Summary
Introduction
Both cervical (McKeown) and intrathoracic (Ivor Lewis) anastomosis of transthoracic esophagectomy are surgical procedures that can be performed for distal esophageal or gastro-esophageal junction (GEJ) cancer. The purpose of this study was to investigate the long-term health-related quality of life (HR-QoL) after McKeown and Ivor Lewis esophagectomy in a tertiary referral center.
Methods
Disease-free patients >1 year following a McKeown or an Ivor Lewis esophagectomy with a two-field lymphadenectomy for a distal or GEJ carcinoma visiting the outpatient clinic between 2014 and 2018 were asked to complete the EORTC QLQ-C30 and EORTC QLQ-OG25 questionnaires. HR-QoL was investigated in both groups.
Results
A total of 89 patients were included after McKeown and 115 after Ivor Lewis esophagectomy. Median follow-up was 2.4 years (IQR 1.7–3.6). Patients after McKeown esophagectomy reported more problems with ‘eating with others’ compared to patients after Ivor Lewis esophagectomy (mean scores: 49.9 vs. 38.8). This difference was both clinically relevant and significant after correction for multiple testing (β = 11.1, 95% CI 3.105–19.127, P = 0.042). Patients in both groups reported a poorer HR-QoL (≥10 points) than the general population with respect to nausea and vomiting, dyspnea, appetite loss, financial difficulties, problems with eating, reflux, eating with others, choked when swallowing, trouble with coughing, and weight loss.
Conclusion
Long-term HR-QoL of disease-free patients following a McKeown or Ivor Lewis esophagectomy for a distal or GEJ carcinoma is largely comparable. Irrespective of the surgical technique, patients’ HR-QoL following esophagectomy is compromised. When given the choice, patients should be informed that after a McKeown esophagectomy more problems while eating with others can occur.
Keywords: esophageal neoplasms, esophagectomy, quality of life, postoperative complications
INTRODUCTION
Treatment of esophageal cancer usually consists of surgery with neoadjuvant or perioperative chemo(radio)therapy. In most cases, a transthoracic esophagectomy with gastric tube reconstruction is performed with either an intrathoracic (Ivor Lewis) or cervical anastomosis (McKeown).1 Whether a cervical or intrathoracic anastomosis is performed mainly depends on the surgeon’s experience, since in the Netherlands, both operations are still standard of care for patients with a distal esophageal or gastro-esophageal junction (GEJ) carcinoma. Both procedures are associated with considerable postoperative morbidity and an impairment in health-related quality of life (HR-QoL) with symptoms of reflux, dysphagia, and fatigue.2–4 Whether HR-QoL differs between a McKeown and Ivor Lewis esophagectomy is not well studied. A recent systematic review found significantly more anastomotic leakages following a McKeown esophagectomy, which may be due to the longer gastric tube with likely a more impaired perfusion at the tip of the gastric tube than in the shorter gastric tube after Ivor Lewis.5 Such postoperative morbidity could have an adverse effect on long-term HR-QoL.6 However, this meta-analysis included mainly small retrospective cohort studies that employed different definitions of outcome parameters. Moreover, during a McKeown esophagectomy the recurrent laryngeal nerve in the cervical region may be damaged, leading to hoarseness and swallowing problems.7 The consequences of recurrent laryngeal nerve injury will likely negatively impact QoL, however, this has not yet been investigated. Studies comparing Ivor Lewis and McKeown with regard to HR-QoL did not find significant differences between the two procedures,4,8 except for one study where significantly more pain and obstipation after Ivor Lewis esophagectomy was observed.9
The aim of this study was to investigate the long-term HR-QoL in disease-free patients having undergone either a transthoracic esophagectomy with gastric tube reconstruction with a cervical anastomosis (McKeown) or an intrathoracic anastomosis (Ivor Lewis) for a distal esophageal or GEJ carcinoma in a tertiary referral center.
METHODS
Study design, patient population, and clinical data
A prospective cohort study was performed in the Amsterdam UMC (location AMC). All patients attending the outpatient clinic >1 year after a McKeown or an Ivor Lewis esophagectomy for a distal esophageal or GEJ carcinoma, in the period between 2014 and 2018, were eligible. After giving oral informed consent, patients were asked to complete quality-of-life questionnaires. Exclusion criteria were a mid- or proximal esophageal tumor, cervical lymph node metastases, salvage esophagectomy, jejunal or colonic interposition, or recurrence or death during follow-up. In these patients with a distal or GEJ tumor, technically both a McKeown and an Ivor Lewis esophagectomy are possible.
All clinical data (baseline patient, tumor, treatment characteristics, and postoperative morbidity variables) for this study were obtained from a prospectively maintained database of all surgical patients with esophageal or gastric cancer from the Amsterdam UMC (location AMC). Age, gender, tumor location, comorbidities (cardiovascular, pulmonal, or metabolic), ASA classification, neoadjuvant therapy (chemotherapy/chemoradiotherapy), surgical approach (open/minimally invasive), cTNM stage, adjuvant therapy (chemotherapy/chemoradiotherapy), histologic tumor type (adenocarcinoma, squamous cell carcinoma, or other), (y)pTNM stage, radicality of surgery, (positive) lymph node yield, and tumor response after neoadjuvant therapy were recorded. Anastomotic leakage, pneumonia, atrial fibrillation, recurrent nerve palsy, other complications, and Clavien-Dindo grade were also recorded according to the ECCG criteria.10
The need for ethical approval was waived by the Institutional Review Board of the Amsterdam UMC (location AMC) and therefore written informed consent was not needed. To strengthen the reporting of results and composition of this article, the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist was used.11
Surgery and (neo)adjuvant therapy
All patients were discussed during the weekly Multidisciplinary Team meeting at the Gastrointestinal Oncology Centre Amsterdam (GIOCA). Operations were minimally invasive or open depending on tumor characteristics (open in case of close relation to trachea or bulky paratracheal lymph nodes), patient characteristics (open in case of previous open surgery or gastric surgery), and time period (before and after implementation of minimally invasive surgery in 2009). A two-field lymphadenectomy (lymph node stations 2 on indication, 4, 7, 8, 9, 15–20 according to the 8th edition of the AJCC) was performed with a gastric tube reconstruction and a cervical or intrathoracic anastomosis. The location of the anastomosis mainly depended on the time period that patients were operated. Before 2013, a McKeown procedure was the preferred operation—also for distal and GEJ cancer. In 2013, the (minimally invasive) Ivor Lewis procedure was adopted and became the standard approach for distal and GEJ cancer. Chemoradiotherapy was administered according to the CROSS schedule if indicated (≥cT2N0-3 M0 or cT1N+).12 If tumor involvement in the stomach was >2 cm, perioperative chemotherapy was generally administered.
Follow-up
All patients completed the questionnaires during postoperative out-patient clinic visits, varying from 1 to 6 years postoperatively. During these visits, a medical history and physical examination were performed with additional imaging only in case of complaints or if disease recurrence was suspected (in accordance with the Dutch guideline13).
Endpoints: HR-QoL
For the evaluation of HR-QoL, the EORTC QLQ-C30, and EORTC QLQ-OG25 questionnaires were used.14 The EORTC QLQ-C30 is validated for cancer patients. It consists of 28 questions employing response categories ranging from 1 (not at all) to 4 (very much) and two questions with response options ranging from 1 (very poor) to 7 (excellent). Fifteen HR-QoL domain scores are calculated from this questionnaire. The EORTC QLQ-OG25 is designed and validated for GEJ cancer patients and consists of 25 questions of which 16 HR-QoL domains are calculated.
The EORTC scoring system was used.15–17 All answers were linearly transformed to scores ranging from 0 to 100. Mean values of HR-QoL domains were calculated for each surgical approach separately. A higher mean score in ‘global health’ and functioning domains represent better QoL and functioning. A higher mean symptom score represents higher level of symptoms.
Statistical analysis
Background (baseline and postoperative morbidity) characteristics were analyzed with Chi square or Fischer’s exact tests when appropriate in case of categorical variables. In case of continuous variables, Student‘s t-test or Mann–Whitney U test was used for, respectively, normally distributed or not normally distributed variables.
The differences in QoL (sub)domains between McKeown and Ivor Lewis esophagectomies were analyzed using univariable and multivariable linear regression analysis. QoL (sub)domains with P < 0.10 in the univariable linear regression analysis were entered in multivariable linear regression analysis. Background variables showing a difference (P < 0.10) between the surgical groups were tested for confounding. If a variable caused a clinically relevant effect (>10% change in regression coefficient), this variable was considered a confounder and was added to the multivariable model. The Bonferroni method was used to correct for multiple testing after multivariable linear regression analysis by multiplying the P-value by the number of multivariable tests performed. The HR-QoL of both groups was compared to the HR-QoL of the general population using the EORTC reference values manual.18,19 Mean score differences of ≥10 points were considered meaningful. Also, a subgroup analysis was performed for patients with no or minor postoperative complications (Clavien-Dindo grade 0–2), to exclude the influence of severe postoperative complications on HR-QoL. Two-sided test was used and statistical significance was set at a P-value <0.05. Again, a difference of ≥10 points in mean scores of the HR-QoL domains was considered clinically relevant according to EORTC guideline.20 SPSS Statistics version 24 was used for all statistical analyses.
RESULTS
Patients
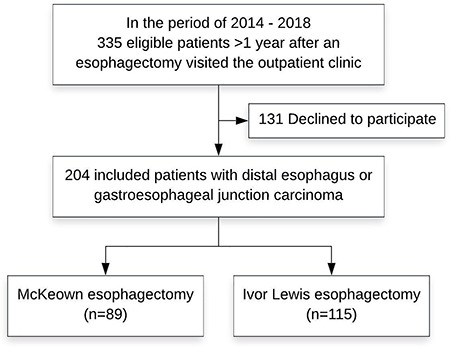
Baseline characteristics are displayed in Table 1. A total of 204 of 335 patients were included (response rate 60.9%). Clinical information of patients who declined participation was not recorded due to data protection regulations. Eighty-nine patients were treated with a McKeown esophagectomy and 115 with an Ivor Lewis esophagectomy (Fig. 1). Median age was 65 years (interquartile range [IQR] 58–71), the majority of patients were men (77.9%) and had a distal esophageal adenocarcinoma (84.8%) and an ASA classification of 2 (48.5%). Median follow-up was 3.3 years (IQR 2.0–4.1) following McKeown and 2.1 (IQR 1.5–2.9) following Ivor Lewis (P < 0.001). There was no significant difference in comorbidities between the two groups. Significantly more minimally invasive surgery was performed in the Ivor Lewis group compared to McKeown group (96.5 vs. 84.3%, P = 0.002). Chemoradiotherapy was the preferred neoadjuvant therapy in both groups (P = 0.649). Significantly more anastomotic leakages (24.7 vs. 8.7%; P = 0.002) and recurrent nerve palsy (7.9 vs. 1.7%, P = 0.043) occurred after a McKeown esophagectomy. No significant difference was found in the incidence of atrial fibrillation, pneumonia or other complications, and Clavien-Dindo grade between the two groups (Table 2).
Table 1.
Background baseline characteristics
| Total | McKeown | Ivor Lewis | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | 204 | 89 | 115 | ||||||
| Age (median (IQR), y) | 65 | (58–71) | 64 | (59.5–72) | 67 | (56–69) | 0.011 | ||
| Gender | Male | 159 | (77.9) | 64 | (71.9) | 95 | (82.6) | 0.068 | |
| Tumor location | Distal esophagus | 173 | (84.8) | 75 | (84.3) | 98 | (85.2) | 0.852 | |
| GEJ | 31 | (15.2) | 14 | (15.7) | 17 | (14.8) | |||
| Comorbidity | No | 103 | (50.5) | 50 | (47.2) | 61 | (53.0) | 0.407 | |
| Cardiovascular | 86 | (42.2) | 39 | (43.8) | 47 | (40.9) | 0.672 | ||
| Pulmonal | 14 | (6.9) | 8 | (9.0) | 6 | (5.2) | 0.291 | ||
| Metabolic | 22 | (10.8) | 10 | (11.2) | 12 | (10.4) | 0.855 | ||
| ASA classification | 1 | 63 | (30.9) | 23 | (25.8) | 40 | (34.8) | 0.240 | |
| 2 | 99 | (48.5) | 49 | (55.1) | 50 | (43.5) | |||
| 3 | 42 | (20.6) | 17 | (19.1) | 25 | (21.7) | |||
| Neoadjuvant therapy | No | 34 | (16.7) | 20 | (22.5) | 14 | (12.2) | 0.059 | |
| Yes | Chemotherapy | 5 | (2.5) | 1 | (1.1) | 4 | (3.5) | 0.649 | |
| Chemoradiotherapy | 165 | (80.9) | 68 | (76.4) | 97 | (84.3) | |||
| Approach | Open | 18 | (8.8) | 14 | (15.7) | 4 | (3.5) | 0.002 | |
| Minimally invasive | 186 | (91.2) | 75 | (84.3) | 111 | (96.5) | |||
| cT | T0 | 6 | (2.9) | 5 | (5.6) | 1 | (0.9) | 0.298 | |
| T1 | 29 | (14.2) | 14 | (15.7) | 15 | (13.0) | |||
| T2 | 53 | (26.0) | 24 | (27.0) | 29 | (25.2) | |||
| T3 | 114 | (55.9) | 44 | (49.4) | 70 | (60.9) | |||
| T4 | 2 | (1.0) | 2 | (2.2) | 0 | 0 | |||
| cN | N0 | 90 | (44.1) | 38 | (42.7) | 52 | (45.2) | 0.680 | |
| N1 | 76 | (37.3) | 32 | (36.0) | 44 | (38.3) | |||
| N2 | 35 | (17.2) | 19 | (21.3) | 16 | (13.9) | |||
| N3 | 3 | (1.5) | 0 | 0 | 3 | (2.6) | |||
| cM | cM0 | 204 | (100) | 89 | (100) | 115 | (100) | 1.000 | |
| Adjuvant therapy | No | 179 | (87.7) | 86 | (96.6) | 93 | (80.9) | 0.001 | |
| Yes | Chemotherapy | 22 | (10.8) | 2 | (2.2) | 20 | (17.4) | 0.330 | |
| Chemoradiotherapy | 3 | (1.5) | 1 | (1.1) | 1 | (1.7) | |||
| Histologic type | Adenocarcinoma | 164 | (80.4) | 60 | (67.4) | 103 | (89.6) | <0.001 | |
| Squamous cell carcinoma | 33 | (16.2) | 24 | (27.0) | 9 | (7.8) | |||
| Other | 7 | (3.4) | 4 | (4.5) | 3 | (2.6) | |||
| pT | T0 | 55 | (27.0) | 23 | (25.8) | 32 | (27.8) | 0.491 | |
| T1 | 55 | (27.0) | 28 | (31.5) | 27 | (23.5) | |||
| T2 | 25 | (12.3) | 12 | (13.5) | 13 | (11.3) | |||
| T3 | 69 | (33.8) | 26 | (29.2) | 43 | (37.4) | |||
| T4 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| pN | N0 | 140 | (68.6) | 64 | (71.9) | 76 | (66.1) | 0.487 | |
| N1 | 45 | (22.1) | 19 | (21.3) | 26 | (22.6) | |||
| N2 | 9 | (4.4) | 4 | (4.5) | 5 | (4.3) | |||
| N3 | 10 | (4.9) | 2 | (2.2) | 8 | (7.0) | |||
| c/pM | cM1 | 2 | (1.0) | 1 | (0.9) | 1 | (1.1) | 1.000 | |
| Radicality | R0 | 202 | (99.0) | 89 | (100) | 113 | (98.3) | 1.000 | |
| R1 | 2 | (1.0) | 0 | 0 | 2 | (1.7) | |||
| Lymph nodes (median (IQR)) | 26 | (20–37) | 23 | (17–31) | 31 | (22–39) | <0.001 | ||
| Lymph node metastases (median (IQR)) | 0 | (0–1) | 0 | (0–1) | 0 | (0–1) | 0.378 | ||
| Tumor response after neoadjuvant therapy | 1 | 45 | (26.5) | 17 | (24.6) | 28 | (27.7) | 0.144 | |
| 2 | 47 | (27.6) | 25 | (36.2) | 22 | (21.8) | |||
| 3 | 42 | (24,7) | 14 | (20.3) | 28 | (27.7) | |||
| 4 | 30 | (17.6) | 11 | (15.9) | 19 | (18.8) | |||
| 5 | 6 | (3.5) | 2 | (2.9) | 4 | (4.0) | |||
n (%) unless otherwise indicated; y, years; IQR, interquartile range. TNM staging classification (AJCC 8th edition). Bold values are significant.
Fig. 1.
Study flow chart 104 × 82 mm (300 × 300 DPI).
Table 2.
Background characteristics: postoperative morbidity
| McKeown (N = 89) | Ivor Lewis (N = 115) | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Postoperative complications | No | 41 | (46.1) | 64 | (55.7) | 0.174 | ||
| Yes | 48 | (53.9) | 51 | (44.3) | ||||
| Anastomotic leakage | 22 | (24.7) | 10 | (8.7) | 0.002 | |||
| Treatment with antibiotics | 3 | (13.6) | 0 | 0 | ||||
| Percutaneous drainage | 11 | (50.0) | 1 | (10.0) | ||||
| Endoscopic management | 3 | (13.6) | 3 | (30.0) | ||||
| Reoperation with preservation of anastomosis | 4 | (18.2) | 6 | (60.0) | ||||
| Reoperation with resection of the anastomosis | 1 | (4.5) | 0 | 0 | ||||
| Atrial fibrillation | 17 | (19.1) | 24 | (20.9) | 0.731 | |||
| Pneumonia | 12 | (13.5) | 13 | (11.3) | 0.638 | |||
| Recurrent nerve palsy | 7 | (7.9) | 2 | (1.7) | 0.043 | |||
| Other | 19 | (21.3) | 22 | (19.1) | 0.695 | |||
| Clavien-Dindo classification | Grade 0 | 39 | (43.8) | 59 | (51.3) | 0.139 | ||
| Grade 1 | 4 | (4.5) | 9 | (7.8) | ||||
| Grade 2 | 24 | (27.0) | 13 | (11.3) | ||||
| Grade 3A | 11 | (12.4) | 16 | (13.9) | ||||
| Grade 3B | 0 | 0 | 1 | (0.9) | ||||
| Grade 4A | 9 | (10.1) | 14 | (12.2) | ||||
| Grade 4B | 2 | (2.2) | 3 | (2.6) | ||||
n (%) unless otherwise indicated. Bold values are significant.
Endpoints: HR-QoL domains
After univariable analysis of all HR-QoL functioning and symptom scores a P-value of <0.10 was found in ‘financial difficulties’, ‘dysphagia’, ‘eating’, ‘odynophagia’, ‘anxiety’, and ‘eating with others’ domains. These HR-QoL domains were then entered in the multivariable analysis and were corrected for age, gender, neoadjuvant therapy (yes/no), surgical approach (open/minimally invasive), adjuvant therapy (yes/no), histologic tumor type (adenocarcinoma, squamous cell carcinoma or other), lymph node yield, anastomotic leakage, recurrent nerve palsy and/or follow-up (Supplementary xref Table S1). Patients after McKeown esophagectomy reported significantly more problems with eating with others compared to patients after Ivor Lewis esophagectomy (mean scores: 49.9 vs. 38.8; P = 0.042). This difference was clinically relevant as the difference between these two scores was 11.1 points. No other domains had significant mean score differences of >10 points (Table 3 and Supplementary data Table S1).
Table 3.
Univariable and multivariable linear regression analysis of EORTC QLQ-C30 and EORTC QLQ-OG25 questionnaires’ domains
| Univariable analysis | Multivariable analysis | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| McKeown | Ivor Lewis | B | 95%CI | P-value | McKeown† | Ivor Lewis† | B | 95% CI | P-value | P-value corrected‡ | |||
| n = 89 | n = 115 | Lower | Upper | n = 89 | n = 115 | Lower | Upper | ||||||
| EORTC QLQ-C30 | |||||||||||||
| Global health | 72.3 (19.5) | 74.3 (19.5) | 2.0 | −3.452 | 7.437 | 0.471 | |||||||
| Functioning | |||||||||||||
| Physical functioning | 79.3 (18.7) | 83 (19.3) | 3.6 | −1.680 | 8.932 | 0.179 | |||||||
| Role functioning | 74.5 (27.6) | 76.7 (27.6) | 2.1 | −5.576 | 9.855 | 0.585 | |||||||
| Emotional functioning | 80.8 (21.8) | 81.4 (23) | 0.5 | −5.739 | 6.816 | 0.866 | |||||||
| Cognitive functioning | 83.2 (20.1) | 84.9 (21.5) | 1.7 | −4.149 | 7.527 | 0.569 | |||||||
| Social functioning | 79.3 (25.9) | 81.1 (25.3) | 1.7 | −5.398 | 8.837 | 0.634 | |||||||
| Symptom scores | |||||||||||||
| Fatigue | 30.5 (24.6) | 32.9 (26.7) | −2.4 | −9.538 | 4.674 | 0.501 | |||||||
| Nausea and vomiting | 15.4 (21.7) | 13.3 (20.3) | −2.1 | −7.888 | 3.784 | 0.489 | |||||||
| Pain | 12.5 (21.3) | 16.9 (22.9) | 4.4 | −1.858 | 10.567 | 0.168 | |||||||
| Dyspnea | 30.3 (32.4) | 24.1 (26.7) | −6.3 | −14.658 | 2.098 | 0.141 | |||||||
| Insomnia | 23.6 (29.8) | 24.7 (30.6) | 1.1 | −7.304 | 9.533 | 0.794 | |||||||
| Appetite loss | 18.9 (28.3) | 15.7 (26) | −3.2 | −10.748 | 4.353 | 0.405 | |||||||
| Constipation | 11.1 (19.5) | 8.4 (17.6) | −2.7 | −7.864 | 2.454 | 0.302 | |||||||
| Diarrhea | 18.6 (24.7) | 15.1 (23.5) | −3.5 | −10.191 | 3.215 | 0.306 | |||||||
| Financial difficulties | 31.4 (31.1) | 23.7 (26.9) | −7.7 | −15.753 | 0.402 | 0.062* | 60.4 | 59.1 | 1.3 | −7.697 | 10.276 | 0.777 | 4.662 |
| EORTC QLQ-OG25 | |||||||||||||
| Functioning | |||||||||||||
| Body image | 84.6 (27.1) | 81.5 (30) | −3.1 | −11.138 | 4.944 | 0.449 | |||||||
| Symptom scores | |||||||||||||
| Dysphagia | 12.9 (17.7) | 8.9 (15.6) | −4.0 | −8.589 | 0.679 | 0.094* | 7.5 | 4.7 | 2.8 | −2.507 | 8.046 | 0.302 | 1.812 |
| Eating | 29.5 (26.4) | 22.5 (23.7) | −7.0 | −13.960 | 0.005 | 0.050* | 45.4 | 38.9 | 6.5 | −1.947 | 15.021 | 0.130 | 0.780 |
| Reflux | 24.6 (29.2) | 20.8 (28.1) | −3.9 | −11.908 | 4.171 | 0.344 | |||||||
| Odynophagia | 12.4 (20.3) | 7.8 (17.3) | −4.6 | −9.957 | 0.813 | 0.096* | 20.0 | 15.0b | 5.0 | −1.454 | 11.441 | 0.128 | 0.768 |
| Pain and discomfort | 16.7 (25.9) | 15.9 (26.1) | −0.8 | −8.063 | 6.547 | 0.838 | |||||||
| Anxiety | 31.8 (30.8) | 24.8 (25.9) | −7.0 | −14.861 | 0.907 | 0.083* | 66.3 | 58.0 | 8.3 | −0.903 | 17.467 | 0.077 | 0.462 |
| Eating with others | 18.2 (28.5) | 7.6 (19) | −10.6 | −17.604 | −3.608 | 0.003* | 49.9 | 38.8 | 11.1 | 3.105 | 19.127 | 0.007 | 0.042 |
| Dry mouth | 19.9 (27.4) | 17.7 (25.9) | −2.1 | −9.601 | 5.374 | 0.578 | |||||||
| Trouble with taste | 11 (22.4) | 11.3 (20.8) | 0.3 | −5.774 | 6.380 | 0.922 | |||||||
| Trouble swallowing saliva | 10.2 (22.8) | 10.2 (21.4) | −0.03 | −6.216 | 6.148 | 0.991 | |||||||
| Choked when swallowing | 14.8 (25.2) | 14.1 (22.3) | −0.7 | −7.341 | 5.976 | 0.840 | |||||||
| Trouble with coughing | 29.2 (29.8) | 31.8 (30.6) | 2.7 | −5.855 | 11.183 | 0.538 | |||||||
| Trouble talking | 9.4 (20.7) | 6 (13.7) | −3.4 | −8.431 | 1.674 | 0.188 | |||||||
| Weight loss | 20.5 (30.3) | 17.6 (26.5) | −3.0 | −10.897 | 5.095 | 0.475 | |||||||
| Hair loss | 19.8 (27.7) | 10.8 (23.4) | −9.1 | −22.377 | 4.223 | 0.177 | |||||||
Better global health and functioning scores but worse symptom scores have high mean values. McKeown and Ivor Lewis data are represented as mean (standard deviation). Regression coefficient (B) with 95% confidence interval (CI).
*HR-QoL domains with P < 0.1 in univariable analysis were entered in multivariable analysis and corrected for confounders (Supplementary data Table S1).
†Mean value of HR-QoL domain after multivariable analysis.
‡Corrected for multiple testing. Bold values represent significance.
When compared to the general population,18,19 patients following McKeown or Ivor Lewis esophagectomies reported a poorer HR-QoL with respect to nausea and vomiting (mean difference is 10.5), dyspnea (mean difference is 15.0), appetite loss (mean difference is 10.4), financial difficulties (mean difference is 17.6), problems with eating (mean difference is 22.7), reflux (mean difference is 15.8), eating with others (mean difference is 11.0), choked when swallowing (mean difference is 10.7), trouble with coughing (mean difference is 17.0) and weight loss (mean difference is 17.1).
A subgroup analysis was performed for patients with no or minor postoperative complications (Clavien-Dindo grade 0–2) (Supplementary data Tables S2 and S3). A total of 148 patients were included: 67 after McKeown and 81 after Ivor Lewis esophagectomy. After univariable analysis of all HR-QoL functioning and symptom scores a P-value of <0.10 was found in ‘global health’, ‘nausea and vomiting’, ‘appetite loss’, ‘financial difficulties’, ‘dysphagia’, ‘eating’, ‘reflux’, ‘odynophagia’, ‘anxiety’, and ‘eating with others’ domains. After multivariable analysis and correction for multiple testing patients with no or minor complications following a McKeown esophagectomy reported significantly more problems with eating with others compared to patients after an Ivor Lewis esophagectomy (mean scores: 47.1 vs. 32.4; P = 0.030). This difference was also clinically relevant with a mean score difference of 14.7 points.
DISCUSSION
This study investigated the long-term HR-QoL in disease-free patients after a McKeown or Ivor Lewis esophagectomy with a two-field lymphadenectomy for tumors where both procedures were technically possible (distal or GEJ carcinoma without the presence of cervical lymph node metastases). The results show that after prolonged follow-up, both surgical patient groups reported a highly comparable HR-QoL. However, after a McKeown esophagectomy, patients reported more problems with eating with others compared to patients after an Ivor Lewis esophagectomy. This difference remained after exclusion of the influence of severe postoperative complications. A subgroup of patients with no or minor postoperative complications also indicated to have trouble with eating with others following a McKeown esophagectomy, a finding that was not affected by major complications. Whereas the results of this study on long-term HR-QoL will not be decisive in choosing the type of surgery, they are useful when informing patients about the possible long-term consequences of these two surgical techniques.
There are only a few studies that investigated the difference in long-term HR-QoL between McKeown and Ivor Lewis esophagectomy in patients with esophageal carcinoma.4,8,9 Overall, our findings are different compared to the literature as we only found one impaired HR-QoL domain after McKeown compared to Ivor Lewis esophagectomy, even in patients with no or minor postoperative complications. In the study of Barbour et al. differences in HR-QoL results were observed after propensity score matching such as more pain and constipation 24 months after open Ivor Lewis esophagectomy compared to thoracoscopically assisted McKeown esophagectomy.9 Two other studies found no significant difference in long-term HR-QoL between open Ivor Lewis and open McKeown. Because the previous studies mainly investigated HR-QoL after open procedures, they do not reflect current practice in most countries. Increasingly, most esophagectomies are performed minimally invasively, since the results of the TIME trial became available showing reduced postoperative morbidity, less pain, and better HR-QoL following a minimally invasive esophagectomy.21 In the current study, a high rate of patients (91.2%) was operated by a minimally invasive approach, thereby reflecting current practice.
Postoperative complications occur in 59–65% of the patients after esophagectomy and the most common complications are anastomotic leakage (11.4–21%), pneumonia (14.6–21%), and atrial dysrhythmia (14.5–15%).22,23 It has recently been shown that complications have a negative impact not only on HR-QoL but also on long-term survival.24 Identifying the surgical approach with the least perioperative morbidity is therefore of great importance, as it may lead to better survival and HR-QoL.6 A recent systematic review with a comprehensive meta-analysis found similar cardiac arrhythmia incidence but a higher incidence of pulmonary complications, anastomotic leakage, and vocal cord injury after minimally invasive McKeown compared to minimally invasive Ivor Lewis esophagectomy.5 This systematic review included patients with esophageal and GEJ carcinoma following minimally invasive McKeown and minimally invasive Ivor Lewis esophagectomies. Likewise, in this current study, all patients had distal or GEJ carcinoma and the majority of patients were operated minimally invasively (91.2%). However, this systematic review did not investigate long-term HR-QoL.
A number of limitations should be addressed. Since this was an observational, nonrandomized study, some of the preoperative characteristics and postoperative morbidity between the two groups were different, including follow-up, age, gender, (neo)adjuvant therapy, surgical approach, histologic tumor type, lymph node yield, occurrence of anastomotic leakage, and occurrence of recurrent nerve palsy. A statistical correction for all of the possible confounders was performed during multivariable linear regression. The majority of the patients (86.4%) following Ivor Lewis esophagectomy received adjuvant chemotherapy because of their participation in the SOX trial (NCT 02347904). Furthermore, this study investigates HR-QoL only in disease-free patients, as patients who did not survive could not have completed the questionnaires. The results of this study are not applicable to patients with cervical lymph node metastases, a more extended radiation field and a more proximal tumor as in these patients an Ivor Lewis esophagectomy could not have been performed, and these patients were excluded from this study. This study applies only to patients in whom both procedures were possible. In addition, station 2 (according to the AJCC 8th edition25) was not a part of the standard lymphadenectomy and was only performed on indication. Unfortunately, it is not possible to exclude selection bias as the reason for patients to decline participation in this study and clinical information of these patients (such as the performed operation) was not recorded, following good clinical practice guidelines, General Data Protection Regulation, and the Medical Contract Bill.26–28 Furthermore, because of the high number of tested outcomes the chance of finding a significant result by coincidence is high. Therefore, a Bonferroni correction for multiple testing was performed. Moreover, clinical relevance of the results was tested. Strengths of the current study are that it has one of the largest sample sizes and one of the longest follow-up compared to other studies.
In conclusion, the present study investigating HR-QoL after Ivor Lewis and McKeown esophagectomy shows a highly comparable HR-QoL in patients with a distal or GEJ carcinoma. Only one HR-QoL domain—more problems in eating with others—was found to be significantly poorer in the McKeown compared to the Ivor Lewis group, even in patients with no or minor postoperative complications. These results apply to disease-free patients in whom both procedures are possible from an oncologic viewpoint. Additionally, irrespective of the surgical technique, patients’ HR-QoL following esophagectomy is compromised. Future studies should not only investigate perioperative morbidity, pathology results and survival, but also long-term HR-QoL in these two procedures in a randomized controlled setting. Currently, such a study is being executed.29
CONFLICT OF INTEREST
E. Jezerskyte, L.M. Saadeh, E.R.C. Hagens, M.A.G. Sprangers, L. Noteboom, W.J. Eshuis, M.C.C.M. Hulshof and S.S. Gisbertz have no conflicts of interest to declare. M.I. van Berge Henegouwen has a consultant role with Mylan, Johnson and Johnson and Medtronic and has research funding from Olympus and Stryker. H.W.M. van Laarhoven has a consultant or advisory role with BMS, Lilly, MSD, Nordic Pharma, Servier and has research funding from Bayer, BMS, Celgene, Janssen, Lilly, Nordic Pharma, Philips, Roche, Servier.
Supplementary Material
References
- 1. DICA Annual Report of the Dutch Upper Gastrointestinal Cancer Audit. Dutch Institute for Clinical Auditing 2019. Available from https://dica.nl/jaarrapportage-2018/duca.
- 2. Smyth E C, Lagergren J, Fitzgerald R C et al. Oesophageal cancer. Nat Rev Dis Primers 2017; 3: 17048–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang Y, Yang X, Geng D, Duan Y, Fu J. The change of health-related quality of life after minimally invasive esophagectomy for esophageal cancer: a meta-analysis. World J Surg Oncol 2018; 16: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Egberts J H, Schniewind B, Bestmann B et al. Impact of the site of anastomosis after oncologic esophagectomy on quality of life—a prospective, longitudinal outcome study. Ann Surg Oncol 2008; 15: 566–75. [DOI] [PubMed] [Google Scholar]
- 5. Deng J, Su Q, Ren Z et al. Comparison of short-term outcomes between minimally invasive McKeown and Ivor Lewis esophagectomy for esophageal or junctional cancer: a systematic review and meta-analysis. Onco Targets Ther 2018; 11: 6057–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anandavadivelan P, Wikman A, Johar A, Lagergren P. Impact of weight loss and eating difficulties on health-related quality of life up to 10 years after oesophagectomy for cancer. Br J Surg 2018; 105(4): 410–8. [DOI] [PubMed] [Google Scholar]
- 7. Mboumi I W, Reddy S, Lidor A O. Complications after esophagectomy. Surg Clin North Am 2019; 99(3): 501–10. [DOI] [PubMed] [Google Scholar]
- 8. Wormald J C, Bennett J, van Leuven M, Lewis M P. Does the site of anastomosis for esophagectomy affect long-term quality of life? Dis Esophagus 2016; 29(1): 93–8. [DOI] [PubMed] [Google Scholar]
- 9. Barbour A P, Cormack O M M, Baker P J et al. Long-term health-related quality of life following esophagectomy: a nonrandomized comparison of thoracoscopically assisted and open surgery. Ann Surg 2017; 265(6): 1158–65. [DOI] [PubMed] [Google Scholar]
- 10. Low D E, Alderson D, Cecconello I et al. International consensus on standardization of data collection for complications associated with esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015; 262(2): 286–94. [DOI] [PubMed] [Google Scholar]
- 11. von Elm E, Altman D G, Egger M, Pocock S J, Gotzsche P C, Vandenbroucke J P. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61(4): 344–9. [DOI] [PubMed] [Google Scholar]
- 12. Shapiro J, van Lanschot J J B, Hulshof M et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015; 16(9): 1090–8. [DOI] [PubMed] [Google Scholar]
- 13. Oncoline Available from: https://www.oncoline.nl/oesofaguscarcinoom. [Accessed June 2019].
- 14. EORTC Available from: http://www.eortc.org/. [Accessed June 2019].
- 15. Lagergren P, Fayers P, Conroy T et al. Clinical and psychometric validation of a questionnaire module, the EORTC QLQ-OG25, to assess health-related quality of life in patients with cancer of the oesophagus, the oesophago-gastric junction and the stomach. Eur J Cancer 2007; 43(14): 2066–73. [DOI] [PubMed] [Google Scholar]
- 16. Fayers P M A N, Bjordal K, Groenvold M, Curran D, Bottomley A, on behalf of the EORTC Quality of Life Group. EORTCQLQ-C30 Scoring Manual, 3rd edn. Brussels, Belgium: European Organisation for Research and Treatment of Cancer, 2001; 2–930064–22-6.
- 17. Aaronson N K, Ahmedzai S, Bergman B et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; 85(5): 365–76. [DOI] [PubMed] [Google Scholar]
- 18. Schaaf M, Derogar M, Lagergren P. Reference values of oesophago-gastric symptoms (EORTCQLQ-OG25) in a population-based setting. Eur J Cancer 2012; 48(11): 1602–7. [DOI] [PubMed] [Google Scholar]
- 19. Scott N W, Fayers P, Aaronson N K, Bottomley A, Graeff A, Groenvold M, EORTC Quality of Life Group . EORTCQLQ-C30 Reference Values Manual, 2nd edn. Brussels, Belgium: European Organisation for Research and Treatment of Cancer, 2008. [Google Scholar]
- 20. Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol 1998; 16(1): 139–44. [DOI] [PubMed] [Google Scholar]
- 21. Straatman J, van der Wielen N, Cuesta M A et al. Minimally invasive versus open esophageal resection: three-year follow-up of the previously reported randomized controlled trial: the TIME trial. Ann Surg 2017; 266(2): 232–6. [DOI] [PubMed] [Google Scholar]
- 22. Werf L R, Busweiler L A D, van Sandick J W, van Berge Henegouwen M I, Wijnhoven B P L. Reporting national outcomes after esophagectomy and gastrectomy according to the Esophageal Complications Consensus Group (ECCG). Ann Surg 2019. Advance online publication. doi: 10.1097/SLA.0000000000003210. [DOI] [PubMed] [Google Scholar]
- 23. Low D E, Kuppusamy M K, Alderson D et al. Benchmarking complications associated with Esophagectomy. Ann Surg 2019; 269(2): 291–8. [DOI] [PubMed] [Google Scholar]
- 24. Werf L R, Wijnhoven B P L, Fransen L F C et al. A national cohort study evaluating the association between short-term outcomes and long-term survival after esophageal and gastric cancer surgery. Ann Surg 2019; 270(5): 868–76. [DOI] [PubMed] [Google Scholar]
- 25. Amin M B E S, Greene F, Byrd D R et al. AJCC Cancer Staging Manual, 8th edn. American Joint Commission on Cancer: Springer International Publishing, 2017. [Google Scholar]
- 26. General Data Protection Regulation (GDPR) 2020. Available from https://gdpr-info.eu/.
- 27. AMC–VUmc Research Code, version effective since October 2013 2020. Available fro: www.amc.nl/researchcode/.
- 28. Good Clinical Practice Guidelines 2020. Available from www.ema.europa.eu.
- 29. Workum F, Bouwense S A, Luyer M D et al. Intrathoracic versus cervical anastomosis after minimally invasive esophagectomy for esophageal cancer: study protocol of the ICAN randomized controlled trial. Trials 2016; 17(1): 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


