Abstract
Cyclospora cayetanensis is an apicomplexan protozoan and is one of the most common pathogens causing chronic diarrhea worldwide. Eight stool samples with diarrheal symptom out of 18 Korean residents who traveled to Nepal were obtained, and examined for 25 enteropathogens including 16 bacterial species, 5 viral species, and 4 protozoans in stool samples as causative agents of water-borne and food-borne disease. Only C. cayetanensis was detected by nested PCR, and 3 PCR-positive samples were sequenced to confirm species identification. However, the oocysts of C. cayetanensis in fecal samples could not be detected by direct microscopy of the stained sample. As far as we know, this is the first report of a group infection with C. cayetanensis from a traveler visiting Nepal, and the second report of a traveler’s diarrhea by C. cayetanensis imported in Korea.
Keywords: Cyclospora cayetanensis, traveler’s diarrhea, protozoa, PCR, Korea
Gut pathogens, including parasites, are the leading cause of infections leading to enteric illness, and the predominant pathogenic organisms may vary temporally and spatially [1]. Cyclospora cayetanensis among these diverse gut pathogens has emerged as one of the most common apicomplexan protozoan causing chronic diarrhea illness worldwide [2]. C. cayetanensis initially known as coccidian-like body, large Cryptosporidium or small isospora-like protozoan. Like other diarrhea-causing pathogens, C. cayetanensis mainly causes acute watery diarrhea and abdominal pain, nausea and low grade of fever. Species of genus Cyclospora is known as host-specific. The human is known to only hosts of C. cayetanensis and become infected by ingesting food or water contaminated by oocysts. Moreover, this pathogen has been associated with cases of international traveler’s diarrhea globally [3]. Epidemiologically, it is seasonal world wide, even though there are regional changes. Its main diagnostic methods were oocyst detection with modified acid-fast staining by microscopy and molecular detection by PCR in fecal samples [4]. Moreover, also, this pathogen has been associated with cases of international traveler’s diarrhea globally [5,6].
In recent years, this pathogen has been increasingly observed both in developed countries, such as the United States of America, United Kingdom and Canada [7–9], and in developing countries such as India, Nepal, Papua New Guinea, Vietnam, and Indonesia [7,10–14]. Especially, Nepal is one of the South-Asia countries with highly prevalent of intestinal pathogens and showed a higher prevalence of C. cayetanensis in drinking water, sewage water, green leafy vegetables, and school children [15–18]. In Korea, only one imported case with cyclosporiasis was reported from an individual infected after visiting Indonesia [19]. We report here the first case of a group infection with C. cayetanensis among Korean travelers returning from Nepal.
Eighteen Korean residents traveled to Nepal for 9 days (April 1–9, 2013) together through a tour company. Among them, one person who had continuous diarrhea after returning to Korea visited the Community Health Care Center (CHCC) in Sokcho-si, Gangwon-do, Korea. Her chief clinical complaint was chronic watery diarrhea continuing for 3–5 days with a frequency of 1–2 times a day. Through a clinical consultation at CHCC, a diarrheal event with her colleagues who traveled to Nepal recognized, and all of them were examined to epidemiological investigation as an outbreak of water-borne or foodborne disease. After the consultation, its outbreak of diarrhea was reported through the surveillance system for mass food poisoning in Korea Centers for Disease Control and Prevention (KCDC).
After consultation of the first patient, additional stool samples were collected from her 7 colleagues with consent to epidemiological investigation about 5 days later. According to Guideline for Water-borne and Foodborne Diseases Control in KCDC, a total of 25 species pathogens, 16 bacterial species including Vibrio cholerae, Shigella spp, and pathogenic Escherichia coli, 5 viral species including Rotavirus and Norovirus, and 4 protozoans including Giardia lamblia and Cryptosporidium parvum/hominis, were targeted to examine [20]. The bacterial and viral pathogens were tested by the Public Health and Environment Research Institute (PHERI) in Gangwon-do, and protozoans were examined by the Division of Vectors & Parasitic Diseases (DVPD), KCDC. To detect water-borne pathogens from the stool samples, all stool samples were smeared on glass slides using the formalin-ether method and stained with modified acid-fast staining (MAFS) under microscopic observation for 3 times repeating the examination. Moreover, molecular detection method, nested PCR was performed for 4 protozoan parasite detection (Cryptosporidium parvum/hominis, Giardia lamblia, Entamoeba histolytica, C. cayetanensis). As a result, C. cayetanensis was identified in 3 out of 8 patients through nested PCR and sequencing analysis.
In Korea, the first case of cyclosporiasis causing traveler’s diarrhea was reported in 2003 in a patient who had traveled to Indonesia [19]. Similarly, in the present case, 8 of the 18 Korean travelers who visited Nepal together on April 1–9, 2013, had enteric symptoms, including diarrhea, upon their return to Korea, and a total of 8 stool samples from 8 patients were collected around April 20. Both Indonesia and Nepal are known as endemic regions of C. cayetanensis, so individuals may be suspected of being unknowingly exposed to C. cayetanensis while traveling. Common symptoms of C. cayetanensis infection are characterized by anorexia, watery diarrhea, fatigue, and low-grade fever; however, it can also be asymptomatic [4]. These and previous cases were similar in that they had diarrhea for 3 to 5 days after returning home as major clinical symptoms. They have all clinical symptoms like diarrhea (75%, 6/8), emesis (12.5%, 1/8), fever (12.5%, 1/8), and stomachache(25%, 2/8) (Table 1). However most of them did not visit a local hospital except 1 patient who visit a clinic at one time.
Table 1.
Clinical symptoms of 8 Korean travelers upon their return from Nepal
| Sample No. | Sex | Age (yr) | Clinical symptoms | |||||
|---|---|---|---|---|---|---|---|---|
| Symptoms exhibited | Diarrhea | Emesis | Fever | Stomachache | Self-medicating | |||
| 1* | Female | 52 | Y | Y | - | Y | N | |
| 2 | Male | 57 | Y | Y | - | - | - | Y |
| 3 | Male | 61 | Y | Y | - | - | - | N |
| 4 | Female | 50 | Y | - | Y | - | Y | N |
| 5 | Male | 46 | Y | Y | - | Y | N | |
| 6 | Female | 55 | Y | - | - | - | - | unknown |
| 7 | Female | 56 | Y | Y | - | - | - | Y |
| 8 | Female | 46 | Y | Y | - | - | - | N |
1 patient only visited a local clinic; Y, yes; N, no.
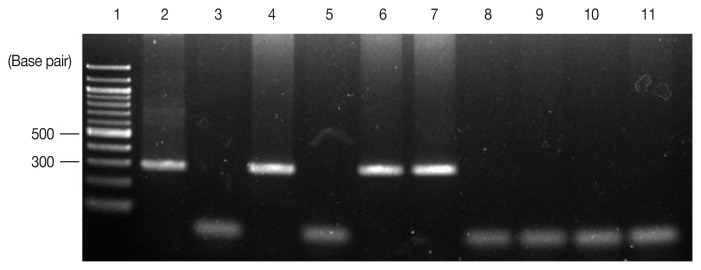
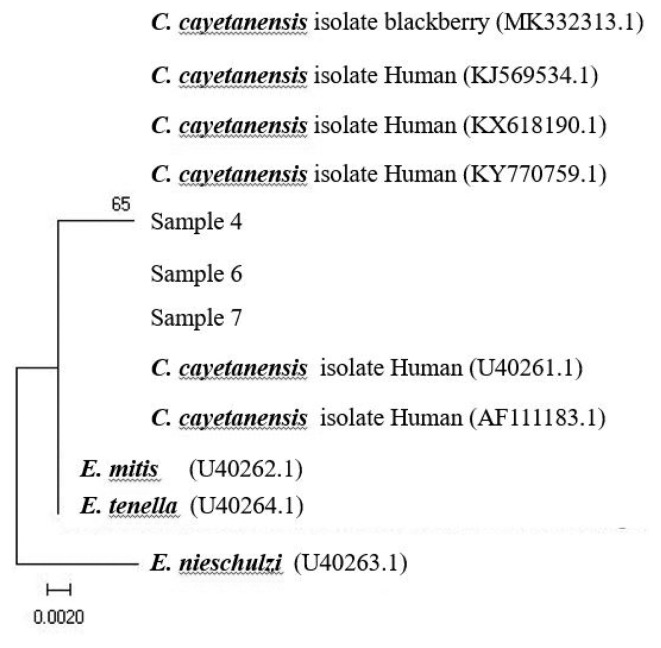
At the present investigation, any virus and bacterial microbes were not detected in all fecal samples through laboratory testing, whereas only the specific gene for C. cayetanensis was detected in 3 out of the 8 samples by nested PCR, showing a 294-bp fragment amplification of the 18S rRNA gene of the pathogen, F3E (5′-CCTTCCGCGCTTCGCTGCGT-3′) and R4B (5′-CGTCTTCAAACCCCCTACTG-3′), and the annealing temperature was 60°C, following PCR procedure to the previsous report [19] (Fig. 1). Using the sequencing data, phylogenetic trees were constructed using the CLUSTAL W multiple sequence alignment computer program (Histon, Cambridgeshire, UK) and the Molecular Evolutionary Genetics Analysis (MEGA) program; the robustness of groupings was assessed using 1,000 bootstrap replicates of the data. In comparative sequencing analysis of 3 PCR positive cases, all sequences showed 98% genetic identity with C. cayetanensis 18S rRNA and showed high similarity to sequences derived from 2 case individuals in Nepal (Accession No. U40261, AF111183) (Fig. 2) [21]. However, unfortunately, it could not be detected any oocysts of C. cayetanensis in all MAFS-stained samples by direct microscopy examination.
Fig. 1.
Detection of Cyclospora cayetanensis by nested PCR of stool samples obtained from 8 patients. To identify C. cayetanensis in feces, nested PCR was performed to amplify a fragment of the 18S rRNA gene of C. cayetanensis, and 294-base pair amplicons were observed by electrophoresis. Lane 1, 100-bp DNA ladder; Lane 2, positive control (DNA extracted from C. cayetanensis used as a template); Lane 3, negative control; Lanes 4–11, patient stool samples.
Fig. 2.
Phylogenetic analysis of the nucleotide sequences of the bands produced from the patients’ stool samples (samples 4,6,7). GenBank accession number U40261, AF111183 was from Nepal; C., Cyclospora; E., Eimeria.
In the first outbreak of cyclosporiasis reported in Korea, the early stage stool samples isolated from patients showing diarrheal symptoms were collected in hospital returning to Korea; thus, oocysts of C. cayetanensis could be possible to more readily detectable [14]. In the present case, the period from occurring clinical symptoms to collecting time of stool samples took about 30 days. Oocysts can intermittently or continuously continue to be shed by non-symptomatic people, and symptoms can also persist in the absence of oocysts in faeces [22]. Thus the reason for the undetectable oocysts in the stool samples may be that they got over illness and clinical symptoms of most of the affected them had disappeared at the time of sample collection.
The prevalence of traveler’s diarrhea of unknown cause among individuals visiting Asia is estimated at 10–56% [1]. Especially, traveler’s diarrhea was the most common clinical symptoms during travel or on arrival and ongoing symptoms were showed by 25% of all travelers on return home [23]. Recently, tourism to Southeast Asian countries such as Nepal and Indonesia as well as Latin America has increased in Korea; thus, the incidence of traveler’s diarrhea caused by enteric pathogens including C. cayetanensis was possible to be increasing unofficially. Therefore, travelers experiencing continuous diarrhea after returning to home from South and Southeast Asia should consider the possibility that they could have been exposed to enteropathogenic parasites while traveling. In this case, C. cayetanensis infection is believed to be through local food intake. As a result of an epidemiological survey over the phone, it was found that the tour group visited the local church and consumed vegetables, water, and fruits. To prevent such infections, all travelers should maintain high standards of personal hygiene and have attention to eating some kinds of local foods while traveling. In addition, travelers with diarrhea of unknown cause after returning home should be appropriately examined at a healthcare facility and be continuously monitored.
The current case represents the second report of imported cyclosporiasis causing traveler’s diarrhea in Korea and the first report of a group infection with C. cayetanensis after visiting Nepal known as endemic country with cyclosporiasis.
ACKNOWLEDGMENT
This research was supported by grants from the Korea Centers for Disease Control and Prevention (4847-302, 2013).
Footnotes
CONFLICT OF INTEREST
We have no conflict of interest related to this work.
REFERENCES
- 1.Al-Abri SS, Beeching NJ, Nye FJ. Traveller’s diarrhoea. Lancet Infect Dis. 2005;5:349–360. doi: 10.1016/S1473-3099(05)70139-0. [DOI] [PubMed] [Google Scholar]
- 2.Chacín-Bonilla L. Epidemiology of Cyclospora cayetanensis: A review focusing in endemic areas. Acta Trop. 2010;115:181–193. doi: 10.1016/j.actatropica.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Ross AG, Olds GR, Cripps AW, Farrar JJ, McManus DP. Enteropathogens and chronic illness in returning travelers. N Engl J Med. 2013;368:1817–1825. doi: 10.1056/NEJMra1207777. [DOI] [PubMed] [Google Scholar]
- 4.Almeria S, Cinar HN, Dubey JP. Cyclospora cayetanensis and Cyclosporiasis: An update. Microorganisms. 2019;7:317. doi: 10.3390/microorganisms7090317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall RL, Jones JL, Hurd S, Smith G, Mahon BE, Herwaldt BL. Population-based active surveillance for Cyclospora infection-United States, foodborne diseases active surveillance network (FoodNet), 1997–2009. Clin Infect Dis. 2012;54(suppl):411–417. doi: 10.1093/cid/cis049. [DOI] [PubMed] [Google Scholar]
- 6.Greenwood Z, Black J, Weld L, O’Brien D, Leder K, Von Sonnenburg F, Pandey P, Schwartz E, Connor BA, Brown G, Freedman DO, Torresi J. Geosentinel surveillance network. Gastrointestinal infection among international travelers globally. J Travel Med. 2008;15:221–228. doi: 10.1111/j.1708-8305.2008.00203.x. [DOI] [PubMed] [Google Scholar]
- 7.Giangaspero A, Gasser RB. Human cyclosporiasis. Lancet Infect Dis. 2019;19:e226–e236. doi: 10.1016/S1473-3099(18)30789-8. [DOI] [PubMed] [Google Scholar]
- 8.Nichols GL, Freedman J, Pollock KG, Rumble C, Chalmers RM, Chiodini P, Hawkins G, Alexander CL, Godbole G, Williams C, Kirkbride HA, Hamel M, Hawker JI. Cyclospora infection linked to travel to Mexico, June to September 2015. Euro Surveill. 2015:20. doi: 10.2807/1560-7917.ES.2015.20.43.30048. [DOI] [PubMed] [Google Scholar]
- 9.Kozak GK, MacDonald D, Landry L, Farber JM. Foodborne outbreaks in Canada linked to produce: 2001 through 2009. J Food Prot. 2013;76:173–183. doi: 10.4315/0362-028X.JFP-12-126. [DOI] [PubMed] [Google Scholar]
- 10.Chiodini PL. A ‘new’ parasite human infection with Cyclospora cayetanensis . Trans R Soc Trop Med Hyg. 1994;88:369–371. doi: 10.1016/0035-9203(94)90385-9. [DOI] [PubMed] [Google Scholar]
- 11.Paudyal R, Tandukar S, Sherchand O, Amatya J, Sherchand JB. Infection of Cyclospora cayetanensis in children under 15 years of age in Kathmandu valley. Scientific World. 2011;9:86–89. [Google Scholar]
- 12.Owen IL. Parasitic zoonoses in Papua New Guinea. J Helminthol. 2005;1:1–14. doi: 10.1079/joh2004266. [DOI] [PubMed] [Google Scholar]
- 13.Tram NT, Hoang L, Cam PD, Chung PT, Fyfe MW, Renton JL, Ong CS. Cyclospora spp. in herbs and water samples collected from markets and farms in Hanoi, Vietnam. Trop Med Int Health. 2008;11:1415–1420. doi: 10.1111/j.1365-3156.2008.02158.x. [DOI] [PubMed] [Google Scholar]
- 14.Blans MC, Ridwan BU, Verweij JJ, Rozenberg-Arska M, Verhoef J. Cyclosporiasis outbreak, Indonesia. Emerg Infect Dis. 2005;9:1453–1455. doi: 10.3201/eid1109.040947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhandari d, Tandukar S, Parajuli H, Thapa P, Chaudhary P, Shresha D, Shah PK, Sherchan JB, Sherchand JB. Cyclospora infection among school children in Kathmandu, Nepal: prevalence and associated risk factors. Trop Med Health. 2015;43:211–216. doi: 10.2149/tmh.2015-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thapa MD, Rai SK, Lekhak B, Rai KR. Study of parasitic infection among children of Sukumbasi Basti in Kathumandu valley. Nepal Med Coll J. 2011;13:7–10. [PubMed] [Google Scholar]
- 17.Kimura K, Rai SK, Rai G, Insisiengmay S, Kawabata M, Karanis P, Uga S. Study on Cyclospora cayetanensis associated with diarrheal disease in Nepal and Loa PDR. Southeast Asian J Trop Med Public Health. 2005;36:1371–1376. [PubMed] [Google Scholar]
- 18.Sherchand JB, Cross JH, Jimba M, Sherchand S, Shrestha MP. Study of Cyclospora cayetanensis in health care facilities, sewage water and green leafy vegetables in Nepal. Southeast Asian J Trop Med Public Health. 1999;30:58–63. [PubMed] [Google Scholar]
- 19.Yu JR, Sohn WM. A case of human cyclosporiasis causing traveler’s diarrhea after visiting Indonesia. J Korean Med Sci. 2003;18:738–741. doi: 10.3346/jkms.2003.18.5.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korea Centers for Disease Control and Prevention. 2018 Guideline for Control of Waterborne and Foodborne Diseases. Cheongju, Korea: KCDC; 2018. [Google Scholar]
- 21.Zhou Y, Lv B, Wang Q, Jian F, Zhang L, Ning C, Fu K, Wang Y, Qi M, Yao H, Zhao J, Zhang X, Sun Y, Shi K, Arrowood MJ, Xiao L. Prevalence and molecular characterization of Cyclospora cayetanensis, Henan, China. Emerg Infect Dis. 2011;10:1887–1890. doi: 10.3201/eid1710.101296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoge CW, Echeverria P, Shlim DR, Rajah R, Shear M, Rabold G, Triplett J. Epidemiology of diarrhoeal illness associated with coccidian-like organism among travelers and foreign residents in Nepal. Lancet. 1993;341:1175–1179. doi: 10.1016/0140-6736(93)91002-4. [DOI] [PubMed] [Google Scholar]
- 23.Vilkman K, Pakkanen SH, Lääveri T, Siikamäki H, Kantele A. Traveler’s health problems and behavior: prospective study with post-travel follow-up. BMC Infect Dis. 2016;16:328. doi: 10.1186/s12879-016-1682-0. [DOI] [PMC free article] [PubMed] [Google Scholar]