge: Cambridge University Press, 45–73.
- 55.Osterholz M, Walter L, Roos C Phylogenetic position of the langur genera Semnopithecus and Trachypithecus among Asian colobines, and genus affiliations of their species groups . BMC Evolutionary Biology. 2008;8:58. doi: 10.1186/1471-2148-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perelman P, Johnson WE, Roos C, Seuánez HN, Horvath JE, Moreira MAM, et al A molecular phylogeny of living primates. PLoS Genetics. 2011;7(3):e1001342. doi: 10.1371/journal.pgen.1001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pocock RI The langurs, or leaf monkeys, of British India. Part II. The Journal of the Bombay Natural History Society. 1928;32(3-4):660–677. [Google Scholar]
- 58.Pocock RI. 1939. The Fauna of British India, Including Ceylon and Burma. Mammalia, Volume 1. London: Taylor and Francis.
- 59.Quyet LK, Lin AK, Oo KN, Naing KK. 2019. Survey of Shan States Langur (Trachypithecus Phayrei ssp. Shanicus) in Panlaung-Pyadalin Cave Wildlife Sanctuary and Myogyi Monastery, Shan State, Myanmar. Yangon: Fauna & Flora International – Myanmar Programme.
- 60.Ram MS, Kittur SM, Biswas J, Nag S, Shil J, Umapathy G Genetic diversity and structure among isolated populations of the endangered Gees Golden Langur in Assam, India. PLoS One. 2016;11(8):e0161866. doi: 10.1371/journal.pone.0161866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rohland N, Siedel H, Hofreiter M Nondestructive DNA extraction method for mitochondrial DNA analyses of museum specimens. BioTechniques. 2004;36(5):814–821. doi: 10.2144/04365ST05. [DOI] [PubMed] [Google Scholar]
- 62.Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, et al MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology. 2012;61(3):539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roos C. 2021. Taxonomic classification of colobine monkeys. In: Matsuda I, Grueter CC, Teichroeb JA. The Colobines: Natural History, Behaviour and Ecological Diversity. Berlin: Springer.
- 64.Roos C, Boonratana R, Supriatna J, Fellowes JR, Groves CP, Nash SD, et al An updated taxonomy and conservation status review of Asian primates. Asian Primates Journal. 2014;4(1):2–38. [Google Scholar]
- 65.Roos C, Kothe M, Alba DM, Delson E, Zinner D The radiation of macaques out of Africa: Evidence from mitogenome divergence times and the fossil record. Journal of Human Evolution. 2019b;133:114–132. doi: 10.1016/j.jhevol.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 66.Roos C, Liedigk R, Thinh VN, Nadler T, Zinner D The hybrid origin of the Indochinese gray langur Trachypithecus crepusculus . International Journal of Primatology. 2019a;40(1):9–27. doi: 10.1007/s10764-017-0008-4. [DOI] [Google Scholar]
- 67.Roos C, Nadler T, Walter L Mitochondrial phylogeny, taxonomy and biogeography of the silvered langur species group (Trachypithecus cristatus) . Molecular Phylogenetics and Evolution. 2008;47(2):629–636. doi: 10.1016/j.ympev.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 68.Roos C, Thanh VN, Walter L, Nadler T Molecular systematics of Indochinese primates. Vietnamese Journal of Primatology. 2007;1(1):41–53. [Google Scholar]
- 69.Roos C, Zinner D. 2021. Molecular phylogeny and phylogeography of colobines. In: Matsuda I, Grueter CC, Teichroeb JA. The Colobines: Natural History, Behaviour and Ecological Diversity. Berlin: Springer.
- 70.Roos C, Zinner D, Kubatko LS, Schwarz C, Yang MY, Meyer D, et al Nuclear versus mitochondrial DNA: evidence for hybridization in colobine monkeys. BMC Evolutionary Biology. 2011;11(1):77. doi: 10.1186/1471-2148-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rowe N, Myers M. 2016. All the World's Primates. New York: Pogonias Press.
- 72.RStudio Team. 2020 [2020-05-18]. RStudio: integrated development for https://www.amazon.de/All-Worlds-Primates-Noel-Rowe/dp/1940496063R. http://www.rstudio.com/.
- 73.Thant NML. 2013. Molecular Phylogenetic Status and Ecological Characteristics of Phayre’s Leaf Monkey (Trachypithecus phayrei) in Myanmar. Ph.D. Dissertation, Seoul National University.
- 74.Thant NML, Kim BJ, Ko HS, Yi KM, Lee WS Molecular phylogenetic analysis of non-invasive samples for the endangered Phayre’s leaf monkey (Trachypithecus phayrei) in Popa Mountain Park, Central Myanmar . Journal of Animal and Veterinary Advances. 2013;12(5):626–632. [Google Scholar]
- 75.Thinh VN, Mootnick AR, Geissmann T, Li M, Ziegler T, Agil M, et al Mitochondrial evidence for multiple radiations in the evolutionary history of small apes. BMC Evolutionary Biology. 2010a;10(1):74. doi: 10.1186/1471-2148-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thinh VN, Rawson B, Hallam C, Kenyon M, Nadler T, Walter L, et al Phylogeny and distribution of crested gibbons (genus Nomascus) based on mitochondrial cytochrome b gene sequence data . American Journal of Primatology. 2010b;72(12):1047–1054. doi: 10.1002/ajp.20861. [DOI] [PubMed] [Google Scholar]
- 77.Wang BS, Zhou XM, Shi FL, Liu ZJ, Roos C, Garber PA, et al Full-length Numt analysis provides evidence for hybridization between the Asian colobine genera Trachypithecus and Semnopithecus . American Journal of Primatology. 2015;77(8):901–910. doi: 10.1002/ajp.22419. [DOI] [PubMed] [Google Scholar]
- 78.Wang XP, Yu L, Roos C, Ting N, Chen CP, Wang J, et al Phylogenetic relationships among the colobine monkeys revisited: new insights from analyses of complete mt genomes and 44 nuclear non-coding markers. PLoS One. 2012;7(4):e36274. doi: 10.1371/journal.pone.0036274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wangchuk T, Inouye DW, Hare MP The emergence of an endangered species: evolution and phylogeny of the Trachypithecus geei of Bhutan . International Journal of Primatology. 2008;29(3):565–582. doi: 10.1007/s10764-008-9258-5. [DOI] [Google Scholar]
- 80.Weitzel V, Groves CP The nomenclature and taxonomy of the colobine monkeys of Java. International Journal of Primatology. 1985;6(4):399–409. doi: 10.1007/BF02736386. [DOI] [Google Scholar]
- 81.Wroughton RC A new “leaf monkey” from the Shan States. The Journal of the Bombay Natural History Society. 1917;25(1):46–48. [Google Scholar]
- 82.Wroughton RC The Shan states langur – a correction. The Journal of the Bombay Natural History Society. 1918;25(3):361. [Google Scholar]
- 83.Wroughton RC Bombay Natural History Society’s mammal survey of India, Burma and Ceylon, Report No.35. The Journal of the Bombay Natural History Society. 1921;27(1):553–554. [Google Scholar]
- 84.Zhang YP, Ryder OA Mitochondrial cytochrome b gene sequences of Old World monkeys: with special reference on evolution of Asian colobines. Primates. 1998;39(1):39–49. doi: 10.1007/BF02557742. [DOI] [Google Scholar]
- 85.Zinner D, Fickenscher GH, Roos C. 2013. Family Cercopithecidae (Old World monkeys). In: Mittermeier RA, Rylands AB, Wilson DE. Handbook of the Mammals of the World. Volume 3: Primates. Barcelona: Lynx Edicions, 550–627.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.
Data Availability Statement
Mitochondrial genome sequences were submitted to GenBank and are available under accession Nos. MT806030–MT806070.