Abstract
Pituitary metastases are rare, a primary tumor of the breasts or lungs are frequently found. The clinical picture is very variable, they can be accidental discovery or revealed by a pituitary dysfunction. The prognosis is generally poor and depends on the anatomopathological type.
We report the observation of a patient with a pituitary metastasis of a breast cancer evolving for 4 years, the diagnosis was suspected in front of pituitary macroadenoma images, a transphenoidal endoscopic biopsy and an anatomopathological confirmation revealing an infiltrating breast adenocarcinoma.
Keywords: Pituitary metastasis, Breast cancer, Hypothalamic-pituitary axis
Highlights
-
•
Pituitary metastases are rare, a primary tumor of the breasts or lungs are frequently found.
-
•
The prognosis of the pituitary metastases is generally poor and depends on the anatomopathological type.
-
•
The clinical picture of the pituitary metastases is very variable, they can be accidental discovery or revealed by a pituitary dysfunction.
1. Introduction
Metastases of the pituitary gland are extremely rare and represent only 1% of pituitary tumor lesions [1], in the majority of cases the primary is of mammary or pulmonary origin [2].
At the time of diagnosis, most patients are elderly and present with advanced cancer disease with multiple metastatic sites [3]. Pituitary metastases can also be the first manifestation of occult primary cancer or the only metastatic site [4].
The clinical picture is dominated by intracranial hypertension syndrome, and visual abnormalities according to a meta-analysis grouping together patients with pituitary metastases from a neo-breast [5]. Thus the rarity and absence of specific radiological and clinical signs make it difficult to distinguish between these tumors and other benign pituitary lesions. The median survival in the case of a primary breast is less than a year [6].
In this report, we describe the case of a patient who had pituitary metastasis from a primitive breast treated successfully for 48 months.
2. Case presentation
A 66-year-old patient, admitted for exploration of a sellar and supra-sellar mass evoking a pituitary Macroadenoma initially discovered in front of a ptosis of the right eyelid and headaches especially nocturnal evolving for 1 month. There was a history of right breast cancer (infiltrating ductal carcinoma SBRII) [[1], [2], [3]], classified Tc4 N1 M0, having expressed estrogen receptors (RE + 80% RP- HER2- Ki67 25%).
The patient underwent a right mastectomy, localized chemotherapy and radiotherapy followed by hormone therapy with Letrozole. The evolution was marked by a deep skin tumor recurrence in the same breast 2 years later, treated with surgery and hormone therapy with Tamoxifene.
The ophthalmological evaluation of the patient objectified a ptosis of the right eye secondary to a partial occulomotor paralysis of the right III, without loss of visual acuity and without abnormality in the fundus or the visual field.
Biological exploration had objectified a hypopituitarism made of a somatotropic, thyrotropic, gonadotropic deficit without corticotropic deficit or diabetes insipidus (Table 1).
Table 1.
Biological exploration.
| Patient | normal value | |
|---|---|---|
| ACTH (pg/mL) | 24,9 | 4,7_48,8 |
| Cortisol de 08h (μg/L) | 375 | 62–180 |
| Urinary free cortisol de 24h: ug/24h | 13 | (10–50) |
| GH (ng/mL) | 1,4 | <6,70 |
| IGF-1 (ng/mL) | 45 | 84–222 |
| FSH (IU/L) | 2,50 | 25-134,8 |
| LH (IU/L) | 1,3 | 7,7–58,5 |
| Estradiol pmol/l | <18,4 | <18–505 (post menopausal period) |
| TSH (mIU/L) | 1,31 | 0,27-4,2 |
| Free T4 (pmol/L) | 9,7 | 12-21,93 |
| Prolactin (ng/mL) | 26 ng/ml | 4,8–23,3 |
| Gonadotropin alpha subunit: HCG, FSH LH/TSH | 0,19 ui/l | <1,3 ui/l (post menopausal period) |
| Antigen CA 15-3 | 28,2 U/mL | <26,4 |
| Vitamin D2+D3 ng/ml | 19 | 30–70 |
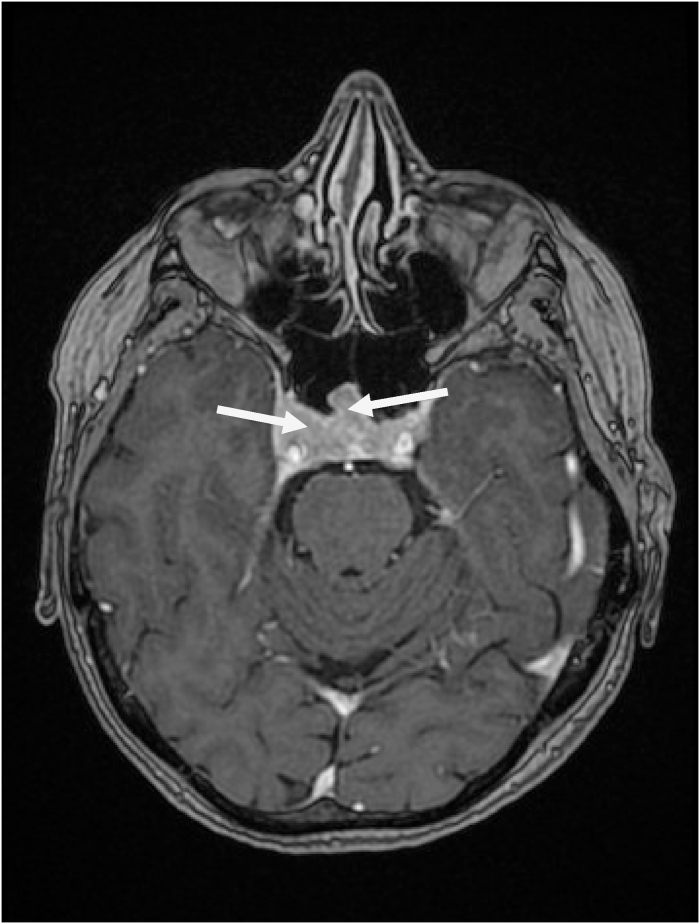
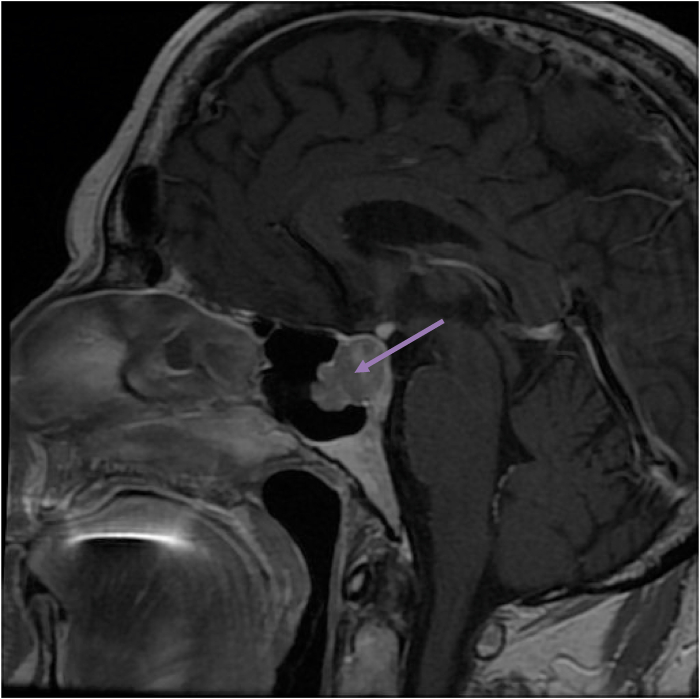
Radiological exploration by a hypothalamic-pituitary MRI has objectified: an intra and supramelar lesion of 21mm H * 15mm L lateralized to the right, initially suggesting a macroadenoma which bulges along the left flank of the stem with extensive infiltration of the right cavernous sinus up to the hard external mother, with erosion of the antero-inferior sellar floor and massively infiltrating the right cavernous sinus, without other secondary intra-cerebral localizations (Fig. 1, Fig. 2).
Fig. 1.
T1-weighted coronal slice injected with hypothalamic pituitary MRI objectifying a pituitary macroadenoma.
Fig. 2.
Sagittal section with hypothalamic-pituitary MRI in T1 injected objectifying a pituitary macroadenoma.
The patient underwent a biopsy of the pituitary mass, the anatomopathological study and the immunohistochemistry objectified a metastasis of the mammary carcinoma, RE 30%, RP 10%, HER 2 negative, the assessment of extension objectified two other hepatic localizations whose biopsy was in favor of an undifferentiated triple negative carcinoma.
Chemotherapy based on (Paclitaxel + Bevacizumab) and pituitary radiotherapy. Were indicated. The evolution after 7 months of the diagnosis of pituitary metastasis the patient presents clinically an asthenia secondary to chemotherapy, with on the performance scale a WHO 1. The hormonal deficits were replaced by l-tyroxine 75 μg, in this active tumor context substitution with GH is not recommended.
3. Discussion
Pituitary metastases are more frequent in the sixth and seventh decades of life [3], the number of case reports increased considerably after the 21st century, following the development of diagnostic and neuroendoscopic technology [7]. Breast and lung tumors are the most common primary sites [2], followed by papillary thyroid cancer [8] and renal adenocarcinoma [9], but others are also described, such as the tract gastrointestinal, prostate, pancreas, lymphomas, leukemias and plasmacytomas.
Regarding the localization of pituitary metastases Mc Cormick et al. Examining the localization of pituitary metastases in 201 cases, found an involvement of the posthypophysis and anterior pituitary in 84.6% of the cases and an involvement of the antehypophysis isolated in 15.4% [10] this higher post-pituitary frequency is explained by the fact that it receives a direct systemic arterial vascularization, while the ante-hypopyse receives its vascularization from a capillary bed coming from the Rathke pouch which would constitute a natural filter for hematogenous metastatic dissemination [11]. This confirms that the hematogenous pathway is the most important mechanism of dissemination, but can also occur by the contiguity of adjacent bone metastases or by meningeal spread through the suprasellar cistern [2].
Clinically, pituitary metastases are only symptomatic in 2.5–18.2% of cases, as they occur in patients with deterioration of the general condition by cancer and its complications thus masking the dysfunction of the anterior pituitary gland explaining that the majority of cases were discovered accidently during autopsies [1].
However, according to a meta-analysis including 20 patients made recently by Mr C. Heng et al. it is objective that the main clinical symptoms observed in the event of pituitary metastases of breast cancer are: an intracranial hypertension syndrome in 45% cases, a decrease in visual acuity and visual field anomalies in 40% of cases, followed by an anterior pituitary endocrine involvement in 35% of cases, diabetes insipidus was present in 30% of patients and lastly paralysis cranial nerves in 5% of cases [5]. Decreased visual acuity and visual field defects are caused by compression of the optic nerve tumor or optic chiasm and ophthalmoplegia by infiltration of the cavernous sinuses. Due to the rapid growth of these metastases, headaches are more common than in patients with benign pituitary adenoma. Hyperprolactinemia is not uncommon so 35% of cases can lead to a false diagnosis of functional pituitary adenoma [5]. in the case of our patient the initial complaint was headache and ophthalmoplegia.
The radiological diagnosis is mainly based on MRI which can highlight an iso-intense or hypo-intense mass on T1-weighted images with a high intensity signal on T2-weighted images, a homogeneous enhancement with gadolinium and loss of the high signal pituitary signal intensity on T1-weighted images. Rapid growth of a sellar tumor with aggressive infiltration of adjacent tissue or a dumbbell-shaped intrasellar and suprasellar tumor [1]. Confirmation of the diagnosis is based on histology and immunohistochemistry.
Since the majority of patients with pituitary metastases also have other extensive metastases, Treatment is essentially palliative ranging from surgery to radiation therapy, including systemic chemotherapy, focusing on the extent and state of the primary tumor.
The role of surgery appears more in symptomatic relief and improvement in quality of life [12], but does not affect survival rates [13]. It often takes place trans-sphenoidally. It can improve visual field anomalies, headaches and ophthalmoplegia in the majority of cases [14], Total resection is not always possible due to the general characteristics of the tumor (tendency to be firm, diffuse, invasive, vascular and hemorrhagic) as well as tumor extension and its relationships [1]. Stereotaxic radiotherapy has been gradually implemented in metastatic pituitary diseases, well tolerated and associated with minimal morbidity and less complications [1].
A small cohort of 18 patients treated with stereotaxic radiosurgery demonstrated that neurological symptoms and diabetes insipidus improved in half of the patients and that tumor progression only occurred in 17% of those treated [15]; other small trials have produced equally promising results [16]. The benefits of chemotherapy for pituitary metastases are not yet fully understood. Certain chemotherapeutic and immunotherapeutic schemes cross the blood-brain barrier and may constitute a survival advantage [17].
Recent studies have shown that anti-HER-2 treatment can facilitate the formation of pituitary metastases [17]. A cohort of 52 patients with primary pituitary metastases from (lung, breast, colon, prostate, liver, Otolaryngological sphere and stomach) all had an average survival of less than 1 year [6]. Another Japanese cohort of 165 cases with pituitary metastases objectified that a younger age, a small pituitary lesion and the use of radiotherapy were all significantly correlated with improved survival [12].
4. Conclusion
Although the pituitary gland is a rare site of metastasis, pituitary metastases should be discussed in the face of any clinical, biochemical hormonal abnormality, in a patient already diagnosed with a neoplastic disease. In addition, the development of diabetes insipidus or ophthalmoplegia from a pituitary lesion suggests metastatic disease, even in patients with no known primary.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Funding
We have no funding for this work.
Author contribution
Dr. EJ, Dr. IJ, and Dr. CB analysed and performed the literature research, Pr. HL performed the examination and performed the scientific validation of the manuscript. Jihane elhabnouny was the major contributors to the writing of the manuscript. All authors read and approved the manuscript.
Registration of research studies
1. Name of the registry
2. Unique Identifying number or registration ID
3. Hyperlink to your specific registration (must be publicly accessible and will be checked)
Guarantor
Dr. Issam Jandou.
Consent
A copy of the written consent is available for review by the Editor-in-Chief of this journal on request”.
Provenance and peer review
Not commissioned, externally peer reviewed.
Declaration of competing interest
All the authors have no conflict of interest neither financial nor ethical.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.amsu.2020.10.054.
Abréviation
- SBR
le grade de Scarff-Bloom et Richardson
- RP
progestérone
- HER
Human Epidermal Growth Factor Receptor-2
- PS
Performance status
- ug
microgramme
- GH
Growth Hormon
- FSH
Follicle Stimulating Hormone
- LH
Lutenizing Hormone
- ACTH
adrenocorticotropic hormone
- TSH
thyroid-stimulating hormone
- HCG
human chorionic gonadotropin
- IGF
insulin-like growth factor
- MRI
Magnetic Resonance Imaging
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Komninos J., Vlassopoulou V., Protopapa D., Korfias S., Kontogeorgos G., Sakas D.E. Tumorsmetastatic to the pituitary gland: case report and literature review. J. Clin. Endocrinol. Metab. 2004;89(2):574–580. doi: 10.1210/jc.2003-030395. [DOI] [PubMed] [Google Scholar]
- 2.Spinelli G.P., Lo Russo G., Miele E., Prinzi N., Tomao F., Antonelli M. Breast cancer metastatic to the pituitary gland: a case report. World J. Surg. Oncol. 2012;10:137. doi: 10.1186/1477-7819-10-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Max M.B., Deck M.D., Rottenberg D.A. Pituitarymetastasis: incidence in cancer patients and clinical differentiation from pituitary adenoma. Neurology. 1981;31(8):998–1002. doi: 10.1212/wnl.31.8.998. [DOI] [PubMed] [Google Scholar]
- 4.Ntyonga-Pono M.P., Thomopoulos P., Luton J.P. Pituitarymetastases. Three cases. Presse Med. 1999;28(29):1567–1571. [PubMed] [Google Scholar]
- 5.C.Heng et Al : ClinicalPresentation and Pathologic Characteristics of Pituitary Metastasis from Breast Carcinoma: Cases and a SystematicReview of the Literature. World Neurosurg., 10.1016/j.wneu.2018.12.126. [DOI] [PubMed]
- 6.Heshmati H.M., Scheithauer B.W., Young W.F. Metastases to the pituitary gland. Endocrinololgist. 2002;12:45–49. doi: 10.1097/00019616-200201000-00010. [DOI] [Google Scholar]
- 7.Duchen L.W. Metastaticcarcinoma in the pituitary gland and hypothalamus. J. Pathol. Bacteriol. 1966;91:347–355. doi: 10.1002/path.1700910208. [DOI] [PubMed] [Google Scholar]
- 8.Stojanović M., Pekić S., Doknić M., Miljić D., Cirić S., Diklić A., Tatić S., Joksimović M., Manojlović-Gačić E., Skender-Gazibara M. What's in the image? Pituitary metastasis from papillarycarcinoma of the thyroid: a case report and a comprehensivere view of the literature. Eur. Thyroid J. 2013;1:277–284. doi: 10.1159/000343910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopan T., Toms S., Prayson R.A., Suh J.H., Hamrahian A.H., Weil R.J. Symptomatic pituitary metastases from renalcellcarcinoma. Pituitary. 2007;10:251–259. doi: 10.1007/s11102-007-0047-5. [DOI] [PubMed] [Google Scholar]
- 10.McCormick P.C., Post K.D., Kandji A.D., Hays A.P. Metastaticcarcinoma to the pituitary gland. Br. J. Neurosurg. 1989;3(1):71–79. doi: 10.3109/02688698909001028. [DOI] [PubMed] [Google Scholar]
- 11.Hardy J., Mohr G. In: Tumeurs de l’hypophyse. Cohadon F., editor. Médecine Sciences Flammarion; Paris: 1989. [Google Scholar]
- 12.Habu M., Tokimura H., Hirano H. Pituitarymetastases: current practice in Japan. J. Neurosurg. 2015;123:998–1007. doi: 10.3171/2014.12.JNS14870. [DOI] [PubMed] [Google Scholar]
- 13.Pinet C., Raholimina V., Ferri R.M., Kleisbauer J.P. Panhypopituitarism secondary to pituitarymetastases. Presse Med. 2000;29:17–18. [PubMed] [Google Scholar]
- 14.Zoli M., Mazzatenta D., Faustini-Fustini M. Pituitarymetastases: role of surgery. World Neurosurg. 2013;79:327–330. doi: 10.1016/j.wneu.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Kano H., Niranjan A., Kondziolka D. Stereotactic radiosurgery for pituitarymetastases. Surg. Neurol. 2009;72:248–255. doi: 10.1016/j.surneu.2008.06.003. (discussion 255–256) [DOI] [PubMed] [Google Scholar]
- 16.Chon H. Hypofractionated stereotactic radiosurgery for pituitarymetastases. J. Neuro Oncol. 2017 doi: 10.1007/s11060-016-2346-z. [DOI] [PubMed] [Google Scholar]
- 17.Kam J., Kam J., Mann G.B. Solitary pituitary metastasis from HER2-positive breast cancer. Asia Pac. J. Clin. Oncol. 2017;13:e181–e184. doi: 10.1111/ajco.12353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.